Abstract
Rationale
Currently available antipsychotics are unsatisfactory given their side effects and limited efficacy for the cognitive symptoms of schizophrenia. Many currently available drugs, such as haloperidol, are T-type calcium channel antagonists in addition to their well-established antagonism of dopamine D2 receptors. Thus, preclinical research into the effects of T-type calcium channel antagonists/blockers in behavioral assays related to schizophrenia may inform novel therapeutic strategies.
Objectives
We explored the effects of a recently developed highly selective T-type calcium channel antagonist, Z944 (2.5, 5.0, 10.0 mg/kg), on the MK-801 (0.15 mg/kg) model of acute psychosis.
Methods
To examine the effects of Z944 on behaviors relevant to schizophrenia, we tested touchscreen-based paired associates learning given its relevance to the cognitive symptoms of the disorder and locomotor activity given its relevance to the positive symptoms.
Results
Acute treatment with Z944 failed to reverse the visuospatial associative memory impairments caused by MK-801 in paired associates learning. The highest dose of drug (10.0 mg/kg) given alone produced subtle impairments on paired associates learning. In contrast, Z944 (5.0 mg/kg) blocked the expected increase in locomotion following MK-801 treatment in a locomotor assay.
Conclusions
These experiments provide support that Z944 may reduce behaviors relevant to positive symptoms of schizophrenia, although additional study of its effects on cognition is required. These findings and other research suggest T-type calcium channel antagonists may be an alternative to currently available antipsychotics with less serious side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia, a psychiatric illness that affects ~ 0.4% of the population, is characterized by abnormal social behavior and an array of positive, negative, and cognitive symptoms (Nicholl et al. 2010). Current medications generally treat the positive symptoms; however, these often produce complicating adverse effects, such as weight gain and extrapyramidal effects, and may have limited therapeutic efficacy in certain populations (Sendt et al. 2015; Stafford et al. 2015; Goff et al. 2017). In addition, current treatments fail to address cognitive symptoms which correlate to patient outcome (Green 2006; Moore et al. 2013). Combined, these factors likely contribute to low compliance in outpatients (Lieberman et al. 2005; Roberts and Velligan 2012) and emphasize the need for new medications with fewer side effects or otherwise increase adherence in order to improve patient care.
One approach to finding new medications is to identify affected proteins which are already the target of approved drugs (Lencz and Malhotra 2015). In the case of schizophrenia, recent analysis of the Psychiatric Genomics Consortium–Schizophrenia Workgroup (PGC–SCZ) genome wide association study (GWAS) suggests that T-type calcium channels encoded by the CACNA1I gene are potential drug targets that should be examined with urgency (Lencz and Malhotra 2015). Low-voltage-activated T-type calcium channels contribute to neuronal excitability and burst firing and are expressed in mesocorticolimbic brain areas implicated in schizophrenia including the prefrontal cortex, nucleus accumbens (NA), thalamus, and ventral tegmental area (Talley et al. 1999). Interestingly, in addition to their role as D2 antagonists (McCormick et al. 2010; Yilmaz et al. 2012), some antipsychotics including haloperidol also act as T-type calcium channel blockers (Enyeart et al. 1992; Santi et al. 2002; Choi and Rhim 2010). These studies raise the possibility that T-type calcium channel antagonists may be effective in treating some aspects of schizophrenia.
A limited number of studies have directly explored the effects of T-type calcium channel antagonists in rodent models related to schizophrenia. One approach to modeling the disorder involves systemic administration of non-competitive NMDA antagonists such as MK-801 (dizocilpine) to produce an acute psychotic state resembling schizophrenia (Andiné et al. 1999; Lins et al. 2015; Cadinu et al. 2018; Winship et al. 2018). In Long-Evans rats, administration of an NMDAR antagonist to slices led to an increase in T-type currents in the nucleus reticularis of the thalamus (nRT), an effect blocked by D2 dopamine receptor antagonism (Zhang et al. 2009). In another rat study, three structurally distinct T-type calcium channel antagonists, TTA-A2, TTA-P1, and TTA-Q6, showed antipsychotic-like properties by blocking stimulant-induced hyperlocomotion in assays predictive of antipsychotic efficacy (Uslaner et al. 2012). Following acute administration of either amphetamine or MK-801, T-type calcium channel antagonists decreased locomotor activity in a dose-dependent manner (Uslaner et al. 2012). Furthermore, in contrast to the typical antipsychotic haloperidol, T-type calcium channel blockade did not produce catalepsy following acute treatment, or exhibit desensitization following 20 days of chronic administration (Uslaner et al. 2012). Together, these studies suggest that T-type antagonists may have less severe side effects compared with current antipsychotics and therefore further investigation of their therapeutic potential is warranted.
Although preclinical data suggest that T-type calcium channel antagonists may be effective for treating positive symptoms, to the best of our knowledge, no studies have evaluated whether these effects extend to treatment of cognitive symptoms. Visuospatial paired associates learning (PAL) is used clinically to assess mild cognitive impairment in humans in conditions such as schizophrenia and Alzheimer’s disease (De Rover et al. 2011). A rodent version has been used to assess the therapeutic potential of currently available antipsychotics (Talpos et al. 2015) and new putative therapeutics in the treatment of the cognitive symptoms associated with schizophrenia (Lins et al. 2015; Lins and Howland 2016). In PAL, visuospatial associative memory is assessed through an animal’s ability to learn object-in-place associations over many sessions (Talpos et al. 2015; Lins et al. 2015; Roschlau et al. 2016; Lins and Howland 2016; Roebuck et al. 2018). Previous work demonstrates the translational potential of PAL within the context of schizophrenia (Bussey et al. 2012; Nithianantharajah et al. 2013; Talpos et al. 2014). Therefore, the task is valuable for preclinical assessment of cognitive impairment, as well as screening of novel drugs, in both humans and animal models (Nithianantharajah et al. 2015).
