Abstract
Rationale
The nucleus accumbens (NAC) is a functionally heterogeneous brain region with respect to its involvement in cocaine-seeking behavior triggered by drug-associated explicit conditioned stimuli, foot shock stress, or cocaine itself in the reinstatement animal model of drug relapse. However, it is not known whether the NAC or its subregions are critical for reinstatement of cocaine-seeking behavior produced by re-exposure to a previously cocaine-paired environmental context.
Objectives
The present study was designed to evaluate potentially unique contributions of the NAC core and shell to this behavior.
Materials and methods
Rats were trained to lever press for unsignaled cocaine infusions (0.15 mg/infusion, intravenous) in a distinct environmental context. Lever responding was then extinguished in a distinctly different environmental context (extinction context) during a minimum of seven daily training sessions. Subsequently, using a counterbalanced testing design, rats were re-exposed to the cocaine-paired context or the extinction context while cocaine seeking (i.e., responding on the previously cocaine-reinforced lever) was assessed. Before each test session, neural activity was inhibited selectively in the NAC core or shell using bilateral microinfusions of the γ-aminobutyric acid agonists, baclofen and muscimol (0/0 or 1.0/0.1 mM; 0.3 μl per hemisphere).
Results
Neural inactivation of the NAC shell or core attenuated responding in the cocaine context and, interestingly, increased responding in the extinction context. Control experiments indicated no effects on general activity or food-reinforced instrumental behavior.
Conclusions
These findings suggest that both subregions of the NAC may promote context-induced reinstatement by facilitating drug context-induced motivation for cocaine and context discrimination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Relapse is the main impediment in the treatment of cocaine addiction. In cocaine users, craving and resumption of drug use can be precipitated by exposure to drug-associated cues, stress, or drug itself (Jaffe et al. 1989; Childress et al. 1993; O’Brien et al. 1998; Sinha 2001). Similarly, in the reinstatement and renewal animal models of drug relapse, cocaine-seeking behavior (i.e., nonreinforced responding on a previously cocaine-paired operandum) can be elicited by exposure to a drug-paired context, explicit conditioned stimuli (CS), foot shock-induced stress, or a cocaine-priming injection (de Wit and Stewart 1981; Carroll and Comer 1996; Fuchs et al. 1998; Crombag and Shaham 2002; Epstein et al. 2006). Studies using these and similar procedures have provided important insights into the neural circuitry of drug relapse and revealed a complex role for the nucleus accumbens (NAC) in cocaine seeking and other goal-directed behaviors (Shalev et al. 2002; Bossert et al. 2005; Everitt and Robbins 2005; See 2005).
The core and shell subregions of the NAC contribute differently to the reinstatement of cocaine seeking depending on the type of trigger used to elicit this behavior. γ-Aminobutyric acid (GABA) agonist-induced functional inactivation of or glutamate antagonism within the NAC core uniformly inhibits all forms of reinstatement (McFarland and Kalivas 2001; Fuchs et al. 2004; McFarland et al. 2004; Peters and Kalivas 2006; Bäckström and Hyytiä 2007). Conversely, NAC shell inactivation fails to alter cocaine-primed reinstatement (McFarland and Kalivas 2001), but the antagonism of dopamine receptors, glutamate receptors, L-type Ca2+ channels, Ca2+/calmodulin-mediated kinase II, or the inhibition of GluR1-containing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor cell surface expression within the NAC shell disrupts this behavior (Anderson et al. 2003; Anderson et al. 2008). NAC shell inactivation fails to alter CS-induced (Fuchs et al. 2004) but inhibits stress-induced reinstatement (McFarland et al. 2004). The NAC shell may also play a permissive role in cocaine-seeking behavior because dopamine receptor stimulation within this brain region is sufficient to produce reinstatement in the absence of any other trigger (Schmidt et al. 2006; Schmidt and Pierce 2006). However, it has not been investigated whether the NAC plays a role in cocaine-seeking behavior produced by re-exposure to a drug-associated context per se.
Supporting the possibility that the NAC mediates some aspects of context-induced cocaine seeking, this brain region has been implicated in a variety of context-induced Pavlovian-conditioned and instrumental goal-directed behaviors. The NAC core but not the shell exhibits Fos protein expression, a marker of neuronal activation, concomitant with expression of cocaine-conditioned place preference (Miller and Marshall 2005), whereas both NAC subregions exhibit Fos protein expression associated with instrumental cocaine-seeking behavior in a cocaine-paired context (Neisewander et al. 2000; Hamlin et al. 2008). However, Fos protein expression in the NAC core is not observed if the act of cocaine seeking is prevented through retraction of the response lever (Neisewander et al. 2000). In addition to this correlational evidence, lesion studies more directly implicate the NAC in context-induced behaviors, including the expression of sucrose-conditioned place preference (Everitt et al. 1991) and contextual fear conditioning (Levita et al. 2002). Furthermore, recent pharmacological studies have demonstrated that D1-dopamine receptor stimulation within the NAC shell and glutamate receptor stimulation in the NAC shell or core are necessary for drug context-induced heroin-seeking behavior (Bossert et al. 2006; Bossert et al. 2007). However, it is unclear whether the same applies to context-induced cocaine-seeking behavior given that partially divergent neural systems mediate some forms of heroin-seeking versus cocaine-seeking behavior (Grimm and See 2000; Fuchs and See 2002).
The present study aimed to directly evaluate general functional contributions of NAC subregions to context-induced cocaine-seeking behavior. To this end, baclofen and muscimol (B/M), GABA agonists that hyperpolarize cell bodies without affecting fibers of passage, were infused into the NAC core or shell of rats prior to a test of cocaine-seeking behavior in the environmental context where cocaine self-administration had occurred previously and in an alternate context in which cocaine had not been available. Given that the NAC has been implicated in motor behavior, the effects of B/M-induced NAC core and shell inactivation were also assessed on locomotor activity and food-reinforced instrumental behavior. Based on the cocaine reinstatement literature, which indicates that the NAC core plays a more critical role than the NAC shell in some other forms of cocaine-seeking behavior, we postulated that functional inactivation of the NAC core would inhibit the expression of context-induced reinstatement to a greater extent than that of the NAC shell. Remarkably, the results from the present study were not fully consistent with these predictions and have led us to re-evaluate our understanding of NAC function.
