Abstract
Regulatory role of vitamin D (VitD) in cognitive memory and learning has been proposed. Here, we examine the behavioral and biochemical effects of VitD in Alzheimer’s disease (AD), as the most common form of dementia, in male Wistar rats. Animals (n = 48) were randomly divided into six groups: control, sham solvent, sham surgery, VitD (by intraperitoneal injection), AD (receiving intrahippocampal injection of amyloid-beta peptide, Aβ), and combination of VitD and Aβ. Learning and memory functions were investigated through the passive avoidance and the Morris water maze (MWM) tasks. Moreover, oxidative stress biomarkers including total antioxidant capacity (TAC), total thiol groups (TTG), lipid peroxidation (LPO), and DNA damage were assessed in hippocampus and serum. In passive avoidance task, Aβ significantly impaired the step-through latency and time in dark compartment. It also increased escape latency and time spent in the target quadrant in the MWM. VitD administration attenuated the Aβ-induced memory impairment in passive avoidance and MWM tests. Furthermore, VitD reduced deleterious biochemical effect of Aβ by enhancing the levels of TAC and TTG in addition to decreasing LPO and DNA damage levels in both hippocampus and serum. We showed, for the first time, that VitD administration improves the impaired Aβ-induced memory and that, by acting as a strong antioxidant, it can attenuate the stress oxidative biomarkers in hippocampus and serum of rats with AD. Altogether, our results provide evidence for further application of VitD in neurodegenerative disorders such as AD to enlighten the involved mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D (VitD) is a secosteroid hormone that has been primarily recognized with participation in bone and calcium homoeostasis. Recent investigations, however, point out to the various functions and actions in a wide range of tissues and cell types (Dankers et al. 2017). Typically, VitD refers to the precursor forms of hormone obtained from dietary sources or through skin’s exposure to sunlight. Over the last decade, a huge body of evidence has revealed the important regulatory effect of VitD in brain and related disorders (Zong et al. 2017). After being absorbed into blood, the VitD can be transformed into the 25(OH)D3 form with a high bioactivity (Cui et al. 2015). The distribution of VitD receptors (VDR) as well as the major rate limiting enzymes involved in synthesis of VitD in the adult brain has been mapped (Eyles et al. 2005). Of particular interest, VDR distribution in specific brain regions has suggested that VitD might influence particular neurotransmitters and cortical function. For instance, the VDR dysregulation was identified in the hippocampus and prefrontal cortex regions required for memory and learning, which are implicated in a wide range of neuropsychiatric disorders (DeLuca et al. 2013).
Alzheimer’s disease (AD) is the prevailing form of dementia. Currently more than 5 million persons in USA, and 35 million individuals worldwide, are estimated to have AD (Keeney and Butterfield 2015). The main pathological hallmarks of AD comprise amyloid-beta peptide (Aβ)-rich plaques, synapse loss as well as brain atrophy in areas associated with memory and higher executive function. Predominantly, its major related clinical signs and symptoms are known as memory loss, cognitive impairment, oxidative stress and brain inflammation (Berridge 2017; Keeney and Butterfield 2015). Oxidative stress defines a cascade reaction recognized by a significant increase in the oxidized components amount, which can result in direct injury to the central nervous system (CNS) (Salim 2017).
Despite the considerable attention regarding effects of VitD, there are still controversial data in conditions such as AD. Taken together, given the limited knowledge in this field, our goal was to evaluate the behavioral and biochemical effects of VitD in a amyloid-beta peptide-induced model of AD.
Materials and methods
Animals
The animals were male Wistar rats, weighing 200–240 g, and obtained from Pasteur Institute of Iran (Tehran, Iran). All rats had free access to the tap water and provided food. They were housed in standard laboratory cages (two or three rats in each cage) in an air-conditioned room with 12 h light / 12 h dark cycles (08:00–20:00 h) at 23 ± 2 °C. All behavioral experiments were performed between 10 a.m. and 4 p.m. The study design was approved by the Ethics Committee Guidelines of Hamadan University of Medical Sciences (IR.UMSHA.REC.1394.486) and, further, was in line with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Experimental procedure
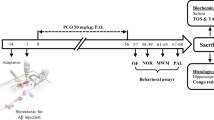
Animals (n = 48) were randomly divided into the following categories (in each 8 rats): control, sham solvent, sham surgery, VitD, AD (receiving amyloid-beta peptide, Aβ), and combination of VitD and Aβ. For AD modeling, we carried out stereotaxic surgery. First, rats were anesthetized by intraperitoneal injection (i.p.) of ketamine/xylazine (90/10 mg/kg) and afterwards their head was mounted on a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA) with incisor bar ±3.3 mm and ear bars positioned symmetrically. The body temperature was maintained at 37 °C by utilizing a heating pad. The scalp was carefully cleaned and incised well in the midline. Then, a burr hole was drilled through the skull and eventually chemicals were injected at coordinates of AP: – 3.8 mm, ML: ± 2.2 mm, and DV: – 2.4 mm, based on the Atlas of Paxinos and Watson (Paxinos and Watson 1986).