A lack of selective T-type calcium channel antagonists has previously limited research in this area. However, recent development of the high affinity pan-T-type calcium channel antagonist Z944 provides new opportunities for preclinical research (Tringham et al. 2012). The present study tested the potential effectiveness of Z944 in blocking the effects of acute MK-801 treatment in two experiments. PAL was initially used to assess the potential of Z944 to alleviate cognitive impairment following acute administration of MK-801. Subsequently, the role of Z944 on MK-801-induced locomotor activity was assessed in a manner comparable to the preclinical work described previously (Uslaner et al. 2012).
Methods
Subjects
Seventy-two adult male Long-Evans rats were used in paired associates learning (n = 24) and for locomotor testing (n = 48) (Charles River Laboratories, Kingston, NY, USA). Upon arrival at the facility, animals were pair housed and left for 1 week undisturbed with food and water ad libitum (Purina Rat Chow). Following acclimatization, animals were single housed and maintained at 90% of free feeding weight. Animals were housed in ventilated plastic home cages in a temperature-controlled vivarium. A 12:12-h lighting cycle was used with lights on at 7:00 am. Animals were given enrichment in the form of a plastic tube throughout the experiment. All experiments were conducted in accordance with the standards of the Canadian Council on Animal Care and the University of Saskatchewan Research Ethics Board.
A subset of animals used for the locomotor activity experiment (n = 32) were used in a separate experiment in which they were trained similarly on PAL and subjected to either a single s.c. injection of corticosterone suspended in vegetable oil (3.0 mg/kg) or the vehicle treatment. Before locomotor testing, animals were given a 2-week free feeding washout period. Analysis of locomotor activity showed no effect of PAL training on locomotor activity (data not shown).
Training apparatus
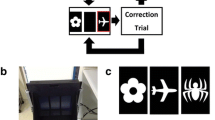
Eight touchscreen-equipped operant conditioning chambers (Lafayette Instruments, Lafayette, IN, USA) were used for PAL (Fig. 1). Each chamber was contained within its own sound-attenuating box, a fan provided background noise and air circulation. A live video feed of animal activity was obtained through a camera mounted within the box above the operant chamber. The chambers were identical to those previously used in our lab (Lins et al. 2015; Roebuck et al. 2018). An interchangeable mask rested on the touchscreen, obscuring the screen entirely except for areas exposed by the response windows. In PAL, the mask had three equally sized rectangular response windows arranged evenly across the mask. Each response window was 15 × 6 cm and images were sized to fit within these windows. The touchscreen windows for PAL sat above a spring-loaded shelf, requiring animals to stand when making a response.
Schematic of the paired associates learning (PAL) and locomotor tests. a Timeline of treatment and events. b PAL. The first trial of PAL begins with illumination of the food port and free delivery of a sucrose pellet. A nose poke initiates the trial and two different stimuli are displayed in the three response windows pseudorandomly with the third window remaining blank. One image is paired to its correct location, in this instance the flower, while the other image is not paired with its correct location, in this instance the airplane. A correct screen touch, the flower, is recorded as a completed selection trial and will result in the food reward followed by a 20-s intertrial period, at the end of which the food port will illuminate and the animal can nose poke to begin a new trial. An incorrect screen touch, the airplane, will not yield a food reward, will cause the house lights to illuminate for 5 s, and will also begin a correction trial. A correction trial consists of the same stimulus pairing and is repeated until the correct selection is made. c Schematic of the locomotor task. Movement was measured during a habituation period (30 min) and following injection of MK-801 (0.15 mg/kg; 120 min)
Touchscreen habituation and pretraining
Habituation, pretraining, and training were conducted according to instructions and protocols established by Lafayette and previous experiments conducted in our lab (Lins et al. 2015; Roebuck et al. 2018). Animals were free to advance through training stages based on their individual performance and ability to fulfill intermediate criteria. Pretraining and training sessions occurred once daily, 6 days a week.
Animals were handled for at least 5 days before touchscreen habituation began. On the first day of habituation, animals were brought from the vivarium to the touchscreen room and left undisturbed in their home cage for 1 h. They were given five reward pellets (Dustless Precision Pellets, 45 mg, Rodent Purified Diet; BioServ, NJ, USA) at the start of the habituation period. During this period, all equipment was on and the lights were dimmed. For all subsequent training days, rats were left undisturbed for 15–20 min following transport to the touchscreen room.
Pretraining consisted of a number of progressive steps beginning with two 30-min chamber habituation sessions in which animals were left undisturbed in the operant chambers and given five reward pellets in the food port. Criterion was reached if all pellets were consumed within 30 min. Rats then began initial touch training in which one of the response windows was illuminated pseudorandomly. The window was illuminated for 30 s and three reward pellets were delivered if the rat correctly touched the illuminated window during this period. One pellet was delivered if the illuminated window was not touched. A 20-s intertrial period followed each trial. Criterion for initial touch was completion of 100 trials in 1 h. Must touch training was administered similarly, with animals receiving one reward pellet for correct touches only. The criterion for must touch training was 100 trials in 1 h. Must initiate training was conducted similarly with the inclusion that the animal must nose poke the food port to initiate a trial. Criterion for the must initiate phase was 100 trials in 1 h. The final stage of pretraining was the punish incorrect stage. Rats were required to initiate each trial by nose poking the food port, which caused one of the response windows to illuminate pseudorandomly. Correct touches to the illuminated window were rewarded with one food pellet; incorrect touches were punished with a 5-s time out and a correction trial. Correction trials are identical to the previous presentation and repeat until successfully completed. The criterion for punish incorrect was 100 trials in 1 h, with greater than 80% correct, with accuracy calculated for the initial presentation only.
