Abstract
Rationale
New pharmacological treatments for the cognitive deficits in schizophrenia are needed. Tetrahydroprotoberberines, such as govadine, are one class of compounds with dopaminergic activities that may be useful in treating some aspects of the cognitive symptoms of the disorder.
Objective
The objective of the present studies was to test the effects of the d- and l-enantiomers of govadine on the impairment in a paired-associate learning (PAL) task produced by acute MK-801 in rats. We also assessed effects of the typical antipsychotic haloperidol as a comparator compound.
Methods
MK-801 (0.05, 0.1, 0.15, and 0.2 mg/kg), d- and l-govadine (0.3, 1.0, and 3.0 mg/kg), and haloperidol (0.05, 0.1, and 0.25 mg/kg) were administered acutely to rats well trained on the PAL task in touchscreen-equipped operant conditioning chambers.
Results
Acute MK-801 impaired performance of PAL in a dose-dependent manner by reducing accuracy and increasing correction trials. l-Govadine (1.0 mg/kg), but not d-govadine, blocked the disruptive effects of MK-801 (0.15 mg/kg) on PAL. Haloperidol failed to affect the MK-801-induced disruption of PAL. Higher doses of l-govadine and haloperidol dramatically impaired performance of the task which confounded interpretation of cognitive outcomes.
Conclusion
l-Govadine appears unique in its ability to improve performance of the MK-801-induced impairment in the PAL task. This behavioral effect may relate the ability of l-govadine to antagonize dopamine D2 receptors while also promoting dopamine efflux. Future research should further characterize the role of the dopamine system in the rodent PAL task to elucidate the mechanisms of its pro-cognitive effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a debilitating psychiatric disorder that affects approximately 1 % of the general population. Cognitive impairment is a hallmark of schizophrenia that is significantly correlated with long-term functional outcomes of patients (Elvevag and Goldberg 2000; Green 2006; Keefe and Fenton 2007; Lewis and Gonzalez-Burgos 2008). Conventional antipsychotic treatments have little to no benefit for treating these symptoms (Marder and Fenton 2004; Green 2006; Young et al. 2009); therefore, determining the efficacy of novel compounds for improving the cognitive symptoms is critical to meet the needs of patients with schizophrenia. Visual learning and memory is one of the seven cognitive domains identified by the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative as commonly affected in the disorder (Nuechterlein et al. 2004). In the laboratory, visual learning and memory is assessed with a number of tasks. Visuospatial paired-associate learning (PAL) is one associative memory task included in the Cambridge Neurological Test Automated Battery (CANTAB) that is impaired in schizophrenia patients (Wood et al. 2002; Barnett et al. 2005). A visuospatial PAL task has been developed for rodents utilizing touchscreen-equipped operant conditioning chambers (Fig. 1; Talpos et al. 2009; Bussey et al. 2012; Horner et al. 2013). While some studies have assessed the neural circuitry and pharmacology underlying performance of the rodent task (Talpos et al. 2009, 2014, 2015; Horner et al. 2013; Delotterie et al. 2015; Kim et al. 2015), the effects of putative antipsychotic compounds not currently used in the clinic have yet to be assessed in the rodent PAL task.
Touchscreen chambers and task schematic. a Flow chart of a trial in the PAL task. See the “Materials and methods” section for procedural details. Note that red rectangles, visible on electronic version of the manuscript, denote the correct and incorrect choices possible after initial presentation of the stimuli in this figure. b The interior of the chamber as it is set up during the PAL task. Note the mask with three windows open to the touchscreen and the spring-loaded response shelf below the windows. The funnel-shaped opening opposite the touchscreen guides the reward pellet to the port when the chamber is closed. c The three images displayed on the touchscreen during PAL. The images are ordered with respect to their correct position in the task
Tetrahydroprotoberberines, including the synthetic compound govadine, have recently shown an interesting profile in preclinical assays relevant to the positive, negative, and cognitive symptoms of schizophrenia (Lapish et al. 2012, 2014; Ashby et al. 2015). The effects of govadine have been assessed following administration as both a racemic mixture and separate enantiomers (d- and l-govadine; Zhai et al. 2012). Both enantiomers share a high affinity for dopamine D1 receptors and enhance dopamine efflux in the medial prefrontal cortex (Lapish et al. 2014); however, l-govadine differs from d-govadine as it has a much higher affinity for dopamine D2 receptors and uniquely increases dopamine efflux in the nucleus accumbens (Lapish et al. 2014). Behavioral studies show that l-govadine reduces amphetamine-induced hyperlocomotion, impairs conditioned avoidance responding, and causes catalepsy at high doses, all of which are characteristics of typical antipsychotics. In contrast, d-govadine improves performance of two prefrontal-dependent memory tasks on the radial arm maze. Both enantiomers reverse amphetamine-disrupted latent inhibition and mitigate social interaction deficits in the neonatal ventral hippocampal lesion (NVHL) model. Taken together, these data suggest that govadine may have a unique ability to improve all three categories of schizophrenia symptoms, which makes it an attractive option for further investigation (Lapish et al. 2012, 2014). Given the different profiles of d- and l-govadine, the goal of the present study was to test the effects of each enantiomer separately in the rodent PAL task both alone and in combination with acute administration of the NMDA receptor antagonist MK-801.