The rats in the control group were kept for 2 weeks in their laboratory cages with free access to taped water and normal food. The sham solvent group received 1 ml/day (i.p.) of distilled water and Tween 80 solvent for 2 weeks. The sham surgery group was under stereotaxic surgery without taking any chemical and also received 1 ml/day (i.p.) of distilled water and Tween 80 solvent for 2 weeks. Rats in VitD group were administered with a dose of 5 μg/kg/day of VitD (Sigma-Aldrich, USA) dissolved in distilled water and Tween 80 solvent by i.p. injections. The animals in Alzheimer’s group received bilateral injections of synthetic Aβ1—40 (Sigma-Aldrich, USA) in the hippocampus with 5 μl of a solution containing Aβ. This group was also treated with 1 ml/day i.p. injection of distilled water and Tween 80 solvent for the next 2 weeks. In the 6th group, first the AD was induced by Aβ injection in the hippocampus, and then for 2 weeks animals received a 5 μg/kg/day dose of VitD (dissolved in distilled water and Tween 80 solvent) by injection. Post-operatively, animals were given appropriate care until spontaneous feeding was revived. Two weeks after Aβ injection, rats were evaluated regarding signs of AD and were assigned for behavioral tests and were examined blind to the treatments by the observer.
Behavioral assessment
Passive avoidance memory test
Apparatus
Basically the apparatus and procedures were the same as our previous studies (Ghahremanitamadon et al. 2014; Jabbarpour et al. 2014). A standard passive avoidance conditioning apparatus (namely a shuttle box) was utilized to train and test the rats. The training was carried out in a conditioning chamber possessing two equal-sized light and dark compartments (20 × 20 × 30 cm). These two compartments were separated by a guillotine-type door (7 × 9 cm) that could easily be lowered or raised by the observer. In order to deliver an electric shock, stainless steel grids (2.5 mm in diameter) were localized (with 1 cm intervals between grids) on the floor of dark compartment. This electric shock was delivered through a stimulator (50 Hz, 2 s, 0.8 mA intensity).
Training
Both training and testing were performed between 10:00 to 13:00. All rats were placed in the experimental room for 30 min before test to habituate. First, each rat was placed in the light compartment for 30 s. Then, the guillotine door was lifted and the latency the rat crossed to the shock (dark) compartment was recorded (step-through latency, STLa).
Those animals who spent more than 120 s to cross to the other compartment were excluded from our experiment. As rat had crossed over in the dark compartment with all four paws, the observer closed the door and the animal was returned to the home cage. This trial of habituation was repeated 30 min later and was pursued after the same interval by the acquisition trial in which the door was closed and an electrical foot shock (50 Hz, 2 s, 0.8 mA intensity) was immediately delivered after the rat entrance to the dark chamber. After 20 s, the animal was removed from apparatus and temporarily placed for 2 min into the home cage. Afterwards, the animal was retested in the same approach as before. If the rat did not cross over into the dark compartment in 120 s, successful acquisition of passive avoidance response was reported. The number of trials to acquisition was recorded in the training phase.
Retention test
Twenty-four hours after training, a retention test was carried out to examine the long-term memory. Each rat was placed in the light compartment and the guillotine door was opened after 5 s. Thereafter, the STL in the retention test (STLr) was measured for animal entrance to the dark compartment. The test session was terminated once the rat either entered the dark compartment or stayed in the light compartment for 600 s, as the criterion for retention. No electric shock was applied in any of all these sessions. Moreover, the time that animal spent in the dark compartment (TDC) was captured as a measure of retention performance.
