Abstract
It has been recognized that drug addiction engages aberrant process of learning and memory, and substantial studies have focused on developing effective treatment to erase the enduring drug memories to reduce the propensity to relapse. Extinction, a behavioral intervention exposing the individuals to the drug-associated cues repeatedly, can weaken the craving and relapse induced by drug-associated cues, but its clinic efficacy is limited. A clear understanding of the neuronal circuitry and molecular mechanism underlying extinction of drug memory will facilitate the successful use of extinction therapy in clinic. As a key component of mesolimbic system, medial prefrontal cortex (mPFC) has received particular attention largely in that PFC stands at the core of neural circuits for memory extinction and manipulating mPFC influences extinction of drug memories and subsequent relapse. Here, we review the recent advances in both animal models of drug abuse and human addicted patients toward the understanding of the mechanistic link between mPFC and drug memory, with particular emphasis on how mPFC contributes to the extinction of drug memory at levels ranging from neuronal architecture, synaptic plasticity to molecular signaling and epigenetic regulation, and discuss the clinic relevance of manipulating the extinction process of drug memory to prevent craving and relapse through enhancing mPFC function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Addiction is a chronic relapsing disorder, and drug-associated cues are crucial contributors to the enduring craving, compulsive drug-taking behaviors and relapse (Baler and Volkow 2006; Everitt and Robbins 2005). Finding effective treatment to prevent the propensity to craving and relapse elicited by drug-associated cues is one of the major goals of addiction research. It has been acknowledged that once entering the brain, the addictive drugs trigger a distorted process of learning and memory during which drug-associated cues (i.e. drugs, drug paraphernalia, environmental context, and et al.) serve as conditioned stimulus (CSs) while drug-associated effects (rewarding or aversive) serve as unconditioned stimulus (UCSs) (Hyman 2005; Milton and Everitt 2012a). Exposure to CSs leads to two processes, reconsolidation and extinction, and both processes reorganize and update the consolidated traces of drug memories (Rich et al. 2016; Taylor et al. 2009). Targeting the mechanisms of reconsolidation and extinction of drug memories is a promising strategy for prevention of drug craving and relapse (Milton and Everitt 2012b; Taylor et al. 2009; Torregrossa and Taylor 2013). Based on the theories of drug memory extinction, cue-exposure has been advocated as a treatment for addiction (Heather and Bradley 1990; Torregrossa and Taylor 2013), but clinically, the efficacy of cue-exposure therapy (CET) is limited. The predominant view is that extinction does not erase the original CS-UCS association. Instead, extinction forms a new association between CS and no UCS, and this new association inhibits the original one (Bouton 2004; Torregrossa and Taylor 2013; Xue et al. 2012). A better understanding of the mechanism underlying extinction of drug memory will facilitate the successful use of CET in practice. In this review, we attempt to unveil the mechanistic link between medial prefrontal cortex (mPFC) and the extinction of drug memories, since mPFC holds a central role in the regulation of both cue-induced drug-seeking and response inhibition. There have been a number of excellent reviews regarding the role of mPFC regulating drug-seeking and relapse (George and Koob 2010; Goldstein and Volkow 2011; Gourley and Taylor 2016; Kalivas 2008; Lasseter et al. 2010; Milton and Everitt 2012a; Moorman et al. 2015; Peters et al. 2009; Schoenbaum et al. 2016; Van den Oever et al. 2010). We here focus on recent research progresses on the circuitry and molecular mechanisms responsible for mPFC engagement in extinction of drug memories. We also summarize the studies on mPFC dysfunction in addicted patients and discuss its clinic relevance with deficit of memory extinction. Lastly, we review the manipulations of memory extinction to in an attempt to erase drug memory and prevent drug craving and relapse through enhancing the mPFC function. Before these, we make a brief summary on the anatomy of mPFC and animal model of extinction memory.
mPFC and its role in extinction of drug memory
mPFC anatomy
A great number of literatures have reviewed the definition, anatomy, and function of mPFC (Heidbreder and Groenewegen 2003; Ongur and Price 2000; Ridderinkhof et al. 2004; Uylings et al. 2003), we only make a brief summary on the anatomy of mPFC. Since Broadmann performed the first topographical study of prefrontal cortex (PFC) across species from rodents to primates, continuous effort has been made to understand this brain region in terms of its definition, boundary, subdivision and function. In humans, the PFC is located in the anterior portion of the frontal lobe, and cytoarchitectonically defined as Broadmann area 8 to 14 and Braodamann area 44 to 47 (Ongur et al. 2003). From the functional perspective, the PFC in human are divided into dorsolateral, dorsomedial, ventromedial, and orbital prefrontal subregions. Based on the anatomical and functional criteria, the rodent PFC includes a medial region, the mPFC, and a lateral region, the orbitofrontal cortex (OFC). The mPFC can be further divided into four subregions including anterior cingulate cortex (ACC), precentral cortex (PrC), prelimbic (PL), infralimbic (IL) (Conde et al. 1995; Moorman et al. 2015; Ongur and Price 2000). Evolutionarily, there are considerable homologies in PFC across species. The IL subregion of mPFC in rodent is homologous to the primate orbitomedial cortex, and the PL subregion in rodent to the primate lateral/dorsolateral cortex in function (Vertes 2006). In the studies we discuss here, the dorsal mPFC is frequently referred to PL and ACC, while ventral mPFC (vmPFC) to IL.
The PL and IL are the two most studied mPFC subregions for memory extinction. There is no clear anatomical boundary between the two subregions. Thus, although the PL and IL function differently, they are not poles apart. The projections from PL and IL to the thalamus (for example, the mediodorsal, intermediodorsal, reuniens, and paraventricular nucleus) and the cortex (for example, the medial orbital cortex and insular cortex) are overall similar (Vertes 2004). However, for some other targets such as nucleus accumbens (NAc), amygdala, and brainstem, the projections from these two brain regions differ drastically. While the PL projects to the core and shell of NAc, the IL projects to the shell only. The PL projects to the central and the basolateral nucleus, while the IL to the central, medial, basomedial, and cortical regions of amygdala (Gabbott et al. 2005; McDonald et al. 1996). The PL projects to the median raphe and dorsal nuclei, and the IL to the solitary and parabrachial nuclei of the brainstem (Gabbott et al. 2005; McDonald et al. 1996; Moorman et al. 2015; Vertes 2004).
Extinction of drug memories and the animal models
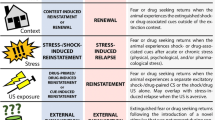
A great number of literatures have excellently reviewed the animal model of drug craving and relapse (Marchant et al. 2013; Nic Dhonnchadha and Kantak 2011; O'Brien and Gardner 2005; Sanchis-Segura and Spanagel 2006; Shaham et al. 2003; Vanderschuren and Ahmed 2013; Venniro et al. 2016); we would like to give a very brief introduction description of the two most used rodent models for studying the extinction of drug memories. The first one is the self-administration model (Bossert et al. 2013; Crombag et al. 2008; Epstein et al. 2006; Shaham et al. 2003). In this model, animals are firstly trained to acquire the association between an action (usually a nose-poke, a lever-press or a chain-pull) and infusion of drug (typically intravenous), a process called conditioning. During conditioning, a cue (or cues) is presented in conjunction with each drug delivery. The cue can be classified as a discrete cue and a discriminative cue (Burattini et al. 2007; Ciccocioppo et al. 2003; Perry et al. 2014). Drug dosing is response-dependent, which mimics human drug addiction. Animals’ motivation for drug is measured by their responses. Then animals go through extinction training in which the same action no longer brings about drug supply, and the self-administration behavior extinguishes gradually. In other cases, animals do not go through extinction training, but are simply enforced to abstain from drug, which is known as withdrawal (Augur et al. 2016; Lu et al. 2004; Tran-Nguyen 1998). Lastly, during the reinstatement test of drug seeking, animals are presented with the same drug-related cues used at the conditioning stage after a passage of time (spontaneous recovery), changed context (renewal) or drug itself (priming), which may trigger the reoccurrence of drug seeking (Luo et al. 2015; Torregrossa et al. 2013; Torregrossa et al. 2010; Xue et al. 2012). The second is the conditioned place preference (CPP)/conditioned place aversion (CPA) model. In the procedure, the event that induces significant motivation (US), typically the drug delivery in CPP, or the drug withdrawal in CPA, is repeatedly presented in a certain environment (CS), and the neutral stimuli (vehicle) are given in a second or third environment. An animal’s preference or aversion for drug is measured by its preference for the specific environment paired with the delivery of drug or drug withdrawal. The procedure of CPP/CPA extinction training was similar to the establishment of CPP/CPA, with the exception that the drugs were replaced by saline. During the relapse test of CPP/CPA, animals are exposed to the CPP/CPA context after a passage of time (spontaneous recovery) or drug itself (priming), which may trigger the re-emergence of CPP/CPA (Cunningham et al. 2006; Moorman et al. 2015; Tzschentke 1998). Similar to extinction memory of fear, extinction responding of drug self-administration or CPP is susceptible to reinstatement, and spontaneous recovery (Xue et al. 2012). The ineffectiveness of CET in clinic may be due to the acute exposure to the addictive drug itself (Jaffe et al. 1989; Venniro et al. 2016), the passage of time (Di Ciano and Everitt 2002; Shaham et al. 1997), changes of environmental context (Bouton and King 1983; Hamlin et al. 2008; Khoo et al. 2017; Marchant et al. 2015). Because CET is conducted in a clinical setting, addicted patients may confront the three factors in the real life. Thus, enhancing the extinction of drug memories may prevent relapse under the above circumstances. For the two kinds of animal models, manipulations can be made before, during or after the extinction training to explore whether the manipulations inhibit or facilitate the extinction training (Chen et al. 2016; Gass and Olive 2009; LaLumiere et al. 2010; Wang et al. 2012) and subsequently, influence the priming-induced reinstatement (Malvaez et al. 2010; Reichel et al. 2011; Xue et al. 2012), renewal (Luo et al. 2015; Torregrossa et al. 2013; Torregrossa et al. 2010) or spontaneous recovery (Degoulet et al. 2016; Malvaez et al. 2013; Peters et al. 2008b; Xue et al. 2014) of drug-seeking behaviors.
