Abstract
Rationale
Aging is the major risk factor for Alzheimer’s disease (AD), and cognitive and memory impairments are common among the elderly. Interestingly, coenzyme Q10 (Q10) levels decline in the brain of aging animals. Q10 is a substantial antioxidant substance, which has an important role in the mitochondria.
Objective
We assessed the possible effects of Q10 on learning and memory and synaptic plasticity in aged β-amyloid (Aβ)-induced AD rats.
Methods
In this study, 40 Wistar rats (24–36 months old; 360–450 g) were randomly assigned to four groups (n = 10 rats/group)—group I: control, group II: Aβ, group III: Q10; 50 mg/kg, and group IV: Q10+Aβ. Q10 was administered orally by gavage daily for 4 weeks before the Aβ injection. The cognitive function and learning and memory of the rats were measured by the novel object recognition (NOR), Morris water maze (MWM), and passive avoidance learning (PAL) tests. Finally, malondialdehyde (MDA), total antioxidant capacity (TAC), total thiol group (TTG), and total oxidant status (TOS) were measured.
Results
Q10 improved the Aβ-related decrease in the discrimination index in the NOR test, spatial learning and memory in the MWM test, passive avoidance learning and memory in the PAL test, and long-term potentiation (LTP) impairment in the hippocampal PP-DG pathway in aged rats. In addition, Aβ injection significantly increased serum MDA and TOS levels. Q10, however, significantly reversed these parameters and also increased TAC and TTG levels in the Aβ+Q10 group.
Conclusions
Our experimental findings suggest that Q10 supplementation can suppress the progression of neurodegeneration that otherwise impairs learning and memory and reduces synaptic plasticity in our experimental animals. Therefore, similar supplemental Q10 treatment given to humans with AD could possibly provide them a better quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease associated with deficits in memory and cognitive functions (Wojsiat et al. 2018). It is the fifth leading cause of death in cases aged 65 years or older (Prasad and Bondy 2014). Aging is the main risk factor for AD, and its incidence has been increasing due to an increase in aging populations worldwide (Winblad et al. 2016). People aged 65 years or older are more vulnerable to developing AD (Prasad and Bondy 2014). In AD, there is a gradual decrease in intellectual function linked to degeneration and death of cerebral cortical neurons, which consequently leads to dementia (Terry and Davies 1980; Prasad and Bondy 2014).
Over the past few decades, different biochemical and genetic events, including elevated oxidative stress (Sultana et al. 2006; Xie et al. 2013), mitochondrial dysfunction (Shoffner et al. 1993; Gibson et al. 2000), chronic inflammation (Yamamoto et al. 2007; Ramirez et al. 2008; Calsolaro and Edison 2016; Marshe et al. 2017), Aß (1–42) peptide production from proteolysis of amyloid precursor protein (APP) (Yankner and Mesulam 1991), inherited mutations in APP, presenilin-1, and presenilin-2 genes (Mohmmad Abdul et al. 2006; Placanica et al. 2009), glia-lymphatic (glymphatic) system impairment (Bosche et al. 2020; Reeves et al. 2020), and hyperphosphorylated tau protein (Prasad and Bondy 2014; Wojsiat et al. 2018; Komaki et al. 2019), have been identified in AD leading to progressive destruction and death of nerve cells. The damaging effects of these factors causing AD become more potent with age. Morphological alterations have been found to be associated with aging in different central nervous system (CNS) areas. Aging plays a crucial role in pathophysiological mechanisms of degenerative disorders, such as AD, Parkinson’s disease (PD), and amyotrophic lateral sclerosis (Matteo and Esposito 2003).
The accurate pathophysiology of AD is still unclear; however, neuroinflammation, oxidative stress, and mitochondrial function may be involved (Prasad and Bondy 2014; Wojsiat et al. 2018; Komaki et al. 2019). An increase in oxidative stress may play an important role in aging (Komaki et al. 2019). Oxidative stress due to free radical production and lipid peroxidation is associated with the pathogenesis of cognitive decline (Berr et al. 2000). The deficiency of antioxidant defenses can affect many brain functions, such as memory (Fukui et al. 2001). Neurons in the brain are highly affected by increased reactive oxygen species (ROS) formation and oxidative damage because of the high oxygen usage and energy generation (Mergenthaler et al. 2013). Enhanced oxidative damage due to free radicals can initiate long-term inflammation in AD (Uttara et al. 2009). Also, increased oxidative stress along with pro-inflammatory cytokines possibly promotes neuronal death in AD (Emerit et al. 2004; Prasad and Bondy 2014).
Due to disruption in the oxidant/antioxidant balance in AD, free radicals scavengers and/or increased oxidative stress defense mechanisms are effective in the treatment of AD (Wojsiat et al. 2018). Using antioxidants for the treatment of neurodegenerative disorders has been widely considered (Emerit et al. 2004; Federico et al. 2012). Studies on animal and AD models using single endogenous antioxidants and herbal remedies have indicated the protection of neurons against oxidative stress-related damage (Prasad and Bondy 2014). Thus, restoring mitochondrial function, reducing oxidative stress levels, and decreasing free radicals using antioxidants are the appropriate strategies to treat AD.
Coenzyme Q10 (Q10) is a natural antioxidant, which is part of the electron transport chain. Several studies have assessed the effect of Q10 on animal models of neurodegenerative disorders (Muthukumaran et al. 2014; Sikorska et al. 2014). As an intracellular antioxidant, it protects the phospholipids of the membrane and membrane protein of the mitochondria that scavenge free radicals (Navas et al. 2007; Lee et al. 2013). Mitochondrial dysfunction causes AD. In addition, Q10 is essential to generate ATP by mitochondria. Therefore, Q10 can be added to different micronutrient preparations to treat AD (Prasad and Bondy 2014; Wojsiat et al. 2018). Q10 has neuroprotection against neurodegenerative disorders (Flint 2002). Treatment with Q10 could reduce markers of oxidative stress in animal AD models (Prasad and Bondy 2014; Zhang et al. 2018). Also, Q10 supplementation affects the cholinergic system and protects cholinergic neurons in AD patients (Majumdar et al. 2014; Yang et al. 2016). On the other hand, Q10 (150 mg/day) reduced interleukin (IL-6) as an inflammatory marker (Lee et al. 2012). Q10 levels decline in the brain of aging animals (Matthews et al. 1998; Ebadi et al. 2001).
LTP occurs most prominently in the hippocampus and is regarded as a proper model to study the mechanisms of long-term changes in the CNS synaptic efficiency, resulting in learning and memory formation (Maren and Baudry 1995; Martin et al. 2000). Aβ peptides in the hippocampus can strongly inhibit synaptic plasticity.
We evaluated the Q10 effects on impaired learning and memory and synaptic plasticity in aged rats that received an intracerebroventricular (ICV) injection of Aβ. In summary, we studied whether Q10 treatment can affect the induction of hippocampal LTP in Aβ-injected old rats. Therefore, besides learning and memory behavioral methods, an in vivo field potential recording method was used to assess the neuroprotective impacts of the oral administration of Q10 on impaired hippocampal synaptic plasticity in aged rats receiving Aβ.
Materials and methods
Ethics statement
The experimental procedures were done according to the Veterinary Ethics Committee of the Hamadan University of Medical Sciences (Ethic code: IR.UMSHA.REC.1394.582), Hamadan, Iran, and conducted following the Guide for Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23, revised 1985).
Animals and experimental design
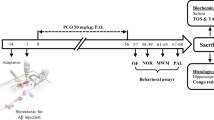
Adult old aged (24–36 months) male Wistar rats (360–450 g) were prepared from the Hamadan University of Medical Sciences. They were kept in clear cages in a room at 23 ± 1 °C with a relative humidity of 50 ± 5%, under a 12/12 h light/dark cycle. They had free access to food and water. Following 7 days of acclimation, animals were randomly divided into four groups (n = 10 animals per group): group I (control group: receiving normal saline by oral gavage daily), group II (Aβ: receiving a unilateral ICV injection of Aβ (1–42), group III (Q10: receiving 50 mg/kg of Q10 by oral gavage daily), and group IV (Aβ+Q10: Q10 administration by oral gavage daily for 4 weeks before ICV injection of Aβ (Komaki et al. 2019). The experiment timeline is shown in Fig. 1.