Assessing spatial memory
The spatial memory was assessed by the Morris water maze (MWM), which is a spatial learning test for rodents (Sharifzadeh et al. 2007). This test mainly relies on the cues to navigate from start locations around the perimeter of an open swimming area to locate a submerged escape platform. In brief, animals were trained to locate a submerged platform in a water pool for 4 days (in each 8 times). The pool was a custom-made black (180 cm 60 cm) filled with water and randomly divided into four equally-sized quadrants (named zones I, II, III, and IV). The maze was surrounded by opaque curtains having high-contrast visual cues (a triangle, an X, a circle, and a square). Escape latency to find hidden platform was measured. After that, probe trial was done in the 5th day without any platform. Percentage of the time spent in the target quadrant was measured (Gharebaghi et al. 2017; Rezvani-Kamran et al. 2017).
Collecting biochemical samples
After behavioral assessments, blood samples were obtained and animals were satisfied with cervical dislocation. Their hippocampi were meticulously removed and immediately rinsed with saline solution. Then, hippocampus was micro-dissected and homogenized in phosphate-buffered saline (PBS) solution (pH = 7.4) for upcoming biochemical assessment of oxidative stress biomarkers. Also, serum samples were collected by centrifugation at 3000 g for 10 min.
Measurement of biomarkers of oxidative stress
Assay of total antioxidant capacity (TAC)
It was measured via ferric reducing ability method in both kind of samples. This approach is according to the ability of reducing Fe3+ to Fe2+ in the presence of TPTZ (Tripyridyl-s-triazin). The reaction of Fe2+ and TPTZ results in a blue color complex with maximum absorbance at 593 nm (Ranjbar et al. 2018).
Assay of total thiol groups (TTG)
To evaluate the total thiol groups in each serum and homogenized hippocampus sample, DTNB (di-thio-nitro-benzoic acid) was utilized as a reagent. DTNB reacts with thiol molecules and makes a yellow complex which has an optimal absorbance at 412 nm in spectrophotometer (Hosseini et al. 2019).
Assay of cellular lipid peroxidation (LPO)
In order to measure the rate of lipid peroxidation, we used thiobarbituric acid (TBA) which reacts with lipid peroxide molecules. Both serum and hippocampus samples were mixed with TCA (Trichloric acetic acid 20%) and the precipitate was dispersed in H2SO4 (0.05 M). TBA (0.2% in sodium sulfate 2 M) was added and then heated for 30 min in boiling water bath. TBARS (Thiobarbituric acid reacting substances) adducts including malonedialdehyde (MDA) were extracted by n-butanol and the absorbance was measured at 532 nm. This reaction occurs in high temperature and acidic pH. The maximum absorption was measured as a pink color at 532 nm (Ghadermazi et al. 2018).
Assay of total protein
Protein concentrations in all samples were measured through the Bradford method by using concentrated Coomassie blue reagent. We used bovine serum albumin as a standard (Bradford 1976).
Assessment of DNA damage level
The level of DNA damage, as evaluated by 8-OHdG content, in the serum and hippocampus samples was investigated through the rat enzyme-linked immunosorbent assay (ELISA) kit (Cell Biolabs, San Diego, CA) that is based on the Biotin double antibody sandwich technology. These particular assay kits were chosen due to their high degree of specificity, sensitivity as well as inter- and intra-assay precision.
Statistical analysis
SPSS version 18 (SPSS Inc., Chicago, Ill., USA) was used to assess the number of trials as well as the STL and TDC between the mentioned experimental groups by utilizing one-way ANOVA followed by a Tukey’s test. The same statistical method was applied to analyze the differences between group means for biochemical investigations. All results were described as mean ± SEM. The level of significance in all experiments was considered p < 0.05.
Results
Effects of VitD and Aβ on passive avoidance memory test in male rats
A one-way ANOVA illustrated that no significant differences were observed in the number of trials to acquisition among all groups (data not shown). Additionally, no significant discrepancies were found in the STLa in the first acquisition trial (before receiving electrical shock) between all groups of this study (data not shown), indicating that the exploratory and native behaviors of the various groups of rats to the dark compartment was not different in our study. As shown in Fig. 1a, the retention test, conducted 24 h after training, demonstrated a significant decrease in the STLr among Aβ and VitD + Aβ groups (both at p < 0.0001) compared with control and both shams. On the other hand, the STLr level in VitD and VitD + Aβ groups was significantly greater than Aβ treatment (both at p < 0.0001).