Acute administration of non-competitive NMDA receptor antagonists such as MK-801 (dizocilpine), ketamine, and phencyclidine has been used to model aspects of schizophrenia in rodents, given the psychotomimetic effects of these drugs in humans (Moghaddam and Krystal 2012). MK-801 disrupts cognition in tasks relevant to schizophrenia when administered acutely (Mathe et al. 1998; Vales et al. 2010). More specifically, acute MK-801 disrupts visual learning and memory in tasks such as spontaneous object recognition (Lyon et al. 2012) and visual discrimination using a touchscreen-based task (Talpos et al. 2012). In a recent paper, Talpos et al. (2015) demonstrate that acute MK-801 administered systemically to rats disrupts PAL. Therefore, the effects of d- and l-govadine on the impairment of PAL by acute systemic MK-801 administration were assessed. To compare the effects observed with govadine to those of typical antipsychotics, we also assessed the effects of acute haloperidol administration on PAL in control and MK-801-treated rats.
Materials and methods
Subjects
Forty male Long-Evans rats were used (Charles River Laboratories, Quebec, Canada). These were trained in two cohorts referred to as squad 1 (n = 16) and squad 2 (n = 24). Throughout training and testing, rats were single housed in clear, ventilated plastic cages in a temperature-controlled vivarium. Lighting was controlled automatically on a 12:12-h cycle with lights on at 7:00 a.m. All handling and experimentation occurred within the light phase. Rats were food restricted to 85 % of their free feeding body weight and maintained on a restricted diet with sufficient intake to support normal growth throughout the experiment. All experiments were performed in accordance with the standards of the Canadian Council on Animal Care and were approved by the University of Saskatchewan Animal Research Ethics Board.
Training apparatus
Training and testing were conducted in eight touchscreen-equipped operant conditioning chambers (Lafayette Instruments, Lafayette, IN, USA). Each operant conditioning chamber is located on a sliding shelf at the base of a sound-attenuating chamber containing a fan to circulate air and create background noise. A second sliding shelf above the operant conditioning chamber holds a pellet dispenser and a video camera, which provides a live feed of the rat’s activity within the chamber on an external monitor. The operant conditioning chambers are trapezoidal in shape with the wide end consisting of a touchscreen monitor covered with a black polycarbonate mask. The PAL mask has three rectangular windows, which allow the rats to contact the touchscreen monitor only in areas where the stimuli are presented. Directly below the three windows is a spring-loaded “response shelf” that forces the rats to intentionally stand and press the shelf down in order to contact the touchscreen monitor and make a selection.
Touchscreen habituation and pretraining
Rats were left undisturbed for at least 5 days following arrival to the animal holding facility and then handled for 5 days before habituation and training. On day 1 of habituation, rats were brought from the vivarium to the testing room. Five reward pellets (Dustless Precision Pellets, 45 mg, Rodent Purified Diet; BioServ, Frenchtown, NJ, USA) were placed in their cages and left undisturbed for 1 h with all testing equipment powered on (eight chambers and two computers). For subsequent days of training, rats were left undisturbed for 15–20 min following transport to the testing room before being introduced to the chambers.
Pretraining protocols were conducted as per the instructions and software that accompanied the Lafayette touchscreen chambers, and each phase was repeated until a criterion was reached. The optional “dPAL acquisition” stage was not used. Training began with 2 days of 30-min habituation periods in which the rats were placed in the chambers with five reward pellets in the food port. Criterion was reached if all pellets were consumed within 30 min. During initial touch training, one of the response windows on the screen was illuminated pseudorandomly such that the same window was not illuminated for three consecutive trials. If the rat touched the illuminated screen, three pellets were delivered. If the illuminated screen was not touched, one pellet was delivered. The stimulus remained illuminated for 30 s or until the rat touched the screen. Each trial was followed by a 20-s inter-trial interval that began when the rat entered the food port to collect the reward. Criterion was completion of 100 trials in 1 h. Must touch training also involved illumination of one window; however, the rat was required to touch the illuminated window to receive one reward pellet (no reward was given if the rat touched a blank window). Criterion was 100 trials completed in 1 h. This was followed by must initiate training where illumination of the window must be triggered by rat nose poking into the illuminated food port. Again, the criterion was 100 trials in 1 h. In the final stage of pretraining (punish incorrect training), rats were required to initiate the trial and touch the illuminated window to receive a reward. An incorrect touch resulted in punishment with a 5-s time out followed by a correction trial. Correction trials were repeated until the illuminated window was touched by the rat, followed by delivery of a food reward. The 20-s inter-trial interval began when the reward was collected. Criterion was 100 trials completed in 1 h with a minimum of 80 % correct for two consecutive days. Once criterion was reached, training on the full version of the task began.