Experimental timeline. After 4 weeks of treatment of experimental groups with Q10 (50 mg/kg), to induce an AD model, xylazine (10 mg/kg) and ketamine (100 mg/kg) were used to anesthetize rats, and they were placed in the stereotaxic apparatus. An intraventricular injection of Aβ (2 μL) was done (1 μL/2 min). Following recovery, open field and novel object recognition (NOR) tasks were performed. To measure spatial and aversive learning and memory following the training trials, Morris water maze (MWM) and shuttle box tests were performed, respectively. Then, electrophysiological recordings were conducted to determine the excitatory postsynaptic potential (EPSP) slope and population spike (PS) amplitude in the dentate gyrus of the hippocampus. Induction of LTP was done through a high-frequency stimulation of the perforant pathway. After the experimental procedures, the levels of the biomarkers of oxidative stress were calculated by serum assessment
ICV injection of Aβ and neurosurgery
To induce an AD model, Aβ (1–42) (100 μg; Tocris Bioscience, UK) was dissolved in PBS (100 μL; vehicle solution), followed by incubation (37 °C/7 days) before usage. This process formed amyloid fibrils, which are toxic to the nervous system (Lorenzo and Yankner 1994; Asadbegi et al. 2017; Asadbegi et al. 2018). Anesthetization of the rats was done with intraperitoneal (IP) injections of ketamine and xylazine (100 and 10 mg/kg, respectively) (Ahmadi et al. 2021a), before transferring to the stereotaxic device (Stoelting Co., USA). After exposing the skull, we drilled holes in the skull on the ventricular region considering the coordinates as follows: 2 mm lateral to the midline, 1.2 mm posterior to bregma, and 4 mm ventral to the cortex surface (Paxinos and Watson 2005). A 10-μL microsyringe (Hamilton Laboratory Products, USA) was used for injections that lasted 5 min. We kept the syringe in place for 5 min following the injection before its removal. The injection volume (5 μL) was administrated slowly (1 μL/min), and the animals had 5–7 days of recovery (Komaki et al. 2019).
Locomotor activity in the open field
The open field (OF) test was applied to measure locomotor activity. The apparatus was made of white acrylic (50 cm (length) × 50 cm (width) × 38 cm (height)). The field was lit by low ambient room lights (Bisagno et al. 2004). An overhead video camera recorded the time spent by animals in an open field area, and the obtained data were analyzed using video track software. Animals were located in the middle of the OF and could explore for 10 min (Drews et al. 2005). The locomotor activity was considered as the total distance traveled and average velocity (Etaee et al. 2017).
Novel object recognition (NOR) test
NOR was first designed by Ennanceur and Delacour according to the animals’ spontaneous behavior to recognize a new object in a familiar environment (Ennaceur and Delacour 1988). Recognition memory as a kind of declarative memory can measure the animal’s ability to judge or discriminate between objects, considering tactile and visual information (Ennaceur and Delacour 1988; Barker et al. 2007; Antunes and Biala 2012). The NOR test measures cortical and hippocampal functioning. Basically, the task was performed as in our previous studies (Ganji et al. 2017) (Fig. 2). The open box has a black wooden floor (50 × 45 × 35 cm) equipped with video recording software.
On the first day, habituation and acquisition phases were performed. Accordingly, two habituation sessions (10 min) in the arena with no object at an interval of 30 min were considered. The training was performed for 30 min following habituation. In this phase, two identical objects were positioned near two adjacent corners of the arena, and the animal was positioned in the middle of the box and had 10 min to find the objects (Cohen and Stackman Jr 2015). The exploration process was regarded as smelling the object. Then, 24 h later, a retention test was done. In this phase, the objects were replaced by a novel (unfamiliar) object, and the animal was placed in the open field for 10 min. The discrimination index (DI) for the new object was measured by dividing the time spent exploring the new object by the total exploration time recorded by video tracking software.
Assessment of spatial memory using Morris water maze (MWM) task
The MWM test as a hippocampal-dependent test assesses spatial learning and memory in rodents (Zarrinkalam et al. 2018; Omidi et al. 2019). As described previously (Karimi et al. 2019; Shekarian et al. 2020; Ahmadi et al. 2021b), the MWM has a black circular pool (diameter: 180 cm; depth: 60 cm) full of water (22 ± 1 °C) to a depth of 35 cm. It is divided into four quadrants at equal distances along the pool rim and has four starting sites. There is a hidden platform (diameter: 10 cm) placed 1 cm below the water surface in the middle of the northern quadrant, which is consistent for all rats throughout the training tasks. In brief, the training session included a block of eight trials daily for 4 consecutive days. The rats were located in the water at one of the four different locations, and the time between the entrance into the water and escape toward the platform (escape latency) was recorded. Each rat was given two blocks of four trials for 60 s at about the same time every day (10:00–12:00) for 4 continuous days. The subjects could stay for 30 s on the platform between the two trials and had 5 min of resting time between the two continuous blocks. When a rat could not find the platform for 60 s, the researcher guided it to the platform and could remain there for 30 s. A video camera (Nikon, Melville, USA) connected to a computer was placed above the pool for recording the parameters, such as the time spent to reach the submerged platform (escape latency), the length of the swimming path (traveled distance), and the time spent in the target quadrant. On day 5, each animal was subjected to one probe trial (60 s) and a visible platform trial. The platform was not available in the probe trials; however, in the visible platform trials, an aluminum foil was used to cover the platform. Escape latency, distance traveled, and the mean swimming speed were recorded in each trial (Zarrinkalam et al. 2018).
Passive avoidance learning (PAL) test
In the PAL task, the subjects learn to avoid an environment where they previously received an adverse stimulus (foot shock). The PAL apparatus, as well as the procedure, were similar to those we previously described (Zarrinkalam et al. 2016; Karimi et al. 2020). In brief, the apparatus is made of transparent plastic and has a rectangular opening guillotine door (6 × 8 cm) to separate the light compartment (20 ×20 ×30 cm) from the dark one (20 × 20 ×30 cm). The dark compartment floor has stainless steel rods (3 mm diameter) placed 1 cm apart. A shock generator is attached to the electrified floor of the dark box. The test was conducted for 2 continuous days. On the first day, after two steps of the habituation trial, training was performed by applying an intermittent electric shock (1.5 s, 0.4 mA intensity) upon the rat’s entrance into the dark chamber through an isolated stimulator. The rat was gently located in the white chamber, and 5 s later, the guillotine door was opened and the rat could enter the dark compartment. Once entering the dark box, the door was closed, followed by delivering an electric shock through the floor grid (acquisition trial). Latency to enter the dark chamber (STLa) and the number of electrical shocks received to the acquisition were noted. Then, animals were transferred to their home cages. On day 2 (memory test), they received no electrical shock. Each animal was again placed in the light compartment (retention trial), and latency to enter the dark chamber (STLr) and the total time spent in the dark compartment (TDC) (as an indicator of inhibitory avoidance behavior) were recorded (Zarrinkalam et al. 2016).
Surgical procedures, electrophysiological recordings, and induction of LTP
The used methods were similar to previous studies (Nazari et al. 2016; Komaki et al. 2017; Omidi et al. 2020a; Omidi et al. 2020b). In brief, after an IP injection of urethane (1.5 g/kg), animals were anesthetized and positioned in the stereotaxic device for surgical procedure and recording. After exposing the skull, we placed a stainless steel concentric bipolar electrode (diameter: 125 μm, insulated with Teflon) in the lateral perforant path (PP) according to the following coordinates: 4.3 mm lateral to the midline, 8.1 mm posterior to bregma, and 3.2 mm ventral below the skull surface (Paxinos and Watson 2005). Also, a bipolar recording electrode (3.8 mm posterior to bregma and 2.3 mm lateral to the midline) was moved to the dentate gyrus (DG), until detecting the maximum field excitatory postsynaptic potentials (EPSP) (commonly, 2.7–3.2 mm ventral).
