Abstract
Finding effective long-lasting treatments for drug addiction has been an elusive goal. Consequently, researchers are beginning to investigate novel treatment strategies including manipulations of drug-associated memories. When environmental stimuli (cues) become associated with drug use, they become powerful motivators of continued drug use and relapse after abstinence. Reducing the strength of these cue–drug memories could decrease the number of factors that induce craving and relapse to aid in the treatment of addiction. Enhancing the consolidation of extinction learning and/or disrupting cue–drug memory reconsolidation are two strategies that have been proposed to reduce the strength of cues in motivating drug-seeking and drug-taking behavior. Here, we review the latest basic and clinical research elucidating the mechanisms underlying consolidation of extinction and reconsolidation of cue–drug memories in the hopes of developing pharmacological tools that exploit these signaling systems to treat addiction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drug addiction is characterized by compulsive use in the face of adverse consequences and repeated cycles of abstinence and relapse. Environmental stimuli (cues) that are repeatedly associated with a drug are known to promote compulsive drug taking and craving and are a primary trigger of relapse (Carter and Tiffany 1999; Shalev et al. 2002; See 2002). Therefore, recent efforts to develop effective treatments for addiction have focused on manipulations of learning and memory processes involved in encoding cue–drug associations.
Under natural conditions, organisms learn about the availability of rewards such as food, water, and mates by their association with specific environmental cues. With repeated associations, the cues are sufficient to elicit emotional and physiological responses and approach behaviors. While it is advantageous for organisms to learn and remember cues that predict natural rewards, these circuits can become abnormally activated in the presence of drugs of abuse. Natural rewards and the cues that predict them increase dopamine release in the nucleus accumbens and prefrontal cortex (Bassareo et al. 2002; Bassareo and Di Chiara 1999; Di Chiara 2002; Torregrossa and Kalivas 2008); however, drugs of abuse produce a much greater dopamine increase that does not habituate over time, potentially enhancing learning and memory consolidation about cues associated with drugs and overshadowing the cues associated with natural rewards (Torregrossa et al. 2011; Hyman et al. 2006; Robbins and Everitt 2002). Indeed, in abstinent smokers, smoking-related cues overshadow neutral cues indicative of monetary reward (Freeman et al. 2012). The resulting enhanced consolidation of drug-associated cues may increase the propensity of the drug–cue memory to strengthen rather than extinguish when cues are encountered in the environment. Therefore, manipulations that inhibit cue memory reconsolidation (a possible mechanism of memory strengthening) or that promote or enhance consolidation of cue extinction have potential therapeutic value for the prevention of relapse in addiction (cf., Taylor et al. 2009; Sorg 2012). Importantly, the same neural circuits that are involved in developing addictive behaviors and that are responsive to dopamine, namely, the nucleus accumbens, amygdala, and prefrontal cortex, are also responsible for the extinction and reconsolidation of drug-associated memories (Jentsch and Taylor 1999; Taylor et al. 2009). Therefore, understanding the molecular mechanisms of learning and memory within this neural circuitry will enhance our understanding of addiction itself. In this review, we will discuss current theories about the interaction of memory extinction and reconsolidation processes, the evidence for specific circuit and molecular mediators of these processes, and evidence that interfering with reconsolidation and/or enhancing extinction of drug cues may provide novel treatments for addiction.
Memory extinction and reconsolidation
Retrieval of a previously consolidated stimulus–reward memory in the absence of reinforcement can lead the memory to undergo two distinct and independent neurobiological processes—extinction and reconsolidation. Extinction involves learning of a new stimulus–no reward association (Bouton 2004) that requires its own consolidation phase, and inhibits or interferes with initial learning, but does not cause forgetting (Bouton 2004; Eisenberg and Dudai 2004). Extinction results in the reduction of the conditioned response to the stimulus. Alternatively, retrieved memories can undergo reconsolidation, which is the process of restabilizing the memory trace after it is retrieved or “reactivated”, possibly by incorporating new information and/or strengthening the memory (Lee 2008; Inda et al. 2011; Tronson et al. 2006) and returning it to long-term storage (Tronson and Taylor 2007). Recent studies have suggested that brief and/or weak exposures to a conditioned stimulus lead to reconsolidation, whereas more prolonged or repeated retrieval events, or weaker conditioning, results in extinction (Pedreira and Maldonado 2003; Eisenberg et al. 2003; Suzuki et al. 2004; Power et al. 2006; Tronson and Taylor 2007). Therefore, deficits in performance following manipulations at the time of retrieval could be interpreted either as a blockade of reconsolidation or a facilitation of extinction. However, when a manipulation produces no observable changes in the rate of extinction, it is more likely that altered reconsolidation has occurred (Tronson and Taylor 2007). Further, demonstrations of memory enhancements following manipulations at the time of retrieval are less easily explained by an altered extinction account. Nevertheless, when studying manipulations to weaken the strength of memories, both reconsolidation and extinction effects should be considered, and the short-term and long-term consequences of these manipulations be examined. Alterations in reconsolidation or extinction that produce only transient mnemonic effects are less likely to be relevant to the very long-lasting role that drug-associated cues play in craving and relapse. Additionally, combinations of treatments targeting both of these putatively distinct processes could be particularly effective in the treatment of addiction. First, we will describe both basic and clinical studies that have explored the mechanisms of extinction and reconsolidation. Then, we will discuss some recent studies attempting to combine manipulations of extinction and reconsolidation to reduce the effects of persistent, maladaptive memories.
Mechanisms of extinction of drug-associated memories
Until recently, the majority of extinction research was conducted by studying the extinction of aversive memories. The importance of extinction of appetitive or reward-related memories has since gained interest as a means of treating addiction-related disorders, and the field has used the information provided by studies of conditioned fear to understand appetitive extinction. Appetitive extinction is generally studied using the conditioned place preference (CPP) or the self-administration/reinstatement models of addiction. In CPP, the place preference is extinguished by repeated exposure to the previously drug-paired context in the absence of reinforcement, while in the self-administration/extinction/reinstatement model, the operant response required to produce a drug infusion is extinguished by withholding the reinforcer after a response (Tzschentke 2007; Shaham et al. 2003). Using these models, appetitive cue extinction has been shown to involve activity of the basolateral amygdala (BLA; Toyomitsu et al. 2002; Lindgren et al. 2003) and the infralimbic region of the medial prefrontal cortex (ILPFC; Peters et al. 2008; Koya et al. 2008). The ILPFC likely mediates extinction of conditioned memories through connections to the BLA (Muller et al. 1997; Wilensky et al. 2006), as the BLA is required for cue-induced reinstatement of cocaine-seeking behavior (Meil and See 1997; McLaughlin and See 2003). Furthermore, renewal of cocaine seeking after extinction in an alternate context is associated with increased Fos expression in both the ILPFC and the BLA (Hamlin et al. 2008).
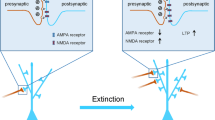
In addition, after extinction of a cocaine-reinforced response, the ability to express the extinction memory (i.e., show low levels of responding in the absence of reinforcement) requires activity in the projection from the ILPFC to the nucleus accumbens (NA) shell (Peters et al. 2008). Moreover, extinction training has been reported to reverse cocaine-induced decreases in the expression of the GluR1 and GluR2/3 subunits of AMPA glutamate receptors in the NAshell, and viral over-expression of GluR1 and GluR2 in the NA enhances extinction of cocaine self-administration (Sutton et al. 2003; Self and Choi 2004). Therefore, extinction learning is sufficient to reverse some aspects of cocaine-induce neuroplasticity, and maintaining the extinction memory appears to require glutamatergic activity in the shell, which may be generated by inputs from the ILPFC and/or BLA. Interestingly, over-expression of AMPA receptor subunits does not alter extinction of responding on a sucrose-paired lever, suggesting that cocaine produces specific neuroadaptations that result in altered extinction learning circuitry (Sutton et al. 2003). Consequently, more research is needed to understand how drugs of abuse alter learning and memory processes, as it is unlikely that the molecular underpinnings of drug-associated memories completely overlap with other forms of memory, such as fear. Drug addiction typically involves many more pairings of cues and contexts with the reinforcer, the pairings can be both predictable and unpredictable, and the pairings are more likely to be spaced over time rather than occurring in a single training session. In addition, extinction of drug cues appears to involve circuitry within the nucleus accumbens to a greater degree than extinction of fear (Peters et al. 2009), suggesting that different strategies may need to be employed to weaken drug-associated memories by extinction.
There are also many potential shortcomings of extinction learning that must be overcome to produce a long-lasting reduction in relapse, including the propensity for extinguished responding to return with the passage of time (spontaneous recovery); after re-exposure to the unconditioned stimulus (reinstatement); or after a change in context (renewal). One strategy for the treatment of addiction is to find ways to enhance and/or maintain extinction learning to overcome these limitations (Taylor et al. 2009). Therefore, several researchers are investigating pharmacological and molecular means of strengthening the consolidation of extinction memory.