PAL task
There are two versions of the rodent PAL task, referred to in the literature as dPAL and sPAL. In dPAL, there are three different stimuli, two of which are presented simultaneously in a given trial. There are three different locations for the stimuli to be presented. Another version, sPAL, is identical to dPAL except that the two stimuli displayed in each trial are identical. We used the dPAL version of the task because it is more sensitive to pharmacological manipulations of the hippocampus (Talpos et al. 2009), an area implicated in schizophrenia pathology (Wood et al. 2002; Lodge and Grace 2007; Jodo 2013). In dPAL, rats are presented with two of the three images in each trial in a pseudorandom order. Each image appears in one of the three windows on the touchscreen monitor. Of these three windows, each image has a correct location and two incorrect locations. The images are flower (f), airplane (a), and spider (s), plus a blank window (b), and are presented as f+/s−/b−, f+/b−/a−, b−/a+/f−, s−/a+/b−, b−/f−/s+, and a−/b−/s+. The flower is always correct when presented in the left window, the airplane is always correct in the middle window, and the spider is always correct in the right window. Selections were made by nose poking directly onto the screen. Correct selections were rewarded with a sugar pellet, and incorrect selections were punished with a 5-s delay. Following an incorrect selection, the rats were given correction trials where the same pair of stimuli was presented repeatedly until the correct selection was made. Correction trials were not included in the number of selection trials completed or task accuracy which, instead, were based only on the first presentation of each stimulus pair. Rats were trained until performance was stable for at least three consecutive days (90 selection trials completed in 1 h with a minimum of 80 % correct). As response and reward latencies provide an additional indirect measure of cognitive and motor function, we assessed latency to touch the screen and make a correct decision (correct response latency), latency to touch the screen and make an incorrect decision (incorrect response latency), and latency to nose poke the food port to collect the reward following a correct decision (reward collection latency) during all sessions.
Drug treatments
Treatments were administered following dPAL training in a counterbalanced order using a within-subjects design. Drugs (MK-801, d- and l-govadine, and haloperidol) were prepared as follows: MK-801 (Abcam, Cambridge, MA, USA) dissolved in distilled water; d- and l-govadine, synthesized by the Sammis Lab at the University of British Columbia as described by Zhai et al. (2012), dissolved in 50 % dimethyl sulfoxide (Sigma, Oakville, ON, Canada) and 50 % distilled water; and haloperidol (Sigma, Oakville, ON, Canada) dissolved in glacial acetic acid diluted with physiological saline and adjusted to a final pH of 5.5 with sodium hydroxide. All drug injection volumes were 1 mL/kg body weight. Govadine (s.c.) and haloperidol (i.p.) or their vehicle treatments were administered approximately 5 min before MK-801 (i.p.) or its vehicle, which was administered 15 min before the start of PAL. Doses were determined from the existing literature (Lecourtier et al. 2007; Uslaner et al. 2009; Stefani and Moghaddam 2010). To establish the effects of MK-801 on dPAL, seven squad 1 and seven squad 2 rats were treated twice each with MK-801 using a range of doses (0.05, 0.1, 0.15, and 0.2 mg/kg). This dose range was chosen as it was previously shown to disrupt cognition in rodents (Mathe et al. 1998; Uslaner et al. 2009; Vales et al. 2010; Stefani and Moghaddam 2010; Fowler et al. 2011; Talpos et al. 2012, 2015; Sullivan et al. 2014). Squads 1 and 2 were then treated with each govadine enantiomer separately plus 0.15 mg/kg MK-801 or vehicle. Squad 1 (n = 14) received 1.0 mg/kg of govadine, while squad 2 was divided into two groups which received either 0.3 mg/kg (n = 11) or 3.0 mg/kg (n = 10). Haloperidol treatments were then conducted on squad 2 rats (n = 12, with balanced numbers from each group previously receiving 0.3 or 3.0 mg/kg govadine treatment). The rats received three doses of haloperidol (0.05, 0.1, and 0.25 mg/kg, doses from Bethus et al. 2006; Banasikowski et al. 2012; Sun et al. 2014) plus 0.15 mg/kg MK-801 and vehicle treatments. It should be noted that squad 1 received another drug treatment (1.0, 3.0, and 10.0 mg/kg CDPPB alone) for a separate experiment prior to the treatments for this experiment. A minimum of 1 week washout was given between all drug treatment schedules.
Statistical analyses
The PAL task is fully automated which eliminates observer bias, and no data scoring is required. All data are presented as group means plus or minus the standard error of the mean (SEM). Dependent measures analyzed were accuracy (% correct selections), number of selection trials completed (first stimuli presentation), number of correction trials completed (repeated errors), total trials completed (selection trials plus correction trials), mean correct response latency, mean incorrect response latency, and mean reward collection latency. Statistics were calculated using SPSS version 21. The MK-801 dose-response data were analyzed using a one-way ANOVA. Two-way repeated measures ANOVAs were used to analyze all other data sets (MK-801 with govadine, d- and l-govadine analyzed separately, and MK-801 with haloperidol). Tukey’s test was used for post hoc analysis. Two rats from squad 1 and one from squad 2 were not involved in any drug treatments because they did not learn the PAL task. Three more rats from squad 1 failed to reach the baseline criterion near the end of the 1.0 mg/kg govadine treatment schedule despite several attempts to re-baseline and did not complete the final treatments. One rat failed to complete any trials when treated with l-govadine and was removed from analysis. The final number of rats included in the govadine analysis was 13 for l-govadine and 12 for d-govadine. Two rats were excluded from the analysis of the haloperidol effects due to completing very few trials with the highest dose (0.25 mg/kg). All rats from other treatments were included in analysis.