Electrophysiological recordings
The electrophysiological recordings, as well as LTP induction, were similar to those previously described (Tahmasebi et al. 2015; Nazari et al. 2016; Komaki et al. 2017; Omidi et al. 2020a; Omidi et al. 2020b). After anesthetization of the animals using an IP injection of urethane (ethyl carbamate; Sigma, USA; 1.5 g/kg), they were located in the stereotaxic device. The skin was incised, and the DG location (ML: 2.3 mm from the midline, AP: −3.8 mm from the bregma, and DV: 2.7–3.2 mm from the skull surface) and perforant pathway (PP) (ML: 4.3 mm from the midline, AP: −8.1 mm from the bregma, and DV: 3.2 mm from the skull surface) were defined (Paxinos and Watson 2005), followed by drilling two small burr holes into the DG and PP. Next, bipolar stimulating and recording electrodes were gently lowered along the cortex into the PP and DG regions, respectively. They moved very gently to observe the maximum field excitatory postsynaptic potentials (fEPSPs). After stimulating the PP, the field potential recordings were recorded in the DG.
By changing the single-pulse stimulation intensity and averaging ten responses for each intensity, we generated the input/output (I/O) response curve. The evoked field potential was 50% of the maximum response in the following stimulations (Omidi et al. 2020b). The stimuli (single 0.1 ms biphasic square wave) characteristics were defined according to our package (eTrace, www.sciencebeam.com) followed by sending to a constant current isolator unit (A365, World Precision Instruments, US) at 0.1 Hz.
After making sure of the base response sustainability (about 45 min), high-frequency stimulation (HFS) was delivered for the induction of LTP. HFS protocol was as follows: 400 Hz, 10 bursts of 20 stimuli, 0.2-ms stimulus duration, and the 10-s interburst interval at a stimulus intensity. The fEPSP slope, as well as two variables of maximal response, were noted at 5, 30, and 60 min following HFS for evaluating changes in the DG neuron’s synaptic response.
Biochemical analysis
At the end of the experiment, 5 ml of blood samples were taken from the portal vein by cardiac puncture and transferred into heparinized tubes. The samples were then centrifuged at 3500 rpm for 10 min at 4 °C. Serum samples were frozen at −80 °C and sent for biochemistry measurement. Finally, plasma measurements were performed for malondialdehyde (MDA), total antioxidant capacity (TAC), total thiol group (TTG), and total oxidant status (TOS) (Komaki et al. 2019).
Measurement of TAC
TAC determination in serum was performed by ferric reducing antioxidant power assay (FRAP) (Benzie and Strain 1999). It can reduce 2,4,6-Tris(2-pyridyl)-s-triazine (Fe III-TPTZ) to Fe II-TPTZ (blue) with biological antioxidants. Alterations in absorbance of the sample at 600 nm were compared with FeSO4 7H2O, which was considered a standard (Salehi et al. 2015).
Measurement of MDA
The MDA generation to assess lipid peroxidation (LPO) was distinguished by reaction with thiobarbituric acid (TBA). Briefly, the determination of the LPO products (MDA) was done through the addition of 1.0 mL of 1% TBA reactive substances and 1.0 mL of 20% trichloroacetic acid to the supernatant (100 μL) and then incubating the solution (100 °C/80 min). The solution was cooled on ice, followed by centrifugation (3000 rpm/20 min), and reading the supernatant absorbance at 532 nm (Salehi et al. 2015).
Determination of the TOS
The Earl’s method determined serum TOS (2005). Briefly, 225 μL of reagent 1 (150 μM xylenol orange, 140 mM NaCl, and 1.35 M glycerol in H2SO4 solution (25 mM; pH1.75)) was mixed with the sample (35 μL). The absorbance was read by a spectrophotometer at 560 nm (sample blank), then, 11 μL of reagent 2 (5 μM ferrous ion and 10 mM o-dianisidine in 25 mM H2SO4 solution) was added to the mixture, and then incubation was done for 3 to 4 min. Afterward, its absorption was read at 560 nm. The analytical sensitivity was considered 0.0076 absorbance/μM. Following calibration of the assay with H2O2, the values were reported as mmol H2O2 equivalent/L. Measurement of the method detection limit was done by evaluating the zero calibrators ten times. The detection limit (the mean TOS of the zero calibrators more than the standard deviation) was 1.13 μmol H2O2 equivalent/L (Akalιn et al. 2007; Aslan et al. 2014).
Measurement of TTG
The Ellman’s reagent (DTNB; 5,5’-dithio-bis-(2-nitrobenzoic acid)) was applied to determine TTG following the Hu method (Hu 1994).
Statistical analysis
Values are provided as mean ± SEM and analyzed using GraphPad Prism® 5.0. One-way analysis of variance (ANOVA) was used to analyze the data obtained from the NOR and PAL tests. Two-way ANOVA was employed to analyze the data obtained from the spatial learning evaluation (training trials) considering days and treatments as repeated measures and between-subjects factors, respectively. One-way and two-way ANOVA followed by Bonferroni and Tukey’s tests were applied to analyze the data obtained from the probe, working, visibility trials, and biochemical data. Electrophysiology results were analyzed by the two-way ANOVA with repeated measures analysis. Tukey’s multiple comparison test was applied to analyze the significance of the differences between the groups, when appropriate. A p-value of smaller than 0.05 was regarded as significant.
Results
Locomotor activity
Based on the results, no significant differences were found in the distance traveled (Fig. 3A) and mean velocity (Fig. 3B) in OF test between the groups [(p > 0.05); F (3, 34) = 2/992)].
NOR test
According to the results, the Aβ group had a significantly lower DI (p < 0.01) in comparison with the control, Q10, and Aβ+Q10 groups (Fig. 4). Therefore, DI increased by Q10 in Aβ-injected rats.
MWM task
In the control, Q10, and Aβ+Q10 rats, escape latency showed a significant decrease (control rats: p < 0.001; Q10 rats: p < 0.001; Aβ+Q10 rats: p > 0.05) on day 4 in comparison with the first day (Fig. 5A). In Aβ-injected rats, escape latency failed to change after 4 days of training (p > 0.05). Therefore, Q10 attenuated Aβ deteriorative impact on memory acquisition. All groups except the Aβ group could learn the invisible platform position following 4 days of training.
The average latency to find the invisible platform (A) and swimming distance (B) vs. training days. CoQ10 significantly decreased the latency and distances. Values are provided as mean ± SEM. * p < 0.05, ** p < 0.01, and *** p < 0.001 versus the control group and # p < 0.05, ## p < 0.01, and ### p < 0.001 versus the Aβ group
The experimental groups except Aβ animals had a shorter swimming path during training (p < 0.05) (Fig. 5B). A significant difference was detected between the experimental groups in escape latency (p < 0.001; [F (9, 84) = 2/247]); two-way ANOVA) and swimming distance (p < 0.001, [F (9, 84) = 3/871]). We used swimming speed to measure the rats’ motor activity, which showed no significant changes through training (p > 0.05, data not shown).
Based on the results, Aβ-injected rats receiving Q10 (Aβ+Q10 group) were observed with a significant reduction in escape latency and moved distance than the Aβ-injected rats (Aβ group) (p < 0.05) (Fig. 5A). Therefore, Q10 decreased the Aβ deteriorative effects on memory acquisition and hence improved Aβ-related reference memory impairment.
In addition, a probe trial was performed 24 h following the last training trial on day 5, in which we removed the platform and noted time spent in each quadrant. In Aβ-treated rats, time in the target zone decreased compared to the other three groups. The Aβ+Q10 group spent a longer time in the target zone than the Aβ group (p <0.05) (control rats: 17.91 ± 0.8 s, n = 9; Q10: 18.35 ± 0.86 s, n = 9; Aβ: 10.07 ± 1.73 s, n = 9; Aβ+Q10: 14.67 ± 0.85 s, n = 9, p < 0.001, [F (3, 32) = 11/41]) (Fig. 6). Thus, Q10 could increase time spent in the target area and improve spatial memory impairment in Aβ-treated animals. There were no significant differences between experimental groups in escape latency to discover the visible platform in the visual discrimination task (data not shown, p > 0.05), which indicates no visual deficit in rats.