Materials and methods
Animals
Experimentally naïve male Sprague–Dawley rats (Charles-River, N = 52), weighing 250–275 g at the start of the experiment, were individually housed in a temperature- and humidity-controlled vivarium on a reversed light–dark cycle. Rats were maintained on 20–25 g of rat chow per day, with water available ad libitum. The housing and treatment of the rats followed the guidelines of the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources, Commission on Life Sciences 1996), and the study protocol was approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Food training
In order to expedite the acquisition of cocaine self-administration, rats were first trained to lever press on a fixed ratio (FR) 1 schedule of food reinforcement (45 mg pellets; Purina, Richmond, IN, USA) in standard sound-attenuated operant-conditioning chambers (26 × 27 × 27 m high; Coulbourn Instruments, Allentown, PA, USA) during a 16-h overnight food training session. The chambers were equipped with two retractable levers and a food pellet dispenser between the levers. During the session, each lever press on the right (active) lever resulted in delivery of a food pellet only. Lever presses on the left (inactive) lever had no programmed consequences. Following food training, food pellet dispensers were removed from the chambers.
Surgery
Forty-eight hours after food training, rats were anesthetized using ketamine hydrochloride and xylazine (66.6 and 1.3 g/kg, respectively, intraperitoneal). Chronic indwelling catheters were constructed using a bent-steel cannula with a screw-type connector (Plastics One, Roanoke, VA, USA), SILASTIC tubing (10 m, inner diameter 0.64 m, outer diameter 1.19 m, Dow Corning, Midland, MI, USA), Prolite monofilament mesh (Atrium Medical, Hudson, NH, USA), and cranioplastic cement, as described previously (Fuchs et al. 2007). The end of the catheter was inserted into the right jugular vein and was secured to surrounding tissue with suture. The catheter ran subcutaneously and exited on the rat’s back, posterior to the shoulder blades.
Immediately after the catheter surgery, the rats were placed into a stereotaxic instrument (Stoelting, Wood Dale, IL, USA). They received stainless steel guide cannulae (26 G, Plastics One), aimed at the NAC core (+1.4 mm anteroposterior [AP], ±3.1 mm mediolateral [ML], −4.8 mm dorsoventral [DV], relative to bregma) or the NAC shell (+1.3 mm AP, ± 2.5 mm ML, −5.9 mm DV, relative to bregma). The cannulae were angled laterally by 10° in order to avoid the lateral ventricles. Three small screws and cranioplastic cement secured the guide cannulae to the skull. Stylets (Plastics One) and Tygon caps were placed into the guide cannulae and catheter to prevent occlusion, respectively. Rats were given 5 days for postoperative recovery before the start of the experiment.
To extend catheter patency, the catheters were flushed through once daily for 5 days following surgery with 0.1 ml of an antibiotic solution of cefazolin (100.0 mg/ml, Schein Pharmaceutical, Florham Park, NJ, USA) dissolved in heparinized saline (70 U/ml; Baxter Healthcare, Deerfield, IL, USA). Thereafter, catheters were flushed with 0.1 ml heparinized saline (10 U/ml) prior to each self-administration session and with 0.1 ml of the cefazolin solution and 0.1 ml of heparinized saline (70 U/ml) after each session. Catheter patency was periodically verified by infusing 0.1 ml of propofol (10 mg/ml, intravenous; Abbot Labs., North Chicago, IL, USA), an ultrashort-acting barbiturate which produces a rapid loss of muscle tone only when administered intravenously.
Cocaine self-administration training
Cocaine self-administration training was conducted during 2-h sessions on a minimum of 10 consecutive days during the rats’ dark cycle. Rats (N = 35) were trained to press a lever according to an FR 1 schedule of cocaine reinforcement (cocaine hydrochloride; 0.15 mg/0.05 ml per infusion, approximately equal to 0.5 mg/kg per infusion; National Institute on Drug Abuse, Research Triangle Park, NC, USA) with a 20-s timeout period. Food reinforcement was no longer available. The catheters were connected to liquid swivels (Instech, Plymouth Meeting, PA, USA) via polyethylene 20 tubing that was encased in steel spring leashes (Plastics One). The swivels were suspended above the operant conditioning chamber and were connected to infusion pumps (Coulbourn). Data collection and reinforcer delivery were controlled using Graphic State Notation software version 2.102 (Coulbourn).
Self-administration training was conducted in operant-conditioning chambers that contained one of two distinctly different sets of visual, auditory, olfactory, and tactile contextual stimuli in addition to the active (right) and inactive (left) levers. Context 1 contained a continuous red houselight (0.4 fc brightness) on the wall opposite to the active lever, intermittent pure tone (80 dB, 1 kHz; 2 s on, 2 s off), pine-scented air freshener strip (4.5 × 2 cm, Car Freshener, Waterton, NY, USA), and wire mesh floor (26 × 27 cm). Context 2 contained an intermittent white stimulus light above the inactive lever (1.2 fc brightness; 2 s on, 2 s off), continuous pure tone (75 dB, 2.5 kHz), vanilla-scented air freshener strip (4.5 × 2 cm, Sopus Products, Moorpark, CA, USA), and a slanted ceramic tile wall that bisected the bar floor (19 cm × 27 cm). Rats had no exposure to these contextual stimuli prior to cocaine self-administration training. The stimuli were presented throughout each session independent of responding, as in our previous studies (Fuchs et al. 2005; Fuchs et al. 2007). Assignment of rats to cocaine self-administration training in Context 1 versus Context 2 was random. Active lever presses resulted in a 2-s activation of the infusion pump only. After each infusion, responses on the active lever had no consequences during the 20-s timeout period. During the sessions, responses on the inactive lever had no programmed consequences but were recorded. Daily self-administration training sessions were continued until a rat reached the acquisition criterion (i.e., ≥10 infusions self-administered/session on a minimum of 10 training days).
Extinction training
Rats underwent 2-h extinction sessions on at least 7 consecutive days, during which responses on either lever had no programmed consequences. Extinction sessions were conducted in Context 2 for rats that had previously self-administered cocaine in Context 1, and vice versa. Rats had no prior exposure to the extinction training context. On extinction day 5, the rats were adapted to the intracranial infusion procedure prior to placement into the chamber. During the adaptation procedure, stainless steel injection cannulae (33 G, Plastics One) were bilaterally inserted into the rat’s guide cannulae to a depth of 2 mm below the tip of the guide cannulae. Rats were held by the experimenter for 4 min, while the injection cannulae were left in place but fluid was not infused through the infusion cannulae. Extinction training was terminated when the rats reached the criterion for extinction (i.e., ≤25 responses per session on the last 2 consecutive days) with a minimum of 7 days of extinction training.