Results
MK-801 impairs PAL performance in a dose-dependent manner
MK-801 (0.05, 0.1, 0.15, and 0.2 mg/kg) was administered 15 min prior to the PAL task. One-way ANOVA revealed a dose-dependent effect of MK-801 in disrupting several measures of PAL performance. Accuracy was reduced (Fig. 2a), and correction trials were increased (Fig. 2b) following MK-801 treatment at the two highest doses [0.15 and 0.2 mg/kg; accuracy: F(4,31) = 8.46, p < 0.001; correction trials: F(4,31) = 4.76, p < 0.01]. Post hoc analyses confirm that vehicle-treated rats performed significantly better than the rats treated with 0.15 and 0.2 mg/kg of MK-801 (p < 0.05). Selection trials (Fig. 2c) were significantly affected by MK-801 treatment (F(4,31) = 5.26, p < 0.01) with rats treated with the highest dose (0.2 mg/kg) completing significantly fewer trials than rats in the other groups (p < 0.05). The reduction in selection trials was due to the performance of increased numbers of correction trials as opposed to a gross motor impairment as there was no effect of MK-801 on total trials completed (Fig. 2d; statistics not shown). Response (Fig. 2e, f) and reward latencies (Fig. 2g) were unaffected following MK-801 treatment, although the high dose of MK-801 tended to impair response latencies for correct and incorrect trials.
Effects of MK-801 (0.05, 0.1, 0.15, and 0.2 mg/kg) on PAL. a Accuracy as measured by the percentage of trials correct. b The number of correction trials completed by rats. c The number of new trials completed by the rats. d Total trials completed (new trials + correction trials). Response latencies for correct trials (e), incorrect trials (f), and reward collection (g). *p < 0.05, between groups as indicated
d-Govadine failed to affect the performance of the PAL task or the impairment caused by acute MK-801
d-Govadine (0.3, 1.0, and 3.0 mg/kg) did not significantly affect PAL task performance in well-trained rats (Fig. 3a–d) nor were significant interactions between MK-801 and d-govadine treatment found for any of the doses (statistics not shown). Similar to the data reported for the rats from the MK-801 dose-response study, MK-801 treatment (0.15 mg/kg) significantly reduced task accuracy (Fig. 3a) in rats that also received 0.3 mg/kg (F(1,10) = 40.94, p < 0.001), 1.0 mg/kg (F(1,11) = 18.30, p < 0.01), or 3.0 mg/kg (F(1,9) = 8.03, p < 0.05) of d-govadine. Correction trials were significantly increased by MK-801 treatment [Fig. 3b; 0.3 mg/kg group: F(1,10) = 40.50, p < 0.001; 1.0 mg/kg group: F(1,11) = 33.86, p < 0.001; 3.0 mg/kg group: F(1,9) = 8.28, p < 0.05]. MK-801 also significantly reduced the number of selection trials completed [Fig. 3c; 0.3 mg/kg group: F(1,10) = 15.98, p < 0.01; 1.0 mg/kg group: F(1,11) = 4.79, p = 0.051; 3.0 mg/kg group: F(1,9) = 3.68, p = 0.09] and significantly increased total trials completed [Fig. 3d; F(1,10) = 9.30, p < 0.05; F(1,11) = 43.79, p < 0.001; F(1,9) = 4.31, p = 0.06]. Neither correct nor incorrect response latencies were affected by MK-801 or d-govadine. Reward collection latency was reduced by MK-801 in the 0.3 and 1.0 mg/kg govadine groups [F(1,10) = 7.85, p < 0.05; F(1,11) = 34.53, p < 0.001; F(1,9) = 2.72, p > 0.05], while d-govadine increased reward latency in rats treated with the 0.3 mg/kg dose (F(1,10) = 20.26, p < 0.01).
Effects of d-govadine (0.3, 1.0, and 3.0 mg/kg) and MK-801 (0.15 mg/kg) on PAL. a MK-801 significantly reduced accuracy, while d-govadine had no effect. b MK-801 reduced trials completed, an effect that was nearly significant (p = 0.051). d-Govadine did not significantly affect trials completed. c MK-801 significantly increased correction trials. d-Govadine was not effective in reversing this effect. d MK-801 increased the number of total trials, and d-govadine had no effect. e MK-801 reduced reward latency without affecting response latencies for correct or incorrect trials. d-Govadine had no effect on latency in this sample. Asterisk indicates a significant effect of MK-801. Number sign indicates a significant effect of d-govadine
l-Govadine affects PAL and blocks the MK-801-induced impairment in task performance