Several studies have examined the ability of the NMDA partial agonist d-cycloserine (DCS), to facilitate extinction of drug memories. DCS is a promising potential therapeutic because it has been shown to facilitate extinction of fear and reduce reinstatement in animal models and humans (Ledgerwood et al. 2003, 2004; Ressler et al. 2004). Likewise, DCS given post-session either systemically or in the BLA facilitates extinction of a cocaine CPP (Botreau et al. 2006), and this effect can be long lasting, indicating less spontaneous recovery, and is resistant to reinstatement (Paolone et al. 2009). DCS also facilitates extinction of responding for self-administered cocaine and reduces re-acquisition of cocaine self-administration in rats and monkeys (Nic Dhonnchadha et al. 2010). Moreover, the full agonist at the glycine modulatory site of the NMDA receptor, d-serine, has also been shown to facilitate extinction of self-administered cocaine and prevent cocaine-induced reinstatement (Kelamangalath et al. 2009). Finally, the glutamatergic modulator, N-acetyl cysteine, enhances extinction of responding on a lever previously paired with heroin self-administration (Zhou and Kalivas 2008). Therefore, facilitating glutamatergic activity at the time of extinction may help prevent relapse to drug use by overcoming some of the weaknesses of extinction training (though see the discussion of clinical studies below).
Manipulating other neurotransmitter systems may also facilitate extinction. For example, extinction of a CPP for amphetamine is enhanced by immediate post-extinction administration of glucose or the muscarinic acetylcholine receptor agonist oxotremorine given either systemically or directly into the BLA (Shroeder and Packard 2003, 2004). The enhancement of extinction was only observed when the compounds were given immediately after an extinction session, not when administered 2 h later, suggesting that the manipulation specifically targeted extinction memory consolidation, and that the window for cholinergic-mediated enhancement of consolidation is short. The possibility of using cholinergic agents to facilitate extinction deserves further study, particularly in drug self-administration models.
To date, most extinction studies in the addiction field have focused on the extinction of the instrumental response used to self-administer drug, but studying ways to extinguish the association between discrete stimuli or cues and the drug of abuse is also desirable because cues are known to be strong mediators of relapse (Carter and Tiffany 1999; Shalev et al. 2002; See 2002). In addition, extinction of cues is highly relevant to the clinical setting where extinction of instrumental responses may be less feasible (e.g., snorting or injecting) and might not reduce the ability of associated cues to drive relapse. However, relatively few preclinical studies have specifically examined mechanisms of drug cue extinction. See and colleagues have reported that glutamatergic activity within the BLA is necessary for the extinction of drug-paired cue memories (Fuchs et al. 2006; Feltenstein and See 2007). In these studies, Pavlovian cue–drug pairings were made in the absence of instrumental responding and the cue’s ability to support responding on a drug-associated lever was assessed in an extinction test (no cocaine delivered). Post-test infusions of the sodium channel blocker tetrodotoxin (TTX) or the NMDA antagonist AP-5 into the BLA inhibited the expression of extinction on subsequent days of testing (Fuchs et al. 2006; Feltenstein and See 2007). While these studies suggest feasibility for modulation of extinction consolidation after cue–drug learning, the passive and discrete (single session) cue–drug pairings given after repeated self-administration does not recapitulate the repeated cue–drug pairings and drug-taking behavior experienced by addicts. Therefore, we tested whether extinction of a cocaine-paired cue in Pavlovian manner, where the cue is presented in the absence of reinforcement or an instrumental response, would be sufficient to reduce later cue-reinforced responding in a cue-induced reinstatement test. We found that Pavlovian extinction of the cue was sufficient to reduce later cue-induced reinstatement of lever responding, similar to clinical studies using exposure therapy (Torregrossa et al. 2010). The development of this animal model will allow for novel preclinical testing of pharmacotherapies that can be paired with exposure therapy to facilitate extinction of drug–cue memories.
Finally, a recent study has examined whether the interoceptive cues associated with cocaine use, as opposed to external environmental cues, could be extinguished to reduce relapse-like behavior in rats self-administering cocaine. The researchers found that repeated priming with cocaine reduced drug-seeking responses when rats were then given access to the lever that had previously produced cocaine. Interestingly, the effect was persistent, occurred in rats that had extended access to cocaine, and the procedure prevented stress-induced reinstatement of cocaine seeking (Mihindou et al. 2011). While the results are intriguing, there are potential clinical limitations as the researchers did not assess whether repeated priming was effective against cue-induced reinstatement or if it could prevent reacquisition of self-administration if cocaine availability became response contingent again. In addition, the effect was context dependent, which will also potentially limit the clinical effectiveness of this treatment strategy as described below. Nonetheless, a repeated drug priming strategy does deserve further study; however, addicts often repeatedly have drug lapses prior to a full relapse of addictive behavior, suggesting that repeated priming may be ineffective clinically (Leri and Stewart 2002).
Extinction of both fear and drug-associated memories is highly context specific (Bouton and Bolles 1979; Parker et al. 2006; Kearns and Weiss 2007)—such that extinction does not generalize to contexts other than that where extinction occurred. Consequently, extinction memories generated in a treatment setting are not likely to generalize to other environments (i.e., drug-taking contexts), contributing to the limited success of extinction-based therapies (Drummond 2000; Bouton 2002; Kalivas et al. 2006). However, the majority of studies aimed at investigating the facilitation of extinction memories have focused on manipulations specifically within the contexts/environments where the original memories were formed. Therefore, experimental methods that make extinction memories generalize to other contexts (i.e., prevent the renewal effect described above) are a highly desirable focus for new research.
For example, the hippocampus is known to be involved in the contextual modulation of extinction (Corcoran and Maren 2001, 2004; Hobin et al. 2006), but it is not yet known if manipulations of hippocampal activity during the acquisition of extinction could later reduce the context specificity of extinction expression for drug-associated memories. Pre-clinical studies should test this hypothesis, and if found to be successful it may be possible to design a clinical treatment that inhibits hippocampal-mediated encoding of context during extinction.
Alternatively, pharmacological enhancement of extinction consolidation using systemic or infralimbic cortex manipulations may alone increase the context generalization of cue extinction by increasing the strength of the extinction memory, thus, producing a greater inhibition of activity in brain regions that promote cue motivated behavior. In support of this hypothesis, we have found that systemic administration of DCS after cue extinction in a novel context inhibits subsequent cue-induced renewal (Torregrossa et al. 2010). However, we did not find that DCS in the infralimbic cortex or BLA affected renewal, suggesting that DCS may not facilitate extinction of drug cues through actions in these regions. Indeed, a recent study found that a profound extinction of conditioned fear could be induced by a single infusion of BDNF into the infralimbic cortex, but it was not sufficient to prevent renewal (Peters et al. 2010). We did, however, find that intra-nucleus accumbens core (NAc) DCS could prevent renewal, recapitulating the systemic effects. This suggests that cocaine cue extinction may involve a circuit including the NAc or that DCS inhibited the contextual encoding of extinction learning, resulting in extinction that generalizes across contexts (Torregrossa et al. 2010).
Importantly, another study has examined the ability of DCS to prevent renewal of responding for a food reinforcer and found that DCS treatment after instrumental extinction did not prevent renewal (Vurbic et al. 2011), similar to previous studies of aversive learning (Woods and Bouton 2006). Therefore, the effect of DCS on extinction of cocaine cues may either be specific to Pavlovian extinction or to the type of reinforcer (i.e., cocaine vs. food vs. shock). Nevertheless, generalization of cue extinction across contexts is important clinically. Chaudhri et al. (2008) found that conducting extinction in multiple, distinct contexts is effective for reducing renewal of behavior associated with alcohol cues in rats, and similar results have been found for fear responses in humans (Vansteenwegen et al. 2007). Manipulations shown to enhance the context generalization of cue extinction will be of tremendous value in augmenting extinction therapies, and we believe that more basic and clinical studies should address this issue.
Finally, it is possible that chronic exposure to drugs of abuse results in neuroplasticity in the PFC, BLA, NAc, and/or other brain regions that make drug-associated cues particularly resistant to extinction. Notably, Weiss and colleagues (2001) reported that renewal of cocaine-seeking behavior induced by a cocaine-paired cue did not diminish even after 34 days of intermittent, repeated testing when extinction would be expected to occur. Likewise, Di Ciano and Everitt (2004) found that the conditioned reinforcing property of drug-paired cues did not diminish with repeated testing, also suggesting that the behaviorally motivating effects of cues are difficult to extinguish. Therefore, enhancing the consolidation of extinction of drug-associated cues may be particularly difficult to accomplish. However, it is promising that extinction of a cocaine CPP can be enhanced by DCS such that there is no reinstatement of preference even more than 20 days after the last extinction session (Paolone et al. 2009). Further, extinction augmentation studies need to examine the effects on all types of reinstatement (relapse) including reinstatement induced by stress, the drug itself, and drug-associated cues. DCS has been successful in reducing reinstatement to cues and to drug, but future studies, including those using other extinction enhancing agents, should address all forms of reinstatement, including stress.