Intracranial Microinfusions
For intracranial microinfusions, the injection cannulae were connected to 10-μl Hamilton syringes (Hamilton, Reno, NV, USA) that were mounted on an infusion pump (KD Scientific, Holliston, MA, USA). A combination of B/M (1.0/0.1 mM; Sigma-Aldrich) or phosphate-buffered saline vehicle (Veh, pH = 7.0 for both) were infused at volumes of 0.3 μl over 2 min, and the injection cannulae were left in place for 1 min prior to and after the infusion. Muscimol, at doses of 1,000 and 20 ng/1 μl, inhibits glucose utilization in a 1.6-mm radius (Martin 1991) and electrophysiological activity in a 1-mm radius (Arikan et al. 2002), respectively. These estimates likely include tissue that exhibits hypoactivity due to reduced synaptic input from inactivated neurons. In the present study, muscimol was administered at 12 ng/0.3 μl; thus, the area of neural inactivation was significantly smaller due to reduced infusion volume. Similar information about the spread of baclofen hydrobromide is not available, but its spread is likely limited by low lipophilicity (Leisen et al. 2003). Importantly, we and others have used this dose of B/M in the past to demonstrate functional differentiation between the NAC core versus shell in CS-induced and cocaine-primed cocaine-seeking behavior (McFarland and Kalivas 2001; Fuchs et al. 2004).
Reinstatement testing
Two 2-h test sessions were conduced using a counterbalanced design. During one test session, rats were re-exposed to the previously cocaine-paired context, whereas during the other test session, they were re-exposed to the extinction context. Immediately prior to placement into each testing context, rats received microinfusions of B/M or Veh into the NAC core or shell using the infusion procedure described above. Assignment to test session order and B/M versus Veh treatment were balanced based on previous cocaine intake because this variable is a significant predictor of the magnitude of CS-induced reinstatement (Kruzich et al. 1999). During both test sessions, lever presses were recorded on the previously active and inactive levers but had no programmed consequences. During the test session in the cocaine context, rats were connected to the infusion apparatus in order to allow for similar perception of and interaction with the spatial/tactile elements of the context (e.g., slanted tile) as during cocaine self-administration training. However, fluid was not infused through the catheter as a consequence of lever pressing. Between the two test sessions, rats received additional extinction training sessions until they reached the extinction criterion (i.e., ≤25 responses per session on minimum 2 consecutive days).
Locomotor activity testing
To further assess possible nonspecific effects of intracranial B/M microinfusions on general activity, we examined the effects of intra-NAC administration of B/M or Veh on locomotion in a novel context. Seventy-two to 94 h following the last test session, rats received bilateral microinfusions of B/M or Veh into the NAC core or shell using the infusion procedures described above. Assignment to the B/M and Veh treatment groups remained the same as in the preceding reinstatement experiment. Immediately after microinfusion, horizontal locomotor activity was measured in novel Plexiglas chambers (42 × 20 × 20 cm high), as described previously (Fuchs et al. 2007). A computerized activity system (San Diego Instruments, San Diego, CA, USA) recorded the number of times eight photobeams were broken by a rat moving in the chamber during a 2-h test session.
Food-reinforced instrumental behavior
To assess possible nonspecific effects of intracranial B/M microinfusions on instrumental behavior per se, we examined the effects of intra-NAC administration of B/M or Veh on lever pressing for a food reinforcer. Experimentally naïve rats (N = 17) received a single overnight food training session and stereotaxic surgery. Rats were food-deprived overnight prior to the food training session and were maintained subsequently on 20–25 g of rodent chow per day, as in the cocaine experiment. After recovery from surgery, rats underwent daily 2-h food self-administration sessions in the presence of the contextual stimuli that made up context 1 or 2. In addition, each chamber was equipped with a feeder and a food pellet dispenser. Active lever presses resulted in the delivery of a single food pellet (45 mg, Purina) into the pellet dispenser according to an FR 1 reinforcement schedule with a 20-s timeout period. Inactive lever presses were recorded but had no programmed consequences. After responding stabilized following minimum 10 days of training (i.e., ≤20% variability in active lever responding across two consecutive sessions), two 2-h test sessions were conducted using a fully counterbalanced within-subjects test design. Prior to each test session, rats received bilateral microinfusions of B/M or Veh into the NAC core or shell using the infusion procedures described above. During the test sessions, active lever presses continued to be reinforced with food pellets according to an FR 1 reinforcement schedule with a 20-s timeout period. Inactive lever presses were recorded but had no programmed consequences. B/M versus Veh treatment order was counterbalanced based on mean food-reinforced responding during the last two training sessions that preceded the first test session. Between the two test sessions, rats received minimum two additional food-reinforced training sessions.
Histological and data analysis
After the last experimental session, rats were fully anesthetized using sodium pentobarbital (100 mg/1 ml, intraperitoneal). Rats were then transcardially perfused using 1× phosphate-buffered saline (Fisher Scientific) and 10% formaldehyde solution (Sigma). The brains were dissected out and sectioned at the coronal plane at a thickness of 75 μm. The sections containing the cannula tracks were mounted on glass slides and were stained using cresyl violet (Kodak, Rochester, NY, USA). Cannula placements were determined using light microscopy. The most ventral portion of each cannula track was mapped onto schematics of appropriate plates from the rat brain atlas (Paxinos and Watson 1997).
Mean cocaine-reinforced and inactive lever presses as well as cocaine intake during the last 3 self-administration training days and the total number of sessions needed to reach the extinction criterion were analyzed separately using 2 × 2 between-subjects analysis of variance (ANOVA) with cannula location (aimed at NAC core, shell) and subsequent treatment (B/M, Veh) as factors. Extinction responding on the active lever was analyzed using separate 2 × 2 × 2 and 2 × 2 × 7 mixed factorial ANOVAs with cannula location and subsequent treatment as between-subjects factors and training day (last self-administration session versus first extinction session; first seven extinction sessions) as a within-subjects factor. Nonreinforced active and inactive lever presses on the test days were analyzed separately using 2 × 2 × 2 mixed-factorial ANOVA with NAC subregion (core, shell) and treatment (Veh, B/M) as between-subjects factors and testing context (extinction, cocaine-paired) as a within-subjects factor. Pearson correlation coefficients were calculated in order to evaluate the relationship between cocaine intake or active lever responding during cocaine self-administration and nonreinforced active lever presses on the reinstatement test day. To further assess treatment effects on context discrimination, nonreinforced active lever presses on the extinction test day and the immediately preceding extinction training day were analyzed using a 2 × 2 × 2 mixed factorial ANOVA with NAC subregion and treatment as between-subject factors and day (training, testing) as the within-subjects factor. Locomotor activity counts were analyzed using a 2 × 2 between-subjects ANOVA with NAC subregion and treatment as factors. Food-reinforced active lever responding was analyzed using a 2 × 2 mixed factorial ANOVA with NAC subregion as a between-subjects factor and treatment as a within-subjects factor. Significant ANOVA main and interaction effects were followed up by simple main-effects tests (t tests for factors with two levels or Tukey LSD post-hoc comparisons for factors with more than two levels), as appropriate. Alpha was set at 0.05.