Roles of different mPFC subregions in drug extinction memories
Pharmacological inactivation of PL causes deficit in pressing for drug during the relapse test, indicating a promoting influence of PL in drug relapse after extinction. The promoting effect of PL in drug relapse was quite similar to its well-established facilitating role in the expression of conditioned fear after extinction. On the other hand, pharmacological inactivation of IL following extinction training augments the drug-seeking behavior, and, activation of this subdivision reduces the relapse, suggesting an active role of IL in limiting drug relapse after extinction (Peters et al. 2008a). In a recent study using cocaine self-administration, it was observed that IL neurons activation via viral-mediated gene transfer of designer receptors (DREADDs) decreased relapse of cocaine seeking triggered by drug-related cues, but only under the condition that previous extinction training was conducted. The findings further support that IL is critical for cocaine memory extinction (Augur et al. 2016).
Though a large number of studies suggest the IL/PL dichotomy in drug extinction/drug seeking, increasing researches report inconsistent results and raises controversies over the IL/PL dichotomy (Gourley and Taylor 2016). It is not surprising, as the mPFC is not a single structure but a massive complexity. A mouse study found that optogenetic activation of IL pyramidal cell promoted the extinction of remote memory rather than recent memory of cocaine, whereas inactivation of the same region blocked extinction learning of recent memory rather than remote memory (Van den Oever et al. 2013), suggesting that the IL’s role in the extinction of conditioned cocaine memory is dual and time-dependent. Another study employed discriminative-stimulus-based self-administration task to measure cocaine-seeking behavior and found that pharmacological inhibition of IL and PL induced greater lever pressing during the CS presentation (Gutman et al. 2017). Clearly, these results are inconsistent with the classic PL/IL dichotomy and indicate that both IL and PL are involved with the inhibitory control of cocaine seeking.
In contrast with the classic view that IL facilitates cocaine memory extinction, the IL, however, may drive heroine seeking. For example, selective pharmacogenetic inactivation of IL neurons enhanced heroine memory extinction (Bossert et al. 2011), as did administration of CB1 antagonist (Alvarez-Jaimes et al. 2008) and GABA receptor agonists (Alvarez-Jaimes et al. 2008; Rogers et al. 2008) in IL. Likewise, for alcohol addiction, the results are inconsistent for the role of IL in extinction. Inactivation with baclofen/muscimol in IL had no impact on the extinction of alcohol memory, but delayed the first response on test in extinction context (Willcocks and McNally 2013). Besides, inactivating IL with GABA receptor agonist resulted in decreased cue-induced reinstatement of extinguished memory of methamphetamine (Rocha and Kalivas 2010). Thus, it appears that the roles of the IL in extinction of drug memory are not consistent among different studies, which may be attributable to different types of drugs and training protocols.
Mechanisms underlying the role of PFC in the extinction of drug memory
Circuit mechanisms
PFC-NAc circuit
Recent studies have begun to examine the PFC-based circuit mechanisms that mediate extinction of drug-seeking behavior. The mPFC-NAc pathway may play an important role in regulating extinction and reinstatement of drug seeking after extinction (Bossert et al. 2013; Li et al. 2015; McFarland et al. 2003). The NAc core subregion receives inputs primarily from the PL, whereas the NAc shell subregion receives input primarily from the IL (Krettek and Price 1977; Sesack et al. 1989). A great number of literatures suggests that projections of PrL-NAc core and IL-NAc shell may play different roles in extinction of drug memories (Chen et al. 2016; Kalivas and O'Brien 2008; Peters et al. 2008a). The glutamatergic projections in mPFC-NAc core are mainly involved in regulating drug-seeking responses (Kalivas 2009; Kalivas and McFarland 2003), and pharmacological or optogenetic inhibition of the projection diminishes cue- or drug-induced reinstatement of drug-seeking behavior and the potentiation of transient synaptic potentiation in medium spiny neurons (MSNs) (LaLumiere and Kalivas 2008; Shen et al. 2014; Stefanik et al. 2016). The glutamatergic projections from mPFC (mainly IL) to the NAc shell are primarily engaged in suppressing conditioned drug seeking after extinction learning (Chen et al. 2016; Peters et al. 2009). Blocking this pathway results in resumption of cocaine seeking (Peters et al. 2008a). Pharmacological inactivation of the NAc shell increases cocaine seeking under extinction conditions (Fuchs et al. 2008; Peters et al. 2008a). Furthermore, by using a retro-DREADD approach to confine the expression Gq-DREADD to mPFC neurons that project to the medial NAc shell, it was found that these neurons are responsible for decreasing cue-induced reinstatement of cocaine seeking. Additionally, the effects of mPFC activation on cue-induced reinstatement depend on prior extinction training of self-administration, suggesting that the glutamatergic input from IL-mPFC to the NAc shell may be responsible for extinction learning (Augur et al. 2016). Moreover, extinction training during withdrawal increases the expression of the GluR1 and GluR2/3 subunits of the AMPAR in NAc shell but not core (Sutton et al. 2003). The increased GluR1 expression in NAc shell is positively associated with the degree of extinction achieved during training and negatively associated with cue-induced relapse (Sutton et al. 2003). These findings suggest that mPFC-NAc shell circuit is required for mediating extinction behavior through both presynaptic and postsynaptic mechanisms.
PFC-VTA circuit
Apart from the NAc, the mPFC also sends dense afferents to ventral tegmental area (VTA). The dopaminergic neurons in VTA can be readily activated by primary rewards (abused drugs) and reward-predicting stimuli (Wise 2009). In animals trained to self-administer cocaine, cocaine-predictive cues trigger glutamate release and dopaminergic activation in VTA (You et al. 2007). Recently, Degoulet et al. 2016 found that isradipine, a general LTCC antagonist, blocked the induction of NMDAR LTP and promoted the reversal of previously induced LTP in the VTA. Furthermore, intra-VTA injection of a CaV1.3 subtype-selective LTCC antagonist before extinction training abolished previously acquired cocaine and alcohol CPP on subsequent days, and the effect lasted at least 2 weeks (Degoulet et al. 2016). TA receives numerous glutamatergic inputs from both the PrL and IL of mPFC (Heidbreder and Groenewegen 2003), and electrical stimulation of these inputs increases glutamate release in the VTA (Rossetti et al. 1998), which may in turn trigger dopamine release. Behaviorally, the glutamatergic transmission from mPFC to VTA plays a pivotal role in reinstatement of drug-seeking behavior after extinction training (Wise 2009). However, whether these specific afferents from PrL and IL to VTA regulate extinction of drug-seeking behavior is not yet known.
PFC-MDH circuit
Medial dorsal hypothalamus (MDH) is another downstream target of mPFC that receives extensive projections from the IL of mPFC (Heidbreder and Groenewegen 2003; Thompson and Swanson 1998). In recent years, the MDH has been shown to be associated with the termination of motivated behaviors and, therefore, is recognized as a logical candidate for regulation of extinction learning. Double labeling of retrograde tracer cholera toxin B subunit (CTb) and Fos revealed recruitment of MDH projecting PFC neurons during extinction expression (Marchant et al. 2010). Infusion of the inhibitory neuropeptides known as cocaine- and amphetamine-regulated transcript (CART) into the MDH prevented extinction expression (Marchant et al. 2010), indicating that mPFC-MDH circuit is also involved in extinction expression.
Neuronal ensemble mechanisms
Neuronal ensemble is a concept which is proposed by Hebb that learned associations are encoded within specific populations of neurons that were selectively activated by environmental cues. Recently, there has been increasing interest in determining the neuronal ensembles in the mesolimbic system control of drug seeking and relapse (Cruz et al. 2013; George and Hope 2017). Current evidence suggests that different neuronal ensembles in mPFC may be responsible for promotion or inhibition of drug-seeking behavior, respectively. It has been found that a fraction of vmPFC neurons were preferentially activated by the heroin-associated context. Selective pharmacogenetic inactivation of these neurons inhibited context-induced drug relapse (Bossert et al. 2011), suggesting that a subset of neuronal ensembles in vmPFC encode the learned associations between heroin reward and heroin-associated contexts, and promote the cue-induced relapse. However, using animal model of alcohol self-administration, Pfarr et al. reported that activity-dependent ablation of neuronal ensemble in the IL but not PL induced excessive alcohol seeking (Pfarr et al. 2015). It seems that the targeted neuronal ensemble were specific for the cue-induced response because nonselective inactivation of IL neurons, using pCAG-lacZ rats, only marginally affected the cue-induced reinstatement task. These results indicate that promotional or inhibitory control over drug seeking is exerted by distinct functional ensembles within IL rather than by a general tone of this region. Indeed, two recent studies have demonstrated that Fos-expressing neuronal ensembles mediating reward and extinction memories are intermingled within the vmPFC. In the first study, Warren et al. found that inactivation of the food reward ensembles decreased food seeking, whereas inactivation of the extinction ensembles increased food seeking (Warren et al. 2016). In the second study, Suto et al. found that the same IL area is capable of controlling both promotion and suppression of reward seeking via different neural ensembles, each selectively reactive to associated or non-associated cues, and disruption of IL neurons activated by either cue exclusively altered the behavioral response as well as neural activation uniquely linked to the targeted cue. The neural ensembles in IL mediating the bidirectional control of reward seeking are most likely mutually exclusive rather than overlapping (Suto et al. 2016). These results could explain the inconsistent results about the role of mPFC in drug memory and extinction memory and promote novel strategy for enhancing extinction memory and prevention of drug relapse.