In contrast to d-govadine, l-govadine had significant dose-dependent effects on the PAL task. As a result, we have presented the effects of the two lower doses (0.3 and 1.0 mg/kg) in Fig. 4 and the higher dose (3.0 mg/kg) in Fig. 5. Specifically, PAL task accuracy was significantly reduced (Fig. 4a) and correction trials were significantly increased (Fig. 4b) following MK-801 treatment [main effect of MK-801 on accuracy: F(1,10) = 23.27, p < 0.01 (0.3 mg/kg l-govadine); F(1,11) = 13.33, p < 0.01 (1.0 mg/kg l-govadine); main effect of MK-801 on correction trials: F(1,10) = 28.63, p < 0.01 (0.3 mg/kg l-govadine); F(1,11) = 20.94, p < 0.01 (1.0 mg/kg l-govadine)]. The low dose of l-govadine did not affect accuracy, correction trials, or selection trials when administered alone or in combination with MK-801 (statistics not shown). However, 1.0 mg/kg of l-govadine blocked the effects of acute MK-801 [main effect of 1.0 mg/kg l-govadine: F(1,11) = 13.44, p < 0.01; interaction: F(1,11) = 12.23, p < 0.01]. Post hoc analysis confirmed that the MK-801-treated group had significantly reduced accuracy compared to the vehicle, 1.0 mg/kg l-govadine, and 1.0 mg/kg l-govadine with MK-801 groups (p < 0.05). In addition, l-govadine (1.0 mg/kg) significantly reduced the number of correction trials committed by the rats given MK-801 [Fig. 4b; main effect of l-govadine: F(1,11) = 30.49, p < 0.001; a significant interaction: F(1,11) = 19.38, p < 0.01; post hoc analysis, p < 0.05]. l-Govadine (1.0 mg/kg) reduced the number of selection trials completed [Fig. 4c; main effect of l-govadine: F(1,11) = 5.73, p < 0.05; main effect of MK-801: F(1,11) = 0.10, p > 0.05; interaction: F(1,11) = 6.89, p < 0.05]. Post hoc analysis revealed the 1.0 mg/kg l-govadine-treated rats completed fewer selection trials than the vehicle-treated group, regardless of treatment with MK-801. MK-801 significantly increased total trials [Fig. 4d; 0.3 mg/kg: F(1,10) = 39.410, p < 0.001; 1.0 mg/kg: F(1,11) = 14.35, p < 0.01], while l-govadine (1.0 mg/kg) significantly reduced total trials completed [F(1,11) = 20.91, p < 0.01; notable effect of 0.3 mg/kg l-govadine: F(1,10) = 4.45, p = 0.06], with no interaction observed for this measure. Neither correct nor incorrect response latencies were significantly affected by MK-801 or l-govadine at either dose, although it should be noted that higher latencies were occasionally observed with l-govadine treatments for the incorrect responses only. MK-801 significantly reduced reward latency [0.3 mg/kg govadine-treated group: F(1,10) = 38.91, p < 0.001; 1.0 mg/kg govadine-treated group: F(1,11) = 25.99, p < 0.001], while l-govadine significantly increased it in both groups [0.3 mg/kg govadine-treated group: F(1,10) = 38.64, p < 0.001; 1.0 mg/kg govadine-treated group: F(1,11) = 40.16, p < 0.001].
Effects of l-govadine (0.3 and 1.0 mg/kg) and MK-801 (0.15 mg/kg) on PAL. a MK-801 significantly reduced task accuracy, an effect that was blocked by l-govadine. b l-Govadine significantly reduced trials completed. c MK-801 significantly increased correction trials. l-Govadine restored correction trials to a number not significantly different from control treatment. d MK-801 significantly increased total trials, while l-govadine decreased total trials. Asterisks were omitted for clarity. e MK-801 reduced reward latency, and l-govadine increased reward latency with no effect on other latency measures. Asterisk indicates a significant effect of MK-801. Number sign indicates a significant effect of d-govadine
The high dose of l-govadine (3.0 mg/kg) caused a highly variable reduction in accuracy (Fig. 5a; F(1,26) = 8.95, p = 0.006) that was confounded by a dramatic reduction in selection trials (Fig. 5c; F(1,26) = 74.12, p < 0.001) and total trials (Fig. 5d; F(1,26) = 81.34, p < 0.001). This reduction in trials is consistent with the large increase in response latencies for correct trials (Fig. 5e; F(1,26) = 4.74, p = 0.039) and incorrect trials (Fig. 5f; F(1,26) = 15.46, p = 0.001) to greater than 100 s. Interestingly, co-administration of MK-801 with l-govadine tended reduced increase in reward collection latencies observed following l-govadine [Fig. 5g; main effect of l-govadine: F(1,26) = 4.63, p = 0.041; MK-801 by l-govadine interaction: F(1,26) = 2.99, p = 0.09].
Haloperidol does not reverse PAL impairments caused by MK-801 treatment
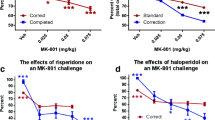
MK-801 (0.15 mg/kg), but not haloperidol (0.05, 0.1, and 0.25 mg/kg), significantly impaired accuracy during the PAL task (Fig. 6a; F(1,9) = 25.47, p = 0.001) and increased correction trials (Fig. 6b; F(1,9) = 40.08, p < 0.001). Haloperidol reduced both selection trials (Fig. 6c; F(3,27) = 7.00, p = 0.001) and total trials (Fig. 6d; F(3,27) = 8.24, p < 0.001), whereas MK-801 increased total trials (F(1,11) = 51.18, p < 0.001) without affecting selection trials. Although no significant effects were seen on correct response latency following the treatments, effects on incorrect response latency were observed following both MK-801 (F(1,9) = 5.44, p = 0.05) and haloperidol (F(3,27) = 3.88, p = 0.02) treatments, along with a significant interaction between the treatments (F(3,27) = 3.47, p = 0.03). Finally, a main effect of MK-801 was seen for reward collection latency (F(1,9) = 6.69, p = 0.03).
