Abstract
The epithelial inner layer of the lower urinary tract, i.e., the urothelium, and other parts of the mucosa are not just a passive barrier but play an active role in the sensing of stretching, neurotransmitters, paracrine mediators, hormones, and growth factors and of changes in the extracellular environment. We review the molecular and cellular mechanisms enabling the urothelium to sense such inputs and how this leads to modulation of smooth muscle contraction and relaxation. The urothelium releases various mediators including ATP, acetylcholine, prostaglandins, nitric oxide, and nerve growth factor. These may affect function and growth of smooth muscle cells and afferent nerves. However, the molecular identity of the urothelium-derived mediator directly modulating contractile and relaxant responses of isolated bladder strips has remained elusive. The morphology and function of the urothelium undergo changes with aging and in many pathophysiological conditions. Therefore, the urothelium may contribute to the therapeutic effects of established drugs to treat lower urinary tract dysfunction and may also serve as a target for novel therapeutics. However, therapeutics may also change urothelial function, and it is not always easy to determine whether such changes are part of the therapeutic response or reflect secondary alterations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The urothelium is the epithelial inner layer of the hollow organs of the lower urinary tract (LUT). While it has long been considered as a passive barrier only, we now know that it is anything but. Firstly, it is much less of a barrier than anticipated and several xenobiotics can be absorbed upon intravesical installation to reach systemically relevant concentrations even in healthy animals (Krege et al. 2004). Second, the urothelium can become leaky under pathophysiological conditions (Parsons 2011). Third, and most importantly, the urothelium is a highly active cell layer, which senses external stimuli, releases various mediators, and thereby modifies smooth muscle tone and the function of other cell types in the bladder wall. We here review the role of the urothelium as a sensor, its modulation of LUT smooth muscle tone, the mediators involved in such modulation, and the regulation of urothelial function in disease and upon treatment. We explicitly wish to refer readers to excellent previous reviews of various aspects of urothelial function (Apodaca et al. 2007; Birder and De Groat 2007; Birder and Andersson 2013; Andersson and McCloskey 2014).
Methodological considerations
The urothelium is part of the mucosa, which also includes the basal membrane and the lamina propria. The latter is a complex structure including afferent nerve endings, interstitial cells similar to those of Cajal in the gut, blood, and lymphatic vessels, and, in some species including humans, a muscularis mucosae (Andersson and McCloskey 2014; Fry and Vahabi 2016). Although the urothelium is only part of the mucosa, some authors have used the terms “urothelium” and “mucosa” as synonyms, and it is not fully clear from reading the respective papers what exactly has been studied. Specifically, “urothelium removal” (for instance to study the role of urothelium-derived mediators on contraction and relaxation) appears to reflect removal of the entire mucosa in many cases. Possibly inappropriate use of terms should be considered when interpreting published data on the role of the urothelium in particular and of the mucosa in general. A summary of key advantages and limitations of various ways to study urothelial function and role is given in Table 1.
Studies on the function of the urothelium rely on a range of approaches, each of them having specific advantages and limitations. For instance, in vitro studies with bladder strips have often applied direct comparisons of strips after removal of the urothelium or entire mucosa with intact strips (see the “Modulation of contractile responses” and “Modulation of relaxant responses” sections). Differences between the two preparations point to the urothelium or mucosa as being involved; however, it does not always become clear whether the urothelial part is in the sensing of the signal and/or the response to it. As there are no approaches to remove the urothelium in vivo, conclusions about its function in a living animal or patient are difficult. A possible alternative, at least in experimental animals, are studies in which a gene of interest is knocked out or overexpressed selectively in the urothelium (Schnegelsberg et al. 2010).
Given these limitations, some investigators have turned to freshly prepared urothelial cells, primary cultures thereof or to urothelium-derived cell lines. Such cell lines can be immortalized such as the non-malignant cell lines UROtsa or TERT-NHUC cells but can also originate from urothelial cancer such as J82 cells. However, the quantitative and qualitative expression patterns of urothelial cells may change in culture, and such changes may be even more extensive in urothelium-derived cell lines (Tyagi et al. 2006), and even more so in cell lines derived from urothelial cancer. Both conclusions were substantiated by later studies exploring a greater variety of receptors and other signaling-related molecules (Ochodnicky et al. 2012; Bahadory et al. 2013). As the main limitation of freshly prepared urothelial cells is the availability of large quantities, various attempts have been made with rat (Kurzrock et al. 2005) and human urothelial cells (Daher et al. 2004) to improve culturing methods, for instance by using matrices or organ-like culture. Nonetheless, the interpretation of data from cultured urothelial cells or urothelium-derived cell lines needs to consider phenotypic changes, which may limit the translational value of these models.