Synaptic mechanisms
Glutamatergic transmission
Glutamate is the primary excitatory neurotransmitter in the brain which acts on the ionotropic glutamate receptors including AMPA receptors (AMPARs), NMDA receptors (NMDARs), kainate receptors and the metabotropic glutamate receptors (mGluRs) (Traynelis et al. 2010). From the behavioral perspective, substantial evidence has existed for the involvement of glutamate system in addiction and extinction of multiple types of abusive drugs. Besides, intra-IL administration of PEPA, a positive allosteric modulator of AMPARs, facilitated extinction of cocaine-seeking (LaLumiere et al. 2010) as well as heroin-seeking behaviors (Chen et al. 2016). For rat model of methamphetamine CPP, transperitoneal administration of ceftriaxone during extinction training, which activates the glutamate transporter (EAAT2), prevents the drug-primed reinstatement with an increase of EAAT2 mRNA expression in mPFC (Abulseoud et al. 2012). However, intracerebroventricular administration of the AMPAR antagonist CNQX augmented the extinction process of rat morphine CPP model, hampered the increased Fos expression and blocked the phosphorylation of cAMP response element-binding protein (CREB) in PFC (Siahposht-Khachaki et al. 2017). The discrepancies of findings between the Siahposht-Khachaki et al and previous studies may be attributable to different types of drugs and routs of administration. The mGluRs are also showed to play roles in drug extinction memories. Infusion of the mGluR1/5 agonist DHPG into the IL of cocaine-experienced rats facilitated extinction of drug seeking (Ben-Shahar et al. 2013). Activation of group I metabotropic glutamate receptor subtype 5 (mGluR5) in IL but not PL facilitates extinction of cue-conditioned alcohol-seeking behavior via potentiation of glutamatergic synaptic plasticity (Gass et al. 2014), suggesting the mGluR5 as a promising drug target for facilitation of extinction learning.
While the importance of the glutamatergic receptors in learning and memory has been long appreciated, recent studies also started to revel how the receptors in mPFC adapt to the extinction of drug memory (Kalivas et al. 2005). Extinction training significantly increases the amplitude of the evoked NMDAR-mediated current in both PL and IL (Fig. 1). In contrast, the AMPAR currents in the PL but not IL are reduced after extinction training (Fig. 1). Not surprisingly, a reduction in the AMPAR/NMDAR current, an indicator of neuronal plasticity, is also found after extinction training in the PL but not IL (Gass et al. 2014). In line with the altered glutamatergic transmission, manipulation of the activity of AMPAR and NMDAR was found to influence the extinction of fear-conditioning as well as drug-seeking behavior (Myers et al. 2011; Peters et al. 2009). Increasing the AMPAR activity in IL via its positive allosteric modulator augments extinction of cocaine seeking (Oliva et al. 2018). Pharmacologically inhibiting NMDAR, particularly those containing the NR2A in IL, facilitates the extinction expression (Hafenbreidel et al. 2017). Few studies investigate how mGluRs in mPFC regulate the extinction of drug memories. A recent study shows that mGlu5R activation significantly reduced calcium activated potassium channel (KCa) currents in layer V PNs of IL. The mGluR5-dependent facilitation of long-term potentiation can be readily prevented by positive modulation of KCa channels in IL (Cannady et al. 2017), suggesting that mGluR5-mediated enhancement of extinction of alcohol-seeking behavior and synaptic plasticity in IL involves functional inhibition of KCa channels.
Schematic showing the synaptic remodeling following extinction of drug memory in the prelimbic subregion (PL) of medial prefrontal cortex. Extinction training causes robust spinogenesis in the projection neurons of PL with redistribution of glutamatergic receptors inside the synapses. It increases the number of AMPA receptors but decrease that of NMDA receptors, accompanied by an enhancement of the long-term potentiation of glutamatergic transmission onto these neurons. Please note that the synaptic remodeling by extinction varies across the mPFC subregions such as the prelimbic and infralimbic regions
In addition to altering the expression and function of glutamatergic receptors in mPFC, extinction of drug memories also causes structural remodeling of glutamatergic synapses in mPFC (Fig. 1). Gass and colleagues demonstrate that extinction training significantly increases the spine density in basal dendrites of layer V PNs in IL as compared with the forced abstinence (Gass et al. 2014). The extinction-associated increase was further potentiated by treatment with mGluR5 positive allosteric modulator which facilitates extinction learning (Gass et al. 2014). The increased spine density in IL following extinction was primarily due to an increase in the number of mature, mushroom spines (Gass et al. 2014) and associated with increased expression of F actin (Toda et al. 2006), suggesting an enhanced glutamatergic transmission. Extinction training activates Rho GTPase Rac1 in the mPFC in a brain-derived neurotrophic factor (BDNF)-dependent manner (Wang et al. 2017), and, both in vivo and vitro studies show that Rac1 plays a crucial role in spine morphogenesis through regulating the size and density of spines in neurons (Luo 2000; Nakayama et al. 2000). Despite this, whether extinction-induced Rac1 activation also contributes to the altered dendritic and spine morphology is not yet known.
The signaling pathways that regulate the glutamatergic receptors trafficking are also suggested to be important for the extinction of drug memories. Neuronal activity-regulated pentraxin (Narp) is an immediate early gene product that is secreted and binds to AMPAR (O'Brien et al. 1999). Although the Narp knockout (KO) mice are intact in instrumental and Pavlovian learning, they are deficient in extinction of morphine CPP (Crombag et al. 2009; Johnson et al. 2007). Blouin et al. suggest that it is the Narp in IL that mediates this phenotype. Viral-mediated expression of a Narp dominant-negative construct in IL of mice blocks extinction of morphine CPP while reintroduction of Narp into IL of KO mice rescues the impaired extinction of morphine CPP (Blouin et al. 2013a). Notably, viral-mediated knockdown of Narp in IL had little effect on the extinction of heroin self-administration, indicating a possibility that Narp differently affects the extinction of memory of different drugs (Blouin et al. 2013b). PKMζ, an autonomously active isozyme of protein kinase C, regulates NSF/GluR2-dependent AMPAR trafficking (Migues et al. 2010; Yao et al. 2008). He et al. showed that inhibiting PKMζ activity in IL but not PL, disrupted the expression of extinction memory of CPP and CPA, indicating that PKMζ in IL is required for the maintenance of extinction memory of morphine reward-related cues and morphine withdrawal-related aversive cues (He et al. 2011).
GABAergic transmissions
Relatively little is known about the role of GABAergic transmission in mPFC in the formation and retention of extinction memory. In the rat model of cocaine self-administration, IL inactivation with the transcranial injection of the GABA receptors agonist baclofen and muscimol during late extinction enhanced drug seeking (Peters et al. 2008a). However, in the animal model of alcohol self-administration, reversible inactivation of IL had no effect on the reinstatement or reacquisition of alcoholic beer-seeking and had no effect on extinction expression, while IL inactivation did, however, increase the latencies with which animals responded on test but only when animals were tested in the extinction context (Willcocks and McNally 2013). In the animal model of cocaine addiction, IL inactivation after extinction leads to the re-emergence of conditioned place preference (Ovari and Leri 2008), an effect which relates to the suppression of GABAergic transmission and facilitation of long-term potentiation (LTP) in vmPFC. Moreover, extinction training decreases the expression of surface GABAAR β3 subunit through dynamin-dependent GABAAR endocytosis (Wang et al. 2017), but has little influence on the AMPAR endocytosis. The extinction training induced GABAAR endocytosis may result from Rac1 activation in the vmPFC via a BDNF-dependent manner (Wang et al. 2017).
Dopaminergic transmission
Despite the extensive dopaminergic innervation of mPFC, the role of dopamine receptor in mPFC subregions in the reinstatement of cocaine seeking is far from being clearly identified. Using rat model of cocaine self-administration, it was found that IL microinfusion of the dopamine receptor 2 (D2)-like agonist quinpirole before extinction attenuated cue-primed relapse in adolescents (Zbukvic et al. 2016). There is evidence that the dysfunction of dopamine signaling may contribute to the deficit in extinction learning and susceptibility to relapse during adolescence. Extinction of drug cue associations was facilitated in adolescents by elevating dopamine and norepinephrine in the PFC with atomoxetine during extinction training. Direct microinjection of the D1 receptor agonist SKF38393 mimicked this effect and facilitated extinction in adolescent subjects (Brenhouse et al. 2010). Furthermore, infusion of quinpirole into IL prior to extinction significantly reduced cue-induced reinstatement in adolescents. This effect was replicated by acute systemic treatment with the atypical antipsychotic aripiprazole (Abilify), a partial D2R-like agonist (Zbukvic et al. 2016).
Adrenergic transmission
Using animal model of self-administration, LaLumiere et al. found that injection of clenbuterol, a β2-adrenergic receptor (AR) agonist, in IL after extinction training facilitates the retention of extinction. Local administration of β2-AR antagonist ICI in IL before extinction training inhibited extinction retention (LaLumiere et al. 2010). Consistent with this, Huang et al. found that β-arrestin-based β-adrenergic signaling in IL regulated extinction learning of cocaine-associated memories using animal model of CPP (Huang et al. 2018). Within 10 min after extinction, the administration of the nonbiased β-AR antagonist propranolol, but not the G protein-biased β-AR antagonist carvedilol, blocked extinction learning of cocaine-conditioned place preference and the associated extracellular signal-regulated kinase (ERK) activation in IL. Genetic deletion of β-arrestin2 in IL, specifically in excitatory neurons, impaired extinction learning of cocaine-conditioned place preference, which was not rescued by carvedilol (Huang et al. 2018). The adrenergic system may also strengthen the extinction memory through increasing the excitability of IL neurons in a β-AR- and PKA-dependent manner. During extinction, blockade of noradrenergic receptors with propranolol in IL prevented the acquisition of extinction memory, and, interfering with noradrenergic receptors, PKA, transcription, or protein synthesis in IL impairs retention of extinction (Mueller et al. 2008).