PAL test
In the training phase, the results showed that there were no significant differences between the experimental groups in STLa (p > 0.05) (Fig. 7A). Moreover, the experimental groups showed a significant difference in the number of trials to acquisition (p < 0.001). Based on the results, the number of trials to acquisition was significantly higher in the Aβ rats in comparison with the control and Q10 groups (p <0.01) (Fig. 7B). Meanwhile, Q10 administration failed to change the number of trials to acquisition in the Aβ+Q10 group in comparison with the Aβ group (p > 0.05) (Fig. 7B).
Effect of CoQ10 oral administration in the training phase (A, B) and the retrieval phase (C, D) of the step-through passive avoidance task. Entrance latency into the dark chamber (A) and the number of trials to acquisition (B) in the training stage, and latency to enter into the dark chamber (C) and the time spent in the dark chamber (D) in the retrieval phase in the experimental groups are displayed. Columns indicate mean ± SEM. * p < 0.05, ** p < 0.01, and *** p < 0.001 versus the control group and ## p < 0.01 and ### p < 0.001 versus the Aβ group
There was a significant difference between experimental groups in the retrieval phase of the PAL and memory test (p < 0.001). The STLr in Aβ rats was significantly lower compared to the control group (p < 0.001). However, the Aβ and Aβ+Q10 groups showed no remarkable differences (p > 0.05) (Fig. 7C). Also, the experimental groups were found with a significant difference in TDC (p < 0.001). The Aβ group showed a longer TDC compared to the control and Q10 groups (p < 0.01 and p < 0.001, respectively) (Fig. 7D). However, no significant difference was found between the Aβ+Q10 and Aβ groups (p >0.05).
Measurement of evoked potentials
Population spike (PS) and fEPSP are two components of the evoked field potential in the DG. The fEPSP slope was considered as 20–80% of the height amplitude (Asadbegi et al. 2016; Komaki et al. 2017; Komaki et al. 2019). The data analysis tool (eTrace, www.sciencebeam.com) was used to measure the fEPSP slope and PS amplitude.
Through the application of HFS to the PP, we induced LTP in the DG to assess the Q10 impacts on the EPSP slopes as well as PS amplitudes of granular cells of DG in AD animals. Figures 8 and 9 display the effects of Aβ and Q10 on the EPSP slope and PS amplitude, respectively. The significant effect of time (p < 0.001; [F (3, 28) = 17/96]) and treatment (p < 0.001; [F (3, 84) = 21/28]) was found on the EPSP slope of the DG granular cells (Fig. 8).
Time-dependent alterations in hippocampal responses to perforant path stimulation after high-frequency stimulation (HFS). Long-term potentiation (LTP) of the EPSP slope in DG granular cell synapses of the hippocampus showed significant differences between groups. Values are provided as mean ± SEM % of the baseline. * p < 0.05; ** p < 0.01, and *** p < 0.001 versus the control group and # p < 0.05 versus the Aβ group
Time-dependent alterations in long-term potentiation (LTP) of population spike (PS) amplitudes in the dentate gyrus (DG) granular cell synapses against perforant pathway stimulation after high-frequency stimulation (HFS). Values are provided as means ± SEM % of the baseline. * p < 0.05, ** p < 0.01, and *** p < 0.001 versus the control group and # p < 0.05, ## p < 0.01, and ### p < 0.001 versus the Aβ group
Aβ could cause a significant reduction in the hippocampal EPSP slope than the control and Q10 groups (Aβ: n = 8, 102.8 ± 6.51; control: n = 8, 149.9 ± 8.39 [p < 0.001]; Q10: n = 8, 132.3 ± 3.62; [p < 0.05]). Q10 administration compensated for a reduction in the EPSP slope than the Aβ group (Q10+Aβ, n = 8, 115.8 ± 5.41; Aβ: 102.8 ± 6.51; but it was not significant at p > 0.05).
The obtained results indicated the significant effect of time (p < 0.001; [F (3, 28) = 196/7]) and treatment (p < 0.001; [F (3, 84) = 24/18]) on the PS amplitude of the DG granular cells (Fig. 9). According to the Tukey’s post hoc results, Aβ could cause a significant impairment in the PS amplitude (Aβ: 172.1 ± 13.52; control: 318.8 ± 24.26; Q10: 301.0+19.37; Aβ versus the control [p < 0.001]). The Aβ and Aβ+Q10 groups were found with significant differences in the PS amplitude following Q10 administration (Aβ: 172.1 ± 13.52; Aβ+Q10: 245.1 ± 13.10, Aβ vs. Aβ+Q10 [p < 0.05]). Furthermore, no significant differences were observed in PS amplitude between the Q10 and control groups (Q10: 301.0 + 19.37; control: 318.8 ± 24.26 [p > 0.05]). These findings demonstrated that Aβ injection (ICV) could impair the LTP of the EPSP slope and PS amplitude, whereas treatment with Q10 could increase them.
Effect of Q10 and Aβ on TAC, TOS, TTG, and MDA
Analysis of TAC indicated a significant difference between experimental groups [F (3, 28) = 77/11, p < 0.001]. Moreover, there was a significant difference in the TOS among the experimental groups [F (3, 24) = 31/86, p < 0.001]. In addition, there was a significant difference in the MDA and the TTG levels between the groups [F (3, 28) = 13/71, p < 0.001] [F (3, 24) = 23/69, p < 0.001] (Fig. 10).
The effect of Aβ and Q10 on plasma parameters of total antioxidant capacity (TAC) (A), total oxidant status (TOS) (B), MDA (C), and total thiol group (TTG) (D) levels. Data are expressed as mean ± SEM. * p <0.05, ** p <0.01, and *** p < 0.001 versus the control group. # p < 0.05, ## p < 0.01, and ### p < 0.001 versus the Aβ group
Discussion
We investigated whether Q10 treatment can prevent the learning, memory, and synaptic plasticity impairment caused by ICV Aβ injection in aged rats. We used old rats to create a better AD model similar to AD patients.
The main findings of our study were as follows: Q10 could improve the Aβ-related decrease in the DI in the NOR test, spatial learning and memory in the MWM test, passive avoidance learning and memory in the PAL test, and synaptic plasticity impairment in the hippocampal PP-DG pathway in aged rats. In addition, Aβ injection significantly increased serum MDA and TOS levels. Q10, however, significantly reversed these parameters and also increased TAC and TTG levels in the Aβ+Q10 group.
Q10 alone showed no significant effectiveness in the learning and memory performance of aged rats (Q10 group) evidenced by the NOR, PAL, and MWM tests, and also synaptic plasticity (LTP induction). In this regard, in normal conditions, treatment with Q10 (Mcdonald et al. 2005; Sumien et al. 2009) or different antioxidant chemicals was not capable of improving memory performance (Monsef et al. 2019). Therefore, an increase in antioxidant concentration and activity in physiological conditions is not effective in cognition and may even cause a decrease in this process (Bouayed and Bohn 2010).
No significant differences were found in the distance traveled between the experimental groups. Therefore, Aβ or treatment with Q10 could not significantly affect the motor activity of animals, which is consistent with the findings of other studies (Dumont et al. 2011).
According to the data obtained from the training stage of the PAL task, no significant differences were found in STLa between the experimental groups. STLa indicates the rats’ natural preference for dark places. Thus, Q10 or Aβ could not significantly affect the rat behavior before delivering the initial shock and initiation of the acquisition process in the PAL test. In the training stage, the Aβ group was found with an elevation in the number of trials to acquisition in comparison with the control group. This result demonstrates that Aβ can impair the acquisition phase of PAL, which is highly associated with the integrity of some brain areas, such as the hippocampus and the amygdala (Gold 2004). Aβ rats treated with Q10 (Aβ+Q10) failed to change the number of trials to acquisition compared to the Aβ group. This finding can be owing to the research design, experiment’s conditions and duration, and Q10 dosage. In the PAL test, STLr and TDC are regarded as indicators of the retrieval phase of passive avoidance learning and memory processing (Zarrinkalam et al. 2018; Omidi et al. 2019). The Aβ group was found with lower STLr and longer TDC than the control group, which indicates a decrease in the retrieval of PAL memory in the Aβ group than the control group. Q10 failed to change the STLr and TDC in Aβ-treated rats.