a Effects of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on the step-through latency (STLr) during retention of passive avoidance test. Data are expressed as means ± SEM. There were eight animals in each of the treated groups. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (*** p < 0.0001 vs. control, sham solvent and sham solvent groups; ### p < 0.0001 vs. Aβ group). b Effects of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on the time spent in the dark compartment (TDC) in comparison to three control groups. Data are expressed as means ± SEM. There were eight animals in each of the treated groups. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (* p < 0.05 and *** p < 0.0001 vs. control, sham solvent and sham solvent groups; ### p < 0.0001 vs. Aβ group)
Variations in the TDC between the experimental groups were observed too (Fig. 1b). We found a statistically significant increase in TDC in the Aβ group (p < 0.0001), compared to the control, sham surgery, and sham solvent groups. Besides, both groups of VitD and Aβ + VitD exhibited significant lowered levels in comparison to the control, sham surgery, and sham solvent groups (both at p < 0.036) and in comparison with Aβ group (p < 0.0001).
Effects of VitD and Aβ on spatial learning and memory in male rats in the Morris water maze test
The data achieved from MWM test revealed that there was no significant discrepancy between study groups in travelled distance (p > 0.05) (Fig. 2a). In addition, an overall shortened trend was detected in the escape latency of all groups after animals were trained. We observed a significant increase in Aβ-treated group (p < 0.0001) in the escape latency of MWM test, compared with control, sham surgery, sham solvent, and VitD groups (Fig. 2b). This indicates the presence of more severe spatial memory deficits by taking longer to find the hidden platform (latency). By contrast, a combination treatment of Aβ and VitD resulted in a decreased latency as compared to rats treated only with Aβ (p < 0.011). Furthermore, a significant decrease (p < 0.025) was found in the time spent in the target quadrant during the probe trial in Aβ group in comparison with control, sham solvent, and sham surgery groups (Fig. 2c). Nonetheless, the increase of this parameter in Aβ and VitD co-administration group did not reach a level of significance in comparison with Aβ alone (p > 0.05).
a Effects of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on the travelled distance in Morris water maze (MWM) test in male rats. Data are expressed as means ± SEM. There were eight animals in each of the treated groups. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (no significant changes were seen between groups). b Effects of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on the escape latency in Morris water maze (MWM) test in male rats, compared with three control groups. Data are expressed as means ± SEM. There were eight animals in each of the treated groups. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (*** p < 0.0001 vs. control, sham solvent and sham solvent groups; # p < 0.05 vs. Aβ + VitD group). c Effects of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on the percentage of time spent in the target quadrant of Morris water maze (MWM) test during probe trial. Data are expressed as means ± SEM. There were eight animals in each of the treated groups. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (* p < 0.05 vs. control, sham solvent and sham solvent groups)
Comparison of TAC level between groups in hippocampus and serum
The Alzheimer’s group (treated with Aβ) demonstrated a significant decrease in TAC level in comparison with groups of control, sham solvent and sham surgery both in hippocampus and serum samples (p < 0.0001). Also, the level of TAC in VitD group was significantly elevated in comparison with the first three groups (p < 0.0001 in hippocampus, p < 0.05 in serum). The groups of VitD and co-administration of Aβ and VitD revealed a statistically increase in the TAC level, compared with Aβ group in hippocampus and serum (p < 0.0001) (Figs. 3 and 4).
Effect of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on mean of total antioxidant capacity (TAC) in hippocampus of male Wistar rats (n = 8 in each group). Data are presented as mean ± SEM. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (*** p < 0.0001 vs. control, sham solvent and sham solvent groups; ### p < 0.0001 vs. Aβ group)
Effect of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on mean of total antioxidant capacity (TAC) in serum of male Wistar rats (n = 8 in each group). Data are presented as mean ± SEM. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (* p < 0.05 and *** p < 0.0001 vs. control, sham solvent and sham solvent groups; ### p < 0.0001 vs. Aβ group)
Comparison of TTG level between groups in hippocampus and serum
For the hippocampus tissues (Fig. 5), the Aβ treated group showed a significantly diminished TTG level, compared with control, sham solvent and sham surgery groups (p < 0.0001). Besides, there was significant increased levels of TTG for VitD (p < 0.0001) and Aβ + VitD (p < 0.01) groups when compared to Alzheimer’s group (Aβ). Also, serum samples of Aβ were significantly reduced as compared to the control, sham solvent and sham surgery groups (p < 0.05). VitD administration lead to a significantly increased serum TTG level in comparison with three control groups of this study (p < 0.0001) and Aβ group (p < 0.0001) (Fig. 6).