Molecular and epigenetic mechanisms
Despite the accumulating literature on the molecular mechanisms underlying extinction of fear memory, little is known on the extinction of drug memory. BDNF is widely known to be critical for synaptic plasticity (Korte et al. 1995; Lohof et al. 1993) and extinction of fear memory (Peters et al. 2010). Otis JM et al. found that IL infusion of BDNF enhanced the extinction of cocaine-CPP, and of ANA-12, an antagonist for BDNF tropomyosin-related kinase B (TrkB) receptor, impaired cocaine-CPP extinction. Consistently, systemic administration of the TrkB receptor agonist facilitated extinction of cocaine-CPP, indicating BDNF signaling as a promising adjunct for extinction therapy (Otis et al. 2014). It is interesting to note that infusion of BDNF in mPFC also suppresses cocaine seeking-induced molecular adaptations within the NAc (Berglind et al. 2007; Berglind et al. 2009; Sun et al. 2014), arguing for a critical role of BDNF in the extinction of drug memory. Previous studies also highlighted BDNF engagement in the extinction of learned fear, such that BDNF infusion into the IL reduced conditioned fear even in the absence of extinction training (Peters et al. 2010). Thus, BDNF appears to act as a co-regulator of the extinction for both drug and fear memory. Other neurotrophic factors, such as basic fibroblast growth factor (bFGF or FGF2) in IL, also showed a role in the formation of extinction memory of addiction. Following cocaine exposure, bFGF is increased in IL, and blocking bFGF in IL-mPFC before extinction training resulted in facilitation of subsequent extinction. However, blocking bFGF alone was not sufficient to facilitate extinction, indicating separate roles of BDNF and bFGF in the extinction of drug memories. In addition, multiple protein kinase signaling pathways, such as cyclin-dependent kinase 5, ERK and Rho GTPase Rac1 are shown to be involved in the extinction of addiction memories (Castino et al. 2018; Wang et al. 2012; Wang et al. 2017). It was found that extinction training of CPA memory led to activation of ERK and CREB in IL and intra-vmPFC infusion of ERK inhibitor U0126 (1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene) before extinction training diminished extinction of CPA behavior and the related epigenetic regulation of BDNF gene transcription (Wang et al. 2012). Extinction of CPA also activates Rho GTPase Rac1 in IL in a BDNF-dependent manner, which affects GABAAR endocytosis via triggering synaptic translocation of activity-regulated cytoskeleton-associated protein (Arc) through facilitating actin polymerization. Knockdown of Rac1 expression within the vmPFC of rats using Rac1-shRNA suppressed GABAAR endocytosis and CPA extinction, whereas expression of a constitutively active form of Rac1 accelerated GABAAR endocytosis and CPA extinction.
Epigenetics refers to the process of altering gene functions without inducing mutations of DNA. The major types of epigenetic modifications include DNA methylation, chromatin modification, non-coding RNAs, post-translational histone regulations, etc. (Nestler 2014). A great number of studies has demonstrated that the persistence of drug memories and relapse propensity are attributed to drug-induced epigenetic mechanisms regulating long-lasting drug-induced molecular alterations (Anier et al. 2010; Garrison and Potenza 2014; Nestler 2014; Pascual et al. 2012; Robison and Nestler 2011; Tian et al. 2012; Wright et al. 2015). Recently, emerging studies have been trying to reveal the epigenetic mechanisms underlying extinction memory and its inhibition on drug relapse. Sadakierska-Chudy et al. studied the effect of extinction training of cocaine self-administration on a few of genes including those encoding histone-modifying enzymes and histone proteins that control the chromatin state. It was found that at the end of extinction training, most of the analyzed genes in the rats that either actively or passively experienced cocaine administration returned to the control level (Sadakierska-Chudy et al. 2017). However, it is still needed to explore the causal link between the changes of histone-modifying enzymes in extinction of cocaine self-administration memory. Using the animal model of CPA, Wang et al. investigated the role of epigenetic regulation of BDNF gene expression in extinction of morphine-associated withdrawal memory (Wang et al. 2012). The results showed that CPA extinction training induced an increase in acetylation of histone H3 at the promoters of BDNF exon I transcript and increased BDNF mRNA and protein expression in the vmPFC of acute morphine-dependent rats. The epigenetic regulation of BDNF gene transcription could be facilitated by intra-vmPFC infusion of HDAC inhibitor trichostatin A before extinction training. Correspondingly, disruption of the epigenetic regulation of BDNF gene transcription blocked extinction of CPA behavior. Histone modifications are also found to be involved in extinction of drug-seeking in rats. A history of nicotine exposure significantly decreased H3K14 acetylation at the BDNF exon IV promoter, and this effect was abolished with extinction training combined with NaB treatment (Castino et al. 2018). Despite these, it is not yet known about the role of DNA methylation and other types of epigenetic modifications in mPFC in the regulation of formation or expression of extinction of drug memories.
Clinic relevance for the role of mPFC in drug extinction memories
Effects of addictive drugs or drug-related cues on PFC activity
Direct drug exposure alters the activity of PFC across species. Intracerebroventricular injection of cocaine in rats induced a significant increase in fMRI blood oxygen level-dependent (BOLD) signal intensity in PFC (Rothbaum and Davis 2003). Non-contingent cocaine administration in drug-naïve rhesus monkeys resulted in activation of dorsolateral PFC (Beylergil et al. 2017). Intravenous cocaine administration to abstinent cocaine-addicted patients improved BOLD responses in anterior prefrontal cortex (aPFC) and OFC (Wolstenholme et al. 2017). Besides, drug cue exposure has pronounced influence on PFC activation. For human nicotine-addictive individuals, fMRI test revealed that cigarette-related cues activated left dorsolateral prefrontal cortex (DLPFC) (Peters et al. 2008a). Similarly, in human abstinent alcoholics, alcohol-related stimuli elicited activation of bilateral ACC and DLPFC (LaLumiere et al. 2012; Ovari and Leri 2008). In cocaine abusers, cocaine cues induced activation of left lateral OFC activation and right DLPFC, whereas deactivation of left mPFC (Ben-Shahar et al. 2013). A PET study showed that when presented with cocaine-related cues, rhesus monkey trained to self-administrate cocaine displayed robust activation in PFC (Beylergil et al. 2017).
Effects of extinction of drug-related cues on mPFC activity
Until now few studies explored the PFC activity during the extinction of drug-associated cues, although substantial evidence has showed that fear extinction critically depends on the vmPFC (Milad et al. 2005; Milad et al. 2007; Mueller et al. 2014; Phelps et al. 2004). Konova et al. studied neural mechanisms of extinguishing drug and pleasant cue associations in human addiction using fMRI. They found that like fear extinction, non-fear-based extinction relies on the vmPFC. Cocaine users showed vmPFC abnormalities for both CSs, which, in the case of the drug-related images, correlated with craving. The study suggests a global deficit in extinction learning in this group that may hinder extinction-based treatment (Konova et al. 2017). The dysfunction of mPFC may underlie the resistance of drug cue associations to extinction in addiction. On the one hand, PFC dysfunction makes individuals vulnerable to drug use. Bechara (Bechara 2005) argued that like individuals with vmPFC lesions, the addicted individuals’ ability of inhibition is impaired, largely due to the relatively weaker function of reflective PFC compared with the impulsive amygdala. A research recruited teenagers with parental history of alcoholism and compared the children who were resilient to alcohol with those vulnerable to alcohol (according to the level of problem drinking). They found that the vulnerable group had greater activation of the dorsomedial PFC in fMRI (Heitzeg et al. 2008).
On the other hand, repeated drug use impairs the PFC function. When compared with rats with short access of cocaine, those with long access performed worse in the sustained attention task, indicating impaired cognitive flexibility. Decrease of D2 receptor mRNA expression and D2 protein levels in the medial prefrontal cortex, and of D2 mRNA in the orbitofrontal cortex were also observed (Briand et al. 2008). A human PET study showed that during Iowa Gambling Task, cocaine-addicted patients have higher activation of right OFC and lower activation of right DLPFC and left mPFC compared with control group (Bolla et al. 2003). This research suggested functional impairment in prefrontal cortex that participates in decision-making exists in drug abusers. Adults rodents with alcohol exposure during adolescence showed weaker baseline connectivity between PFC-striatum and among PFC subregions, as well as alterations in the expression of genes related with myelin and histone demethylation in PFC (Wolstenholme et al. 2017).
Potential application of extinction combined with mPFC modulation in the treatment of drug abuse
CET includes repeated presentation of drug-related stimuli without reinforcement. The goal of CET is to form new associations between drug cues and the absence of drug, thus inhibiting the expression of drug memory and preventing cue-induced drug seeking. However, the efficacy of CET appears to be limited (Conklin and Tiffany 2002). It is not surprising considering that extinction is a new learning but not an erasure of drug-related memory (Torregrossa and Taylor 2013). Compared with drug-related memory, extinction memory is unstable and vulnerable to forgetting (Myers et al. 2011). During and after CET, drug memory remains intact, and tends to reemerge with the passage of time, or exposure to drug or drug-related cues (Conklin and Tiffany 2002). Given the role of mPFC in the extinction of drug memories in animal models mentioned above, combination of extinction therapy with mPFC modulation may help to reduce craving and relapse in addicted patients.