Finally, caution needs to be taken in using DCS to facilitate extinction in drug addicts, as one study has found that DCS given after 30 cocaine cue presentations produced an increase in cue-induced reinstatement, suggesting an enhancement in reconsolidation, rather than a facilitation of extinction (Lee et al. 2009). Therefore, if there is not sufficient cue exposure to induce extinction learning, reconsolidation could be facilitated instead, worsening the clinical outcome. Indeed, clinical studies using DCS in combination with extinction in addicts have been unsuccessful (see below), possibly due in part to reconsolidation effects. Moreover, due to the fact that drug-associated cues may be particularly difficult to extinguish, clinical studies should be designed to ensure extinction occurs before administering a cognitive enhancer such as DCS.
Mechanisms of reconsolidation of drug-associated memories
In addition to understanding mechanisms of extinction, investigations of reconsolidation processes have intensified in recent years (Tronson and Taylor 2007). Reconsolidation of fear memories, like consolidation, depends upon protein synthesis (e.g., Nader et al. 2000; Dudai 2004; Alberini 2005) and several other parallel signaling mechanisms (Kida et al. 2002; Bozon et al. 2003). Likewise, reconsolidation of memories for alcohol- and cocaine-associated cues requires both protein synthesis and NMDA receptor activity (von der Goltz et al. 2009; Lee et al. 2005; Milton et al. 2008a). However, the requirement for NMDA receptor activation may be time-limited as only pre-session infusions of NMDA antagonists were effective in blocking reconsolidation of cocaine cues (Milton et al. 2008a) and post-session NMDA antagonism was only marginally effective in preventing reconsolidation of alcohol cue memories (Wouda et al. 2010). Moreover, one study has shown that NMDA antagonism only blocks reconsolidation to prevent cocaine-primed reinstatement for a place preference and not in a self-administration paradigm (Brown et al. 2008).
In addition to protein synthesis and NMDA signaling, we have recently demonstrated that reconsolidation of cue memories associated with cocaine requires amygdalar PKA activation in a similar manner to conditioned fear (Sanchez et al. 2010; Tronson et al. 2006). This observation is particularly intriguing given that chronic cocaine exposure persistently increases PKA activity (Pollandt et al. 2006; Lynch and Taylor 2005; Nestler 2004; Terwilliger et al. 1991), possibly resulting in cue–drug memory strengthening through enhanced reconsolidation.
A growing body of research has identified additional molecular mediators of cue–drug memory reconsolidation. Notably, Lee and Everitt first demonstrated that cue-induced reinstatement of cocaine seeking, cue-maintained cocaine seeking under a second-order schedule of reinforcement, and the acquisition of a new response reinforced by drug-associated cues (conditioned reinforcement) are all disrupted if the immediate-early gene Zif268 is knocked down in the BLA at the time of cue retrieval (Lee et al. 2005, 2006). Moreover, Zif268 knockdown by oligodeoxynucleotides during cue retrieval is sufficient to reduce cue-mediated drug seeking for 27 days, making reconsolidation disruption a promising strategy for the long-term treatment of addiction.
Developing clinical treatments for addiction requires finding agents that can disrupt reconsolidation when given systemically, making the report demonstrating that systemic propranolol can disrupt the ability of both cocaine- and food-paired cues to act as conditioned reinforcers in rats particularly exciting (Milton et al. 2008b). In addition, propranolol has been shown to block reconsolidation of both cocaine and morphine conditioned place preference (Bernardi et al. 2006; Robinson and Franklin 2007). Together, these studies suggest that adrenergic signaling is important for reconsolidation of appetitive memories, much like has been shown for fear reconsolidation and might be useful clinically (Debiec and LeDoux 2006; Debiec et al. 2011). Interestingly though, a recent study has found that propranolol given 20 min prior to a cocaine CPP test inhibited retrieval of the CPP memory, and that this deficit in retrieval persisted over several tests and was not subject to reinstatement (Otis and Mueller 2011). The effect of propranolol in this study was not easily explained as a reconsolidation effect as it was apparent without any memory reactivation. It is also unclear why retrieval of the CPP memory would be disrupted once the propranolol had worn off, but regardless of the mechanism, it suggests that some of the effects of propranolol could be due to long-lasting retrieval deficits as opposed to reconsolidation effects. Nevertheless, a retrieval disrupting effect could also be useful clinically.
However, other reports suggest that propranolol does not disrupt reconsolidation (or retrieval) for all forms of cue-related learning, particularly for alcohol (Milton et al. 2012; Font and Cunningham 2012). Likewise, propranolol is only effective in disrupting reconsolidation for some but not all forms of aversive conditioning (Muravieva and Alberini 2010), possibly suggesting that propranolol affects Pavlovian aspects of memory to a greater degree than learned instrumental associations. Moreover, a series of studies examining a conditioned place preference for morphine has determined that propranolol is most effective in blocking reconsolidation when the drug-associated memory is weakly conditioned, time passes between the last drug conditioning session and the memory reactivation, if the animal is not morphine dependent, and if the reactivation conditions are novel. When these conditions are not met, then propranolol is not effective in disrupting reconsolidation and the place preference is maintained (Robinson and Franklin 2007, 2010; Robinson et al. 2011a, b). Therefore, clinical studies are needed to directly test the ability of propranolol in conjunction with drug memory reactivation, or possibly prior to drug memory retrieval, to prevent relapse and determine the reactivation conditions required for effective propranolol use.
Using disruption of memory reconsolidation as a treatment for psychiatric disorders has been somewhat controversial because of the potential that inhibiting reconsolidation could result in memory erasure. However, most manipulations of conditioned fear and cue–drug reconsolidation have not produced a complete loss of the associated behavior, and other studies using fear conditioning have shown that the efficacy of reconsolidation manipulations is very specific to the memory that is reactivated with non-reactivated memories remaining intact (Debiec et al. 2006; Doyere et al. 2007). Though, notably, a recent study has found that animals trained to learn both a cocaine conditioned place preference and a passive avoidance response reduced expression of both memories when intra-amygdala lidocaine was given after retrieval of either memory (Tzeng et al. 2012). This study suggests that either some manipulations are capable of disrupting even non-reactivated memories or that multiple memories can be encoded in the same circuits such that disrupting one memory in the circuit disrupts them all. Future research should examine these issues, though one speculative explanation for the above results is that if the same researcher handled the animals during each phase of the experiment, the researcher became an occasion–setting cue associated with both memories, and thus exposure to the researcher caused both memories to become labile simultaneously. It will certainly be important moving forward to determine the specificity of reconsolidation manipulations before wide scale use in clinical populations.
To date, the majority of reconsolidation manipulations do not appear to completely erase the memory, and indeed, studies in humans indicate that manipulating reconsolidation can inhibit conditioned emotional responses to fearful stimuli, but not the declarative memory about the stimulus–shock association (Kindt et al. 2009). However, in animal studies, memory erasure appears to be possible as inhibiting the protein kinase C (PKC) isoform PKMzeta has been shown to persistently block the expression of a long-term aversive (Shema et al. 2007) and conditioned place preference memory (Li et al. 2011; Shabashov et al. 2011). While a conditioned place preference memory could be apparently ablated, this did not impede re-learning the association (Li et al. 2011). Reactivation of the drug or aversive memory was not required for the effect of PKMzeta inhibition, but it has been shown that extinction of drug memories is associated with decreases in PKMzeta expression in the amygdala, suggesting the protein is dynamically regulated based on the current strength of memory expression (Xue et al. 2012). In the clinical situation, PKMzeta inhibition might erase multiple memories, but it is possible that in combination with memory reactivation, a PKMzeta inhibitor might be able to produce a selective loss of a drug-associated memory. However, this hypothesis still needs to be explicitly tested.
While a complete erasure of memory may not be ideal in the clinical treatment setting, manipulations that profoundly weaken cue–drug associations could be efficacious in reducing craving and relapse induced by drug-associated cues. Importantly, we have shown that reconsolidation processes can be context independent, such that manipulations of reconsolidation can occur in novel contexts and be successful in reducing reinstatement in the drug-taking context (Sanchez et al. 2010). Therefore, reconsolidation manipulations may have greater applicability and utility to the clinical setting.