PAL required the animal to differentiate between two different stimuli presented simultaneously in two of the three response windows pseudorandomly (Fig. 1). Each stimulus was correct only when paired with its respective location. Negative images of a flower, airplane, and spider were used as stimuli. The flower was always correct in the left position, the airplane in the center position, and the spider in the right position. Correct responses were rewarded and punished in the same manner as the pretraining stages. Animals were trained until they could successfully complete 90 correct selection trials in 1 h, with greater than 80% accuracy, for three consecutive days. Selection trials were the initial trial or subsequent trials following a correction trial. Correction trials were not included in the number of selection trials completed. Accuracy was calculated for the initial presentation only.
Locomotor activity testing
Locomotor testing occurred in a different room than paired associates learning that was novel to all animals. Testing occurred in 40 × 40 × 60 cm arenas constructed from white corrugated plastic (Fig. 1). A ceiling mounted camera recorded activity of individual animals run simultaneously in four separate arenas. Analysis was conducted using EthoVision software (Noldus Information Technology, Wageningen, The Netherlands). Animals were first recorded for 30 min to establish a baseline level of spontaneous activity. Animals were then randomly assigned to a group, treated, and returned to the arena for 120 min. Analysis was conducted to determine the total distance traveled in m.
Drug treatment
Preparation
The characterization and synthesis of Z944 is described in Tringham et al. (2012). For all treatments, Z944 was prepared daily in a solution of 10% dimethyl sulfoxide (DMSO; Sigma-Aldrich, MO, USA) and 90% sodium carboxymethyl cellulose (0.5% in saline; Sigma-Aldrich). MK-801 (Sigma-Aldrich) was dissolved in saline and delivered i.p. at a dose of 0.15 mg/kg. This dose was determined from a previous dose-response curve generated in our lab which found it to be the lowest dose to reliably impair performance in PAL (Lins et al. 2015).
PAL
Following completion of PAL training, animals were randomly assigned to one of two experimental groups: the Z944 dose-response curve group (n = 10) or the Z944/MK-801 treatment group (n = 14). One animal was removed from the Z944/MK-801 group due to a health concern.
To the best of our knowledge, no previous experiments have used Z944 in PAL; therefore, a dose-response curve was produced. A within-subjects design was used with three different doses: 2.5 mg/kg, 5.0 mg/kg, 10.0 mg/kg, and a vehicle treatment. Z944 was delivered i.p. 15 min before PAL. These doses were based on previous behavioral experiments in our lab (Marks et al. 2016a; 2018; Henbid et al. 2017). The order of treatments was randomized such that no two animals followed the same treatment order. Each animal received treatments twice weekly, with 2 days of PAL between treatments. Animals were trained 6 days/week during this period. Two animals received an additional treatment due to technical issues.
A within-subjects design with six different treatments was used for the Z944/MK-801 experiment: vehicle + saline, vehicle + 0.15 mg/kg MK-801, 2.5 mg/kg Z944 + saline, 5.0 mg/kg Z944 + saline, 2.5 mg/kg Z944 + 0.15 mg/kg MK-801, and 5.0 mg/kg Z944 + 0.15 mg/kg MK-801. Treatments on a given day always began with the Z944 or vehicle followed immediately by the MK-801 or saline. Both drugs were delivered i.p., and PAL began 15 min after injection. The order of treatments was randomized such that no more than two animals followed the same treatment order. Each animal received treatments twice weekly, with 2 days of training as normal between treatments. Animals were trained 6 days/week during this period. One animal received an additional treatment due to technical issues.
Locomotor activity
Based on previous experiments conducted in our lab and the dose-response curve generated for Z944 in PAL, the 5.0 mg/kg dose of Z944 (Marks et al. 2016a; 2018; Henbid et al. 2017) and the 0.15 mg/kg dose of MK-801 (Lins et al. 2015) were used for the locomotor experiment. Animals were randomly assigned to one of four groups: Z944 + MK-801 (n = 12), Z944 + vehicle (n = 12), vehicle + MK-801 (n = 12), vehicle + vehicle (n = 12). Injections were delivered i.p. immediately following the baseline locomotor measurement. One animal was removed from the MK-801 + vehicle group as they were determined to be an outlier with locomotor activity greater than two standard deviations from the mean.
Statistical analysis
All data were automatically collected and are presented as group means plus or minus the standard error of the mean (SEM). Several measures were used to asses PAL performance. Animals were compared on the number of selection trials completed, task accuracy, and number of correction trials required. Latency was assessed through four separate measures. The correct response latency (CRL) was the time from nose poke initiation to correct stimulus selection. The incorrect response latency (IRL) was the time from nose poke initiation to incorrect stimulus selection. The reward collection latency (RCL) was the time from correct selection to reward collection. Lastly, the total task time was used as measure of overall activity. Statistics were calculated with SPSS version 21 for Windows. The dose-response effects of Z944 on PAL were analyzed using within-subjects repeated measures ANOVAs (Z944 dose as the factor). Mixed factors repeated measures ANOVAs were used to analyze the effects of Z944 and MK-801 on PAL (Z944 dose and MK-801 as factors). The locomotor data was analyzed using mixed factors repeated measures ANOVAs (Z944, MK-801, and time bin as factors). Greenhouse-Geisser corrections were made for violations of sphericity (Mauchley’s test) for all repeated measures ANOVAs. Post hoc analyses were performed using Tukey’s test. The effect size was calculated with partial η2 which demonstrates the total variability in each dependent variable that can be attributed to the independent variables. Partial η2 values of 0.01, 0.06, and 0.14 are considered to be small, medium, and large effect sizes. Non-significant main effects and interactions are not reported. Statistical significance was set at p ≤ 0.05 unless stated otherwise.