Results
Histology
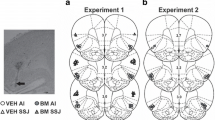
A schematic illustrating the distribution of injection cannula placements and photomicrographs showing representative cannula tracts are shown in Fig. 1. The target brain region was defined as the NAC core and the medial NAC shell with no distinction between dorsal versus ventral subareas of the NAC shell. The most ventral point of each injection cannula track was located bilaterally within the NAC core or in the medial NAC shell, including the NAC shell–olfactory tubercle transition zone, for the following number of rats per group: NAC core Veh (N = 8), NAC core B/M (N = 10), NAC shell Veh (N = 8), NAC shell B/M (N = 9), NAC core food control (N = 9; not shown), and NAC shell food control (N = 8; not shown).
Schematic and photographic representation of injection cannula placements within the NAC core (a) or shell (b). The symbols represent the most ventral point of the infusion cannula tracts for rats that received bilateral vehicle (Veh) or baclofen plus muscimol (B/M) infusions into the NAC core (open and filled triangles, respectively) or shell (open and filled circles, respectively), on schematics from the rat brain atlas of Paxinos and Watson (1997). Numbers indicate the distance from bregma in millimeters. The arrows on the photomicrographs identify the most ventral point of the infusion cannula tracts on representative cresyl violet-stained sections
Self-administration and extinction history
NAC core- and NAC shell-cannulated rats exhibited stable responding on the active lever during the last 3 self-administration training days with a within-subject variability of less than 10% in daily cocaine intake. Collapsed across groups, the mean daily cocaine intake (+SEM) was 25.67 + 1.84 infusions (approximately 17.11 + 1.22 mg/kg per session), respectively. There was no pre-existing difference between the four groups in active lever responding (cannula location and subsequent treatment group main and interaction effects, F (1, 31) = 0.002–1.32, p = 0.25–0.96) or inactive lever responding (main and interaction effects, F (1, 31) = 0.14–0.92, p = 0.34–0.71). However, the NAC core-cannulated groups exhibited a strong trend toward higher cocaine intake relative to the NAC shell-cannulated groups (cannula location main effect F (1, 31) = 3.96, p = 0.06) independent of subsequent treatment group (subsequent treatment group main effect and interaction effect, F (1, 31) = 0.06–0.23, p = 0.63–0.81). This trend occurred despite the fact that NAC core and shell experiments were conducted simultaneously, using identical procedures and three internal replications.
The mean active lever responding on the last day of self-administration training was 59.13 ± 7.02. Relative to this day, responding did not decrease significantly on extinction day 1 (mean = 42.82 ± 6.5; all-day main and interaction effects, F (1, 22) = 0.09–0.66, p = 0.42–0.76). The microinfusion adaptation procedure failed to alter responding on extinction day 5 (data not shown). Active lever responding gradually extinguished to criterion (see “Materials and methods”) in all groups (time main effect F (6, 144) = 7.65, p = 0.0001). There was no pre-existing difference between the groups in active lever responding during extinction training (cannula location and subsequent treatment main and interaction effects, F (6, 144) = 0.39–0.78, p = 0.53–0.88) or in the mean number of daily sessions needed to reach the extinction criterion (mean = 7.63 + 0.21; F (1, 31) = 0.02–0.97, p = 0.33–0.88). The mean active lever responding on the last day before the test session conducted in the extinction context and the cocaine context was 6.54 + 2.15 (EXTb shown in Fig. 2a) and 5.91 + 0.89 lever presses per session, respectively.
Responses (mean/2 h + SEM) on the active (a) and inactive (b) levers during self-administration (SA; last 3 days of training), extinction training (EXTb, last session before the extinction context test), as well as during testing conducted in the extinction (EXT CTX) and cocaine-paired contexts (COC CTX). Microinfusions of B/M (black bars; 1.0/0.1 mM, 0.3 μl per site) or Veh (open bars; 0.3 μl per site) were administered into the NAC core (N = 8–10 per group) or shell (N = 8–9 per group) immediately before each test session. In the main panel, symbols represent significant difference relative to the extinction context test (asterisk, ANOVA context simple main effect, p = 0.0001), relative to Veh treatment (cross, ANOVA treatment simple main effect, p = 0.002–0.045), and relative to EXTb (double cross, ANOVA day simple main effect, p = 0.03). In the insets, symbols represent significant difference relative to the extinction context test (number sign, ANOVA context simple main effect, p = 0.002–0.01) and relative to Veh treatment (carat sign, a ANOVA treatment simple main effect, p = 0.04; b ANOVA treatment main effect, p = 0.001)
Effects of NAC core or shell functional inactivation on context-induced cocaine seeking
Cocaine-seeking behavior
B/M-induced neuronal inactivation of the NAC shell or core attenuated cocaine-seeking behavior in the cocaine-paired context and increased responding in the extinction context, relative to Veh treatment (Fig. 2a). The 2 × 2 × 2 ANOVA of active lever presses revealed a significant treatment × context interaction (F (1, 31) = 16.60, p = 0.0001) and significant context main (F (1, 31) = 21.21, p = 0.0001) and treatment main effects (F (1, 31) = 4.67, p = 0.03) but no NAC subregion main or interaction effects. Collapsed across NAC subregions (Fig. 2a main panel), exposure to the cocaine-paired context produced greater active responding than exposure to the extinction context in the groups that received Veh treatment (ANOVA context simple main-effects test, p = 0.0001). Active lever responding in these groups in the cocaine-paired context was similar in magnitude to that during self-administration with a strong trend for more robust responding overall in NAC core-cannulated rats (trends for context × NAC subregion interaction and NAC subregion main effects, F (1, 31) = 3.89, p = 0.058 and F (1, 31) = 3.84, p = 0.06, respectively), relative to the NAC shell-cannulated rats. However, overall, in Veh-treated rats, the magnitude of active lever responding in the cocaine-paired context did not correlate with previous cocaine intake (R (16) = 0.012, p = 0.96) or cocaine-reinforced active lever responding (R (16) = 0.037, p = 0.89). Collapsed across NAC subregions, B/M treatment significantly attenuated active lever responding in the cocaine-paired context (ANOVA treatment simple main-effects test, p = 0.002) but increased responding in the extinction context (p = 0.045), relative to Veh treatment. Thus, the B/M-treated groups exhibited similar rates of responding upon exposure to the cocaine-paired and extinction contexts (ANOVA context simple main-effects test, p = 0.58). Similar to the omnibus ANOVA, separate treatment × context ANOVAs for the NAC core- or shell-cannulated groups revealed significant context main and context × treatment interaction effects (NAC core, F (1, 16) = 16.41–6.48, p = 0.001–0.02; NAC shell, F (1, 15) = 5.41–12.58, p = 0.03–0.003). Thus, exposure to the cocaine-paired context increased active lever responding in both the NAC core-cannulated (p = 0.01) and NAC shell-cannulated Veh-treated groups (p = 0.002). B/M treatment attenuated this behavior in the cocaine-paired context (NAC core, p = 0.04; NAC shell, p = 0.001), relative to Veh treatment.