According to the data obtained from the memory retrieval of the PAL and NOR tasks, Q10 could improve memory and cognitive deficit in the Aβ+Q10 group in the NOR test, and Q10 could enhance the DI in the NOR test in the Aβ+Q10 group, which indicates the greater effectiveness of Q10 in the brain NOR-processing regions compared to PAL-related areas. Q10 alone caused no significant effect in both PAL or NOR tests in the Q10 group. These findings demonstrate that Q10 was not capable of enhancing learning and memory in the Q10 group.
According to the results of the NOR test, the Aβ group showed less time exploring the new object compared to the three other groups, which can result from neophobia as one of the pitfalls in assessing memory in non-verbal animals (Thorpe et al. 2004). Medial temporal lobe systems, like the hippocampus and amygdala, are associated with emotion-associated memory. The amygdala plays an important role in the processing of emotion, whereas the hippocampus is involved in episodic memory (Yang and Wang 2017). The amygdala modifies both the storage and encoding of hippocampal-dependent memories (Phelps 2004). The hippocampus and basolateral amygdala groups (BLA) can work separately to exhibit their various functions in emotion and memory. Nonetheless, synergism in the amygdala-hippocampus axis is involved in emotion-regulated memories (Yang and Wang 2017). The amygdala is important to learn the valence of new objects from the emotional expressions of others (Blair et al. 2008). Also, amygdala atrophy occurs in early AD (Poulin et al. 2011). Lesions of the amygdala in monkeys could disrupt emotional behavior in some new objects (Zola-Morgan et al. 1991; Sarowar et al. 2017). Thus, amygdala atrophy because of Aβ-induced AD can impair emotional behavior regarding new objects (Zola-Morgan et al. 1991; Sarowar et al. 2017) in AD. Therefore, the shorter exploration time of the Aβ group can be due to memory impairment, not neophobia. The time exploring the novel object was similar in transgenic and wild-type AD mice (Baazaoui and Iqbal 2017). Both groups were similar regarding non-spatial working memory and exploration of a new object. Hence, the neophobia effect on the exploration of new objects in our research might be eliminated.
DI to new objects increased by Q10 in Aβ-treated animals. Thus, Q10 improved non-spatial memory deficit in Aβ-treated rats. Muthukumaran et al. (2018) reported that transgenic AD mice, whether or not they were treated with Q10, acted similarly to non-transgenic mice in the NOR test. Nonetheless, only transgenic mice that received Q10 recognized the familiar object moving to a new location (the NLR test) similar to non-transgenic mice (Muthukumaran et al. 2018)).
The current research on the Q10 neuroprotective impact on cognition in Aβ-injected aged male rats showed that Aβ could significantly diminish spatial memory acquisition and impair spatial memory retention. Q10 could increase the level of memory acquisition and retention, which was found in Aβ rats. Treatment with Q10 attenuated Aβ deteriorative effect on memory and learning in aged male rats. Therefore, supplementation with Q10 improved learning and memory in Aβ-treated rats. Furthermore, the Q10 alone did not affect swimming speed, which suggests that locomotion improvement cannot lead to spatial learning improvement. AD causes a remarkable impairment in memory (Selkoe 2002). Aβ impairs alternation behavior as well as passive avoidance task scores in rats (Rasoolijazi et al. 2013). Also, Aβ intrahippocampal injection induced significant learning impairments in PAL and MWM tests (Asadbegi et al. 2017; Komaki et al. 2019). Our findings are consistent with other studies on Aβ-induced memory impairment (Asadbegi et al. 2017; Shekarian et al. 2020). The following mechanisms are possibly involved in memory and cognitive impairments in AD: (1) changes in synapses and neurons in brain areas associated with learning and memory functions, such as the basal forebrain, the hippocampus, entorhinal cortex, and neocortical association cortices (Mattson et al. 1999) and (2) Aβ neurotoxicity that increases apoptosis, inflammatory responses, the cell wall permeability, and free radical damage (Clippingdale et al. 2001) because an increase in Aβ (Aß 1–42) is involved in the AD pathogenesis (Prasad and Bondy 2014).
In our experiment, Aβ injection significantly increased serum MDA and TOS levels, indicating an increase in serum ROS and oxidant components. Q10 supplementation, however, significantly reversed these parameters and increased TAC and TTG levels, which indicated that Q10 treatment could alter the oxidant/antioxidant balance, in favor of antioxidants. There is a significant reduction in the antioxidant enzyme activities, such as catalase, glutathione peroxidase, and superoxide dismutase in various brain areas in AD (Venkateshappa et al. 2012). Increased oxidant events can increase the generation and accumulation of Aβ. Increased oxidative events accelerate the intracellular buildup of Aβ in neurons (Misonou et al. 2000; Prasad and Bondy 2014). Consequently, Aβ oligomerization, overproduction, and aggregation increase oxidative stress. After the initiation of the positive feedback loop, where ROS generation and Aβ are connected, this loop gently can enhance and accelerate brain pathology (Wojsiat et al. 2018).
Q10 can prevent memory and cognitive impairments in different conditions (Sandhir et al. 2014). Q10 also could improve spatial learning, reduce oxidative injury, and delay early aging (Papucci et al. 2003). Also, Q10 improved cognitive deficit, in experimental models of AD and PD, through an improvement in electron transport within the MC membrane (Sandhir et al. 2014). Q10 possibly can be used for the treatment of AD. Treatment with Q10 in STZ-infused rats could reverse oxidative damage and decline ATP levels in the hippocampus as well as the cerebral cortex of rats injected with STZ (ICV). Q10 modulated cognitive deficit caused by STZ injection (ICV) in rats in the MWM and PAL tests (Ishrat et al. 2006). Cholinergic signaling plays a role in learning and memory formation, and its modification causes cognitive impairment in AD (Cummings 2000). Q10 supplementation improves memory processing through an improvement in hippocampal cholinergic function by an increase in choline acetyltransferase and a decrease in acetylcholinesterase activities (Ishrat et al. 2006), neurogenesis by a reduction in Aβ formation (Muthukumaran et al. 2018) and oxidative stress markers, and an increase in ATP amounts in the hippocampus and cortex (Ishrat et al. 2006). In diabetic and ischemic injury model rats, pre-treatment with Q10 (a strong antioxidant and free radical scavenger in the hippocampus and DG) significantly decreased neuronal loss because of ischemia and decreased activity of caspase-3 associated with apoptotic cell death (Young et al. 2007).
The elevated levels of oxidative stress, mitochondrial impairment, and metabolic aberrancies are the main factors to induce AD (Mattson et al. 1999). In this study, we found that Q10 prevented the memory and cognitive deficit caused by Aβ in aged rats; however, it was weaker compared to adult rats. More ROS are generated in the mitochondria of aged animals, and also, their mitochondria are characterized by higher amounts of lipid peroxides and losses of polyunsaturated fatty acids, suggesting elevated oxidative stress in old age. Therefore, ROS are highly involved in the aging process (Ebadi et al. 2001). There are several factors promoting progressive neurodegeneration, and their deleterious effects become more potent in aging. For example, the glymphatic system causes waste removal and brain fluid clearance during sleep through glia-supported perivascular channels. The impairment of the glymphatic system reduced the clearance of AD-associated proteins. Human studies have reported a diminish in the glymphatic function in AD (Reeves et al. 2020; Silva et al. 2021).
LTP is a persistent elevation in synaptic strength in the hippocampus and has been a famous model to investigate learning and memory formation (Komaki et al. 2019). Aβ caused impairments in synaptic plasticity in the hippocampus in a rat model of AD (Asadbegi et al. 2016). Measurements of hippocampal LTP, as a cellular mechanism of learning and memory, indicate the probable mechanisms of Q10 function in such situations. We found that Aβ attenuated hippocampal LTP induction in aged rats, which is in line with the idea that intrahippocampal injection of Aβ can reduce LTP induction in adult rats (Asadbegi et al. 2016). Aβ injection decreased LTP, whereas treatment with Q10 enhanced LTP in Aβ-injected adult rats (Komaki et al. 2019). The hippocampus is highly associated with learning and memory. AD is initiated with slight changes in synaptic efficacy in the hippocampus leading to noticeable neuronal degeneration (Selkoe 2002).