Other limitations exist that are not specific for research on the urothelium. The presence of receptors, channels, and other molecules can be assessed and quantified at the mRNA level with relative ease; however, it remains unclear for most genes how predictive mRNA levels are for those of functional protein. Detection at the protein level has been based on radioligand binding in some studies on muscarinic receptors (Mansfield et al. 2005; Braverman et al. 2007; Anisuzzaman et al. 2008; Mansfield et al. 2009) but has relied additionally (for muscarinic receptors) and predominantly (for other receptors) on antibodies. Of note, the vast majority of antibodies against G protein-coupled receptors and ligand-gated ion channels lack useful target specificity (Michel et al. 2009), but some carefully validated receptor antibodies are emerging (see below). While radioligands allow a more definitive detection of receptors, the ligands used for discrimination of subtypes typically had only moderate selectivity. Thus, either method has limitations that need to be considered.
Finally, the presence of a given protein or other molecule does not necessarily demonstrate that it is involved in a given functional response. Dissection of pathways leading to functional responses requires use of activators or, even better, inhibitors of specific steps leading from sensing of signal to cellular response. Such dissection can be done using genetic tools, such as siRNA, antibodies, or small molecules; again, each of these tools comes with its own limitations as extensively discussed elsewhere (Michel and Seifert 2015).
Urothelium as sensor
The physiological amount of urine in the bladder immediately after a void is close to 0 ml in humans but may be higher in species using urine for territorial marking behavior such as dogs. On the other hand, the healthy human bladder can store about 500 ml, which means that it undergoes major stretching during the micturition cycle. This requires sensing mechanisms that inform the brain about the filling state of the bladder and allow it to decide whether to continue storing or to void. The urothelium appears to play a central role in such sensing. It is also involved in the sensing of thermal and chemical stimuli (Birder and Andersson 2013). Such sensing involves various ion channels and receptors and leads to the release of mediators such as ATP, acetylcholine (Ach), and nitric oxide (NO, see below). For instance, stretching can stimulate the release of mediators including Ach (Yoshida et al. 2006) and ATP (Ochodnicky et al. 2013) from the urothelium. Stretch-induced release can be further augmented by mediators such as bradykinin (Ochodnicky et al. 2013).
Urothelial cells express a range of cation channels. These include the transient receptor potential channels (TRPs) TRPV1 and TRPV4. Expression of TRPV1 protein was reportedly greater in the superficial than in the basal cells, and much less expression was found in malignant tissue (Lazzeri et al. 2005). In contrast, TRPV4 was preferentially found close to adherence junctions, with similar expression in healthy and malignant human tissue and in healthy mice (Janssen et al. 2011). Such TRP channels are likely to be involved in the sensing of stretching and some pathophysiological conditions of the bladder (Nilius and Szallasi 2014). The urinary bladder also expresses several big, small, and intermediate conductance Ca2+-activated K+ channels (Chen et al. 2004), but at least for the SK2 channel expression appears to exist at a lower level in urothelium as compared to bladder smooth muscle (Thorneloe et al. 2008). A difference in contractile responses in the presence of the K+ channel opener rimalkalim in the absence and presence of urothelium in mouse, pig, and human bladder strips (Wuest et al. 2005) has been taken as indirect evidence for a functional role of K+ channels in the urothelium (although these experiments most likely reflect a role in the mucosa in general). In line with this caveat, direct evidence for a functional role of KCNQ channels in the interstitial cells of the lamina propria has been obtained in guinea pigs (Anderson et al. 2009).