Effect of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on mean of total thiol groups (TTG) in hippocampus of male Wistar rats (n = 8 in each group). Data are presented as mean ± SEM. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (*** p < 0.0001 vs. control, sham solvent and sham solvent groups; ## p < 0.01 and ### p < 0.0001 vs. Aβ group)
Effect of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on mean of total thiol groups (TTG) in serum of male Wistar rats (n = 8 in each group). Data are presented as mean ± SEM. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (* p < 0.05 and *** p < 0.0001 vs. control, sham solvent and sham solvent groups; ### p < 0.0001 vs. Aβ group)
Comparison of LPO level between groups in hippocampus and serum
The level of MDA was considered as a biomarker for cellular lipid peroxidation. In both hippocampus and serum samples (Figs. 7 and 8), the level of MDA was significantly promoted in comparison with control and two sham groups (both at p < 0.0001). In line, MDA level of Aβ + VitD group was enhanced in hippocampus (p < 0.0001) and serum (p < 0.01) samples as compared to the same three groups. By comparing to the Alzheimer’s group, both treatments of VitD and Aβ + VitD caused significant declined levels (p < 0.0001) in hippocampus and serum of animals.
Effect of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on mean of lipid peroxidation (LPO) in hippocampus of male Wistar rats (n = 8 in each group). Data are presented as mean ± SEM. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (** p < 0.01 and *** p < 0.0001 vs. control, sham solvent and sham solvent groups; ### p < 0.0001 vs. Aβ group). MDA: malonedialdehyde
Effect of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on mean of lipid peroxidation (LPO) in serum of male Wistar rats (n = 8 in each group). Data are presented as mean ± SEM. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (*** p < 0.0001 vs. control, sham solvent and sham solvent groups; ### p < 0.0001 vs. Aβ group). MDA: malonedialdehyde
Comparison of DNA damage between groups in hippocampus and serum
The amount of DNA damage was significantly enhanced in Aβ and Aβ + VitD groups in serum (both at p < 0.0001). In a corresponding way, Aβ group in hippocampi tissues was elevated too (p < 0.0001). Furthermore, the level of DNA damage in VitD and Aβ + VitD groups in hippocampus and VitD group of serum samples was significantly lower than that of Aβ group (all at p < 0.0001) (Figs. 9 and 10).
Effect of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on mean of DNA damage in hippocampus of male Wistar rats (n = 8 in each group). Data are presented as mean ± SEM. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (*** p < 0.0001 vs. control, sham solvent and sham solvent groups; ### p < 0.0001 vs. Aβ group)
Effect of vitamin D (VitD), amyloid-beta peptide (Aβ) and their co-administration on mean of DNA damage in serum of male Wistar rats (n = 8 in each group). Data are presented as mean ± SEM. Comparisons were obtained with a one-way ANOVA, which was followed by a post hoc Tukey test (*** p < 0.0001 vs. control, sham solvent and sham solvent groups; ### p < 0.0001 vs. Aβ group)
Discussion
In the present study, we aimed to investigate the behavioral and biochemical aspects of VitD effect in an animal model of AD. We only involved male rats to exclude the possible hormonal effects and to unify the results in our study. In addition, there are evidences that male rats demonstrate higher levels of novelty-seeking behavior than females (Cyrenne and Brown 2011). By recruiting passive avoidance memory and spatial memory tests, we demonstrated that VitD administration could improve the impaired Aβ-induced memory in Alzheimer’s group of male rats. Moreover, we observed the beneficial effects of VitD on biochemical biomarkers of stress oxidative such as TAC, TTG, LPO, and DNA damage in the hippocampus and serum samples of the same animals.
The plasma levels of antioxidants comprising vitamin E, vitamin A, and vitamin C were previously reported to be declined in AD patients (Wang et al. 2014). Further evidence in support of causative role of oxidative imbalance in the AD pathogenesis arise from experiments illustrating that antioxidant vitamin deficiency alone is capable of inducing neurological deficits similar to those in AD (Aslam et al. 2004). Recent studies have highlighted the various roles of VitD in the nervous system, exclusively in crucial survival mechanisms such as regulation of oxidative stress and immune functions (Gezen-Ak et al. 2014). VitD has illustrated various biological targets mediated by VDRs including CNS (Annweiler et al. 2013). VDRs exist in neurons and glial cells influencing multiple physiological processes in all areas essential for cognition such as hippocampus, cortex, subcortex and hypothalamus (Pogge 2015). Though AD is mainly characterized via abnormal accumulation of the neurotoxic oligomer Aβ peptide as the neuropathological diagnostic criterion of the disease; however, these phenomena are predominantly initiated and augmented by oxidative stress, a process in which an imbalance occurs between antioxidants and oxidants in favor of the latter (Huang et al. 2016). It is well-known that there is a long latent period in AD before appearance of symptoms and reaching to a diagnosis. This interim phase is known as mild cognitive impairment (MCI) in which no significant increase of senile plaques is observed. Importantly, MCI individuals exhibit significant oxidative imbalance by comparing to age-matched healthy subjects (Wang et al. 2014). This can result in cellular degeneration and interactions of reactive oxygen species (ROS) with proteins, lipids, nucleic acids, and other molecules altering their structure and function (Jiang et al. 2016).