Results
The effects of Z944 on PAL task performance are dose dependent
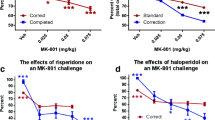
We initially examined the dose-dependent effects of Z944 (2.5, 5.0, and 10.0 mg/kg) on PAL performance (Fig. 2). Analyses revealed a significant main effect of Z944 on three measures of PAL performance: accuracy (F(3,27) = 6.79, p = 0.001, partial η2 = 0.430; Fig. 2a), selection trials (F(3,27) = 3.69, p = 0.024, partial η2 = 0.291; Fig. 2c), and total time to complete the task (F(3,27) = 12.28, p < 0.001, partial η2 = 0.577; Fig. 2h). Post hoc analyses determined that the highest dose of Z944 significantly decreased PAL performance for each measure. Rats treated with the 10.0 mg/kg dose of Z944 had significantly decreased accuracy relative to vehicle and 2.5 mg/kg Z944-treated rats (p < 0.05). Further, the 10.0 mg/kg dose of Z944 significantly decreased the number of selection trials and increased the total time spent to complete the task relative to all other treatment groups (p < 0.05). Post hoc analyses further revealed that the 5.0 mg/kg dose of Z944 significantly decreased accuracy relative to vehicle-treated rats (p < 0.05).
Effects of Z944 (2.5, 5, and 10 mg/kg) on PAL (n = 12). a Accuracy determined by the percentage of trials correct. Rats treated with the 10 mg/kg dose of Z944 had significantly decreased accuracy relative to vehicle and 2.5 mg/kg Z944-treated rats (*p < 0.05). The 5 mg/kg dose of Z944 significantly decreased accuracy relative to vehicle-treated rats (#p < 0.05). b The number of correction trials. c The number of selection trials completed by the rats. The 10 mg/kg dose significantly decreased the number of selection trials relative to all other Z944 doses (*p < 0.05). d Total trials completed (selection trials + correction trials). The response latency for correct trials (e), incorrect trials (f), and reward collection (g). h The total time to complete the task. The 10 mg/kg dose of Z944 significantly increased the total time spent to complete the task relative to all other treatment groups (*p < 0.05)
Z944 failed to block MK-801-induced impairments in the PAL task
As expected, MK-801 impaired PAL performance (Fig. 3) as reflected through a significant decrease in accuracy (F(1,12) = 22.12, p = 0.001, partial η2 = 0.648; Fig. 3a), increase in correction trials (F(1,12) = 21.20, p = 0.001, partial η2 = 0.639; Fig. 3b), decrease in selection trials (F(1,12) = 22.76, p < 0.001, partial η2 = 0.655; Fig. 3d), and increase in total time to complete the task (F(1,12) = 20.25, p = 0.001, partial η2 = 0.628; Fig. 3h). Although a significant main effect of Z944 treatment was found for accuracy (F(2,24) = 7.21, p = 0.004, partial η2 = 0.375), selection trials (F(2,24) = 7.03, p = 0.004, partial η2 = 0.369), total trials (F(2,24) = 6.35, p = 0.006, partial η2 = 0.346), and total time to complete the task (F(2,24) = 8.03, p = 0.002, partial η2 = 0.401), Tukey’s post hoc analyses did not reveal a significant effect of Z944 dose on any of these measures (p > 0.05). No interactions between MK-801 and Z944 were found to be significant for any measures taken (all p ≥ 0.05). While the combination of MK-801 and the higher dose of Z944 (5.0 mg/kg) increased response latencies (Fig. 3e, f), we note that this effect was related to dramatic changes in only 2 of 13 rats, and was not significantly different than the other treatments administered.
Effects of MK-801 (0.15 mg/kg) and Z944 (2.5 and 5 mg/kg) on PAL (n = 13). MK-801 significantly impaired PAL performance as seen by a reduced accuracy (*p < 0.05), b increased number of correction trials (*p < 0.05), c reduced number of selection trials (*p < 0.05), and h increased total time to complete the task (*p < 0.05). No significant effects were noted for d total trials, e correct response latency, f incorrect response latency, or g reward collection latency
Z944 decreases locomotor activity in MK-801-treated rats
Statistical analyses revealed a significant within-subjects main effect of time bin during the habituation phase indicating that all rats similarly acclimatized to the testing arena (F(2,86) = 419.71, p < 0.001, partial η2 = 0.907; Fig. 4a, b). Analysis of the locomotor data during the testing phase (Fig. 4c, d) revealed a significant within-subjects main effect of time bin (F(11,473) = 33.60, p < 0.001, partial η2 = 0.439) indicating that the locomotor activity of all rats changed over time. A significant within-subjects time bin by MK-801 by Z944 interaction (F(5.83,250.92) = 2.27, p = 0.039, partial η2 = 0.050) was also found. A significant between-subjects main effect of Z944 (F(1,43) = 26.38, p < 0.001, partial η2 = 0.380) driven by a significant MK-801 by Z944 interaction (F(1,43) = 15.38, p < 0.001, partial η2 = 0.263) was observed. Post hoc analyses determined that rats treated with MK-801 + vehicle showed significantly increased locomotor activity relative to all other treatment groups (p < 0.05). Post hoc tests also showed that rats treated with MK-801 + Z944 had significantly decreased locomotor activity relative to vehicle + vehicle-treated rats (p < 0.05).