Several of the studies described above specifically manipulated the reconsolidation of drug-associated cues, which is a method that can easily translate to the clinic. Several additional studies have investigated the molecular mechanisms of reconsolidation of contextual drug associations, which is a bit more difficult to translate to the clinic, but provides a wealth of knowledge about signaling molecules that may be involved in many types of memory reconsolidation. Using the conditioned place preference (CPP) paradigm, muscarinic acetylcholine and NMDA receptors, including specifically the glycine site of NMDA receptors (Kelley et al. 2007; Sadler et al. 2007, Sakurai et al. 2007; Brown et al. 2008; Zhai et al. 2008; Zhou et al. 2011), calcium/calmodulin-dependent protein kinase II (CaMKII; Sakurai et al. 2007), matrix metalloproteinases (Brown et al. 2007), and neuronal nitric oxide synthase (Itzhak and Anderson 2007) have all been shown to be necessary for the reconsolidation of CPP memories using multiple drugs of abuse. In addition, reactivation of a CPP memory has been shown to activate extracellular regulated protein kinase (ERK) in the nucleus accumbens core, and inhibition of ERK after reactivation persistently reduces the expression of CPP for up to 14 days (Miller and Marshall 2005). Likewise, systemic inhibition of ERK or protein synthesis after cocaine or morphine CPP reactivation is sufficient to reduce subsequent expression of CPP (Valjent et al. 2006). Moreover, an inhibitor of the cyclin-dependent kinase 5 (CDK5) specifically in the BLA can also inhibit reconsolidation of a cocaine CPP memory that is also maintained for 14 days and cannot be reinstated by a priming dose of cocaine (Li et al. 2010). Similar findings have also been reported for glycogen synthase kinase 3β (GSK3β) inhibitors (Wu et al. 2011). Finally, stress, via actions at glucocorticoid receptors in the BLA, can inhibit the reconsolidation of a morphine CPP memory (Wang et al. 2008). Therefore, there are many potential targets available for the development of pharmacotherapeutics that can take advantage of reconsolidation processes to treat addiction.
In some studies, the drug of abuse must be administered when the animal is placed into the conditioned context to see an effect of a particular protein on reconsolidation processes (e.g., matrix metalloproteinases, systemic ERK inhibition), suggesting that reconsolidation of contextual associations may involve distinct processes depending on whether the individual is under the influence of that drug (Brown et al. 2007; Valjent et al. 2006). However, NMDA antagonists can prevent reconsolidation of a CPP, either when the drug of abuse is given (Brown et al. 2008) or when it is omitted (e.g., Kelley et al. 2007). The requirement for administration of the conditioning drug to observe effects on reconsolidation appears to be specific to the signaling cascade that is manipulated, rather than dependent on the type of drug of abuse used for conditioning. However, to date, not all studies have tested memory reactivation in both the presence and the absence of the drug of abuse, so it is possible that some negative findings may be due to the absence of the conditioning drug, while some positive findings may only be found under very specific reactivation conditions. The drug of abuse itself may induce the activation of certain signaling cascades that impinge on memory processes themselves, resulting in a subset of molecules that are only required for reconsolidation in the presence of the drug. Future studies testing a variety of clinically applicable reconsolidation paradigms, with or without exposure to the drug of abuse, in combination with these molecular manipulations will establish which targets are most valuable for clinical development. Interestingly, a study from the fear literature suggests that memory reactivation of the US (shock) followed by administration of a protein synthesis inhibitor results in reduced memory expression for multiple non-reactivated cues that had been associated with the shock (Debiec et al. 2010). Therefore, US reactivation using the drug itself may enable disruption of reconsolidation of multiple drug-associated memories at once, which could be very useful clinically. However, substantial preclinical validation of this hypothesis is required to overcome the ethical dilemma surrounding re-exposing addicts to the drug of abuse.
Finally, a recent series of studies has examined anatomical and molecular mediators of reconsolidation of a drug self-administration contextual memory using the context-induced renewal model. In this model, animals are trained to self-administer a drug, but rather than manipulating cue-associated reconsolidation, the memory of the self-administration context is manipulated. These experiments have established that reconsolidation of this form of drug-associated memory requires non-protein synthesis dependent neuronal activity within the dorsal hippocampus (Ramirez et al. 2009) and protein synthesis in the BLA (Fuchs et al. 2009). While these studies eliminated a primary role for the dorsomedial prefrontal cortex and caudate–putamen in contextual reconsolidation, the necessity of other signaling molecules (e.g., those mentioned above) and the role of the nucleus accumbens has yet to be determined (Ramirez et al. 2009). Overall, these exciting studies are rapidly leading to the development of novel learning and memory-based treatments for addiction.
Clinical use of extinction and reconsolidation-based therapies
Several researchers have attempted to use extinction learning, known as cue exposure therapy clinically, to treat addiction to a variety of drugs of abuse (Conklin and Tiffany 2002). Cue exposure therapy (CET) is based on the assumption that when environmental stimuli are repeatedly associated with a drug, the stimuli will become sufficient to elicit conditioned responses that lead to craving and relapse, and that these conditioned responses can be extinguished. This assumption was explicitly tested by Foltin and Haney (2000) who showed that in a laboratory setting, cues explicitly associated with smoked cocaine could elicit conditioned responses such as changes in heart rate, skin temperature, and desire for cocaine when presented alone. These researches also demonstrated that the conditioned responses could extinguish when repeatedly presented in the absence of cocaine. Similar results have been found for alcohol-associated cues (Field and Duka 2002). Therefore, drug-associated cues can elicit craving responses that can be extinguished, verifying that extinction is a plausible therapy for addiction.
Indeed, CET has been shown to increase the latency to relapse and reduce consumption in alcohol-dependent subjects (Drummond and Glautier 1994), and O’Brien and colleagues (1990) found that extinction of cocaine cues successfully reduced craving in the laboratory and prolonged the period of abstinence compared to non-extinguished controls. Unfortunately, many clinical studies have not found CET to be an effective treatment for addiction (Conklin and Tiffany 2002). For example, a randomized controlled trial of CET in opiate addicts found that while CET reduced physiological responses and craving in the laboratory, the subjects were more likely than controls to relapse (Marissen et al. 2007). Another study of opiate addicts found a reduction in cue reactivity after CET that lasted up to 6 weeks (Franken et al. 1999), but the testing was conducted in the laboratory where the extinction training occurred and no assessments were made of outcomes outside of the laboratory.
Determining the effectiveness of CET once the subjects return to the natural environment is very important as extinction is known to be a context-dependent phenomena (Bouton 2002), such that extinction training conducted in a clinical context is unlikely to transfer to the drug-taking environment, as described above. Therefore, there is a need for alternative strategies such as pharmacological enhancement of extinction, reducing the contextual encoding of extinction, or manipulations of reconsolidation to more effectively treat addiction. One strategy that has been used to reduce the influence of the laboratory context on extinction learning is to conduct extinction in an immersive virtual reality environment. Virtual reality extinction has been effective in reducing cue-elicited craving (Lee et al. 2007) and is more effective in eliciting conditioned responses than traditional slides or videos (Kuntze et al. 2001). However, the long-term effectiveness of this strategy to maintain abstinence still needs to be determined.
Recently, several studies have examined whether a pharmacological agent can augment extinction of drug memories to reduce craving and relapse. The cognitive enhancing agent DCS has been successful in facilitating extinction in animal models of addiction such that reinstatement, reacquisition, spontaneous recovery, and renewal are all reduced (Nic Dhonnchadha et al. 2010; Paolone et al. 2009; Torregrossa et al. 2010). Moreover, DCS has been successful in augmenting exposure therapy in phobic clinical populations (Ressler et al. 2004). Therefore, several groups have examined whether DCS could enhance extinction of drug-associated cues. To date, DCS has been tested as an augmentation to exposure therapy in smokers, alcohol-dependent/heavy drinking subjects, and cocaine addicts. In these studies, DCS was given prior to extinction/exposure sessions. In general, DCS alone produced mild stimulant/euphoric effects that increased craving in some subjects. Then, during the first extinction session, craving and other physiological measures (depending on the study) increased in response to exposure to drug-related stimuli, but these measures decreased with continued exposure. Unfortunately, DCS did not facilitate extinction or reduce drug use for cocaine (Price et al. 2012) or alcohol (Watson et al. 2011; Kamboj et al. 2011a; Hofmann et al. 2012). Only in studies of cigarette smokers has DCS shown any promise, with one study finding that DCS reduced smoking cue reactivity (Santa Ana et al. 2009) and another finding that DCS reduced emotionality on a Tobacco Craving Questionnaire at a 2-week follow-up (Kamboj et al. 2011b). DCS has been given pre-session in the clinical studies to obtain sufficient blood levels by the end of the exposure session; however, the potential for producing stimulant effects on its own suggests that it might be better to administer DCS after some extinction learning has occurred to determine if it can facilitate the consolidation of extinction. It may also be that DCS is more effective in treating anxiety disorders where producing stimulant effects would not be confounding.