In behavioral neuroscience, the MWM and passive avoidance tests are the most common approach to study the learning and memory abilities (Zong et al. 2017). The MWM test is recognized as a broadly used approach to examine the spatial learning and memory in rats by providing a highly reliable form of cognitive evaluation (Vouros et al. 2018). Here, we found that the Alzheimer’s group (animals treated with Aβ) had an increased escape latency in acquisition (days 2 to 4) and that the time spent in the target quadrant for this group was also significantly reduced, suggesting the Aβ deleterious effect on memory retention. On the other hand, the combined treatment of VitD and Aβ seemed to improve this impaired spatial learning and memory close to the control groups. This finding confirms the previous studies showing neuroprotective aspect of VitD in multiple related diseases (Latimer et al. 2014; Yu et al. 2011).
Similar results were also achieved in the passive avoidance test and it was demonstrated that Aβ alters cognitive performance (Shankar et al. 2008; Wang et al. 2012). The effect of VitD, alone or combined with Aβ, in increasing the STLr and reducing the TDC during retention test illustrates facilitatory effects of VitD on the memory retention both in healthy and Alzheimer subjects. A large body of research explains the relationship between VitD status and risk of brain disorders. These investigations, including cross-sectional studies in case-control designs and longitudinal cohort studies linking the later incidence of brain disorders and baseline VitD status, provide key contribution of VitD in dementia and cognitive function (Annweiler et al. 2009; Annweiler et al. 2013; Balion et al. 2012), Parkinson’s disease (Knekt et al. 2010), schizophrenia or psychosis (Murri et al. 2013; Valipour et al. 2014), depression (Anglin et al. 2013) and autism (Eyles 2010). The results of these papers are by no means consistent; however, they offer sufficient evidence for VitD involvement. Findings obtained from behavioral tests in this study confirm the effects of VitD as an effective antioxidant agent on learning and memory in a passive avoidance task in male rats in the Aβ protein-induced AD animal model.
Moreover, neurochemical changes have been well documented as potential biomarkers in neurodegenerative disorders (Rapoport and Nelson 2011). Our data supports these results by showing significant elevated level of LPO and DNA damage along with decrease in TAC and TTG (as an indicator of GSH/GSSG ratio) levels in rats with AD as compared to the control, sham solvent, and sham surgery groups. The two sham groups were recruited to investigate the validity of our measurements. We also confirmed these results in serum of animals treated with Aβ, emphasizing the significance of dysregulated oxidative stress biomarkers in this condition.
In particular, we observed that levels of LPO and DNA damage were increased in Aβ group and co-administration of VitD + Aβ resulted in their lowered levels. Free radicals attack to the brain membrane phospholipids that are composed of polyunsaturated fatty acids and highly vulnerable to ROS. This leads to the enhanced LPO levels that is the most outstanding characteristic in which degenerative changes are most executed in the AD brain (Huang et al. 2016; Markesbery 1997). As well, DNA damage can be a consequence of oxidation in the brain, which will produce DNA-protein crosslinking, strand breaks, sister chromatid exchange, and base modification (Cooke et al. 2003). Our findings are consisted with preceding studies showing greater levels of these oxidative biomarkers in AD patients (Butterfield et al. 2010a; Butterfield et al. 2010b). Furthermore, VitD caused the improved levels of TAC and TTG in group of VitD + Aβ as compared to Aβ group. Oxidation of proteins in brain may be a pivotal event owing to the fact that it affects enzymes critical to the glial and neural functions (Butterfield et al. 1997). Protein oxidation also can provoke the formation of advanced glycation end products as a post-translational modification of proteins that motivate non-enzymatic reaction of proteins with monosaccharides (Huang et al. 2016). Our results are well in line with that of Calvello et al. study (Calvello et al. 2017) depicting beneficial features of VitD treatment in an animal model of Parkinson’s disease. Finally, although oxidative damage is an important element of AD pathogenesis, there are certainly other driving forces of the disease progression. It should be noted that in addition to the antioxidant effects of VitD, the anti-inflammatory activity of VitD can also retain positive effects (Briones and Darwish 2012) and this can thoroughly be considered for future studies in this field to pave the road for enhancing current clinical and pharmacological designs. As the next steps, we can suggest to investigate the combined influence of VitD and well-known chemicals in other cellular mechanisms to seek the potential improved results.