To further assess the effects of B/M on responding in the extinction context, a separate analysis compared active lever responding in the four groups during the test day that was conducted in the extinction context compared with responding exhibited on the last extinction training day before this test day (see EXTb in Fig. 2a). This 2 × 2 × 2 ANOVA of active lever presses revealed a treatment × day interaction effect only (F (1, 31) = 5.52, p = 0.025) and no NAC subregion main or interaction effects. Interestingly, the source of the interaction effect was that the B/M-treated groups exhibited a significant increase in responding during the test session in the extinction context relative to the preceding extinction session (ANOVA day simple main effect, p = 0.028), whereas the Veh-treated groups did not (p = 0.38). Similar to the omnibus ANOVA, a separate treatment × context ANOVA for the NAC core-cannulated groups revealed a treatment × day interaction effect (F (1, 16) = 4.61, p = 0.048), but pair-wise comparisons failed to indicate statistically significant treatment or day simple main effects (p = 0.08–0.52; Fig. 2a insets). Furthermore, a separate ANOVA for the NAC shell-cannulated groups did not indicate significant treatment or day main or interaction effects.
During the test sessions, inactive lever responding was slightly increased by intra-NAC core but not intra-NAC shell and B/M treatment relative to Veh treatment (Fig. 2b insets). The 2 × 2 × 2 ANOVA of inactive lever presses did not indicate any significant main or interaction effects besides a marginally significant NAC subregion × treatment interaction effect (F (1, 31) = 3.89, p = 0.054). Contrary to the omnibus ANOVA, a separate treatment × context ANOVA for the NAC core-cannulated groups revealed a significant treatment main effect (F (1, 16) = 4.47, p = 0.050), indicating that B/M treatment in the NAC core increased inactive lever responding in both contexts, relative to the Veh (Fig. 2b insets). The ANOVA for the NAC shell-cannulated groups revealed a significant treatment × context interaction effect (F (1, 15) = 6.93, p = 0.02), but pair-wise comparisons failed to indicate statistically significant treatment or day simple main effects (p = 0.09–0.66).
Effects of NAC core or shell inactivation on locomotor activity and food-reinforced instrumental behavior
B/M treatment failed to alter locomotor activity in a novel context (Fig. 3a) or food-reinforced lever responding in contexts 1 and 2 (Fig. 3b), relative to Veh treatment. Consistent with this, the 2 × 2 ANOVA of photobeam interruptions failed to indicate NAC subregion or treatment main or interaction effects (F (1,31) = 0.14–3.18, p = 0.08–0.71). Similarly, separate 2 × 2 ANOVAs of active lever presses, inactive lever presses, and food pellets obtained during the two food self-administration test sessions failed to indicate NAC subregion or treatment main or interaction effects (F (1,15) = 0.002–3.04, p = 0.10–0.96). Thus, B/M failed to alter motor or instrumental performance, relative to Veh. Separate analyses for the NAC core- or shell-cannulated groups yielded similar results as the omnibus ANOVAs.
Locomotor activity (a; photobeam breaks/2 h + SEM) in a novel context and food-reinforced instrumental behavior (b; active lever presses/2 h + SEM) in contexts 1 or 2. Immediately before the test session, B/M (1.0/0.1 mM, 0.3 μl per site) or Veh (0.3 μl per site) were administered bilaterally into the NAC core (gray bars; N = 8–10 per group) or shell (hatched bars; N = 8–9 per group)
Discussion
While the contribution of the NAC has been extensively investigated with respect to goal-directed behavior, the present study provides the first demonstration that the functional integrity of this brain region is necessary for the ability of a cocaine-paired context to produce reinstatement of cocaine-seeking behavior. Briefly, passive re-exposure to a cocaine-paired context was sufficient to reinstate extinguished cocaine-seeking behavior relative to responding in the extinction context. Reinstatement likely reflected context-induced motivation for cocaine as opposed to dishabituation produced by a context shift, since exposure to a novel context fails to elicit an increase in lever responding in this animal model (Fuchs et al. 2005). GABA agonist-induced neural inhibition within the core or shell subregions of the NAC disrupted this behavior, which was indicated by a cocaine context-specific and lever-specific decrease in responding following B/M treatment relative to Veh treatment. B/M-treated groups responded at a similar magnitude in the cocaine-paired and extinction contexts. Remarkably, this was in part due to a B/M-induced increase in responding on the active lever in the extinction context relative to Veh and relative to the extinction baseline. This mitigates the possibility that the B/M-induced decrease in reinstatement was due to a performance deficit, especially since B/M failed to disrupt locomotor activity or food-reinforced instrumental behavior. Instead, these findings suggest that the functional integrity of the NAC is necessary for context-induced cocaine-seeking behavior.