Currently, to our knowledge, only two studies have pharmacologically manipulated reconsolidation in drug addicts. These studies found that heroin addicts exposed to a stressor (Zhao et al. 2009) or propranolol (Zhao et al. 2011) after retrieval of a learned drug-associated word list had reduced word recall the following day. However, these studies did not examine whether this apparent inhibition of reconsolidation had any effect on heroin craving or other treatment outcomes. On the other hand, this strategy has been used for treating anxiety disorders such as post-traumatic stress disorder (PTSD) and phobias (McCleery and Harvey 2004; Debiec and LeDoux 2006). Patients with PTSD often have extreme symptoms of anxiety when exposed to stimuli that remind them of the traumatic experience. When these stimuli are presented to patients in the clinical setting to induce fear, the reconsolidation process can be inhibited by glucocorticoid exposure (similar to the stress-induced inhibition of reconsolidation described above for heroin-associated words) and by propranolol. Glucocorticoid treated PTSD and phobic patients have reported reduced severity of fear and anxiety in response to these stimuli when encountered in the outside environment (de Quervain 2008; de Quervain and Margraf 2008), and propranolol-treated subjects have decreased physiological fear responses when later asked to recall the traumatic event (Brunet et al. 2008). In addition, propranolol given with repeated memory reactivations produces a greater in magnitude and more persistent reduction in PTSD symptoms, with most participants no longer meeting diagnostic criteria for PTSD (Brunet et al. 2011).
In the 2008 study by Brunet and colleagues, propranolol was given after reactivation of the traumatic memory, suggesting that reconsolidation processes were specifically targeted, though additional controls, including non-reactivation and delayed administration groups, would strengthen a reconsolidation interpretation of the effect. One human fear conditioning study does support a reconsolidation interpretation as the study found that propranolol given prior to reactivation blocked emotional fear expression, but that there was no effect of propranolol in a no reactivation control group (Kindt et al. 2009). Interestingly, the declarative memory about the fear-conditioned stimulus was not affected, providing evidence for dissociable memory systems for specific aspects of fear that can be selectively modulated by a reconsolidation manipulation (Kindt et al. 2009). On the other hand, in the glucocorticoid studies, glucocorticoids were given prior to and during memory reactivation, making it difficult to interpret the exact mechanism by which the fear memory was disrupted. Though, considering that corticosterone can disrupt reconsolidation of a morphine CPP in rats (Wang et al. 2008), it is possible that the glucocorticoid effect in this clinical population was mediated through disrupted reconsolidation. Regardless of the mechanism underlying the results described above, disruption of reconsolidation of a cue-related memory appears to be a feasible clinical treatment strategy. However, the mechanisms by which drug–cue memories are reconsolidated still need to be elucidated, and the effectiveness of this treatment strategy remains to be explicitly tested in human drug addicts. Nonetheless, it seems possible that a substance like propranolol could be used during treatment sessions where craving could be elicited by asking addicts to reactivate cue–drug memories. It is critical for future researchers to address this hypothesis.
Interactions between extinction and reconsolidation
Finally, a combined approach, where both reconsolidation is inhibited and extinction is enhanced, might produce a long-lasting prevention of relapse. Current theories suggest that extinction and reconsolidation are separate processes that are both initiated upon unreinforced presentation of a conditioned cue. The reconsolidation process first involves activation of the original memory trace such that it is in a labile state and, depending on the circumstances of reactivation, the memory is updated with new associations or is strengthened and returned to a stable, consolidated state (Lee 2008; Winters et al. 2009; Inda et al. 2011). If the reactivation event is long, occurs repeatedly, or the memory is old, then extinction learning is likely to occur, where a new memory is formed encoding that in the current context the cue no longer predicts the outcome. Thus, according to this theory, both reconsolidation and extinction can occur simultaneously, and later behavioral expression in response to the cue is dependent on the context in which it is encountered and the strength of the extinction memory (i.e., whether the original memory or extinction memory is the dominant trace) (Eisenberg et al. 2003; Eisenberg and Dudai 2004; Eisenhardt and Menzel 2007). If the extinction memory actively inhibits expression of the reconsolidated memory, then it may be possible to manipulate both processes independently. However, it should be noted that both extinction and reconsolidation require the activity of certain molecules (e.g., NMDA receptors), while other molecules are oppositely regulated by extinction and reconsolidation (e.g., NFkB) (Merlo et al. 2005; Merlo and Romano 2008; de la Fuente et al. 2011). Therefore, if the two memory processes engage the same signaling cascades manipulating them in combination may become complicated. However, there is evidence from hippocampal-dependent contextual fear conditioning that while both extinction and reconsolidation regulate NFkB, extinction selectively engages activity of the transcription factor, nuclear factor of activated T cells (NFAT), such that manipulations of NFAT only affect extinction and not reconsolidation (de la Fuente et al. 2011). Further studies analyzing the signaling cascades that are differentially activated by extinction and reconsolidation will increase our ability to selectively modulate the two processes.
While it will be interesting for future research to clarify how these memory processes interact, it may be useful to treat addiction through a combination of reconsolidation and extinction therapies. Indeed, in studies of conditioned fear, researchers have found that if the conditioned cue memory was reactivated 10–60 min before an extinction session, the cue was subsequently less likely to produce spontaneous recovery, reinstatement, and, critically, renewal when the cue was presented back in the original training context (Monfils et al. 2009). These results suggest that by making the original memory labile through reactivation, extinction learning was able to “overwrite” the original memory. These studies have been extended to human subjects and appear to require time- and mGluR1-dependent removal of calcium permeable (GluR2 lacking) AMPA receptors from lateral amygdala synapses (Schiller et al. 2010; Clem and Huganir 2010). However, it should be noted that additional studies have not been able to replicate this “retrieval-extinction” effect using very similar parameters in both rats (Chan et al. 2010) and humans (Kindt and Soeter 2011). Nonetheless, studies using animal models of addiction have found that the retrieval-extinction procedure greatly reduces conditioned place preference and reinstatement to heroin and cocaine (Ma et al. 2011; Xue et al. 2012). In addition, the procedure successfully reduced reported craving in heroin addicts (Xue et al. 2012). It is unclear why there have been inconsistent results across laboratories. Even within laboratories, Flavell and colleagues (2011) did not find a persistent reduction in classically conditioned fear using the retrieval-extinction paradigm, but did observe a reduction of contextual fear and were able to use the paradigm to reduce the conditioned rewarding properties of a food-paired cue. Therefore, future studies should determine the exact parameters of retrieval-extinction required to observe a reduction in fear or craving and test whether it is effective in reducing actual drug use.
In addition, the two processes might be targeted independently, even with the same manipulation, by conducting extinction in one context and reactivating the memory in a separate context. The different contexts may allow specific manipulations of the two processes due to the context specificity of extinction. Alternatively, it may be possible to manipulate a signaling cascade that is regulated in opposite directions by the two processes, like NFkB, because inhibiting NFkB might inhibit reconsolidation and enhance extinction.
Concluding remarks
Drug addiction is a chronic, relapsing disorder in part due to the strong associations formed between drugs and the stimuli associated with drug use. These stimuli become strong drivers of continued use and relapse after abstinence. These drug-associated memories may be particularly strong because drugs increase the activity of circuits sub-serving normal reward-related learning. Therefore, reducing the strength of drug-associated memories through enhanced extinction learning and/or inhibition of reconsolidation holds promise for the treatment of addictive disorders. Clinical studies employing drug memory manipulations are just beginning and have yet to demonstrate much success. However, the exact parameters required to sufficiently extinguish memories in addicts are still being elucidated. Likewise, inhibiting the reconsolidation of drug-associated memories has yet to be directly tested in the clinic, but both preclinical addiction studies and clinical studies of PTSD suggest that inhibiting reconsolidation could be a successful addiction treatment strategy. Finally, combining memory retrieval with extinction learning might be an effective, drug-free method for persistently reducing the strength of drug memories to prevent relapse. In conclusion, manipulations of drug-associated memories have much promise for the treatment of addiction; however, much more research needs to be conducted to find the ideal conditions and pharmacological agents to safely and effectively treat human addicts.