The effects of haloperidol (0.05, 0.1, and 0.25 mg/kg) and MK-801 (0.15 mg/kg) on PAL. a Haloperidol caused a dose-dependent decrease in accuracy, although this effect failed to reach significance. Haloperidol dose-dependently decreased the number of trials completed (b), the number of correction trials (c), and the total number of trials completed. e Haloperidol (0.1 mg/kg) increased response and reward latencies. Significantly effects were noted for correct and incorrect response latency. Asterisk indicates a significant effect of MK-801. Number sign indicates a significant effect of d-govadine
Discussion
The PAL task, as adapted for use in rats and mice with touchscreen-equipped operant conditioning chambers, has been selected by CNTRICS as a useful drug development assay for the treatment of the cognitive symptoms of schizophrenia. We utilized the PAL task to assess the acute effects of systemic MK-801 in rats as well as the effects of three drugs relevant to schizophrenia. We found that systemic MK-801 treatment reduced task accuracy and increased correction trials in two independent samples of rats. The l-enantiomer (Fig. 4), but not the d-enantiomer (Fig. 3), of govadine successfully blocked the impairment in PAL performance caused by MK-801. Baseline performance of rats on the PAL task was dramatically impaired following a high dose (3.0 mg/kg) of l-govadine (Fig. 5). Haloperidol (Fig. 6) was ineffective in normalizing PAL task performance following treatment with MK-801.
MK-801-induced disruption of PAL
Acute administration of NMDA receptor antagonists including MK-801, ketamine, and phencyclidine has been used to induce a state resembling some aspects of schizophrenia in humans, primates, and rodents (Moghaddam and Krystal 2012). In rodents, MK-801 increases locomotor activity (Mathe et al. 1998; Homayoun et al. 2004; Howland et al. 2012), disrupts prepulse inhibition (Geyer et al. 2001), and impairs various types of learning and memory relevant to the cognitive symptoms of schizophrenia (Uslaner et al. 2009; Stefani and Moghaddam 2010; Lyon et al. 2012). Previous research has shown that phencyclidine and MK-801, but not ketamine, disrupted performance of PAL independently from changes in trials completed, although response latencies were increased by ketamine (Talpos et al. 2014, 2015). Although differences exist in the selectivity of the effects of NMDA receptor antagonists for associative visuospatial memory in the absence of gross locomotor disturbances which has been observed for other behavioral tasks (Dix et al. 2010; Gilmour et al. 2012), interestingly, this is not the case for visual discrimination using stimuli presented on touchscreens (Talpos et al. 2012).
Previous rodent studies using the PAL task have used task accuracy and trials completed as measures of cognitive performance and responding, respectively (Talpos et al. 2009, 2014). In the present experiments, we also analyzed correction trials and total trials completed (the sum of selection trials completed and correction trials) to provide more information regarding the behavior of the rats. Correction trials occurred following an incorrect response where the same stimuli are presented repeatedly until the correct choice is made (Fig. 1) and may be analogous to perseverative errors in other cognitive assays (Ragozzino 2002; Floresco et al. 2009; Stefani and Moghaddam 2010; Zhang et al. 2012). The increases in correction trials following acute MK-801 were of particular relevance to schizophrenia as increased perseveration is noted in both patients with this disorder (Pantelis et al. 1999; Brown et al. 2009) and those with damage to the prefrontal cortex (Pantelis et al. 1999).
Interpretation of the results must take into consideration the side effects of acute MK-801 treatment. In the present study, a robust disruption of PAL was observed following 0.15 mg/kg MK-801 without consistent effects on total trials completed (selection trials completed plus correction trials) or response/reward latencies. This suggests that locomotor activity was not altered in animals performing this task. In a recent study, doses of MK-801 from 0.025 to 0.075 mg/kg impaired accuracy on PAL and increased correction trials (Talpos et al. 2015). Increased response latencies and impaired performance on a visual discrimination task using touchscreens were also observed in hooded rats given 0.1 mg/kg of MK-801 (Talpos et al. 2012). In our hands, doses of MK-801 lower than 0.15 mg/kg did not have significant effects on PAL. In addition to effects on motor function, NMDA receptor blockade may have impaired visual perception required for accurate performance of the touchscreen task. To address this question, Talpos et al. (2012) progressively “morphed” the visual stimuli to appear more similar in a discrimination task. Importantly, deterioration of performance did not interact with MK-801 treatment (0.1 mg/kg).
Intracranial infusions of MK-801 or dorsal hippocampus lesions impair PAL in rodents (Talpos et al. 2009; Kim et al. 2015), raising the possibility that the systemic MK-801 treatment we employed also altered processing in the hippocampus. Cortical regions also appear to be involved in the rodent PAL task (Oomen et al. 2012), consistent with findings from human studies (Owen et al. 1995). The rodent PAL task differs from those used in humans and primates as it requires several weeks of training before a criterion is reached (Horner et al. 2013). In the early phases of training, operant conditioning tasks such as PAL are likely goal oriented but become habit driven and controlled by striatal circuits following extensive training (O’Tousa and Grahame 2014). Thus, the disruption in PAL following acute MK-801 treatment may also be related to the effects of the drug in the striatum (Delotterie et al. 2015).