The urothelium does not only sense filling of the bladder but can also sense presence of surrounding neurotransmitters, paracrine mediators, and hormones. The primary physiological transmitter of the parasympathetic nerves innervating the human bladder is Ach (ATP may be a primary physiological parasympathetic transmitter in several non-human mammals; (Burnstock 2014). Moreover, Ach can be formed non-neuronally in the urothelium (Yoshida et al. 2006). Some studies have demonstrated expression of some subtypes of nicotinic Ach receptors at the mRNA level. In mice, α2, α4, α5, α6, α7, α9, and α10, but not α3 subunits were detected; while α9 was found only in the umbrella cells, α4, α7, and α10 subunits were also seen in the intermediate and basal cell layers (Zarghooni et al. 2007). The same group reported that the α7 subunit was much more abundant than the α9 and α10 subunits in human urothelium (Bschleipfer et al. 2007). However, most studies have explored the presence of muscarinic Ach receptors in the urothelium. Muscarinic receptors have been detected in the urothelium of every mammalian species investigated, including mouse (Zarghooni et al. 2007), rat (Cheng et al. 2007; Anisuzzaman et al. 2008), guinea pig (Grol et al. 2009), pig (Bahadory et al. 2013), and man (Mansfield et al. 2005; Tyagi et al. 2006; Braverman et al. 2007; Bschleipfer et al. 2007; Mansfield et al. 2009; Ochodnicky et al. 2012). Studies in rat, pig, and human have generally reported a lower expression of muscarinic receptors in urothelium than in detrusor (Mansfield et al. 2005; Tyagi et al. 2006; Braverman et al. 2007; Cheng et al. 2007; Anisuzzaman et al. 2008; Bahadory et al. 2013), although this is not unchallenged (Hawthorne et al. 2000). The suburothelial layer also stained positive for muscarinic receptors, but other parts of the lamina propria did not (Grol et al. 2009; Bahadory et al. 2013). The most abundantly expressed subtypes at the mRNA and protein levels appear to be M2 and M3 receptors (Braverman et al. 2007; Bschleipfer et al. 2007; Anisuzzaman et al. 2008; Ochodnicky et al. 2012), but some subtypes may exhibit a rather restricted expression with M1 restricted to basal cells, M2 largely to umbrella cells, M3 and M4 homogenously distributed, and M5 exhibiting a decreasing expression from luminal to basal (Bschleipfer et al. 2007). However, most of these findings were generated with antibodies shown to lack proper target selectivity (Jositsch et al. 2009). As most clinically used muscarinic antagonists exhibit moderate selectivity for one subtype at best, the clinical relevance of differential expression of muscarinic receptor subtypes within the mucosa remains to be elucidated.
Adrenoceptors are also expressed and functionally active in the urothelium. β-Adrenoceptor subtype mRNA is present in the urothelium of rats (Kullmann et al. 2011) and humans (Otsuka et al. 2008; Tyagi et al. 2009a; Ochodnicky et al. 2012), and in human urothelium-derived cell lines (Harmon et al. 2005). The studies providing quantitative evaluation suggest that the β2-adrenoceptor subtype is expressed most prominently in both species (Kullmann et al. 2011; Ochodnicky et al. 2012). Expression of all three β-adrenoceptor subtypes at the protein level has been reported based on immunohistochemistry in humans (Otsuka et al. 2008; Kullmann et al. 2011; Otsuka et al. 2013), but only some of these studies have applied validated antibodies. However, it is notable that the staining was more intense in urothelium than in smooth muscle in all of these studies, which is opposite to the expression pattern of muscarinic receptors (see above). One study reported that interstitial cells exhibit less expression than urothelium, but more than bladder smooth muscle (Otsuka et al. 2013). α1-Adrenoceptors have low abundance in the overall human or porcine bladder (Goepel et al. 1997), and in situ hybridization experiments did not detect α1A-adrenoceptor subtype mRNA in human urothelium (Walden et al. 1997); the same study, however, reported a high density in the urothelium of rat and monkey bladder. An expression profiling study in freshly prepared human urothelial cells reported lack of mRNA for α1B-, moderate amounts of α1A-, and strongest abundance of α1D-adrenoceptors (Ochodnicky et al. 2012). In contrast, the same study detected all three subtypes of α2-adrenoceptors. α1D-Adrenoceptors were also detected by immunoblots in rat urothelium (Ishihama et al. 2006), albeit based on a non-validated antibody.