References
Alberini CM (2005) Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci 28:51–56. doi:10.1016/j.tins.2004.11.001
Bassareo V, Di Chiara G (1999) Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation of motivational state. Eur J Neurosci 11:4389–4397. doi:10.1046/j.1460-9568.1999.00843.x
Bassareo V, De Luca MA, Di Chiara G (2002) Differential expression of motivational stimulus properties of by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci 22:4709–4719
Bernardi RE, Lattal KM, Berger SP (2006) Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport 17:1443–1447. doi:10.1097/01.wnr.0000233098.20655.26
Botreau F, Paolone G, Stewart J (2006) D-cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res 15:173–178. doi:10.1016/j.bbr.2006.05.012
Bouton ME (2002) Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry 52:976–986. doi:10.1016/S0006-3223(02)01546-9
Bouton ME (2004) Context and behavioral processes in extinction. Learn Mem 11:485–494. doi:10.1101/lm.78804
Bouton ME, Bolles RC (1979) Role of conditioned contextual stimuli in reinstatement of conditioned fear. J Exp Psychol Anim Behav Process 5:368–378
Bozon B, Davis S, Laroche S (2003) A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron 40:695–701. doi:10.1016/S0896-6273(03)00674-3
Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA (2007) Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem 14:214–223. doi:10.1101/lm.476207
Brown TE, Lee BR, Sorg BA (2008) The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem 15:857–865. doi:10.1101/lm.1152808
Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK (2008) Effect of post-retrieval propranolol on pscyhophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatry Res 42:503–506. doi:10.1016/j.jpsychires.2007.05.006
Brunet A, Poundja J, Tremblay J, Bui E, Thomas E, Orr S, Azzoug A, Birmes P, Pitman RK (2011) Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J Clin Psychopharmacol 31:547–550. doi:10.1097/JCP.0b013e318222f360
Carter BL, Tiffany ST (1999) Meta-analysis of cue-reactivity in addiction research. Addiction 94:327–340. doi:10.1046/j.1360-0443.1999.9433273.x
Chan WY, Leung HT, Westbrook RF, McNally GP (2010) Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn Mem 17:512–521. doi:10.1101/lm.1912510
Chaudhri N, Sahuque LL, Janak PH (2008) Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry 64:203–210. doi:10.1016/j.biopsych.2008.03.007
Clem RL, Huganir RL (2010) Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330:1108–1112. doi:10.1126/science.1195298
Conklin CA, Tiffany ST (2002) Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97:155–167. doi:10.1046/j.1360-0443.2002.00014.x
Corcoran KA, Maren S (2001) Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci 21:1720–1726
Corcoran KA, Maren S (2004) Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem 11:598–603. doi:10.1101/lm.78704
de la Fuente V, Freudenthal R, Romano A (2011) Reconsolidation or extinction: transcription factor switch in the determination of memory course after retrieval. J Neurosci 31:5562–5573. doi:10.1523/JNEUROSCI.6066-10.2011
de Quervain DJ (2008) Glucocorticoid-induced reduction of traumatic memories: implications for the treatment of PTSD. Prog Brain Res 167:239–247. doi:10.1016/S0079-6123(07)67017-4
de Quervain DJ, Margraf J (2008) Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: a novel therapeutic approach. Eur J Pharmacol 583:365–371. doi:10.1016/j.ejphar.2007.11.068
Debiec J, LeDoux JE (2006) Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: treatment implications for PTSD. Ann NY Acad Sci 1071:521–524. doi:10.1196/annals.1364.056
Debiec J, Doyere V, Nader K, LeDoux JE (2006) Directly reactivated, but not indirectly reactivated memories undergo reconsolidation in the amygdala. Proc Natl Acad Sci USA 103:3428–3433. doi:10.1073/pnas.0507168103
Debiec J, Diaz-Mataix L, Bush DEA, Doyere V, LeDoux JE (2010) The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci 13:536–537. doi:10.1038/nn.2520
Debiec J, Bush DE, LeDoux JE (2011) Noradrenergic enhancement of reconsolidation in the amygdala impairs extinction of conditioned fear in rats—a possible mechanism for the persistence of traumatic memories in PTSD. Depress Anxiety 28:186–193. doi:10.1002/da.20803
Di Chiara G (2002) Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res 137:75–114. doi:10.1016/S0166-4328(02)00286-3
Di Ciano P, Everitt BJ (2004) Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin, or sucrose: implications for the persistence of addictive behaviour. Neuropharmacol 47:202–213. doi:10.1016/jneuropharm.2004.06.005
Doyere V, Debiec J, Monfils MH, Schafe GE, LeDoux JE (2007) Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci 10:414–416. doi:10.1038/nn1871
Drummond DC (2000) What does cue-reactivity have to offer clinical research? Addiction 95:S129–144
Drummond DC, Glautier S (1994) A controlled trial of cue exposure treatment in alcohol dependence. J Consult Clin Psychol 62:809–817. doi:10.1037/0022-006X.62.4.809
Dudai Y (2004) The neurobiology of consolidations, or, how stable is the engram? Ann Rev Psychol 55:51–86. doi:10.1146/annurev.psych.55.090902.142050
Eisenberg M, Dudai Y (2004) Reconsolidation of fresh, remote, and extinguished fear memory in medaka: old fears don’t die. Eur J Neurosci 20:3397–3403. doi:10.1111/j.1460-9568.2004.03818.x
Eisenberg M, Kobilo T, Berman DE, Dudai Y (2003) Stability of retrieved memory: inverse correlation with trace dominance. Science 301:1102–1104. doi:10.1126/science.1086881
Eisenhardt D, Menzel R (2007) Extinction learning, reconsolidation and the internal reinforcement hypothesis. Neurobiol Learn Mem 87:167–173. doi:10.1016/j.nlm.2006.09.005
Feltenstein MW, See RE (2007) NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus–reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol Learn Mem 88:435–444. doi:10.1016/j.nlm.2007.05.006
Field M, Duka T (2002) Cues paired with a low dose of alcohol acquire conditioned incentive properties in social drinkers. Psychopharmacol 159:325–334. doi:10.1007/s00213-001-0923-z
Flavell CR, Barber DJ, Lee JL (2011) Behavioural memory reconsolidation of food and fear memories. Nat Commun 2:504. doi:10.1038/ncomms1515
Foltin RW, Haney M (2000) Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacol 149:24–33. doi:10.1007/s002139900340
Font L, Cunningham CL (2012) Post-retrieval propranolol treatment does not modulate reconsolidation or extinction of ethanol-induced conditioned place preference. Pharmacol Biochem Behav 101:222–230. doi:10.1016/j.pbb.2012.01.009
Franken IH, de Haan HA, van der Meer CW, Haffmans PM, Hendriks VM (1999) Cue reactivity and effects of cue exposure in abstinent posttreatment drug users. J Subst Abuse Treat 16:81–85, PII: S0740-5472(98)00004-X
Freeman TP, Morgan CJ, Beesley T, Curran HV (2012) Drug cue induced overshadowing: selective disruption of natural reward processing by cigarette cues amongst abstinent but not satiated smokers. Psychol Med 42:161–171. doi:10.1017/S0033291711001139
Fuchs RA, Feltenstein MW, See RE (2006) The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci 23:2809–2813. doi:10.1111/j.1460-9568.2006.04806.x
Fuchs RA, Bell GH, Ramirez DR, Eaddy JL, Su ZI (2009) Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci 30:889–900. doi:10.1111/j.1460-9568.2009.06888.x
Hamlin AS, Clemens KJ, McNally GP (2008) Renewal of extinguished cocaine-seeking. Neurosci 151:650–670. doi:10.1016/j.neuroscience.2007.11.018
Hobin JA, Ji J, Maren S (2006) Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus 16:174–182. doi:10.1002/hipo.20144
Hofmann SG, Huweler R, Mackillop J, Kantak KM (2012) Effects of d-cycloserine on craving to alcohol cues in problem drinkers: preliminary findings. Am J Drug Alcohol Abuse 38:101–107. doi:10.3109/00952990.2011.600396
Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598. doi:10.1146/annurev.neuro.29.051605.113009
Inda MC, Muravieva EV, Alberini CM (2011) Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J Neurosci 31:1635–1643. doi:10.1523/JNEUROSCI.4736-10.2011
Itzhak Y, Anderson KL (2007) Memory reconsolidation of cocaine-associated context requires nitric oxide signaling. Synapse 61:1002–1005. doi:10.1002/syn.20446
Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Pychopharmacol 146:373–390. doi:10.1007/PL00005483
Kalivas PW, Peters J, Knackstedt L (2006) Animal models and brain circuits in drug addiction. Mol Interven 6:339–344
Kamboj SK, Massey-Chase R, Rodney L, Das R, Almahdi B, Curran HV, Morgan CJA (2011a) Changes in cue reactivity and attentional bias following experimental cue exposure and response prevention: a laboratory of the effects of d-cycloserine in heavy drinkers. Psychopharmacol 217:25–37. doi:10.1007/s00213-011-2254-z
Kamboj SK, Joye A, Das RK, Gibson AJW, Morgan CJA, Curran HV (2011b) Cue exposure and response prevention with heavy smokers: a laboratory-based randomized placebo-controlled trial examining the effects of d-cycloserine on cue reactivity and attentional bias. Psychopharmacol. doi:10.1007/s00213-011-2571-2