Interestingly, the observed effects were not subregion specific. The failure to observe subregion-specific B/M effects on reinstatement was not likely due to a trend for greater cocaine intake in the NAC core-cannulated subjects. Neither cocaine intake nor cocaine-reinforced lever responding correlated with the magnitude of cocaine context-induced reinstatement in Veh control subjects, even though reinstatement responding appeared to be proportional to cocaine-reinforced responding. Furthermore, high reinstatement responding in the NAC core-cannulated Veh control group was unlikely to increase the sensitivity to detect a B/M-induced decrease in responding because the subtle effects of cannula placement on responding appeared to affect equally the Veh and B/M-treated groups. In fact, effect sizes appeared to be greater in the NAC shell (79% inhibition) than in the NAC core (57% inhibition), which is consistent with previous studies that revealed a selective role for dopamine D1 receptor stimulation in the NAC shell but a role for glutamate neurotransmission in both the NAC shell and core in context-induced renewal of heroin-seeking behavior (Bossert et al. 2006, 2007). Thus, it appears that the ventral striatum represents a point of convergence between the neural substrates for context-induced cocaine-seeking and heroin-seeking behaviors assessed using the reinstatement and renewal paradigms. To further evaluate the extent of this convergence, future studies will be needed to assess the pharmacological mechanisms of context-induced cocaine-seeking behavior within the NAC.
The lack of subregion-specific effects in the present study was not likely due to B/M spread between the NAC core and shell. The exact spread of GABA agonists in brain tissue is difficult to estimate because radioactive and fluorescent tags alter diffusion properties and measures of neural activity overestimate spread due to the synaptic inhibition of neurons in contact with GABA-inactivated cells (for a review, see Martin 1991; Martin and Ghez 1999). According to one such study, 20 ng muscimol delivered in a larger (1 μl) infusion volume than that used in the present study impairs electrophysiological activity directly and indirectly, via the inhibition of postsynaptic neurons, in a 1-mm radius (Arikan et al. 2002). In contrast, in the present study, B/M (including 12 ng muscimol) was administered at a volume of 0.3 μl into the NAC core or shell approximately 1.25 mm apart, thus outside of likely drug spread. More importantly, using the same B/M dose and microinfusion parameters, we and others have demonstrated functional differentiation between the NAC core and shell in CS-induced and cocaine-primed cocaine-seeking behavior, with effects observed in the NAC core only (McFarland and Kalivas 2001; Fuchs et al. 2004). These findings diminish the possibility that the lack of NAC subregion-specific B/M effects on reinstatement was due to drug spread between the NAC core and shell. However, the effects of intra-NAC shell B/M treatment may have been partially mediated by the adjacent medial olfactory tubercle, which was within the area of likely B/M diffusion. In fact, post-hoc analyses revealed that B/M treatment attenuated reinstatement to a similar extent relative to Veh regardless of placement proximity to the olfactory tubercle (data not shown). Similar to the NAC shell, the olfactory tubercle has been implicated in the rewarding and primary reinforcing effects of cocaine, morphine, and electrical self-stimulation (Fibiger et al. 1987; Kornetsky et al. 1991; Ikemoto 2003; Di Ciano et al. 2008; Sellings et al. 2006; Ikemoto 2007). Also, fundamental similarities exist between the anatomical connectivity of the NAC shell and olfactory tubercle, which suggests that the NAC shell–olfactory tubercle complex may comprise a functional unit with respect to context-induced goal-directed behavior (Sesack et al. 1989; Bossert et al. 2007; Ikemoto 2007).
The opposite effects of B/M treatment on lever responding in the cocaine-paired versus extinction context in the present study suggest that the ventral striatum may contribute to context-induced reinstatement by promoting drug context-induced motivation and context discrimination. The robust B/M-induced decrease in responding in the cocaine-paired context likely reflected a decrease in the incentive motivational effects of this environment. Indeed, previous studies have shown that the NAC is necessary for the expression of discriminative stimulus-induced or context-induced motivated behaviors. For instance, nonselective dopamine receptor antagonism in the NAC core inhibits Pavlovian approach to a food-predictive CS+ without altering the approach to a nonreward-predictive CS− (Di Ciano et al. 2001). Dopamine D1 receptor antagonism in the NAC as a whole attenuates instrumental responding selectively in the presence of a food-predictive discriminative stimulus (Yun et al. 2004). Furthermore, dopamine D1 receptor antagonism in the NAC shell or group 2 metabotropic glutamate autoreceptor stimulation in the NAC shell or core impairs reinstatement of heroin-seeking behavior in a heroin-paired context without altering responding in the extinction context (Bossert et al. 2006, 2007). This not withstanding, impairment in context discrimination may account for the B/M-induced modest increase in extinction responding observed in the present study. Similarly, 6-hydroxy-dopamine or neurotoxic lesions of the NAC as a whole or AMPA/kainate receptor antagonism in the NAC core disrupt discriminated conditioned approach behavior in rodents, as indicated by an increase in the approach to a nonreward-predictive CS− or a decrease in the response latency to an aversive outcome (Di Ciano et al. 2001; Parkinson et al. 2002; Schoenbaum and Setlow 2003). Perhaps, most relevant to the present study, tetrodotoxin-induced or ionotropic glutamate receptor antagonist-induced neural inactivation of the NAC disrupts discriminative stimulus control over food-seeking behavior by increasing the latency to respond to a food-predictive discriminative stimulus and increasing responding in the absence of cues and on the inactive lever (Yun et al. 2004). A B/M-induced context discrimination deficit could impair response suppression in the extinction context as well as response initiation in the cocaine-paired context without altering the motivation for cocaine. A discrimination deficit could reflect impairment in the ability to adjust behavior according to accurate context-based outcome expectancies. Alternatively, the same could result from context recognition deficit, since dopamine D2 or N-methyl-d-aspartic acid receptor blockade in the NAC disrupts spatial and object recognition memory in cocaine-naïve rats and mice (Léna et al. 2001; Sargolini et al. 2003). However, in the present study, B/M produced a greater decrease in responding in the cocaine context than the increase in responding in the extinction context. The context discrimination deficit hypothesis alone does not fully explain this pattern of findings. Accordingly, we postulate that B/M reduced context-induced motivation for cocaine and produced a discrimination deficit, which further attenuated residual goal-directed behavior in the cocaine-paired context and augmented it in the extinction context. Interestingly, SCH23390 or LY379268 administered into the NAC selectively disrupts drug context-induced motivation for heroin-seeking behavior (Bossert et al. 2006, 2007). Thus, it is possible that distinct ventral striatal subcircuits mediate context-induced motivation and context discrimination and the latter are inhibited by B/M but not by SCH23390 or LY379268.