Mounting evidence suggests that repetitive transcranial magnetic stimulation (rTMS) localized at the DLPFC, which is more superficial than the vmPFC and functionally connected with vmPFC, is effective in treating human with substance addiction (Bellamoli et al. 2014; Jansen et al. 2013; Salling and Martinez 2016). The mechanisms are still unclear, one of the possibilities is the regulation of activity in the brain regions related with addiction behaviors (Gorelick et al. 2014). To our knowledge, no human research has combined TMS with extinction training for the intervention of drug addiction, but in the field of fear memory, a study found that rTMS with imaginal exposure therapy attenuated hyperarousal symptoms, and altered the catecholamine and hormone levels in posttraumatic stress disorder (PTSD) patients (Osuch et al. 2009). Transcranial direct current stimulation (tDCS) has been proved to enhance abstinent rate in crack-cocaine-addicted patients (Batista et al. 2015). Alcohol-dependent patients who underwent bilateral tDCS of the DLPFC had lower subjective craving for alcohol as well as higher startle amplitudes, in comparison with those receiving placebo tDCS. In this study, the startle amplitude is the objective measurement for cue reactivity, and furthermore, the objective measurement for craving level (Wietschorke et al. 2016). Similarly, deep brain stimulation (DBS) has been proved as a future treatment for addiction (Peisker et al. 2018; Salling and Martinez 2016), yet more studies are needed to reveal the underlying mechanisms and how those physical interventions can be co-used with extinction therapy to yield more satisfactory treatment outcomes.
There are some other extinction-based interventions for drug relapse, such as memory retrieval-extinction procedure and combination of extinction training and vagus nerve stimulation (VNS). These interventions are related to the changes of mPFC activities, although mPFC are not directly stimulated. A retrieval-extinction procedure is referred to giving an extinction training after a memory retrieval manipulation. The effects only emerged when the interval between retrieval and extinction training is shorter than the reconsolidation time window. The memory retrieval-extinction procedure has been shown to reduce drug craving and relapse, both in abstinent human individuals addicted with heroin and nicotine, and rat model of cocaine, morphine, heroin, and alcohol relapse (Germeroth et al. 2017; Millan et al. 2013; Sartor and Aston-Jones 2014; Xue et al. 2012). The mechanism underlying memory retrieval-extinction procedure may at least be partially due to the alterations of mPFC activities (Xue et al. 2012). In rats self-administered with cocaine, VNS conducted during extinction attenuated cue-elicited reinstatement, and decreased the expression of the phosphorylated transcription factor CREB (pCREB) in the PFC (Childs et al. 2017), which regulates drug-seeking behaviors (Zhou and Zhu 2006). With the development of transcutaneous VNS in a broader range of neuropsychological disorders (Ben-Menachem et al. 2015; Genheimer et al. 2017; Jin and Kong 2017; Kong et al. 2018; Nichols et al. 2011; Shi et al. 2013), pairing VNS with extinction has begun to show potential for the treatment of drug addiction in clinic.
Conclusive remarks
Aberrant drug memories of the association between the drug-taking behavior and drug-related environmental cues contribute to the high rate of relapse after abstinence. Memory extinction weakens the strength of drug memories and reduces the propensity to relapse. However, since extinction training only causes temporary suppression but not permanent erasure of memories, drug memories are often spontaneously recovered after a long period of abstinence, reinstated by a priming dose of drugs, or renewed after exposure to drug-associated stimulus in a new environment. The ultimate goal of exploring the mechanisms underlying extinction of drug memories is to augment the persistence of memory extinction or prevent the original drug memories from relapse under the above-mentioned circumstances. Converging evidence from clinical and animal studies have suggested a critical role of mPFC, especially the IL-mPFC, in the extinction of drug memories, and manipulation of neural activity, synaptic plasticity or signaling pathway in IL-mPFC is effective in altering the persistence of extinction memory of drugs. To better understand the role of mPFC in the extinction memory of drugs and enhance the clinic translation for the use of memory extinction therapy, future studies in the following directions are needed. First, the causal relationship between mPFC and extinction memory in drug-addicted patients remains to be identified. Second, clinical and preclinical studies should be developed to ascertain whether manipulating the function of mPFC may be sufficient to reduce the reappearances of drug memories in addicted patients. Lastly, given the sufficiency of memory retrieval-extinction behavioral procedure in reducing the drug-seeking behavior and relapse, it is urgent to understand whether mPFC participates in this process, and if yes, how to enhance the anti-relapse effect of the behavioral procedure in both animal models and addicted patients through manipulating the mPFC function.
References
Abulseoud OA, Miller JD, Wu J, Choi DS, Holschneider DP (2012) Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain Res 1456:14–21. https://doi.org/10.1016/j.brainres.2012.03.045
Alvarez-Jaimes L, Polis I, Parsons LH (2008) Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology 33:2483–2493. https://doi.org/10.1038/sj.npp.1301630
Anier K, Malinovskaja K, Aonurm-Helm A, Zharkovsky A, Kalda A (2010) DNA methylation regulates cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology 35:2450–2461. https://doi.org/10.1038/npp.2010.128
Augur IF, Wyckoff AR, Aston-Jones G, Kalivas PW, Peters J (2016) Chemogenetic activation of an extinction neural circuit reduces Cue-induced reinstatement of cocaine seeking. J Neurosci 36:10174–10180. https://doi.org/10.1523/JNEUROSCI.0773-16.2016
Baler RD, Volkow ND (2006) Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med 12:559–566. https://doi.org/10.1016/j.molmed.2006.10.005
Batista EK, Klauss J, Fregni F, Nitsche MA, Nakamura-Palacios EM (2015) A randomized placebo-controlled trial of targeted prefrontal cortex modulation with bilateral tDCS in patients with crack-cocaine dependence. Int J Neuropsychopharmacol 18(12):pyv066. https://doi.org/10.1093/ijnp/pyv066
Bellamoli E, Manganotti P, Schwartz RP, Rimondo C, Gomma M, Serpelloni G (2014) rTMS in the treatment of drug addiction: an update about human studies. Behav Neurol 2014:815215–815211. https://doi.org/10.1155/2014/815215
Ben-Menachem E, Revesz D, Simon BJ, Silberstein S (2015) Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol 22:1260–1268. https://doi.org/10.1111/ene.12629
Ben-Shahar O, Sacramento AD, Miller BW, Webb SM, Wroten MG, Silva HE, Caruana AL, Gordon EJ, Ploense KL, Ditzhazy J, Kippin TE, Szumlinski KK (2013) Deficits in ventromedial prefrontal cortex group 1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci 33:495–506. https://doi.org/10.1523/jneurosci.3710-12.2013
Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW Jr, Miller SW, McGinty JF (2007) A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci 26:757–766. https://doi.org/10.1111/j.1460-9568.2007.05692.x
Berglind WJ, Whitfield TW Jr, LaLumiere RT, Kalivas PW, McGinty JF (2009) A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci 29:3715–3719. https://doi.org/10.1523/JNEUROSCI.5457-08.2009
Beylergil SB, Beck A, Deserno L, Lorenz RC, Rapp MA, Schlagenhauf F, Heinz A, Obermayer K (2017) Dorsolateral prefrontal cortex contributes to the impaired behavioral adaptation in alcohol dependence. Neuroimage Clin 15:80–94. https://doi.org/10.1016/j.nicl.2017.04.010
Blouin AM, Han S, Pearce AM, Cheng K, Lee JJ, Johnson AW, Wang C, During MJ, Holland PC, Shaham Y, Baraban JM, Reti IM (2013a) Role of medial prefrontal cortex Narp in the extinction of morphine conditioned place preference. Learn Mem 20:75–79. https://doi.org/10.1101/lm.028621.112
Blouin AM, Stern AL, Han S, Theberge FR, Wang C, During MJ, Baraban JM, Reti IM (2013b) Neuronal activity-regulated pentraxin expressed in medial prefrontal cortex neurons is not necessary for extinction of heroin self-administration. Behav Pharmacol 24:332–336. https://doi.org/10.1097/FBP.0b013e328363367b
Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y (2011) Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci 14:420–422. https://doi.org/10.1038/nn.2758
Bossert JM, Marchant NJ, Calu DJ, Shaham Y (2013) The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology 229:453–476. https://doi.org/10.1007/s00213-013-3120-y
Bouton ME (2004) Context and behavioral processes in extinction. Learn Mem 11:485–494. https://doi.org/10.1101/lm.78804
Bouton ME, King DA (1983) Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J Exp Psychol Anim Behav Process 9:248–265. https://doi.org/10.1037/0097-7403.9.3.248
Brenhouse HC, Dumais K, Andersen SL (2010) Enhancing the salience of dullness: behavioral and pharmacological strategies to facilitate extinction of drug-cue associations in adolescent rats. Neuroscience 169:628–636. https://doi.org/10.1016/j.neuroscience.2010.05.063
Burattini C, Burbassi S, Aicardi G, Cervo L (2007) Effects of naltrexone on cocaine- and sucrose-seeking behaviour in response to associated stimuli in rats. Int J Neuropsychopharmacol 11. https://doi.org/10.1017/S1461145707007705