Kearns DN, Weiss SJ (2007) Contextual renewal of cocaine seeking in rats and its attenuation by the conditioned effects of an alternative reinforcer. Drug Alcohol Depend 90:193–202. doi:10.1016/j.drugalcdep.2007.03.006
Kelamangalath L, Seymour CM, Wagner JJ (2009) D-serine facilitates the effects of extinction to reduce cocaine-primed reinstatement of drug-seeking behavior. Neurobiol Learn Mem 92:544–551. doi:10.1016/j.nlm.2009.07.004
Kelley JB, Anderson KL, Itzhak Y (2007) Long-term memory of cocaine-associated context: disruption and reinstatement. Neuroreport 18:777–780. doi:10.1097/WNR.0b013e3280c1e2e7
Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, Silva AJ (2002) CREB required for the stability of new and reactivated fear memories. Nat Neurosci 5:348–355. doi:10.1038/nn819
Kindt M, Soeter M (2011) Reconsolidation in a human fear conditioning study: a test of extinction as updating mechanism. Biol Psychol. doi:10.1016/j.biopsycho.2011.09.016
Kindt M, Soeter M, Vervliet B (2009) Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci 12:256–258. doi:10.1038/nn.2271
Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y (2008) Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacol 56(Suppl 1):177–185. doi:10.1016/j.neuropharm.2008.04.022
Kuntze MF, Stoermer R, Mager R, Roessler A, Mueller-Spahn F, Bullinger AH (2001) Immersive virtual environments in cue exposure. Cyberpsychol Behav 4:497–501. doi:10.1089/109493101750527051
Ledgerwood L, Richardson R, Cranney J (2003) Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci 117:341–349. doi:10.1037/0735-7044.117.2.341
Ledgerwood L, Richardson R, Cranney J (2004) D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci 118:505–513. doi:10.1037/0735-7044.118.3.505
Lee JL (2008) Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci 11:1264–1266. doi:10.1038/nn.2205
Lee JL, Di Ciano P, Thomas KL, Everitt BJ (2005) Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 47:795–801. doi:10.1016/j.neuron.2005.08.007
Lee JL, Milton AL, Everitt BJ (2006) Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci 26:5881–5887. doi:10.1523/JNEUROSCI.0323-06.2006
Lee J-H, Kwon H, Choi J, Yang B-H (2007) Cue-exposure therapy to decrease alcohol craving in virtual environment. Cyberpsychol Behav 10:617–623. doi:10.1089/cpb.2007.9978
Lee JL, Gardner RJ, Butler VJ, Everitt BJ (2009) D-cycloserine potentiates the reconsolidation of cocaine-associated memories. Learn Mem 16:82–85. doi:10.1101/lm.1186609
Leri F, Stewart J (2002) The consequences of different “lapses” on relapse to heroin seeking in rats. Exp Clin Psychopharmacol 10:339–349. doi:10.1037/1064-1297.10.4.339
Li FQ, Xue YX, Wang JS, Fang Q, Li YQ, Zhu WL, He YY, Liu JF, Xue LF, Shaham Y, Lu L (2010) Basolateral amygdala cdk5 activity mediates consolidation and reconsolidation of memories for cocaine cues. J Neurosci 30:10451–10359. doi:10.1523/JNEUROSCI.2112-10.2010
Li YQ, Xue YX, He YY, Li FQ, Xue LF, Xu CM, Sacktor TC, Shaham Y, Lu L (2011) Inhibition of PKMzeta in nucleus accumbens core abolishes long-term drug reward memory. J Neurosci 31:5436–5446. doi:10.1523/JNEUROSCI.5884-10.2011
Lindgren JL, Gallagher M, Holland PC (2003) Lesions of basolateral amygdala impair extinction of CS motivational value, but not of explicit conditioned responses, in Pavlovian appetitive second-order conditioning. Eur J Neurosci 17:160–166. doi:10.1046/j.1460-9568.2003.02421.x
Lynch WJ, Taylor JR (2005) Persistent changes in motivation to self-administer cocaine following modulation of cyclic AMP-dependent kinase A (PKA) activity in the nucleus accumbens. Eur J Neurosci 22:1214–1220. doi:10.1111/j.1460-9568.2005.04305.x
Ma X, Zhang JJ, Yu LC (2011) Post-retrieval extinction training enhances or hinders the extinction of morphine-induced conditioned place preference in rats dependent on the retrieval–extinction interval. Psychopharmacol. doi:10.1007/s00213-011-2545-4
Marissen MA, Franken IH, Blanken P, van den Brink W, Hendriks VM (2007) Cue exposure therapy for the treatment of opiate addiction: results of a randomized controlled clinical trial. Psychother Psychosom 76:97–105. doi:10.1159/000097968
McCleery JM, Harvey AG (2004) Integration of psychological and biological approaches to trauma memory: implications for pharmacological prevention of PTSD. J Trauma Stress 17:485–496
McLaughlin J, See RE (2003) Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacol 168:57–65. doi:10.1007/s00213-002-1196-x
Meil WM, See RE (1997) Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res 87:139–148. doi:10.1016/S0166-4328(96)02270-X
Merlo E, Romano A (2008) Memory extinction entails the inhibition of transcription factor NF-kappaB. PLoS One 3:e3687. doi:10.1371/journal.pone.0003687
Merlo E, Freudenthal R, Maldonado H, Romano A (2005) Activation of the transcription factor NF-kappaB by retrieval is required for long-term memory reconsolidation. Learn Mem 12:23–29. doi:10.1101/lm.82705
Mihinidou C, Vouillac C, Koob GF, Ahmed SH (2011) Preclinical validation of a novel cocaine exposure therapy for relapse prevention. Biol Psychiatry 70:593–598. doi:10.1016/j.biopsych.2011.03.036
Miller CA, Marshall JF (2005) Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 47:873–884. doi:10.1016/j.neuron.2005.08.006
Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ (2008a) Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci 28:8230–8237. doi:10.1523/JNEUROSCI.1723-08.2008
Milton AL, Lee JLC, Everitt BJ (2008b) Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on B-adrenergic receptors. Learn Mem 15:88–92. doi:10.1101/lm.825008
Milton AL, Schramm MJW, Wawrzynski JR, Gore F, Oikonomou-Mpegeti F, Wang NQ, Samuel D, Economidou D, Everitt BJ (2012) Antagonism at NMDA receptors, but not beta-adrenergic receptors, disrupts the reconsolidation of Pavlovian conditioned approach and instrumental transfer for ethanol-associated conditioned stimuli. Psychopharmacol 219:751–761. doi:10.1007/s00213-011-2399-9
Monfils MH, Cowansage KK, Klann E, LeDoux JE (2009) Extinction–reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324:951–955. doi:10.1126/science.1167975
Muller J, Corodimas KP, Fridel Z, LeDoux JE (1997) Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci 111:683–691
Muravieva EV, Alberini CM (2010) Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem 17:306–313. doi:10.1101/lm.1794710
Nader K, Schafe GE, LeDoux JE (2000) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406:722–726. doi:10.1038/35021052
Nestler EJ (2004) Molecular mechanisms of drug addiction. Neuropharmacol 47:24–32. doi:10.1016/j.neuropharm.2004.06.031
Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM (2010) D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacol 35:357–367. doi:10.1038/npp.2009.139
O’Brien CP, Childress AR, McLellan T, Ehrman R (1990) Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addictive Behav 15:355–365
Otis JM, Mueller D (2011) Inhibition of beta-adrenergic receptors induces a persistent deficit in retrieval of a cocaine-associated memory providing protection against reinstatement. Neuropsychopharmacol 36:1912–1920. doi:10.1038/npp.2011.77
Paolone G, Botreau F, Stewart J (2009) The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacol 202:403–409. doi:10.1007/s00213-008-1280-y
Parker LA, Limebeer CL, Slomke J (2006) Renewal effect: context-dependent extinction of a cocaine- and a morphine-induced conditioned floor preference. Psychopharmacol 187:133–137. doi:10.1007/s00213-006-0422-3
Pedreira ME, Maldonado H (2003) Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 38:863–869. doi:10.1016/S0896-6273(03)00352-0
Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28:6046–6053. doi:10.1523/JNEUROSCI.1045-08.2008
Peters J, Kalivas PW, Quirk GJ (2009) Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16:279–288. doi:10.1101/lm.1041309
Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ (2010) Induction of fear extinction with hippocampal–infralimbic BDNF. Science 328:1288–1290
Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P (2006) Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci 24:1733–1743. doi:10.1111/j.1460-9568.2006.05049.x
Power AE, Berlau DJ, McGaugh JL, Steward O (2006) Anisomycin infused into the hippocampus fails to block "reconsolidation" but impairs extinction: the role of re-exposure duration. Learn Mem 13:27–34. doi:10.1101/lm.91206
Price KL, Baker NL, McRae-Clark AL, Saladin ME, DeSantis SM, Santa Ana EJ, Brady KT (2012) A randomized, placebo-controlled laboratory study of the effects of d-cycloserine on craving in cocaine-dependent individuals. Psychopharmacol. doi:10.1007/s00213-011-2592-x
Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, Fuchs RA (2009) Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci 30:901–912. doi:10.1111/j.1460-9568.2009.06889.x
Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M (2004) Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 61:1136–1144
Robbins TW, Everitt BJ (2002) Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem 78:149–163. doi:10.1006/nlme.2002.4103
Robinson MJ, Franklin KB (2007) Central but not peripheral beta-adrenergic antagonism blocks reconsolidation for a morphine place preference. Behav Brain Res 182:129–134. doi:10.1016/j.bbr.2007.05.023