From another perspective, elevated chronic inflammatory processes are considered an important factor to initiate and promote neurodegeneration in AD and associated neurological diseases regarding inflammatory endothelium activation (Bentz et al. 2010; Bosche et al. 2020; Haupt et al. 2020; Tso et al. 2021). Many investigations using non-steroidal anti-inflammatory drugs, such as aspirin and ibuprofen, indicated their effectiveness in patients with idiopathic AD (Rich et al. 1995). Therefore, using anti-inflammatory agents combined with antioxidants can be used to reduce the progression of AD (Prasad and Bondy 2014). The Q10 level showed a negative correlation with inflammatory markers, such as TNF-α and IL-6 (Lee et al. 2013). Also, Q10 exerted anti-inflammation activities by a decrease in nuclear factor-κB (NF-κB)-dependent gene expression. NF-κB is activated by the ROS leading to up-regulation of the expression levels of pro-inflammatory cytokines. Nonetheless, this NF-κB-activating cascade is suppressed by antioxidants, such as Q10 (Lee et al. 2013).
Improvement in the CoQ10 bioavailability and consequently its efficiency can be involved in better protection against Aβ-related injury. Although the oil-soluble Q10 exhibits effective neuroprotection in animal models of neurodegenerative disorders, it showed no effect in clinical trials of PD, amyotrophic lateral sclerosis, and Huntington’s disease (Muthukumaran et al. 2018).
Such clinical failures were probably due the low bioavailability in such lipid-solubility CoQ10 formulations at comparable doses used on preclinical trials not allowed in FDA-approved human clinical trials. Because oil-soluble CoQ10 at our low dosage could have also been a limiting factor in our research, we tried to improve the Q10 bioavailability with a water-soluble formulation (produced by or obtained from the study of Muthukumaran et al. (2018)). Therefore, clinical trials may have failed because of FDA required much lower doses administrated to participants. Hence, a lower dose of Q10 (50 mg/kg/day) in our research may be allowed for a clinical trial.
Our study may not be a preclinical test on the efficacy of Q10 in preventing AD, but this formulation can be a promising contender for such an effect. MSW or PAL tests are applied in preclinical research and are not appropriate indicants of cognition not caused by AD-induced heightened adverse emotional effects that this treatment might alleviate. Therefore, it can be expected that the clinical trials have provided much less evidence of preserved cognition in AD participants tested in non-threatening contexts. Accordingly, preclinical trials of Q10 can be conducted only on tests, such as NOR/NLR in non-threatening settings. As mentioned, neophobia as a pitfall in the measurement of memory can be eliminated.
Our study was associated with some limitations. Our used model is still not representative of the clinical situation of patients with AD and leads to no direct deductive conclusion. Also, several compounds exhibiting preclinical prevention of AD precursors in the brain of rodents or preventing cognitive-behavioral deficits could not pass clinical trials.
Conclusion
Supplementation with Q10 significantly improved learning and memory deficits and synaptic plasticity impairment induced by Aβ in aged rats. The Q10 concentration is decreased in the brain of aging animals and administration of Q10 minimized Aβ-induced AD severity. Such protective effects can be due to the antioxidant, anti-inflammatory, anti-aging, and antiapoptotic effects of Q10 and also maintenance of mitochondrial integrity and prevention of membrane damage. Accordingly, Q10 is a possible neuroprotective agent against age-related neurodegeneration diseases, such as AD. However, further investigation is necessary to elucidate the possible neuroprotection mechanisms of Q10 on learning and memory and also synaptic plasticity in aged subjects.
References
Ahmadi N, Mirazi N, Komaki A, Safari S, Hosseini A (2021a) Vanillic acid attenuates amyloid β1-40-induced long-term potentiation deficit in male rats: an in vivo investigation. Neurol Res 1-8
Ahmadi N, Safari S, Mirazi N, Karimi SA, Komaki A (2021b) Effects of vanillic acid on Aβ1-40-induced oxidative stress and learning and memory deficit in male rats. Brain Res Bull 170:264–273
Akalιn FA, Baltacιoğlu E, Alver A, Karabulut E (2007) Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J Clin Periodontol 34:558–565
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13:93–110
Asadbegi M, Komaki A, Salehi I, Yaghmaei P, Ebrahim-Habibi A, Shahidi S, Sarihi A, Asl SS, Golipoor Z (2018) Effects of thymol on amyloid-β-induced impairments in hippocampal synaptic plasticity in rats fed a high-fat diet. Brain Res Bull 137:338–350
Asadbegi M, Yaghmaei P, Salehi I, Ebrahim-Habibi A, Komaki A (2016) Neuroprotective effects of metformin against Aβ-mediated inhibition of long-term potentiation in rats fed a high-fat diet. Brain Res Bull 121:178–185
Asadbegi M, Yaghmaei P, Salehi I, Komaki A, Ebrahim-Habibi A (2017) Investigation of thymol effect on learning and memory impairment induced by intrahippocampal injection of amyloid beta peptide in high fat diet-fed rats. Metab Brain Dis 32:827–839
Aslan R, Kutlu R, Civi S, Tasyurek E (2014) The correlation of the total antioxidant status (TAS), total oxidant status (TOS) and paraoxonase activity (PON1) with smoking. Clin Biochem 47:393–397
Baazaoui N, Iqbal K (2017) Prevention of dendritic and synaptic deficits and cognitive impairment with a neurotrophic compound. Alzheimers Res Ther 9:1–15
Barker GR, Bird F, Alexander V, Warburton EC (2007) Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci 27:2948–2957
Bentz K, Molcanyi M, Schneider A, Riess P, Maegele M, Bosche B, Hampl JA, Hescheler J, Patz S, Schäfer U (2010) Extract derived from rat brains in the acute phase following traumatic brain injury impairs survival of undifferentiated stem cells and induces rapid differentiation of surviving cells. Cell Physiol Biochem 26:821–830
Benzie IF, Strain J (1999) [2] Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27
Berr C, Balansard B, Arnaud J, Roussel AM, Alpérovitch A, Group ES (2000) Cognitive decline is associated with systemic oxidative stress: the EVA study. J Am Geriatr Soc 48:1285–1291
Bisagno V, Grillo CA, Piroli GG, Giraldo P, McEwen B, Luine VN (2004) Chronic stress alters amphetamine effects on behavior and synaptophysin levels in female rats. Pharmacol Biochem Behav 78:541–550
Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, Ng P, Hollon N, Jones M, Blair R (2008) Neural response to self-and other referential praise and criticism in generalized social phobia. Arch Gen Psychiatry 65:1176–1184
Bosche B, Mergenthaler P, Doeppner TR, Hescheler J, Molcanyi M (2020) Complex clearance mechanisms after intraventricular hemorrhage and rt-PA treatment—a review on clinical trials. Transl Stroke Res 11:337–344
Bouayed J, Bohn T (2010) Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev 3:228–237
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement 12:719–732
Clippingdale AB, Wade JD, Barrow CJ (2001) The amyloid-β peptide and its role in Alzheimer’s disease. J Pept Sci 7:227–249
Cohen SJ, Stackman RW Jr (2015) Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285:105–117
Cummings JL (2000) The role of cholinergic agents in the management of behavioural disturbances in Alzheimer’s disease. Int J Neuropsychopharmacol 3:S21–S29
Drews E, Schneider M, Koch M (2005) Effects of the cannabinoid receptor agonist WIN 55,212-2 on operant behavior and locomotor activity in rats. Pharmacol Biochem Behav 80:145–150