Cannady R, McGonigal JT, Newsom RJ, Woodward JJ, Mulholland PJ, Gass JT (2017) Prefrontal cortex KCa2 channels regulate mGlu5-dependent plasticity and extinction of alcohol-seeking behavior. J Neurosci 37:4359–4369. https://doi.org/10.1523/jneurosci.2873-16.2017
Castino MR, Baker-Andresen D, Ratnu VS, Shevchenko G, Morris KV, Bredy TW, Youngson NA, Clemens KJ (2018) Persistent histone modifications at the BDNF and Cdk-5 promoters following extinction of nicotine-seeking in rats. Genes Brain Behav 17:98–106. https://doi.org/10.1111/gbb.12421
Chen W, Wang Y, Sun A, Zhou L, Xu W, Zhu H, Zhuang D, Lai M, Zhang F, Zhou W, Liu H (2016) Activation of AMPA receptor in the infralimbic cortex facilitates extinction and attenuates the heroin-seeking behavior in rats. Neurosci Lett 612:126–131. https://doi.org/10.1016/j.neulet.2015.11.024
Childs JE, DeLeon J, Nickel E, Kroener S (2017) Vagus nerve stimulation reduces cocaine seeking and alters plasticity in the extinction network. Learn Mem 24:35–42. https://doi.org/10.1101/lm.043539.116
Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F (2003) Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology 168:208–215. https://doi.org/10.1007/s00213-002-1380-z
Conde F, Maire-Lepoivre E, Audinat E, Crepel F (1995) Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol 352:567–593. https://doi.org/10.1002/cne.903520407
Conklin CA, Tiffany ST (2002) Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97:155–167. https://doi.org/10.1046/j.1360-0443.2002.00014.x
Crombag HS, Bossert JM, Koya E, Shaham Y (2008) Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond Ser B Biol Sci 363:3233–3243. https://doi.org/10.1098/rstb.2008.0090
Crombag HS, Dickson M, Dinenna M, Johnson AW, Perin MS, Holland PC, Baraban JM, Reti IM (2009) Narp deletion blocks extinction of morphine place preference conditioning. Neuropsychopharmacology 34:857–866. https://doi.org/10.1038/npp.2008.80
Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, Hope BT (2013) New technologies for examining neuronal ensembles in drug addiction and fear. Nat Rev Neurosci 14:743–754. https://doi.org/10.1038/nrn3597
Cunningham CL, Gremel CM, Groblewski PA (2006) Drug-induced conditioned place preference and aversion in mice. Nat Protoc 1:1662–1670. https://doi.org/10.1038/nprot.2006.279
Degoulet M, Stelly CE, Ahn KC, Morikawa H (2016) L-type Ca(2)(+) channel blockade with antihypertensive medication disrupts VTA synaptic plasticity and drug-associated contextual memory. Mol Psychiatry 21:394–402. https://doi.org/10.1038/mp.2015.84
Di Ciano P, Everitt BJ (2002) Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol 13:397–405. https://doi.org/10.1097/00008877-200209000-00013
Epstein DH, Preston KL, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology 189:1–16. https://doi.org/10.1007/s00213-006-0529-6
Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489. https://doi.org/10.1038/nn1579
Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O'Brien CP, Childress AR (2002) Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry 51:134–142. https://doi.org/10.1016/S0006-3223(01)01269-0
Fuchs RA, Ramirez DR, Bell GH (2008) Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology 200:545–556. https://doi.org/10.1007/s00213-008-1234-4
Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ (2005) Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492:145–177. https://doi.org/10.1002/cne.20738
Garrison KA, Potenza MN (2014) Neuroimaging and biomarkers in addiction treatment. Curr Psychiatry Rep 16:513. https://doi.org/10.1007/s11920-014-0513-5
Gass JT, Olive MF (2009) Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry 65:717–720. https://doi.org/10.1016/j.biopsych.2008.11.001
Gass JT, Trantham-Davidson H, Kassab AS, Glen WB Jr, Olive MF, Chandler LJ (2014) Enhancement of extinction learning attenuates ethanol-seeking behavior and alters plasticity in the prefrontal cortex. J Neurosci 34:7562–7574. https://doi.org/10.1523/jneurosci.5616-12.2014
Genheimer H, Andreatta M, Asan E, Pauli P (2017) Reinstatement of contextual conditioned anxiety in virtual reality and the effects of transcutaneous vagus nerve stimulation in humans. Sci Rep 7:17886. https://doi.org/10.1038/s41598-017-18183-3
George O, Hope BT (2017) Cortical and amygdalar neuronal ensembles in alcohol seeking, drinking and withdrawal. Neuropharmacology 122:107–114. https://doi.org/10.1016/j.neuropharm.2017.04.031
George O, Koob GF (2010) Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev 35:232–247. https://doi.org/10.1016/j.neubiorev.2010.05.002
Germeroth LJ, Carpenter MJ, Baker NL, Froeliger B, LaRowe SD, Saladin ME (2017) Effect of a brief memory updating intervention on smoking behavior: a randomized clinical trial. JAMA Psychiatry 74:214–223. https://doi.org/10.1001/jamapsychiatry.2016.3148
Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669. https://doi.org/10.1038/nrn3119
Gorelick DA, Zangen A, George MS (2014) Transcranial magnetic stimulation (TMS) in the treatment of substance addiction. Ann N Y Acad Sci 1327:79–93. https://doi.org/10.1111/nyas.12479
Gourley SL, Taylor JR (2016) Going and stopping: dichotomies in behavioral control by the prefrontal cortex. Nat Neurosci 19:656–664. https://doi.org/10.1038/nn.4275
Gutman AL, Ewald VA, Cosme CV, Worth WR, LaLumiere RT (2017) The infralimbic and prelimbic cortices contribute to the inhibitory control of cocaine-seeking behavior during a discriminative stimulus task in rats. Addict Biol 22:1719–1730. https://doi.org/10.1111/adb.12434
Hafenbreidel M, Rafa Todd C, Mueller D (2017) Infralimbic GluN2A-containing NMDA receptors modulate reconsolidation of cocaine self-administration memory. Neuropsychopharmacology 42:1113–1125. https://doi.org/10.1038/npp.2016.288
Hamlin AS, Clemens KJ, McNally GP (2008) Renewal of extinguished cocaine-seeking. Neuroscience 151:659–670. https://doi.org/10.1016/j.neuroscience.2007.11.018
He Y-Y, Xue Y-X, J-s W, Fang Q, Liu J-F, Xue L-F, Lu L (2011) PKMζ maintains drug reward and aversion memory in the basolateral amygdala and extinction memory in the Infralimbic cortex. Neuropsychopharmacology 36:1972–1981. https://doi.org/10.1038/npp.2011.63
Heather N, Bradley BP (1990) Cue exposure as a practical treatment for addictive disorders: why are we waiting? Addict Behav 15:335–337. https://doi.org/10.1016/0306-4603(90)90043-W
Heidbreder CA, Groenewegen HJ (2003) The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27:555–579. https://doi.org/10.1016/j.neubiorev.2003.09.003
Huang B, Li Y, Cheng D, He G, Liu X, Ma L (2018) β-Arrestin-biased beta-adrenergic signaling promotes extinction learning of cocaine reward memory. Sci Signal 11. https://doi.org/10.1126/scisignal.aam5402
Hyman SE (2005) Addiction: a disease of learning and memory. Am J Psychiatry 162:1414–1422. https://doi.org/10.1176/appi.ajp.162.8.1414
Jaffe JH, Cascella NG, Kumor KM, Sherer MA (1989) Cocaine-induced cocaine craving. Psychopharmacology (Berl) 97:59–64. https://doi.org/10.1007/BF00443414
Jansen JM, Daams JG, Koeter MW, Veltman DJ, van den Brink W, Goudriaan AE (2013) Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci Biobehav Rev 37:2472–2480. https://doi.org/10.1016/j.neubiorev.2013.07.009
Jin Y, Kong J (2017) Transcutaneous Vagus nerve stimulation: a promising method for treatment of autism Spectrum disorders. Front Neurosci 10:609. https://doi.org/10.3389/fnins.2016.00609
Johnson AW, Crombag HS, Takamiya K, Baraban JM, Holland PC, Huganir RL, Reti IM (2007) A selective role for neuronal activity regulated pentraxin in the processing of sensory-specific incentive value. J Neurosci 27:13430–13435. https://doi.org/10.1523/JNEUROSCI.4320-07.2007
Kalivas PW (2008) Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res 14:185–189. https://doi.org/10.1007/BF03033809
Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572. https://doi.org/10.1038/nrn2515
Kalivas PW, McFarland K (2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 168:44–56. https://doi.org/10.1007/s00213-003-1393-2
Kalivas PW, O'Brien C (2008) Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33:166–180. https://doi.org/10.1038/sj.npp.1301564
Kalivas PW, Volkow N, Seamans J (2005) Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45:647–650. https://doi.org/10.1016/j.neuron.2005.02.005
Khoo SY, Gibson GD, Prasad AA, McNally GP (2017) How contexts promote and prevent relapse to drug seeking. Genes Brain Behav 16:185–204. https://doi.org/10.1111/gbb.12328
Kong J, Fang J, Park J, Li S, Rong P (2018) Treating depression with transcutaneous auricular vagus nerve stimulation: state of the art and future perspectives. Front Psychiatry 9:20. https://doi.org/10.3389/fpsyt.2018.00020
Konova AB, Parvaz MA, Bernstein V, Zilverstand A, Moeller SJ, Delgado MR, Alia-Klein N, Goldstein RZ (2017) Neural mechanisms of extinguishing drug and pleasant cue associations in human addiction: role of the VMPFC. Addict Biol. https://doi.org/10.1111/adb.12545
Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T (1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A 92:8856–8860. https://doi.org/10.1073/pnas.92.19.8856
Krettek JE, Price JL (1977) The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171:157–191. https://doi.org/10.1002/cne.901710204
LaLumiere RT, Kalivas PW (2008) Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci 28:3170–3177. https://doi.org/10.1523/JNEUROSCI.5129-07.2008
LaLumiere RT, Niehoff KE, Kalivas PW (2010) The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem 17:168–175. https://doi.org/10.1101/lm.1576810
LaLumiere RT, Smith KC, Kalivas PW (2012) Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci 35:614–622. https://doi.org/10.1111/j.1460-9568.2012.07991.x
Lasseter HC, Xie X, Ramirez DR, Fuchs RA (2010) Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci 3:101–117. https://doi.org/10.1007/7854_2009_19