Conclusion
Briefly, for the first time we showed that VitD administration could improve the impaired Aβ-induced memory in Alzheimer’s group of male rats. Furthermore, we observed the beneficial effects of VitD on biochemical biomarkers of stress oxidative such as TAC, TTG, LPO, and DNA damage in the hippocampus and serum samples of the same animals. Altogether, our results provide new avenues for further assessment and application of VitD in neurodegenerative disorders such as AD to enlighten the involved mechanisms.
References
Anglin RE, Samaan Z, Walter SD, McDonald SD (2013) Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry 202:100–107
Annweiler C, Allali G, Allain P, Bridenbaugh S, Schott AM, Kressig RW, Beauchet O (2009) Vitamin D and cognitive performance in adults: a systematic review. Eur J Neurol 16:1083–1089
Annweiler C, Llewellyn DJ, Beauchet O (2013) Low serum vitamin D concentrations in Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 33:659–674
Aslam A, Misbah S, Talbot K, Chapel H (2004) Vitamin E deficiency induced neurological disease in common variable immunodeficiency: two cases and a review of the literature of vitamin E deficiency. Clin Immunol 112:24–29
Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, Llewellyn DJ, Raina P (2012) Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology 79:1397–1405
Berridge MJ (2017) Vitamin D deficiency accelerates ageing and age-related diseases: a novel hypothesis. J Physiol 595:6825–6836
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Briones TL, Darwish H (2012) Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J Neuroinflammation 9:244
Butterfield DA et al (1997) Oxidatively induced structural alteration of glutamine synthetase assessed by analysis of spin label incorporation kinetics: relevance to Alzheimer’s disease. J Neurochem 68:2451–2457
Butterfield DA et al (2010a) In vivo oxidative stress in brain of Alzheimer disease transgenic mice: requirement for methionine 35 in amyloid β-peptide of APP. Free Radic Biol Med 48:136–144
Butterfield DA, Lange MLB, Sultana R (2010b) Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer’s disease. Biochim Biophys Acta 1801:924–929
Calvello R et al (2017) Vitamin D treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson’s disease, shifting M1 to M2 microglia responses. J Neuroimmune Pharmacol 12:327–339
Cooke MS, Evans MD, Dizdaroglu M, Lunec J (2003) Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17:1195–1214
Cui X, Gooch H, Groves NJ, Sah P, Burne TH, Eyles DW, McGrath JJ (2015) Vitamin D and the brain: key questions for future research. J Steroid Biochem Mol Biol 148:305–309
Cyrenne DL, Brown GR (2011) Effects of suppressing gonadal hormones on response to novel objects in adolescent rats. Horm Behav 60:625–631
Dankers W, Colin EM, van Hamburg JP, Lubberts E (2017) Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol 7:697
DeLuca G, Kimball S, Kolasinski J, Ramagopalan S, Ebers G (2013) The role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol 39:458–484
Eyles DW (2010) Vitamin D and autism: does skin colour modify risk? Acta Paediatr 99:645–647
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ (2005) Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat 29:21–30
Gezen-Ak D, Yılmazer S, Dursun E (2014) Why vitamin D in Alzheimer’s disease? The hypothesis. J Alzheimers Dis 40:257–269
Ghadermazi R et al (2018) Hepatoprotective effect of tempol on oxidative toxic stress in STZ-induced diabetic rats. Toxin Rev 37:82–86
Ghahremanitamadon F, Shahidi S, Zargooshnia S, Nikkhah A, Ranjbar A, Soleimani Asl S (2014) Protective effects of Borago officinalis extract on amyloid β-peptide (25–35)-induced memory impairment in male rats: a behavioral study. Biomed Res Int 2014:1–8
Gharebaghi A et al (2017) Treadmill exercise attenuates 3, 4-methylenedioxymethamphetamine-induced memory impairment through a decrease apoptosis in male rat hippocampus. J Neurosci Res 95:2448–2455