Effects of MK-801 (0.15 mg/kg) and Z944 (5 mg/kg) on locomotor activity (n = 12/group). a Distance traveled (m) during the 30-min habituation period divided into 10 min time bins. b Total distance traveled (m) during the 30-min habituation period. c Distance traveled (m) during the 2-h post-treatment period divided into 10 min time bins. Rats treated with MK-801 alone showed significantly increased locomotor activity relative to all other treatment groups (*p < 0.05). Rats treated with MK-801 + Z944 showed significantly reduced locomotor activity relative to vehicle alone treatment (#p < 0.05). d Total distance traveled (m) during the 2-h post-treatment period. Rats treated with MK-801 alone showed significantly increased locomotor activity relative to all other treatment groups (*p < 0.05). Rats treated with MK-801 + Z944 showed significantly reduced locomotor activity relative to vehicle treatment alone (#p < 0.05)
Discussion
In the present study, two experiments assessed the effectiveness of the T-type calcium channel antagonist Z944 in preventing the effects of acute MK-801 on PAL and locomotor activity. Z944 failed to reduce the cognitive impairment caused by acute injection of MK-801 in PAL. However, Z944 significantly reduced the MK-801-induced increase in locomotor activity, an assay commonly used to assess the effectiveness of novel compounds in treating the positive symptoms of schizophrenia. Together, these data support continued research regarding the use of T-type calcium antagonists to treat positive symptoms.
Z944 dose dependently impairs PAL and does not block MK-801-induced impairment
This experiment revealed a dose-dependent effect of Z944 on PAL performance (Fig. 2). The vehicle and low dose had no effect on task performance, while accuracy was moderately impaired at the medium dose, with a broader impairment identified at the highest dose (10.0 mg/kg). These results fit well with previous behavioral studies which have found that higher acute doses (> 5.0 mg/kg) of Z944 decrease activity during behavioral tasks and impair cognitive performance (Marks et al. 2016a, b; Henbid et al. 2017). Interestingly, Z944 did not increase the number of correction trials, which has been interpreted as a reflection of increased perseveration (Bussey et al. 2012; Lins et al. 2015). Considering these data, the cognitive impairment introduced by moderate doses of Z944 alone is mild, but may indicate decreased wakefulness, an effect shown previously with some T-type antagonists (Kraus et al. 2010; Tringham et al. 2012). Alternatively, previous studies have found that deleting the Cav3.2 T-type calcium channel gene impaired hippocampal-dependent spatial memory (Gangarossa et al. 2014), fear conditioning (Chen et al. 2012), and passive avoidance tasks (Chen et al. 2012). As PAL performance relies on hippocampal spatial memory (Kim et al. 2015), blockade of T-type channels may impair this task. However, whether impairment in PAL from Z944 represents decreased wakefulness or hippocampal disruption cannot be determined from this experiment alone.
Consistent with previous studies (Lins et al. 2015; Lins and Howland 2016; Talpos et al. 2015), we found that MK-801 significantly impaired several measures of PAL performance (Fig. 3). Selection trials, accuracy, and total time to complete the task were all decreased while correction trials were increased. There were no significant effects in total trial completion or latency suggesting that while MK-801 decreased performance on many measures, animals still continued to respond when trials were presented. Following an incorrect selection, animals are presented with the same stimulus-location pairing until a successful trial is completed (i.e., a correction trial); therefore, the number of correction trials are unlimited. However, dramatic increases in correction trials may represent more than a failure to remember the correct stimuli, also indicating an increase in perseveration. Successive repeated errors and failure to adapt are of particular interest as increased perseveration is seen in patients with schizophrenia (Brown et al. 2009; Leeson et al. 2009).
Based on the dose-response profile for Z944 alone, both the 2.5 and 5.0 mg/kg doses were tested against MK-801 in PAL. In contrast to the data produced during the dose-response curve, there was no impairment of PAL performance on any measure following Z944 and vehicle for either dose of Z944. This appears to support our interpretation that low to moderate doses of Z944 produce only mild cognitive impairment in PAL. However, neither dose of Z944 reduced visuospatial deficits associated with acute administration of MK-801. Furthermore, rats treated with the 5.0 mg/kg dose of Z944 PAL appeared further impaired than those treated with MK-801 alone. This may indicate an interaction between these two drugs. A substantial decrease in selection trials, increase in total time, and near significant decrease in total trials may also indicate a combined sedative effect; however, further work must carefully investigate this potential interaction. This result, although disappointing, was not entirely unexpected as previously tested antipsychotics including haloperidol and risperidone that are effective in treating the positive symptoms also failed to reverse MK-801-induced impairments in PAL (Lins et al. 2015; Talpos et al. 2015). It remains to be determined whether other atypical antipsychotics, such as clozapine, may improve the MK-801-induced impairments in PAL. In addition, testing the potential benefit of Z944 on other cognitive assays related to schizophrenia may prove more fruitful.
Z944 blocks the MK-801-induced increase in locomotor activity
To assess whether Z944 may normalize behaviors which relate to positive symptoms of schizophrenia, locomotor activity was tested in the presence and absence of MK-801. As expected, MK-801 significantly increased locomotor activity as previously shown (Fig. 4; Howland et al. 2012; Uslaner et al. 2012). Z944 alone had no effect on locomotor activity. When both drugs were given, locomotor activity was significantly decreased relative to MK-801- and saline-treated animals. This effect is consistent with previous work demonstrating that T-type calcium channel antagonists reduce hyperlocomotion associated psychostimulants such as amphetamine (Uslaner et al. 2012; Gangarossa et al. 2014), cocaine (Bisagno et al. 2010; Gangarossa et al. 2014), and MK-801 (Uslaner et al. 2012). Furthermore, hyperlocomotion and increased sensitivity to stimulants are significantly reduced in Cav3.2 knockout mice (Gangarossa et al. 2014).