In a previous study, GABA agonist-induced neural inactivation of the NAC shell failed to inhibit CS-induced reinstatement of cocaine-seeking behavior (Fuchs et al. 2004). The apparent discrepancy between this and the present findings may be related to the maintenance of CS-induced and context-induced reinstatement by conditioned reinforcers and occasion setters, respectively. Conditioned reinforcers signal imminent drug reinforcement and can become the end goal of responding, whereas occasion setters are discriminative stimuli that indicate reinforcement availability contingent upon drug-seeking behavior (Gordon and Klein 1994; Ciccocioppo et al. 2001). Consistent with this distinction, partially divergent neural substrates appear to mediate the motivational effects of these cues (Fuchs et al. 2005). The NAC shell may mediate context-induced cocaine-seeking behavior by interacting with the dorsal hippocampus, the only known NAC shell efferent that selectively mediates context-induced cocaine-seeking behavior via interaction with the basolateral amygdala (BLA; van Groen and Wyss 1990; Amaral and Witter 1995; Ferbinteanu and McDonald 2001; Fuchs et al. 2005, 2007). Other sources of efferent input to the NAC shell may include elements of the shared context/CS and context/CS/drug reinstatement circuitries, such as the BLA, ventromedial prefrontal cortex (vmPFC), and ventral hippocampus (Sesack et al. 1989; McDonald 1991; McDonald 1991; McLaughlin and See 2003; Fuchs et al. 2005; Fuchs et al. 2007; Rogers and See 2007; Peters et al. 2008; Fuchs, unpublished data). In turn, the NAC shell may influence the activity of the context-induced reinstatement circuitry via its afferent projections to the lateral hypothalamus (Hamlin et al. 2008) and reciprocal connections with the NAC core and the ventral tegmental area (VTA; Heimer et al. 1991; McFarland and Kalivas 2001; Crombag et al. 2002; van Dongen et al. 2005). Orexin/hypocretin-containing neurons in the lateral hypothalamus project to the BLA, dorsomedial prefrontal cortex (dmPFC), and VTA, and promote discriminative stimulus-induced ethanol-seeking behavior as well as morphine-conditioned place preference (Fadel and Deutch 2002; Narita et al. 2006; Dayas et al. 2008). Furthermore, dopamine neurons in the VTA innervate almost all elements of the known reinstatement circuitry and influence its activity, since dopamine receptor blockade systemically or selectively in the NAC shell disrupts context-induced renewal of cocaine- and heroin-seeking behavior, respectively (Crombag et al. 2002; Bossert et al. 2007).
Unlike in the NAC shell, B/M treatment in the NAC core inhibits both CS-induced and context-induced reinstatement (Fuchs et al. 2004; present study). Behavioral and anatomical evidence suggests that the NAC core may initiate context-induced or CS-induced cocaine-seeking behavior based on input from several of its afferents that play a critical role in these forms of cocaine-seeking behavior. These brain regions include the BLA, dmPFC, dorsolateral caudate-putamen (dlCPu), and the reciprocally connected VTA (Heimer et al. 1991; McDonald 1991; Crombag et al. 2002; Heidbreder and Groenewegen 2003; McLaughlin and See 2003; Fuchs et al. 2005; Fuchs et al. 2007). Disconnection of the NAC core from the BLA or dlCPu disrupts CS-induced cocaine-seeking behavior or responding on a second-order schedule of cocaine reinforcement (Di Ciano and Everitt 2004; Belin and Everitt 2008). However, similar disconnection studies have yet to be conducted to verify functionally significant interactions between the NAC and the above brain regions with respect to context-induced reinstatement.
It has been theorized that integration of information from the hippocampus, BLA, and other elements of the reinstatement circuitry occurs at the level of the dmPFC and vmPFC, which are theorized to coregulate the display of cocaine-seeking behavior via inputs to the NAC core and NAC shell, respectively (Kalivas and McFarland 2003; Peters et al. 2007, 2008). The complex anatomy of the reinstatement circuitry and recent electrophysiological data suggest that additional computations based on information from the BLA and hippocampus may also occur at the level of the NAC, increasing the influence of this brain region (O’Donnell and Grace 1995; Goto and O’Donnell 2002; Di Ciano and Everitt 2004). While the present study did not reveal NAC subregion-specific effects on context-induced reinstatement, it is probable that distinct neuronal subcircuits and neurochemical mechanisms within the NAC core and shell, as well as the larger reinstatement circuitry, mediate context-induced versus other forms of cocaine-seeking behavior, and these subcircuits may be differentially susceptible to cocaine-induced and experience-based neuroadaptations (Neisewander et al. 2004; Bossert et al. 2006; Fuchs et al. 2006; See et al. 2007). Therefore, future studies will need to investigate the neurobiology of these subcircuits to better understand and treat cocaine addiction.
References
Amaral DG, Witter MP (1995) Hippocampal formation. In: Paxinos, G (eds) The rat nervous system. Academic, Los Angeles, CA, pp 443–493
Anderson SM, Bari AA, Pierce RC (2003) Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 168:132–138
Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha J-HJ, Pierce RC (2008) CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci 11:344–353
Arikan R, Blake NM, Erinjeri JP, Woolsey TA, Giraud L, Highstein SM (2002) A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. J Neurosci Methods 118:51–57
Bäckström P, Hyytiä P (2007) Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology 192(4):571–580
Belin D, Everitt BJ (2008) Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57:432–441
Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y (2005) Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol 526:36–50
Bossert JM, Gray SM, Lu L, Shaham Y (2006) Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology 31:2197–2209
Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y (2007) Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci 27:12655–12663
Carroll ME, Comer SD (1996) Animal models of relapse. Exp Clin Psychopharmacol 4:11–18
Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP (1993) Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 137:73–95
Ciccocioppo R, Sanna PP, Weiss F (2001) Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA 98:1976–1981
Crombag HS, Shaham Y (2002) Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci 116:169–173
Crombag HS, Grimm JW, Shaham Y (2002) Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology 27:1006–1015
Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F (2008) Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry 63:152–157
de Wit H, Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 75:134–143
Di Ciano P, Everitt BJ (2004) Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci 24:7167–7173
Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ (2001) Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of Pavlovian approach behavior. J Neurosci 21:9471–9477
Di Ciano P, Robbins TW, Everitt BJ (2008) Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology 33:1413–25
Epstein DH, Preston KL, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189:1–16
Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489
Everitt BJ, Morris KA, O’Brien A, Robbins TW (1991) The basolateral amygdale–ventral striatal system and conditioned place preference: further evidence of limbic–striatal interactions underlying reward-related processes. Neuroscience 42:1–18
Fadel J, Deutch AY (2002) Anatomical substrates of orexin–dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience 111:379–387
Ferbinteanu J, McDonald RJ (2001) Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus 11:187–200
Fibiger HC, LePaine FG, Jakubovic A, Phillips AG (1987) The role of dopamine in intracranial self-stimulation of the ventral tegmental area. J Neurosci 7:3888–3896
Fuchs RA, See RE (2002) Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 160:425–433
Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL (1998) Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology (Berl) 135:151–160
Fuchs RA, Evans KA, Parker MC, See RE (2004) Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 176:459–65
Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30:296–309
Fuchs RA, Branham RK, See RE (2006) Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci 26:3584–3588
Fuchs RA, Eaddy JL, Su ZI, Bell GH (2007) Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci 26:487–498
Gordon WC, Klein RL (1994) In: Animal memory. The effects of context change on retention performance. 2nd edn. Academic, San Diego, CA
Goto Y, O’Donnell P (2002) Timing-dependent limbic-motor synaptic integration in the nucleus accumbens. Proc Natl Acad Sci USA 99:13189–13193
Grimm JW, See RE (2000) Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology 22:473–479
Hamlin AS, Clemens KJ, McNally GP (2008) Renewal of extinguished cocaine-seeking. Neuroscience 151:659–670
Heidbreder CA, Groenewegen HJ (2003) The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27:555–579
Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C (1991) Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience 41:89–125
Ikemoto S (2003) Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci 23:9305–11
Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res Rev 56:27–78
Institute of Laboratory Animal Resources, Commission on Life Sciences (1996) Guide for the Care and Use of Laboratory Animals. National Academy Press, Washington D.C.