Hosseini SA, Saidijam M, Karimi J, Azari RY, Hosseini V, Ranjbar A (2019) Cerium oxide nanoparticle effects on paraoxonase-1 activity and oxidative toxic stress induced by malathion: a potential antioxidant compound, yes or no? Indian J Clin Biochem 34:1–6
Huang WJ, Zhang X, Chen WW (2016) Role of oxidative stress in Alzheimer’s disease. Biomed Rep 4:519–522
Jabbarpour Z, Shahidi S, Saidijam M, Sarihi A, Hassanzadeh T, Esmaeili R (2014) Effect of tempol on the passive avoidance and novel object recognition task in diabetic rats. Brain Res Bull 101:51–56
Jiang T, Sun Q, Chen S (2016) Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog Neurobiol 147:1–19
Keeney JT, Butterfield DA (2015) Vitamin D deficiency and Alzheimer disease: Common links. Neurobiol Dis 84:84–98
Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Sääksjärvi K, Heliövaara M (2010) Serum vitamin D and the risk of Parkinson disease. Arch Neurol 67:808–811
Latimer CS et al (2014) Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci 111:E4359–E4366
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med 23:134–147
Murri MB, Respino M, Masotti M, Innamorati M, Mondelli V, Pariante C, Amore M (2013) Vitamin D and psychosis: mini meta-analysis. Schizophr Res 150:235–239
Paxinos G, Watson C (1986) Atlas of the rat brain in stereotaxic coordinates. Academic, New York
Pogge E (2015) Vitamin D for the prevention of Alzheimer’s disease. In: Bioactive nutraceuticals and dietary supplements in neurological and brain disease. Elsevier, pp 475–479
Ranjbar A, Asl SS, Firozian F, Dartoti HH, Seyedabadi S, Azandariani MT, Ganji M (2018) Role of cerium oxide nanoparticles in a paraquat-induced model of oxidative stress: emergence of neuroprotective results in the brain. J Mol Neurosci 66:420–427
Rapoport SI, Nelson PT (2011) Biomarkers and evolution in Alzheimer disease. Prog Neurobiol 95:510–513
Rezvani-Kamran A, Salehi I, Shahidi S, Zarei M, Moradkhani S, Komaki A (2017) Effects of the hydroalcoholic extract of Rosa damascena on learning and memory in male rats consuming a high-fat diet. Pharm Biol 55:2065–2073
Salim S (2017) Oxidative stress and the central nervous system. J Pharmacol Exp Ther 360:201–205
Shankar GM et al (2008) Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14:837
Sharifzadeh M et al (2007) Protective effects of chronic lithium treatment against spatial memory retention deficits induced by the protein kinase AII inhibitor H-89 in rats. Pharmacology (Basel) 80:158–165
Valipour G, Saneei P, Esmaillzadeh A (2014) Serum vitamin D levels in relation to schizophrenia: a systematic review and meta-analysis of observational studies. J Clin Endocrinol Metabol 99:3863–3872
Vouros A et al (2018) A generalised framework for detailed classification of swimming paths inside the Morris Water Maze. Sci Rep 8:15089
Wang C et al (2012) The phosphodiesterase-4 inhibitor rolipram reverses Aβ-induced cognitive impairment and neuroinflammatory and apoptotic responses in rats. Int J Neuropsychopharmacol 15:749–766
Wang X, Wang W, Li L, Perry G, Lee H-G, Zhu X (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta 1842:1240–1247
Yu J, Gattoni-Celli M, Zhu H, Bhat NR, Sambamurti K, Gattoni-Celli S, Kindy MS (2011) Vitamin D 3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J Alzheimers Dis 25:295–307
Zong L, Chu P, Huang P, Guo Y, Lv Y (2017) Effect of vitamin D on the learning and memory ability of FGR rat and NMDA receptor expression in hippocampus. Exp Ther Med 14:581–586
Acknowledgements
This study was financially supported by the Vice-Chancellor for research and technology of Hamadan University of Medical Sciences (Grant Number: 9412116950).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehri, N., Haddadi, R., Ganji, M. et al. Effects of vitamin D in an animal model of Alzheimer’s disease: behavioral assessment with biochemical investigation of Hippocampus and serum. Metab Brain Dis 35, 263–274 (2020). https://doi.org/10.1007/s11011-019-00529-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-019-00529-7