As discussed above, one possible explanation for the effects of Z944 may be the demonstrated role of T-type channel antagonists reducing wakefulness and arousal (Crunelli et al. 2014; Kraus et al. 2010). However, in the case of Z944, systemic administration of 10.0 mg/kg did not produce delta rhythms associated with drowsiness, nor produce behavioral measures of sedation (Tringham et al. 2012). Although we did not find a significant decrease in locomotor activity from Z944 alone, some evidence to support this can be found in the present study by examining the dose-response relationship in PAL. At the highest dose (10.0 mg/kg), animals required significantly more time to complete the task. However, this effect is relatively mild, and may be due to increased sensitivity in PAL. The effect on total time was moderate, subject to influence from decreased accuracy resulting in more correction trials, and not accompanied by significant increases on measures of latency.
Together, these experiments suggest an interaction between Z944 and MK-801 providing evidence that Z944 and T-type calcium channel antagonists may be effective in treating the positive symptoms. The mechanism by which this effect is produced is not fully evident. Previous research demonstrates that MK-801 increases glutamate efflux in the NA, an effect blocked by systemic administration of the T-type antagonist TTA-A2 (Uslaner et al. 2012). Glutamatergic efferents exist from the thalamus to the NA (Robinson and Beart 1988) and irregularities within the NA have been associated with schizophrenia pathology (McCollum and Roberts 2015). This led to a theory proposed by Uslaner et al. (2012) that NMDA receptor blockade increases T-type currents in the thalamus leading to excitation of the NA and subsequent hyperlocomotion. The reduction of locomotor activity by Z944 and other structurally distinct T-type antagonists (Uslaner et al. 2012) provide further support for this theory. Notably however, synaptic Cav3.2 T-type channels have recently been shown to control the strength of NMDA receptor-mediated transmission suggesting that there exists a tight regulation between T-type channels and NMDA receptors and thus the situation is likely more complex than originally proposed (Wang et al. 2015). PAL performance is impaired through disruption of the dorsal hippocampus (Kim et al. 2015), and through administration of MK-801 (Lins and Howland 2016). As PAL is thought to involve concurrent hippocampal and prefrontal activation, minimal cortical involvement in this proposed mechanism may explain why there was less of an effect of Z944 on PAL, but a more substantial effect in the locomotor task.
The theory proposed by Uslaner et al. (2012) was used to inform a double-blinded clinical study in which the efficacy of the T-type antagonist MK-8998 was evaluated in comparison with a placebo and the atypical antipsychotic olanzapine over a 4-week period in acutely psychotic inpatients with a diagnosis of schizophrenia (Egan et al. 2013). However, although both active treatments were generally well tolerated, neither MK-8998 nor olanzapine was more effective than placebo on the Positive and Negative Syndrome Scale (PANSS) (Egan et al. 2013). While these data did not support a role of T-type calcium channel antagonists in the treatment of positive symptoms, the failure of the active comparator in the trial makes interpretation of these results difficult. Importantly, at doses used preclinically (Uslaner et al. 2012, Marks et al. 2016a; 2018) and in human clinical trials (Egan et al. 2013, Lee 2014), T-type antagonists do not appear to produce extrapyramidal side effects often associated with current antipsychotics. In one human trial, MK-8998 was generally well tolerated with a comparable number of incidents to the control drug, olanzapine. The primary complaint of MK-8998 was insomnia, with no serious adverse events reported within the 4-week period (Egan et al. 2013). In a phase I trial, Z944 was reported as safe and well tolerated with mild to moderate side effects following oral doses of 20, 40, and 80 mg (Lee 2014). Together, these studies suggest T-type antagonists are relatively well tolerated at therapeutic levels, suggesting they may be alternatives to current antipsychotics.
Conclusion
While little research to date has directly explored the role of T-type calcium channels in schizophrenia, there is considerable interest in this area (Choi 2013; Lencz and Malhotra 2015 Siegrist et al. 2016; Zamponi 2016; Joksimovic et al. 2017). T-type calcium channel antagonists may have similar therapeutic efficacy when compared to clinical antipsychotics, but the potential for reduced side effects makes them an appealing target for future research. In agreement with previous work using other T-type calcium channel antagonists, we found that Z944 reduced hyperlocomotion associated with acute administration of MK-801 in rats but not the disruptive effects of MK-801 on PAL. Considering these findings and the functional roles and distribution of T-type calcium channels in the brain, continued research in this area may lead to the development of novel therapeutics for schizophrenia and related disorders.