Dumont M, Kipiani K, Yu F, Wille E, Katz M, Calingasan NY, Gouras GK, Lin MT, Beal MF (2011) Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis 27:211–223
Ebadi M, Govitrapong P, Sharma S, Muralikrishnan D, Shavali S, Pellett L, Schafer R, Albano C, Eken J (2001) Ubiquinone (coenzyme q10) and mitochondria in oxidative stress of Parkinson’s disease. Neurosignals 10:224–253
Emerit J, Edeas M, Bricaire F (2004) Neurodegenerative diseases and oxidative stress. Biomed Pharmacother 58:39–46
Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31:47–59
Etaee F, Asadbegi M, Taslimi Z, Shahidi S, Sarihi A, Asl SS, Komaki A (2017) The effects of methamphetamine and buprenorphine, and their interaction on anxiety-like behavior and locomotion in male rats. Neurosci Lett 655:172–178
Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E (2012) Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci 322:254–262
Flint BM (2002) Coenzyme Q 10 as a possible treatment for neurodegenerative diseases. Free Radic Res 36:455–460
Fukui K, Onodera K, Shinkai T, Suzuki S, Urano S (2001) Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Ann N Y Acad Sci 928:168–175
Ganji A, Salehi I, Nazari M, Taheri M, Komaki A (2017) Effects of Hypericum scabrum extract on learning and memory and oxidant/antioxidant status in rats fed a long-term high-fat diet. Metab Brain Dis 32:1255–1265
Gibson G, Haroutunian V, Zhang H, Park L, Shi Q, Lesser M, Mohs R, Sheu RKF, Blass J (2000) Mitochondrial damage in Alzheimer’s disease varies with apolipoprotein E genotype. Ann Neurol 48:297–303
Gold PE (2004) Coordination of multiple memory systems. Neurobiol Learn Mem 82:230–242
Haupt M, Zechmeister B, Bosche B, Lieschke S, Zheng X, Zhang L, Venkataramani V, Jin F, Hein K, Weber MS (2020) Lithium enhances post-stroke blood-brain barrier integrity, activates the MAPK/ERK1/2 pathway and alters immune cell migration in mice. Neuropharmacology 181:108357
Hu M-L (1994) [41] Measurement of protein thiol groups and glutathione in plasma. In: Methods in enzymology. Elsevier, pp 380–385
Ishrat T, Khan MB, Hoda MN, Yousuf S, Ahmad M, Ansari MA, Ahmad AS, Islam F (2006) Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav Brain Res 171:9–16
Karimi SA, Salehi I, Shykhi T, Zare S, Komaki A (2019) Effects of exposure to extremely low-frequency electromagnetic fields on spatial and passive avoidance learning and memory, anxiety-like behavior and oxidative stress in male rats. Behav Brain Res 359:630–638
Karimi SA, Salehi I, Taheri M, Faraji N, Komaki A (2020) Effects of regular exercise on diabetes-induced memory deficits and biochemical parameters in male rats. J Mol Neurosci 1-8
Komaki H, Faraji N, Komaki A, Shahidi S, Etaee F, Raoufi S, Mirzaei F (2019) Investigation of protective effects of coenzyme Q10 on impaired synaptic plasticity in a male rat model of Alzheimer’s disease. Brain Res Bull 147:14–21
Komaki H, Saadat F, Shahidi S, Sarihi A, Hasanein P, Komaki A (2017) The interactive role of CB1 receptors and L-type calcium channels in hippocampal long-term potentiation in rats. Brain Res Bull 131:168–175
Lee B-J, Huang Y-C, Chen S-J, Lin P-T (2012) Effects of coenzyme Q10 supplementation on inflammatory markers (high-sensitivity C-reactive protein, interleukin-6, and homocysteine) in patients with coronary artery disease. Nutrition 28:767–772
Lee B-J, Tseng Y-F, Yen C-H, Lin P-T (2013) Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: a randomized, placebo-controlled trial. Nutr J 12:1–9
Lorenzo A, Yankner BA (1994) Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc Natl Acad Sci 91:12243–12247
Majumdar A, Nirwane A, Kamble R (2014) New evidences of neurotoxicity of aroclor 1254 in mice brain: potential of coenzyme q10 in abating the detrimental outcomes. Environ Health Toxicol 29
Maren S, Baudry M (1995) Properties and mechanisms of long-term synaptic plasticity in the mammalian brain: relationships to learning and memory. Neurobiol Learn Mem 63:1–18
Marshe VS, Pira S, Mantere O, Bosche B, Looper KJ, Herrmann N, Mueller DJ, Rej S (2017) C-reactive protein and cardiovascular risk in bipolar disorder patients: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry 79:442–451
Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711
Matteo V, Esposito E (2003) Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. CNS Neurol Disord Drug Targets 2:95–107
Matthews RT, Yang L, Browne S, Baik M, Beal MF (1998) Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci 95:8892–8897
Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S (1999) Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Ann N Y Acad Sci 893:154–175
Mcdonald SR, Sohal RS, Forster MJ (2005) Concurrent administration of coenzyme Q10 and α-tocopherol improves learning in aged mice. Free Radic Biol Med 38:729–736
Mergenthaler P, Lindauer U, Dienel GA, Meisel A (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36:587–597
Misonou H, Morishima-Kawashima M, Ihara Y (2000) Oxidative stress induces intracellular accumulation of amyloid β-protein (Aβ) in human neuroblastoma cells. Biochemistry 39:6951–6959
Mohmmad Abdul H, Sultana R, Keller JN, St. Clair DK, Markesbery WR, Butterfield DA. (2006) Mutations in amyloid precursor protein and presenilin-1 genes increase the basal oxidative stress in murine neuronal cells and lead to increased sensitivity to oxidative stress mediated by amyloid β-peptide (1–42), H2O2 and kainic acid: implications for Alzheimer’ disease. J Neurochem 96:1322–1335
Monsef A, Shahidi S, Komaki A (2019) Influence of chronic coenzyme Q10 supplementation on cognitive function, learning, and memory in healthy and diabetic middle-aged rats. Neuropsychobiology 77:92–100
Muthukumaran K, Kanwar A, Vegh C, Marginean A, Elliott A, Guilbeault N, Badour A, Sikorska M, Cohen J, Pandey S (2018) Ubisol-Q 10 (a nanomicellar water-soluble formulation of CoQ 10) treatment inhibits Alzheimer-type behavioral and pathological symptoms in a double transgenic mouse (TgAPEswe, PSEN1dE9) model of Alzheimer’s disease. J Alzheimers Dis 61:221–236
Muthukumaran K, Leahy S, Harrison K, Sikorska M, Sandhu JK, Cohen J, Keshan C, Lopatin D, Miller H, Borowy-Borowski H (2014) Orally delivered water soluble Coenzyme Q 10 (Ubisol-Q 10) blocks on-going neurodegeneration in rats exposed to paraquat: potential for therapeutic application in Parkinson’s disease. BMC Neurosci 15:1–11
Navas P, Villalba JM, de Cabo R (2007) The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion 7:S34–S40
Nazari M, Komaki A, Karamian R, Shahidi S, Sarihi A, Asadbegi M (2016) The interactive role of CB1 and GABAB receptors in hippocampal synaptic plasticity in rats. Brain Res Bull 120:123–130
Omidi G, Karimi SA, Rezvani-Kamran A, Monsef A, Shahidi S, Komaki A (2019) Effect of coenzyme Q10 supplementation on diabetes induced memory deficits in rats. Metab Brain Dis 34:833–840
Omidi G, Karimi SA, Shahidi S, Faraji N, Komaki A (2020a) Coenzyme Q10 supplementation reverses diabetes-related impairments in long-term potentiation induction in hippocampal dentate gyrus granular cells: an in vivo study. Brain Res 1726:146475
Omidi G, Rezvani-Kamran A, Ganji A, Komaki S, Etaee F, Asadbegi M, Komaki A (2020b) Effects of Hypericum scabrum extract on dentate gyrus synaptic plasticity in high fat diet-fed rats. J Physiol Sci 70:1–8
Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, Formigli L, Zecchi-Orlandini S, Orlandini G, Carella G (2003) Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem 278:28220–28228