Li X, Caprioli D, Marchant NJ (2015) Recent updates on incubation of drug craving: a mini-review. Addict Biol 20:872–876. https://doi.org/10.1111/adb.12205
Lohof AM, Ip NY, Poo MM (1993) Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 363:350–353. https://doi.org/10.1038/363350a0
Lu L, Grimm JW, Hope BT, Shaham Y (2004) Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47:214–226. https://doi.org/10.1016/j.neuropharm.2004.06.027
Luo L (2000) Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci 1:173–180. https://doi.org/10.1038/35044547
Luo YX, Xue YX, Liu JF, Shi HS, Jian M, Han Y, Zhu WL, Bao YP, Wu P, Ding ZB, Shen HW, Shi J, Shaham Y, Lu L (2015) A novel UCS memory retrieval-extinction procedure to inhibit relapse to drug seeking. Nat Commun 6:7675. https://doi.org/10.1038/ncomms8675
Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA (2010) Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry 67:36–43. https://doi.org/10.1016/j.biopsych.2009.07.032
Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, Rusche JR, Wood MA (2013) HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci U S A 110:2647–2652. https://doi.org/10.1073/pnas.1213364110
Marchant NJ, Furlong TM, McNally GP (2010) Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci 30:14102–14115. https://doi.org/10.1523/JNEUROSCI.4079-10.2010
Marchant NJ, Li X, Shaham Y (2013) Recent developments in animal models of drug relapse. Curr Opin Neurobiol 23:675–683. https://doi.org/10.1016/j.conb.2013.01.003
Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM (2015) Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res 1628:219–232. https://doi.org/10.1016/j.brainres.2014.09.004
McDonald AJ, Mascagni F, Guo L (1996) Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71:55–75. https://doi.org/10.1016/0306-4522(95)00417-3
McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23:3531–3537. https://doi.org/10.1523/JNEUROSCI.23-08-03531.2003
Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K (2010) PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci 13:630–634. https://doi.org/10.1038/nn.2531
Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL (2005) Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A 102:10706–10711. https://doi.org/10.1073/pnas.0502441102
Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL (2007) Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 62:446–454. https://doi.org/10.1016/j.biopsych.2006.10.011
Millan EZ, Milligan-Saville J, McNally GP (2013) Memory retrieval, extinction, and reinstatement of alcohol seeking. Neurobiol Learn Mem 101:26–32. https://doi.org/10.1016/j.nlm.2012.12.010
Milton AL, Everitt BJ (2012a) The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev 36:1119–1139. https://doi.org/10.1016/j.neubiorev.2012.01.002
Milton AL, Everitt BJ (2012b) Wiping Drug Memories. Science 336:167–168. https://doi.org/10.1126/science.1221691
Moorman DE, James MH, McGlinchey EM, Aston-Jones G (2015) Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res 1628:130–146. https://doi.org/10.1016/j.brainres.2014.12.024
Mueller D, Porter JT, Quirk GJ (2008) Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci 28:369–375. https://doi.org/10.1523/jneurosci.3248-07.2008
Mueller EM, Panitz C, Hermann C, Pizzagalli DA (2014) Prefrontal oscillations during recall of conditioned and extinguished fear in humans. J Neurosci 34:7059–7066. https://doi.org/10.1523/JNEUROSCI.3427-13.2014
Myers KM, Carlezon WA, Davis M (2011) Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology 36:274–293. https://doi.org/10.1038/npp.2010.88
Nakayama AY, Harms MB, Luo L (2000) Small GTPases Rac and rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci 20:5329–5338. https://doi.org/10.1523/JNEUROSCI.20-14-05329.2000
Nestler EJ (2014) Epigenetic mechanisms of drug addiction. Neuropharmacology 76:259–268. https://doi.org/10.1016/j.neuropharm.2013.04.004
Nic Dhonnchadha BA, Kantak KM (2011) Cognitive enhancers for facilitating drug cue extinction: insights from animal models. Pharmacol Biochem Behav 99:229–244. https://doi.org/10.1016/j.pbb.2011.01.018
Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M (2011) Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience 189:207–214. https://doi.org/10.1016/j.neuroscience.2011.05.024
O'Brien CP, Gardner EL (2005) Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther 108:18–58. https://doi.org/10.1016/j.pharmthera.2005.06.018
O'Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P (1999) Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron 23:309–323. https://doi.org/10.1016/S0896-6273(00)80782-5
Oliva V, Cartoni E, Latagliata EC, Puglisi-Allegra S, Baldassarre G (2018) Interplay of prefrontal cortex and amygdala during extinction of drug seeking. Brain Struct Funct 223:1071–1089. https://doi.org/10.1007/s00429-017-1533-9
Ongur D, Price JL (2000) The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10:206–219. https://doi.org/10.1093/cercor/10.3.206
Ongur D, Ferry AT, Price JL (2003) Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460:425–449. https://doi.org/10.1002/cne.10609
Osuch EA, Benson BE, Luckenbaugh DA, Geraci M, Post RM, McCann U (2009) Repetitive TMS combined with exposure therapy for PTSD: a preliminary study. J Anxiety Disord 23:54–59. https://doi.org/10.1016/j.janxdis.2008.03.015
Otis JM, Fitzgerald MK, Mueller D (2014) Infralimbic BDNF/TrkB enhancement of GluN2B currents facilitates extinction of a cocaine-conditioned place preference. J Neurosci 34:6057–6064. https://doi.org/10.1523/JNEUROSCI.4980-13.2014
Ovari J, Leri F (2008) Inactivation of the ventromedial prefrontal cortex mimics re-emergence of heroin seeking caused by heroin reconditioning. Neurosci Lett 444:52–55. https://doi.org/10.1016/j.neulet.2008.08.015
Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C (2012) Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology 62:2309–2319. https://doi.org/10.1016/j.neuropharm.2012.01.011
Peisker CB, Schüller T, Peters J, Wagner BJ, Schilbach L, Müller UJ, Visser-Vandewalle V, Kuhn J (2018) Nucleus accumbens deep brain stimulation in patients with substance use disorders and delay discounting. Brain Sci 8:21. https://doi.org/10.3390/brainsci8020021
Perry CJ, Zbukvic I, Kim JH, Lawrence AJ (2014) Role of cues and contexts on drug-seeking behaviour. Br J Pharmacol 171:4636–4672. https://doi.org/10.1111/bph.12735
Peters J, LaLumiere RT, Kalivas PW (2008a) Infralimbic Prefrontal Cortex is Responsible for Inhibiting Cocaine Seeking in Extinguished Rats. J Neurosci 28:6046–6053. https://doi.org/10.1523/JNEUROSCI
Peters J, Vallone J, Laurendi K, Kalivas PW (2008b) Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology (Berl) 197:319–326. https://doi.org/10.1007/s00213-007-1034-2
Peters J, Kalivas PW, Quirk GJ (2009) Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16:279–288. https://doi.org/10.1101/lm.1041309
Peters J, Dieppa-Perea LM, Melendez LM, Melendez LM, Quirk GJ (2010) Induction of fear extinction with hippocampal-infralimbic BDNF. Science 328:1288–1290. https://doi.org/10.1126/science.1186909
Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schonig K, Bartsch D, Hope BT, Spanagel R, Sommer WH (2015) Losing control: excessive alcohol seeking after selective inactivation of cue-responsive neurons in the infralimbic cortex. J Neurosci 35:10750–10761. https://doi.org/10.1523/jneurosci.0684-15.2015
Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004) Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43:897–905. https://doi.org/10.1016/j.neuron.2004.08.042
Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE (2011) Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J Pharmacol Exp Ther 337:487–493. https://doi.org/10.1124/jpet.111.179317
Rich MT, Abbott TB, Chung L, Gulcicek EE, Stone KL, Colangelo CM, Lam TT, Nairn AC, Taylor JR, Torregrossa MM (2016) Phosphoproteomic analysis reveals a novel mechanism of CaMKIIalpha regulation inversely induced by cocaine memory extinction versus reconsolidation. J Neurosci 36:7613–7627. https://doi.org/10.1523/JNEUROSCI.1108-16.2016
Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004) The role of the medial frontal cortex in cognitive control. Science 306:443–447. https://doi.org/10.1126/science.1100301
Robison AJ, Nestler EJ (2011) Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 12:623–637. https://doi.org/10.1038/nrn3111
Rocha A, Kalivas PW (2010) Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci 31:903–909. https://doi.org/10.1111/j.1460-9568.2010.07134.x
Rogers JL, Ghee S, See RE (2008) The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience 151:579–588. https://doi.org/10.1016/j.neuroscience.2007.10.012
Rossetti ZL, Marcangione C, Wise RA (1998) Increase of extracellular glutamate and expression of Fos-like immunoreactivity in the ventral tegmental area in response to electrical stimulation of the prefrontal cortex. J Neurochem 70:1503–1512. https://doi.org/10.1046/j.1471-4159.1998.70041503.x
Rothbaum BO, Davis M (2003) Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci 1008:112–121. https://doi.org/10.1196/annals.1301.012
Sadakierska-Chudy A, Frankowska M, Jastrzebska J, Wydra K, Miszkiel J, Sanak M, Filip M (2017) Cocaine administration and its withdrawal enhance the expression of genes encoding histone-modifying enzymes and histone acetylation in the rat prefrontal cortex. Neurotox Res 32:141–150. https://doi.org/10.1007/s12640-017-9728-7
Salling MC, Martinez D (2016) Brain stimulation in addiction. Neuropsychopharmacology 41:2798–2809. https://doi.org/10.1038/npp.2016.80
Sanchis-Segura C, Spanagel R (2006) Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol 11:2–38. https://doi.org/10.1111/j.1369-1600.2006.00012.x