Robinson MJ, Franklin KB (2010) Reconsolidation of a morphine place preference: impact of the strength and age of memory on disruption by propranolol and midazolam. Behav Brain Res 213:201–207. doi:10.1016/j.bbr.2010.04.056
Robinson MJ, Ross EC, Franklin KB (2011a) The effect of propranolol dose and novelty of the reactivation procedure on the reconsolidation of a morphine place preference. Behav Brain Res 216:281–284. doi:10.1016/j.bbr.2010.08.009
Robinson MJ, Armson M, Franklin KB (2011b) The effect of propranolol and midazolam on the reconsolidation of a morphine place preference in chronically treated rats. Front Behav Neurosci 5:42. doi:10.3389/fnbeh.2011.00042
Sadler R, Herzig V, Schmidt WJ (2007) Repeated treatment with the NMDA antagonist MK-801 disrupts reconsolidation of memory for amphetamine-conditioned place preference. Behav Pharmacol 18:699–703. doi:10.1097/FBP.0b013e3282effb81
Sakurai S, Yu L, Tan SE (2007) Roles of hippocampal N-methyl-D-aspartate receptors and calcium/calmodulin-dependent protein kinase II in amphetamine-produced conditioned place preference in rats. Behav Pharmacol 18:497–506. doi:10.1097/FBP.0b013e3282ee7b62
Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR (2010) Reconsolidation of a cocaine-paired stimulus requires amygdalar protein kinase A. J Neurosci 30:4401–4407. doi:10.1523/JNEUROSCI.3149-09.2010
Santa Ana EJ, Rounsaville BJ, Frankforter TL, Nich C, Babuscio T, Poling J, Gonsai K, Hill KP, Carroll KM (2009) D-cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug Alcohol Depend 104:220–227. doi:10.1016/j.drugalcdep.2009.04.023
Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA (2010) Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463:49–53. doi:10.1038/nature08637
Schroeder JP, Packard MG (2003) Systemic or intra-amygdala injections of glucose facilitate memory consolidation for extinction of drug-induced conditioned reward. Eur J Neurosci 17:1482–1488. doi:10.1046/j.1460-9568.2003.02578.x
Schroeder JP, Packard MG (2004) Facilitation of memory for extinction of drug-induced conditioned reward: role of amygdala and acetylcholine. Learn Mem 11:641–647. doi:10.1101/lm.78504
See RE (2002) Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav 71:517–529. doi:10.1016/S0091-3057(01)00682-7
Self DW, Choi KH (2004) Extinction-induced neuroplasticity attenuates stress-induced cocaine seeking: a state-dependent learning hypothesis. Stress 7:145–155. doi:10.1080/10253890400012677
Shabashov D, Shohami E, Yaka R (2011) Inactivation of PKMzeta in the NAc shell abolished cocaine-conditioned reward. J Mol Neurosci. doi:10.1007/s12031-011-9671-7
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacol 168:3–20. doi:10.1007/s00213-002-1224-x
Shalev U, Grimm JW, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42. doi:10.1124/pr.54.1.1
Shema R, Sacktor TC, Dudai Y (2007) Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science 317:951–953. doi:10.1126/science.1144334
Sorg BA (2012) Reconsolidation of drug memories. Neurosci Biobehav Rev 36:1400–1417. doi:10.1016/j.neubiorev.2012.02.004
Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW (2003) Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature 421:70–75. doi:10.1038/nature01249
Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S (2004) Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24:4787–4795. doi:10.1523/JNEUROSCI.5491-03.2004
Taylor JR, Olausson P, Quinn JJ, Torregrossa MM (2009) Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology 56(Suppl 1):186–195. doi:10.1016/j.neuropharm.2008.07.027
Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ (1991) A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res 548:100–110. doi:10.1016/0006-8993(91)91111-D
Torregrossa MM, Kalivas PW (2008) Microdialysis and the neurochemistry of addiction. Pharmacol Biochem Behav 90:261–272. doi:10.1016/j.pbb.2007.09.001
Torregrossa MM, Sanchez H, Taylor JR (2010) D-cycloserine reduces the context specificity of Pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci 30:10526–10533. doi:10.1523/JNEUROSCI.2523-10.2010
Torregrossa MM, Corlett PR, Taylor JR (2011) Aberrant learning and memory in addiction. Neurobiol Learn Mem 96:609–623. doi:10.1016/j.nlm.2011.02.014
Toyomitsu Y, Nishijo H, Uwano T, Kuratsu J, Ono T (2002) Neuronal responses of the rat amygdala during extinction and reassociation learning in elementary and configural associative tasks. Eur J Neurosci 15:753–768. doi:10.1046/j.1460-9568.2002.01889.x
Tronson NC, Taylor JR (2007) Molecular mechanisms of memory reconsolidation. Nature Rev Neurosci 8:262–275. doi:10.1038/nrn2090
Tronson NC, Wiseman SL, Olausson P, Taylor JR (2006) Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci 9:167–169. doi:10.1038/nn1628
Tzeng WY, Chang WT, Chuang JY, Lin KY, Cherng CG, Yu L (2012) Disruption of memory reconsolidation impairs storage of other, non-reactivated memory. Neurobiol Learn Mem 97:241–249. doi:10.1016/j.nlm.2012.01.001
Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462. doi:10.1111/j.1369-1600.2007.00070.x
Valjent E, Corbillé AG, Bertran-Gonzalez J, Hervé D, Girault JA (2006) Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Nat Acad Sci 103:2932–2937. doi:10.1073/pnas.0511030103
Vansteenwegen D, Vervliet B, Iberico C, Baeyens F, Van den Bergh O, Hermans D (2007) The repeated confrontation with videotapes of spiders in multiple contexts attenuates renewal of fear in spider-anxious students. Behav Res Ther 45:1169–1179. doi:10.1016/j.brat.2006.08.023
von der Goltz C, Vengeliene V, Bilbao A, Perreau-Lenz S, Pawlak CR, Kiefer F, Spanagel R (2009) Cue-induced alcohol-seeking behavior is reduced by disrupting the reconsolidation of alcohol-related memories. Psychopharmacol 205:389–397. doi:10.1007/s00213-009-1544-1
Vurbic D, Gold B, Bouton ME (2011) Effects of D-cycloserine on the extinction of appetitive operant learning. Behav Neurosci 125:551–559. doi:10.1037/a0024-403
Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L (2008) Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci 28:5602–5610. doi:10.1523/JNEUROSCI.0750-08.2008
Watson BJ, Wilson S, Griffin L, Kalk NJ, Taylor LG, Munafo MR, Lingford-Hughes AR, Nutt DJ (2011) A pilot study of the effectiveness of d-cycloserine during cue-exposure therapy in abstinent alcohol-dependent subjects. Psychopharmacol 216:121–129. doi:10.1007/s00213-011-2199-2
Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O (2001) Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacol 25:361–372. doi:361-372.10.1038/S0893-133X(01)00238-X
Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE (2006) Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 26:12387–12396. doi:10.1523/JNEUROSCI.4316-06.2006
Winters BD, Tucci MC, DaCosta-Furtado M (2009) Older and stronger object memories are selectively destabilized by reactivation in the presence of new information. Learn Mem 16:545–553. doi:10.1101/lm.1509909
Woods AM, Bouton ME (2006) D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci 120:1159–1162. doi:10.1037/0735-7044.120.5.1159
Wouda JA, Diergaarde L, Riga D, van Mourik Y, Schoffelmeer AN, De Vries TJ (2010) Disruption of long-term alcohol-related memory reconsolidation: role of beta-adrenoceptors and NMDA receptors. Front Behav Neurosci 4:179. doi:10.3389/fnbeh.2010.00179
Wu P, Xue YX, Ding ZB, Xue LF, Xu CM, Lu L (2011) Glycogen synthase kinase 3B in the basolateral amygdala is critical for the reconsolidation of cocaine reward memory. J Neurochem 118:113–125. doi:10.1111/j.1471-4159.2011.07277.x
Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, Epstein DH, Shaham Y, Lu L (2012) A memory retrieval–extinction procedure to prevent drug craving and relapse. Science 336:241–245. doi:10.1126/science.1215070
Zhai H, Wu P, Chen S, Li F, Liu Y, Lu L (2008) Effects of scopolamine and ketamine on reconsolidation of morphine conditioned place preference in rats. Behav Pharmacol 19:211–216. doi:10.1097/FBP.0b013e3282fe88a0
Zhao LY, Zhang XL, Shi J, Epstein DH, Lu L (2009) Psychosocial stress after reactivation of drug-related memory impairs later recall in abstinent heroin addicts. Psychopharmacol 203:599–608. doi:10.1007/s00213-008-1406-2
Zhao LY, Sun LL, Shi J, Li P, Zhang Y, Lu L (2011) Effects of beta-adrenergic receptor blockade on drug-related memory reconsolidation in abstinent heroin addicts. Drug Alcohol Depend 118:224–229. doi:10.1016/j.drugalcdep.2011.03.025
Zhou W, Kalivas PW (2008) N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry 63:338–340. doi:10.1016/j.biopsych.2007.06.008
Zhou SJ, Xue LF, Wang XY, Jiang WG, Xue YX, Liu JF, He YY, Luo YX, Lu L (2011) NMDA receptor glycine modulatory site in the ventral tegmental area regulates the acquisition, retrieval, and reconsolidation of cocaine reward memory. Psychopharmacol. doi:10.1007/s00213-011-2551-6
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Torregrossa, M.M., Taylor, J.R. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology 226, 659–672 (2013). https://doi.org/10.1007/s00213-012-2750-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2750-9