References
Andiné P, Widermark N, Axelsson R, Nyberg G, Olofsson U, Mårtensson E, Sandberg M (1999) Characterization of MK-801-induced behavior as a putative rat model of psychosis. J Pharmacol Exp Ther 290(3):1393–1408
Bisagno V, Raineri M, Peskin V, Wikinski SI, Uchitel OD, Llinás RR, Urbano FJ (2010) Effects of T-type calcium channel blockers on cocaine-induced hyperlocomotion and thalamocortical GABAergic abnormalities in mice. Psychopharmacology 212(2):205–214
Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, … Schaefer CA (2009) Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiatr 166(6):683–690
Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KAL, Nithianantharajah J, … Saksida LM (2012) New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology 62(3):1191–1203
Cadinu D, Grayson B, Podda G, Harte MK, Doostdar N, Neill JC (2018) NMDA receptor antagonist rodent models for cognition in schizophrenia and identification of novel drug treatments, an update. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2017.11.045
Chen CC, Shen JW, Chung NC, Min MY, Cheng SJ, Liu IY (2012) Retrieval of context-associated memory is dependent on the Ca v3.2 T-type calcium channel. PLoS One 7(1):1–10
Choi K-H (2013) The design and discovery of T-type calcium channel inhibitors for the treatment of central nervous system disorders. Expert Opin Drug Discovery 8(8):919–931
Choi KH, Rhim H (2010) Inhibition of recombinant Cav3.1 (α1G) T-type calcium channels by the antipsychotic drug clozapine. Eur J Pharmacol 626(2–3):123–130
Crunelli V, David F, Leresche N, Lambert RC (2014) Role for T-type Ca2+ channels in sleep waves. Pflugers Arch Eur J Physiol 466(4):735–745
De Rover M, Pironti VA, McCabe JA, Acosta-Cabronero J, Arana FS, Morein-Zamir S, … Sahakian BJ (2011) Hippocampal dysfunction in patients with mild cognitive impairment: a functional neuroimaging study of a visuospatial paired associates learning task. Neuropsychologia 49(7):2060–2070
Egan MF, Zhao X, Smith A, Troyer MD, Uebele VN, Pidkorytov V, Cox K, Murphy M, Snavely D, Lines C, Michelson D (2013) Randomized controlled study of the T-type calcium channel antagonist MK-8998 for the treatment of acute psychosis in patients with schizophrenia. Hum Psychopharmacol Clin Exp 28(2):124–133
Enyeart JJ, Biagi B a, Mlinar B (1992) Preferential block of T-type calcium channels by neuroleptics in neural crest-derived rat and human C cell lines. Mol Pharmacol 42(2):364–372
Gangarossa G, Laffray S, Bourinet E, Valjent E (2014) T-type calcium channel Cav3.2 deficient mice show elevated anxiety, impaired memory and reduced sensitivity to psychostimulants. Front Behav Neurosci 8(March):1–12
Goff DC, Falkai P, Fleischhacker WW, Girgis RR, Kahn RM, Uchida H, … Lieberman JA (2017) The long-term effects of antipsychotic medication on clinical course in schizophrenia. Am J Psychiatr 174(9):840–849
Green MF (2006) Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry 67(SUPPL. 9):3–8
Henbid MT, Marks WN, Collins MJ, Cain SM, Snutch TP, Howland JG (2017) Sociability impairments in genetic absence epilepsy rats from Strasbourg: reversal by the T-type calcium channel antagonist Z944. Exp Neurol 296:16–22
Howland JG, Cazakoff BN, Zhang Y (2012) Altered object-in-place recognition memory, prepulse inhibition, and locomotor activity in the offspring of rats exposed to a viral mimetic during pregnancy. Neuroscience 201:184–198
Joksimovic SM, Eggan P, Izumi Y, Joksimovic SL, Tesic V, Dietz RM, … Todorovic SM (2017) The role of T-type calcium channels in the subiculum: to burst or not to burst? J Physiol 595(19):6327–6348
Kim CH, Heath CJ, Kent BA, Bussey TJ, Saksida LM (2015) The role of the dorsal hippocampus in two versions of the touchscreen automated paired associates learning (PAL) task for mice. Psychopharmacology 232(21–22):3899–3910
Kraus RL, Li Y, Gregan Y, Gotter AL, Uebele VN, Fox SV, … Renger JJ (2010) In vitro characterization of T-type calcium channel antagonist TTA-A2 and in vivo effects on arousal in mice. J Pharmacol Exp Ther 335(2):409–417
Lee M (2014) Z944: a first in class T-type calcium channel modulator for the treatment of pain. J Peripher Nerv Syst 19(S2):S11–S12
Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, Joyce EM (2009) Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry 66(6):586–593
Lencz T, Malhotra AK (2015) Targeting the schizophrenia genome: a fast track strategy from GWAS to clinic. Mol Psychiatry 20(7):820–826
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353(12):1209–1223
Lins BR, Howland JG (2016) Effects of the metabotropic glutamate receptor 5 positive allosteric modulator CDPPB on rats tested with the paired associates learning task in touchscreen-equipped operant conditioning chambers. Behav Brain Res 301:152–160
Lins BR, Phillips AG, Howland JG (2015) Effects of D- and L-govadine on the disruption of touchscreen object-location paired associates learning in rats by acute MK-801 treatment. Psychopharmacology 232(23):4371–4382
Marks WN, Cain SM, Snutch TP, Howland JG (2016a) The T-type calcium channel antagonist Z944 rescues impairments in crossmodal and visual recognition memory in genetic absence epilepsy rats from Strasbourg. Neurobiol Dis 94:106–115
Marks WN, Greba Q, Cain SM, Snutch TP, Howland JG (2016b) The T-type calcium channel antagonist Z944 disrupts prepulse inhibition in both epileptic and non-epileptic rats. Neuroscience 332:121–129
Marks WN, Parker ME, Zabder NK, Greba Q, Snutch TP, Howland JG (2018) T-type calcium channels in the orbitofrontal cortex mediate sensory integration as measured using a spontaneous oddity task in rats. Learn Mem 25(7):317–324
McCollum LA, Roberts RC (2015) Uncovering the role of the nucleus accumbens in schizophrenia: a postmortem analysis of tyrosine hydroxylase and vesicular glutamate transporters. Schizophr Res 169(1–3):369–373
McCormick PN, Kapur S, Graff-Guerrero A, Raymond R, Nobrega JN, Wilson AA (2010) The antipsychotics olanzapine, risperidone, clozapine, and haloperidol are d2-selective ex vivo but not in vitro. Neuropsychopharmacology 35(8):1826–1835