l-Govadine, but not d-govadine, blocks the impairment of PAL caused by acute MK-801 treatment
When administered with acute MK-801, l-govadine attenuated deficits in PAL seen with MK-801 alone (Fig. 4); however, d-govadine had no effect (Fig. 3). Previous research showed that d- and l-govadine have distinct but complementary profiles. Effects of l-govadine appeared similar to typical antipsychotics and improved behavior in tests related to positive symptoms of schizophrenia, whereas d-govadine enhanced cognition in frontal-dependent memory tasks (Lapish et al. 2014). l-Govadine has greater affinity for D2 receptors than d-govadine; whereas l-govadine administration increased DA efflux in the nucleus accumbens and medial prefrontal cortex, d-govadine treatment increased DA efflux only in the prefrontal cortex (Lapish et al. 2014). Accordingly, the impairment in PAL caused by acute MK-801 may be due to effects on the dopamine system. Consistent with this hypothesis, acute MK-801 treatment (0.1 mg/kg) increased dopamine efflux in the nucleus accumbens and medial prefrontal cortex (Homayoun et al. 2004). Furthermore, systemic administration of the indirect dopamine agonist amphetamine dramatically impaired performance of PAL over a range of doses that did not affect completed trials or response latencies (Talpos et al. 2014, 2015). However, our observation that the D2 antagonist haloperidol failed to prevent the MK-801-induced disruption of PAL (Fig. 6 of the present study and Talpos et al. 2015) suggests that increased dopamine is not solely responsible for the impairment. Interestingly, the amphetamine-induced disruption of PAL is blocked by haloperidol, the dopamine D1 receptor antagonist SCH-23390, and the atypical antipsychotic risperidone (Talpos et al. 2015), which suggests that different mechanisms mediate the effects of MK-801 and amphetamine on PAL. Whether the effect of amphetamine on PAL is also sensitive to l-govadine treatment will require further experimentation. The observed effects of higher doses of l-govadine and haloperidol on response latencies in the PAL task indicate that careful attention must be paid to the selection of the doses of drugs used with this task.
Conclusion
The present results demonstrate that acute administration of MK-801 disrupts performance of PAL in rats. We found that l-govadine, a compound with properties similar to established antipsychotics, restored PAL performance to control levels following systemic MK-801, within a narrow dose range. These data also suggest that the MK-801-induced disruption of PAL is a useful procedure to advance the study of putative antipsychotic drug effects in rodent models of schizophrenia.
References
Ashby DM, Lapish CC, Phillips AG (2015) Stability of avoidance behaviour following repeated intermittent treatment with clozapine, olanzapine or D, L-govadine. Behav Pharmacol 26:133–138
Banasikowski TJ, MacLeod LS, Beninger RJ (2012) Comparison of nafadotride, CNQX, and haloperidol on acquisition versus expression of amphetamine-conditioned place preference in rats. Behav Pharmacol 23:89–97
Barnett JH, Sahakian BJ, Werners U, Hill KE, Brazil R, Gallagher O et al (2005) Visuospatial learning and executive function are independently impaired in first-episode psychosis. Psychol Med 35:1031–1041
Bethus I, Muscat R, Goodall G (2006) Dopamine manipulations limited to preexposure are sufficient to modulate latent inhibition. Behav Neurosci 120:554–562
Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD et al (2009) Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiatry 166:683–690
Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J et al (2012) New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology 62:1191–1203
Delotterie DF, Mathis C, Cassel JC, Rosenbrock H, Dorner-Ciossek C, Marti A (2015) Touchscreen tasks in mice to demonstrate differences between hippocampal and striatal functions. Neurobiol Learn Mem 120:16–27
Dix S, Gilmour G, Potts S, Smith JW, Tricklebank M (2010) A within-subject cognitive battery in the rat: differential effects of NMDA receptor antagonists. Psychopharmacology (Berl) 212:227–242
Elvevag B, Goldberg TE (2000) Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol 14:1–21
Floresco SB, Zhang Y, Enomoto T (2009) Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res 204:396–409
Fowler SW, Ramsey AK, Walker JM, Serfozo P, Olive MF, Schachtman TR et al (2011) Functional interaction of mGlu5 and NMDA receptors in aversive learning in rats. Neurobiol Learn Mem 95:73–79
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR (2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 156:117–154
Gilmour G, Dix S, Fellini L, Gastambide F, Plath N, Steckler T et al (2012) NMDA receptors, cognition and schizophrenia—testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology 62:1401–1412
Green MF (2006) Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry 67:e12
Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B (2004) Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology 29:1259–1269
Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, Nilsson SR et al (2013) The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc 8:1961–1984
Howland JG, Cazakoff BN, Zhang Y (2012) Altered object-in-place recognition memory, prepulse inhibition, and locomotor activity in the offspring of rats exposed to a viral mimetic during pregnancy. Neuroscience 201:184–198
Jodo E (2013) The role of the hippocampo-prefrontal cortex system in phencyclidine-induced psychosis: a model for schizophrenia. J Physiol Paris 107:434–440
Keefe RS, Fenton WS (2007) How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull 33:912–920
Kim CH, Heath CJ, Kent BA, Bussey TJ, Saksida LM (2015) The role of the dorsal hippocampus in two versions of the touchscreen automated paired associates learning (PAL) task for mice. Psychopharmacology (Berl)