Jaffe JH, Cascella NG, Kumor KM, Sherer MA (1989) Cocaine-induced cocaine craving. Psychopharmacology 97:59–64
Kalivas PW, McFarland K (2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 168:44–56
Kornetsky C, Huston-Lyons D, Porrino LJ (1991) The role of the olfactory tubercle in the effects of cocaine, morphine and brain-stimulation reward. Brain Res 541:75–81
Kruzich PJ, Grimm JW, Rustay NR, Parks CD, See RE (1999) Predicting relapse to cocaine-seeking behavior: a multiple regression approach. Behav Pharmacol 10:513–521
Leisen C, Langguth P, Herbert B, Dressler C, Koggel A, Spahn-Langguth H (2003) Lipophilicities of baclofen ester prodrugs correlate with affinities to the ATP-dependent efflux pump P-glycoprotein: relevance for their permeation across the blood–brain barrier? Pharm Res 20:772–778
Léna I, Dh tel H, Garbay C, Dauqé V (2001) Involvement of D2 dopamine receptors in the opposing effects of two CCK-B agonists in a spatial recognition memory task: role of the anterior nucleus accumbens. Psychopharmacology (Berl) 153:170–179
Levita L, Dalley JW, Robbins TW (2002) Disruption of Pavlovian contextual conditioning by excitotoxic lesions of the nucleus accumbens core. Behav Neurosci 116:539–552
Martin JH (1991) Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett 127:160–164
Martin JH, Ghez C (1999) Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods 86:145–159
McDonald AJ (1991) Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience 44:15–33
McFarland K, Kalivas PW (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–8663
McFarland K, Davidge SB, Lapish CC, Kalivas PW (2004) Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci 24:1551–1560
McLaughlin J, See RE (2003) Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 168:57–65
Miller CA, Marshall JF (2005) Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 47:873–884
Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T (2006) Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci 26:398–405
Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF (2000) Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20:798–805
Neisewander JL, Fuchs RA, Tran-Nguyen LT, Webber SM, Coffee GP, Joyce JN (2004) Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology 29:1479–1487
O’Brien CP, Childress AR, Ehrman R, Robbins SJ (1998) Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12:15–22
O’Donnell P, Grace AA (1995) Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 15:3622–3639
Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ (2002) Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res 137:149–163
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates, 3rd edn. Academic, Los Angeles, CA
Peters J, Kalivas PW (2006) The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology 186:143–149
Peters J, Vallone J, Laurendi K, Kalivas PW (2007) Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology (Berl) 197:319–326
Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28:6046–6053
Rogers JL, See RE (2007) Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem 87:688–692
Sargolini F, Roullet P, Oliverio A, Mele A (2003) Effects of intra-accumbens focal administration of glutamate antagonists on object recognition memory in mice. Behav Brain Res 138:153–63
Schmidt HD, Pierce RC (2006) Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience 142:451–461
Schmidt HD, Anderson SM, Pierce RC (2006) Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci 23:219–228
Schoenbaum G, Setlow B (2003) Lesions of nucleus accumbens disrupt learning about aversive outcomes. J Neurosci 23:9833–9841
See RE (2005) Neural substrates of cocaine–cue associations that trigger relapse. Eur J Pharmacol 526:140–146
See RE, Elliott JC, Feltenstein MW (2007) The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 194:321–31
Sellings LH, McQuade LE, Clarke PB (2006) Evidence for multiple sites within rat ventral striatum mediating cocaine-conditioned place preference and locomotor activation. J Pharmacol Exp Ther 317:1178–87
Sesack SR, Deutch AY, Roth RH, Bunney BS (1989) Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290:213–242
Shalev U, Grimm JW, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158:343–359
van Dongen YC, Deniau JM, Pennartz CM, Galis-de Graaf Y, Voorn P, Thierry AM, Groenewegen HJ (2005) Anatomical evidence for direct connections between the shell and core subregions of the rat nucleus accumbens. Neuroscience 136:1049–71
van Groen T, Wyss JM (1990) Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol 302:515–528
Yun IA, Nicola SM, Fields HL (2004) Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci 20:249–263
Acknowledgments
The authors would like to thank Heather Lasseter, Stephanie Traina, Dr. Xiaohu Xie, Jessica L. Eaddy, and Zu-In Su for excellent technical assistance and for insightful comments on an earlier version of this manuscript. This work was supported by National Institute on Drug Abuse grant R01 DA017673 (R.A.F), a NIDA R01 grant supplement to promote diversity in health-related research (DA017673-S1, D.R.R.), the University of North Carolina at Chapel Hill Junior Faculty Development Award (R.A.F.), and the Mason and Linda Stephenson Faculty Award (R.A.F.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuchs, R.A., Ramirez, D.R. & Bell, G.H. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology 200, 545–556 (2008). https://doi.org/10.1007/s00213-008-1234-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1234-4