Moore H, Geyer MA, Carter CS, Barch DM (2013) Harnessing cognitive neuroscience to develop new treatments for improving cognition in schizophrenia: CNTRICS selected cognitive paradigms for animal models. Neurosci Biobehav Rev 37(9):2087–2091
Nicholl D, Akhras KS, Diels J, Schadrack J (2010) Burden of schizophrenia in recently diagnosed patients: healthcare utilisation and cost perspective. Curr Med Res Opin 26(4):943–955
Nithianantharajah J, Komiyama NH, McKechanie A, Johnstone M, Blackwood DH, Clair DS, … Grant SGN (2013) Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nat Neurosci 16(1):16–24
Nithianantharajah J, McKechanie AG, Stewart TJ, Johnstone M, Blackwood DH, St Clair D, … Saksida LM (2015) Bridging the translational divide: identical cognitive touchscreen testing in mice and humans carrying mutations in a disease-relevant homologous gene. Sci Rep 5(August):14613
Roberts DL, Velligan DI (2012) Medication adherence in schizophrenia. Drug Discovery Today: Therapeutic Strategies 8(1–2):11–15
Robinson TG, Beart PM (1988) Excitant amino acid projections from rat amygdala and thalamus to nucleus accumbens. Brain Res Bull 20(4):467–471
Roebuck AJ, Liu MC, Lins BR, Scott GA, Howland JG (2018) Acute stress, but not corticosterone, facilitates acquisition of paired associates learning in rats using touchscreen-equipped operant conditioning chambers. Behav Brain Res 348:139–149
Roschlau C, Votteler A, Hauber W (2016) Stimulant drug effects on touchscreen automated paired-associates learning (PAL) in rats. Learn Mem 23(8):422–426
Santi CM, Cayabyab FS, Sutton KG, McRory JE, Mezeyova J, Hamming KS, … Snutch TP (2002) Differential inhibition of T-type calcium channels by neuroleptics. J Neurosci 22(2):396–403
Sendt KV, Tracy DK, Bhattacharyya S (2015) A systematic review of factors influencing adherence to antipsychotic medication in schizophrenia-spectrum disorders. Psychiatry Res 225(1–2):14–30
Siegrist R, Pozzi D, Jacob G, Torrisi C, Colas K, Braibant B, … Bezençon O (2016) Structure-activity relationship, drug metabolism and pharmacokinetics properties optimization, and in vivo studies of new brain penetrant triple T-type calcium channel blockers. J Med Chem 59(23):10661–10675
Stafford MR, Mayo-Wilson E, Loucas CE, James A, Hollis C, Birchwood M, Kendall T (2015) Efficacy and safety of pharmacological and psychological interventions for the treatment of psychosis and schizophrenia in children, adolescents and young adults: a systematic review and meta-analysis. PLoS One 10(2):1–17
Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA (1999) Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 19(6):1895–1911
Talpos JC, Aerts N, Fellini L, Steckler T (2014) A touch-screen based paired-associates learning (PAL) task for the rat may provide a translatable pharmacological model of human cognitive impairment. Pharmacol Biochem Behav 122:97–106
Talpos J, Aerts N, Waddell J, Steckler T (2015) MK-801 and amphetamine result in dissociable profiles of cognitive impairment in a rodent paired associates learning task with relevance for schizophrenia. Psychopharmacology 232(21–22):3911–3920
Tringham E, Powell KL, Cain SM, Kuplast K, Mezeyova J, Weerapura M, … Snutch TP (2012) T-type calcium channel blockers that attenuate thalamic burst firing and suppress absence seizures. Sci Transl Med 4(121):121ra19–121ra19
Uslaner JM, Smith SM, Huszar SL, Pachmerhiwala R, Hinchliffe RM, Vardigan JD, … Hutson PH (2012) T-type calcium channel antagonism produces antipsychotic-like effects and reduces stimulant-induced glutamate release in the nucleus accumbens of rats. Neuropharmacology 62(3):1413–1421
Wang G, Bochorishvili G, Chen Y, Salvati KA, Zhang P, Dubel SJ, Perez-Reyes E, Snutch TP, Stornetta RL, Deisseroth K, Erisir A, Todorovic SM, Luo JH, Kapur J, Beenhakker MP, Zhu JJ (2015) CaV3.2 calcium channels control NMDA receptor-mediated transmission: a new mechanism for absence epilepsy. Genes Dev 29:1535–1551
Winship IR, Dursun SM, Baker GB, Balista PA, Kandratavicius L, Maia-de-oliveira JP, … Howland JG (2018) An overview of animal models related to schizophrenia. Can J Psychiatr in press
Yilmaz Z, Zai CC, Hwang R, Mann S, Arenovich T, Remington G, Daskalakis ZJ (2012) Antipsychotics, dopamine D2receptor occupancy and clinical improvement in schizophrenia: a meta-analysis. Schizophr Res 140(1–3):214–220
Zamponi GW (2016) Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat Publ Group 15(1):19–34
Zhang Y, Llinas RR, Lisman JE (2009) Inhibition of NMDARs in the nucleus reticularis of the thalamus produces delta frequency bursting. Frontiers in Neural Circuits 3(November):1–9
Acknowledgements
This research was supported by an operating grant from the Canadian Institutes of Health Research (CIHR) to JGH (#125984). Work in the laboratory of TPS is supported by an operating grant from CIHR (#10677), the Canada Research Chair in Biotechnology and Genomics-Neurobiology and the Koerner Foundation. Matching funds for these experiments were provided by the Saskatchewan Health Research Foundation and the University of Saskatchewan. AJR received salary support from the Natural Sciences and Engineering Research Council of Canada Collaborative Research and Training Program (NSERC CREATE). WNM is supported by funding from the Saskatchewan Health Research Foundation. MCL received an award from the Natural Sciences and Engineering Research Council of Canada Undergraduate Student Research Award Program. NBT received salary support from the College of Medicine, University of Saskatchewan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Roebuck, A.J., Marks, W.N., Liu, M.C. et al. Effects of the T-type calcium channel antagonist Z944 on paired associates learning and locomotor activity in rats treated with the NMDA receptor antagonist MK-801. Psychopharmacology 235, 3339–3350 (2018). https://doi.org/10.1007/s00213-018-5040-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5040-3