Sartor GC, Aston-Jones G (2014) Post-retrieval extinction attenuates cocaine memories. Neuropsychopharmacology 39:1059–1065. https://doi.org/10.1038/npp.2013.323
Schoenbaum G, Chang CY, Lucantonio F, Takahashi YK (2016) Thinking outside the box: orbitofrontal cortex, imagination, and how we can treat addiction. Neuropsychopharmacology 41:2966–2976. https://doi.org/10.1038/npp.2016.147
Sesack SR, Deutch AY, Roth RH, Bunney BS (1989) Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290:213–242. https://doi.org/10.1002/cne.902900205
Shaham Y, Adamson LK, Grocki S, Corrigall WA (1997) Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology (Berl) 130:396–403. https://doi.org/10.1007/s002130050256
Shaham Y, Shalev U, Lu L, de Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168:3–20. https://doi.org/10.1007/s00213-002-1224-x
Shen HW, Gipson CD, Huits M, Kalivas PW (2014) Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology 39:1169–1177. https://doi.org/10.1038/npp.2013.318
Shi C, Flanagan SR, Samadani U (2013) Vagus nerve stimulation to augment recovery from severe traumatic brain injury impeding consciousness: a prospective pilot clinical trial. Neurol Res 35:263–276. https://doi.org/10.1179/1743132813Y.0000000167
Siahposht-Khachaki A, Fatahi Z, Yans A, Khodagholi F, Haghparast A (2017) Involvement of AMPA/kainate glutamate receptor in the extinction and reinstatement of morphine-induced conditioned place preference: a behavioral and molecular study. Cell Mol Neurobiol 37:315–328. https://doi.org/10.1007/s10571-016-0371-2
Stefanik MT, Kupchik YM, Kalivas PW (2016) Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct Funct 221:1681–1689. https://doi.org/10.1007/s00429-015-0997-8
Sun WL, Eisenstein SA, Zelek-Molik A, McGinty JF (2014) A single brain-derived neurotrophic factor infusion into the dorsomedial prefrontal cortex attenuates cocaine self-administration-induced phosphorylation of synapsin in the nucleus accumbens during early withdrawal. Int J Neuropsychopharmacol 18:pyu049. https://doi.org/10.1093/ijnp/pyu049
Suto N, Laque A, De Ness GL, Wagner GE, Watry D, Kerr T, Koya E, Mayford MR, Hope BT, Weiss F (2016) Distinct memory engrams in the infralimbic cortex of rats control opposing environmental actions on a learned behavior. eLife 5:e21920. https://doi.org/10.7554/eLife.21920
Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW (2003) Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature 421:70–75. https://doi.org/10.1038/nature01249
Taylor JR, Olausson P, Quinn JJ, Torregrossa MM (2009) Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology 56:186–195. https://doi.org/10.1016/j.neuropharm.2008.07.027
Thompson RH, Swanson LW (1998) Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with fluorogold and PHAL in the rat. Brain Res Brain Res Rev 27:89–118. https://doi.org/10.1016/S0165-0173(98)00010-1
Tian W, Zhao M, Li M, Song T, Zhang M, Quan L, Li S, Sun ZS (2012) Reversal of cocaine-conditioned place preference through methyl supplementation in mice: altering global DNA methylation in the prefrontal cortex. PLoS One 7:e33435. https://doi.org/10.1371/journal.pone.0033435
Toda S, Shen HW, Peters J, Cagle S, Kalivas PW (2006) Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci 26:1579–1587. https://doi.org/10.1523/JNEUROSCI.4132-05.2006
Torregrossa MM, Taylor JR (2013) Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology 226:659–672. https://doi.org/10.1007/s00213-012-2750-9
Torregrossa MM, Sanchez H, Taylor JR (2010) D-cycloserine reduces the context specificity of Pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci 30:10526–10533. https://doi.org/10.1523/JNEUROSCI.2523-10.2010
Torregrossa MM, Gordon J, Taylor JR (2013) Double dissociation between the anterior cingulate cortex and nucleus accumbens core in encoding the context versus the content of pavlovian cocaine cue extinction. J Neurosci 33:8370–8377. https://doi.org/10.1523/JNEUROSCI.0489-13.2013
Tran-Nguyen PDL (1998) Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology 19:48–59. https://doi.org/10.1016/S0893-133X(97)00205-4
Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62:405–496. https://doi.org/10.1124/pr.109.002451
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
Uylings HBM, Groenewegen HJ, Kolb B (2003) Do rats have a prefrontal cortex? Behav Brain Res 146:3–17. https://doi.org/10.1016/j.bbr.2003.09.028
Van den Oever MC, Spijker S, Smit AB, De Vries TJ (2010) Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev 35:276–284. https://doi.org/10.1016/j.neubiorev.2009.11.016
Van den Oever MC, Rotaru DC, Heinsbroek JA, Gouwenberg Y, Deisseroth K, Stuber GD, Mansvelder HD, Smit AB (2013) Ventromedial prefrontal cortex pyramidal cells have a temporal dynamic role in recall and extinction of cocaine-associated memory. J Neurosci 33:18225–18233. https://doi.org/10.1523/JNEUROSCI.2412-13.2013
Vanderschuren LJ, Ahmed SH (2013) Animal studies of addictive behavior. Cold Spring Harb Perspect Med 3:a011932. https://doi.org/10.1101/cshperspect.a011932
Venniro M, Caprioli D, Shaham Y (2016) Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res 224:25–52. https://doi.org/10.1016/bs.pbr.2015.08.004
Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58. https://doi.org/10.1002/syn.10279
Vertes RP (2006) Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142:1–20. https://doi.org/10.1016/j.neuroscience.2006.06.027
Wang W-S, Kang S, Liu W-T, Li M, Liu Y, Yu C, Chen J, Chi Z-Q, He L, Liu J-G (2012) Extinction of aversive memories associated with morphine withdrawal requires ERK-mediated epigenetic regulation of brain-derived neurotrophic factor transcription in the rat ventromedial prefrontal cortex. J Neurosci 32:13763–13775. https://doi.org/10.1523/JNEUROSCI.1991-12.2012
Wang W, Ju YY, Zhou QX, Tang JX, Li M, Zhang L, Kang S, Chen ZG, Wang YJ, Ji H, Ding YQ, Xu L, Liu JG (2017) The small GTPase Rac1 contributes to extinction of aversive memories of drug withdrawal by facilitating GABAA receptor endocytosis in the vmPFC. J Neurosci 37:7096–7110. https://doi.org/10.1523/JNEUROSCI.3859-16.2017
Warren BL, Mendoza MP, Cruz FC, Leao RM, Caprioli D, Rubio FJ, Whitaker LR, McPherson KB, Bossert JM, Shaham Y, Hope BT (2016) Distinct Fos-expressing neuronal ensembles in the ventromedial prefrontal cortex mediate food reward and extinction memories. J Neurosci 36:6691–6703. https://doi.org/10.1523/JNEUROSCI.0140-16.2016
Wietschorke K, Lippold J, Jacob C, Polak T, Herrmann MJ (2016) Transcranial direct current stimulation of the prefrontal cortex reduces cue-reactivity in alcohol-dependent patients. J Neural Transm (Vienna) 123:1173–1178. https://doi.org/10.1007/s00702-016-1541-6
Willcocks AL, McNally GP (2013) The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci 37:259–268. https://doi.org/10.1111/ejn.12031
Wise RA (2009) Ventral tegmental glutamate: a role in stress-, cue-, and cocaine-induced reinstatement of cocaine-seeking. Neuropharmacology 56(Suppl 1):174–176. https://doi.org/10.1016/j.neuropharm.2008.06.008
Wolstenholme JT, Mahmood T, Harris GM, Abbas S, Miles MF (2017) Intermittent ethanol during adolescence leads to lasting behavioral changes in adulthood and alters gene expression and histone methylation in the PFC. Front Mol Neurosci 10:307. https://doi.org/10.3389/fnmol.2017.00307
Wright KN, Hollis F, Duclot F, Dossat AM, Strong CE, Francis TC, Mercer R, Feng J, Dietz DM, Lobo MK, Nestler EJ, Kabbaj M (2015) Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. J Neurosci 35:8948–8958. https://doi.org/10.1523/jneurosci.5227-14.2015
Xue Y-X, Luo Y-X, Wu P, Shi H-S, Xue L-F, Chen C, Zhu W-L, Ding Z-B, Bao Y-p, Shi J, Epstein DH, Shaham Y, Lu L (2012) A memory retrieval-extinction procedure to prevent drug craving and relapse. Science 336:241–245. https://doi.org/10.1126/science.1215070
Xue YX, Xue LF, Liu JF, He J, Deng JH, Sun SC, Han HB, Luo YX, Xu LZ, Wu P, Lu L (2014) Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J Neurosci 34:6647–6658. https://doi.org/10.1523/JNEUROSCI.5390-13.2014
Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU, Sacktor TC (2008) PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci 28:7820–7827. https://doi.org/10.1523/JNEUROSCI.0223-08.2008
You ZB, Wang B, Zitzman D, Azari S, Wise RA (2007) A role for conditioned ventral tegmental glutamate release in cocaine seeking. J Neurosci 27:10546–10555. https://doi.org/10.1523/JNEUROSCI.2967-07.2007
Zbukvic IC, Ganella DE, Perry CJ, Madsen HB, Bye CR, Lawrence AJ, Kim JH (2016) Role of dopamine 2 receptor in impaired drug-cue extinction in adolescent rats. Cereb Cortex 26:2895–2904. https://doi.org/10.1093/cercor/bhw051
Zhou L-F, Zhu Y-P (2006) Changes of CREB in rat hippocampus, prefrontal cortex and nucleus accumbens during three phases of morphine induced conditioned place preference in rats. J Zhejiang Univ Sci B 7:107–113. https://doi.org/10.1631/jzus.2006.B0107
Funding
This work was supported by grants from the National Basic Research Program of China (2014CB846100, 2015CB559200), National Natural Science Foundation of China (81601179, 81503079, 81741759, 31700916), and the National Program for Support of Top-notch Young Professionals and Natural Science Foundation of Jiangxi Province (20143ACB21002, 20172BCB22005, KJLD14013, 20161BAB215204).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article belongs to a Special Issue on Psychopharmacology of Extinction
Rights and permissions
About this article
Cite this article
Zhang, WH., Cao, KX., Ding, ZB. et al. Role of prefrontal cortex in the extinction of drug memories. Psychopharmacology 236, 463–477 (2019). https://doi.org/10.1007/s00213-018-5069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5069-3