Lapish CC, Belardetti F, Ashby DM, Ahn S, Butts KA, So K et al (2012) A preclinical assessment of d.l-govadine as a potential antipsychotic and cognitive enhancer. Int J Neuropsychopharmacol 15:1441–1455
Lapish CC, Ahn KC, Chambers RA, Ashby DM, Ahn S, Phillips AG (2014) Selective effects of D- and L-govadine in preclinical tests of positive, negative, and cognitive symptoms of schizophrenia. Neuropsychopharmacology 39:1754–1762
Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B (2007) Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biol Psychiatry 62:739–746
Lewis DA, Gonzalez-Burgos G (2008) Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology 33:141–165
Lodge DJ, Grace AA (2007) Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci 27:11424–11430
Lyon L, Saksida LM, Bussey TJ (2012) Spontaneous object recognition and its relevance to schizophrenia: a review of findings from pharmacological, genetic, lesion and developmental rodent models. Psychopharmacology (Berl) 220:647–672
Marder SR, Fenton W (2004) Measurement and treatment research to improve cognition in schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res 72:5–9
Mathe JM, Nomikos GG, Schilstrom B, Svensson TH (1998) Non-NMDA excitatory amino acid receptors in the ventral tegmental area mediate systemic dizocilpine (MK-801) induced hyperlocomotion and dopamine release in the nucleus accumbens. J Neurosci Res 51:583–592
Moghaddam B, Krystal JH (2012) Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr Bull 38:942–949
Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK (2004) Identification of separable cognitive factors in schizophrenia. Schizophr Res 72:29–39
Oomen CA, Saksida LM, Bussey TJ (2012) The retrosplenial cortex is involved in object-place paired associates learning in the rat touchscreen operant chamber
O’Tousa D, Grahame N (2014) Habit formation: implications for alcoholism research. Alcohol 48:327–335
Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW (1995) Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 33:1–24
Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW (1999) Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res 37:251–270
Ragozzino ME (2002) The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn Mem 9:18–28
Stefani MR, Moghaddam B (2010) Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur J Pharmacol 639:26–32
Sullivan EM, Timi P, Hong LE, O’Donnell P (2014). Reverse translation of clinical electrophysiological biomarkers in behaving rodents under acute and chronic NMDA receptor antagonism. Neuropsychopharmacology
Sun T, Liu X, Li M (2014) Effect of environmental cues on the behavioral efficacy of haloperidol, olanzapine, and clozapine in rats. Behav Pharmacol 25:277–286
Talpos JC, Winters BD, Dias R, Saksida LM, Bussey TJ (2009) A novel touchscreen-automated paired-associate learning (PAL) task sensitive to pharmacological manipulation of the hippocampus: a translational rodent model of cognitive impairments in neurodegenerative disease. Psychopharmacology (Berl) 205:157–168
Talpos JC, Fletcher AC, Circelli C, Tricklebank MD, Dix SL (2012) The pharmacological sensitivity of a touchscreen-based visual discrimination task in the rat using simple and perceptually challenging stimuli. Psychopharmacology (Berl) 221:437–449
Talpos JC, Aerts N, Fellini L, Steckler T (2014) A touch-screen based paired-associates learning (PAL) task for the rat may provide a translatable pharmacological model of human cognitive impairment. Pharmacol Biochem Behav 122:97–106
Talpos J, Aerts N, Waddell J, Steckler T (2015) MK-801 and amphetamine result in dissociable profiles of cognitive impairment in a rodent paired associates learning task with relevance for schizophrenia. Psychopharmacology (Berl). In press
Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH et al (2009) Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology 57:531–538
Vales K, Svoboda J, Benkovicova K, Bubenikova-Valesova V, Stuchlik A (2010) The difference in effect of mGlu2/3 and mGlu5 receptor agonists on cognitive impairment induced by MK-801. Eur J Pharmacol 639:91–98
Wood SJ, Proffitt T, Mahony K, Smith DJ, Buchanan JA, Brewer W et al (2002) Visuospatial memory and learning in first-episode schizophreniform psychosis and established schizophrenia: a functional correlate of hippocampal pathology? Psychol Med 32:429–438
Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA (2009) Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther 122:150–202
Zhai H, Miller J, Sammis G (2012) First enantioselective syntheses of the dopamine D1 and D2 receptor modulators, (+)- and (−)-govadine. Bioorg Med Chem Lett 22:1557–1559
Zhang Y, Cazakoff BN, Thai CA, Howland JG (2012) Prenatal exposure to a viral mimetic alters behavioural flexibility in male, but not female, rats. Neuropharmacology 62:1299–1307
Acknowledgments
This work was supported by an Operating Grant from the Canadian Institutes for Health Research (CIHR) and a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada to JGH. JGH is a CIHR New Investigator. BRL received salary support from the College of Medicine at the University of Saskatchewan.
Conflict of interest
BRL and JGH declare no conflict of interest. AGP who served until July 2013 on the board of directors of Allon Therapeutics declares a patent pending related to the use of d-govadine (PCT/CA2012/050526) and a pending patent (PCT/CA2004/001813) for an IV formulation of the interference peptide Tat-GluA23Y.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lins, B.R., Phillips, A.G. & Howland, J.G. Effects of D- and L-govadine on the disruption of touchscreen object-location paired associates learning in rats by acute MK-801 treatment. Psychopharmacology 232, 4371–4382 (2015). https://doi.org/10.1007/s00213-015-4064-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4064-1