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Elsevier Academic Press, San Diego, CA
Phelps EA (2004) Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol 14:198–202
Placanica L, Tarassishin L, Yang G, Peethumnongsin E, Kim S-H, Zheng H, Sisodia SS, Li Y-M (2009) Pen2 and presenilin-1 modulate the dynamic equilibrium of presenilin-1 and presenilin-2 γ-secretase complexes. J Biol Chem 284:2967–2977
Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC, Initiative AsDN (2011) Amygdala atrophy is prominent in early Alzheimer’ disease and relates to symptom severity. Psychiatry Res Neuroimaging 194:7–13
Prasad KN, Bondy SC (2014) Inhibition of early upstream events in prodromal Alzheimer’s disease by use of targeted antioxidants. Curr Aging Sci 7:77–90
Ramirez G, Rey S, von Bernhardi R (2008) Proinflammatory stimuli are needed for induction of microglial cell-mediated AβPP 244–C and Aβ-neurotoxicity in hippocampal cultures. J Alzheimers Dis 15:45–59
Rasoolijazi H, Azad N, Joghataei M, Kerdari M, Nikbakht F, Soleimani M (2013) The protective role of carnosic acid against beta-amyloid toxicity in rats. Scientific World Journal 2013
Reeves BC, Karimy JK, Kundishora AJ, Mestre H, Cerci HM, Matouk C, Alper SL, Lundgaard I, Nedergaard M, Kahle KT (2020) Glymphatic system impairment in Alzheimer’s disease and idiopathic normal pressure hydrocephalus. Trends Mol Med 26:285–295
Rich JB, Rasmusson D, Folstein M, Carson K, Kawas C, Brandt J (1995) Nonsteroidal anti-inflammatory drugs in Alzheimer’s disease. Neurology 45:51–55
Salehi I, Karamian R, Komaki A, Tahmasebi L, Taheri M, Nazari M, Shahidi S, Sarihi A (2015) Effects of vitamin E on lead-induced impairments in hippocampal synaptic plasticity. Brain Res 1629:270–281
Sandhir R, Sethi N, Aggarwal A, Khera A (2014) Coenzyme Q10 treatment ameliorates cognitive deficits by modulating mitochondrial functions in surgically induced menopause. Neurochem Int 74:16–23
Sarowar T, Grabrucker S, Boeckers TM, Grabrucker AM (2017) Object phobia and altered RhoA signaling in amygdala of mice lacking RICH2. Front Mol Neurosci 10:180
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298:789–791
Shekarian M, Komaki A, Shahidi S, Sarihi A, Salehi I, Raoufi S (2020) The protective and therapeutic effects of vinpocetine, a PDE1 inhibitor, on oxidative stress and learning and memory impairment induced by an intracerebroventricular (ICV) injection of amyloid beta (aβ) peptide. Behav Brain Res 383:112512
Shoffner JM, Brown MD, Torroni A, Lott MT, Cabell MF, Mirra SS, Beal MF, Yang C-C, Gearing M, Salvo R (1993) Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics 17:171–184
Sikorska M, Lanthier P, Miller H, Beyers M, Sodja C, Zurakowski B, Gangaraju S, Pandey S, Sandhu JK (2014) Nanomicellar formulation of coenzyme Q10 (Ubisol-Q10) effectively blocks ongoing neurodegeneration in the mouse 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine model: potential use as an adjuvant treatment in Parkinson’s disease. Neurobiol Aging 35:2329–2346
Silva I, Silva J, Ferreira R, Trigo D (2021) Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurol Res Pract 3:1–9
Sultana R, Perluigi M, Butterfield DA (2006) Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer’s disease: insights into mechanism of neurodegeneration from redox proteomics. Antioxid Redox Signal 8:2021–2037
Sumien N, Heinrich KR, Shetty RA, Sohal RS, Forster MJ (2009) Prolonged intake of coenzyme Q10 impairs cognitive functions in mice. J Nutr 139:1926–1932
Tahmasebi L, Komaki A, Karamian R, Shahidi S, Sarihi A, Salehi I, Nikkhah A (2015) The interactive role of cannabinoid and vanilloid systems in hippocampal synaptic plasticity in rats. Eur J Pharmacol 757:68–73
Terry RD, Davies P (1980) Dementia of the Alzheimer type. Annu Rev Neurosci 3:77–95
Thorpe CM, Jacova C, Wilkie DM (2004) Some pitfalls in measuring memory in animals. Neurosci Biobehav Rev 28:711–718
Tso MK, Turgeon P, Bosche B, Lee CK, Nie T, D’Abbondanza J, Ai J, Marsden PA, Macdonald RL (2021) Gene expression profiling of brain endothelial cells after experimental subarachnoid haemorrhage. Sci Rep 11:1–16
Uttara B, Singh AV, Zamboni P, Mahajan R (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74
Venkateshappa C, Harish G, Mahadevan A, Bharath MS, Shankar S (2012) Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: implications for neurodegeneration in Alzheimer’s disease. Neurochem Res 37:1601–1614
Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B, Edvardsson D, Feldman H (2016) Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol 15:455–532
Wojsiat J, Zoltowska KM, Laskowska-Kaszub K, Wojda U (2018) Oxidant/antioxidant imbalance in Alzheimer’s disease: therapeutic and diagnostic prospects. Oxid Med Cell Longev 2018
Xie H, Hou S, Jiang J, Sekutowicz M, Kelly J, Bacskai BJ (2013) Rapid cell death is preceded by amyloid plaque-mediated oxidative stress. Proc Natl Acad Sci 110:7904–7909
Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, Ikezu T (2007) Interferon-γ and tumor necrosis factor-α regulate amyloid-β plaque deposition and β-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol 170:680–692
Yang X, Zhang Y, Xu H, Luo X, Yu J, Liu J, Chang RC-C (2016) Neuroprotection of coenzyme Q10 in neurodegenerative diseases. Curr Top Med Chem 16:858–866
Yang Y, Wang J-Z (2017) From structure to behavior in basolateral amygdala-hippocampus circuits. Front Neural Circuits 11:86
Yankner BA, Mesulam M-M (1991) β-amyloid and the pathogenesis of Alzheimer's disease. N Engl J Med 325:1849–1857
Young AJ, Johnson S, Steffens DC, Doraiswamy PM (2007) Coenzyme Q10: a review of its promise as a neuroprotectant. CNS Spectr 12:62–68
Zarrinkalam E, Heidarianpour A, Salehi I, Ranjbar K, Komaki A (2016) Effects of endurance, resistance, and concurrent exercise on learning and memory after morphine withdrawal in rats. Life Sci 157:19–24
Zarrinkalam E, Ranjbar K, Salehi I, Kheiripour N, Komaki A (2018) Resistance training and hawthorn extract ameliorate cognitive deficits in streptozotocin-induced diabetic rats. Biomed Pharmacother 97:503–510
Zhang S-y, Yang K-l, Zeng L-t, Wu X-h, Huang H-y (2018) Effectiveness of coenzyme Q10 supplementation for type 2 diabetes mellitus: a systematic review and meta-analysis. Int J Endocrinol 2018
Zola-Morgan S, Squire LR, Clower RP, Alvarez-Royo P (1991) Independence of memory functions and emotional behavior: separate contributions of the hippocampal formation and the amygdala. Hippocampus 1:207–220
Acknowledgements
This work was supported by a grant (Grant No.: 9412257459) of Hamadan University of Medical Sciences, Iran. This Center supported us in study design, in the collection, analysis, and interpretation of data. The authors are grateful to the staff of the Neurophysiology Research Center, Hamadan University of Medical Sciences for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All of the experiments and animal care methods were confirmed by the Veterinary Ethics Board of the Hamadan University of Medical Science and carried out according to the Guidelines of the National Institutes of Health on the principles of laboratory animal care (NIH Publication 80-23, 1996).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asadbegi, M., Komaki, H., Faraji, N. et al. Effectiveness of coenzyme Q10 on learning and memory and synaptic plasticity impairment in an aged Aβ-induced rat model of Alzheimer’s disease: a behavioral, biochemical, and electrophysiological study. Psychopharmacology 240, 951–967 (2023). https://doi.org/10.1007/s00213-023-06338-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06338-2