Presence of a wide range of other receptors has been explored in isolated studies. For instance, freshly isolated human urothelial cells expressed mRNA for serotonin 5HT2A but not 5HT1B receptors, angiotensin II type 1 but not type 2 receptors, high levels of endothelin ETA and ETB receptors, and of all five subtypes of sphingosine-1-phosphate receptors, as well as the sphingosine kinases 1 and 2 generating their ligand based on array technology (Ochodnicky et al. 2012) but not verified by PCR based on validated primers. Bradykinin B1 and B2 receptor mRNA was found in human urothelial cells (Ochodnicky et al. 2012); in contrast, only B2 mRNA was present in normal rat urothelium but B1 receptors became detectable in cyclophosphamide-induced cystitis (Chopra et al. 2005). Another study at the whole bladder level found upregulation of B1 receptor mRNA upon spinal cord injury; immunohistochemical experiments showed an increased expression of B1 receptor protein in the urothelium (Forner et al. 2012), but this was based on antibodies without proper validation. Freshly isolated human urothelial cells expressed mRNA for substance P (NK1), substance K (NK2), and neuromedin K receptors (Ochodnicky et al. 2012). Pig bladder has a similar expression of substance K receptor mRNA in smooth muscle and suburothelium, with less but detectable expression in the urothelium (Bahadory et al. 2013). P2Y6 receptor mRNA is also found in the urothelium but at lower abundance than in suburothelium and smooth muscle, whereas P2X1 mRNA is only present in suburothelium and smooth muscle and not in urothelium. While prostaglandin EP1 receptors exhibited only weak staining in urothelium and suburothelium of the guinea pig bladder, strong staining of EP2 receptors was found in both layers, albeit on limited validation of the antibodies being used (Rahnama'i et al. 2010). Cannabinoid CB1 and CB2 receptor mRNA was found in human urothelium, possibly with higher expression than in smooth muscle (Tyagi et al. 2009b). The expression level of CB1 appeared higher than that of CB2, and this was confirmed in a study of freshly isolated human urothelial cells (Ochodnicky et al. 2012).
Nerve growth factor (NGF) is a key modulator of bladder function (Ochodnicky et al. 2011). Urothelium produces and releases NGF (see below) and expresses receptors for it. Freshly isolated human urothelial cells expressed mRNA for its low-affinity receptor p75 and, to a lesser extent, for the high-affinity receptor trkA (Ochodnicky et al. 2012). Immunohistochemical studies confirmed strong expression of p75 in human urothelium (Vaidyanathan et al. 1998; Eryildirim et al. 2006), albeit with poorly validated antibodies.
Modulation of contractile responses
Alongside the sensory functions, it has become clear that the urothelium has a local and direct influence on the contractility of the underlying smooth muscle in the lower urinary tract. This modulatory action was initially demonstrated as an inhibitory effect on bladder detrusor contractility and was first reported in the guinea pig bladder, in which substance P-induced contractions were enhanced in mucosa-free bladder strips compared to their mucosa-intact counterparts (Maggi et al. 1987). Thereafter, this inhibitory effect of the urothelium on detrusor smooth muscle contraction has been reported in bladders from many species, including mouse (Wuest et al. 2005; Meng et al. 2008; Canda et al. 2009), guinea pig (Guan et al. 2014), cat (Levin et al.), dog (Saban et al. 1992), pig (Hawthorn et al. 2000; Buckner et al. 2002; Sadananda et al. 2008), and human (Chaiyaprasithi et al. 2003; Wuest et al. 2005; Propping et al. 2013). In 2000, Hawthorn et al. confirmed, using a bioassay experiment in which a urothelium-intact strip was coincubated with a denuded strip, that the inhibitory effect was due to a diffusible urothelium-derived inhibitory factor (UDIF) which was released from the pig bladder urothelium (Hawthorn et al. 2000). The effect observed is an inhibition of contractions rather than a direct relaxation. Although unidentified in nature, UDIF was released in response to muscarinic stimulation, but not in response to elevated extracellular KCl or neurokinin A (Hawthorn et al. 2000). Muscarinic-receptor-mediated release of the inhibitory factor is consistent across most species, apart from dog (Saban et al. 1992). However, an inhibitory effect of the urothelium has also been demonstrated following stimulation with KCl in the mouse (Wuest et al. 2005), cat (Levin et al.), and human bladder (Wuest et al. 2005, Propping et al. 2013), in response to ATP in the cat bladder (Levin et al.), and in response to neurokinin A in the dog bladder (Saban et al. 1992) and following nerve-stimulation in cat (Levin et al.) and human bladder (Chaiyaprasithi et al. 2003). Thus, the stimulus for release may be species dependent. Moreover, a species-specific controversy appears to exist in the rat bladder with respect to UDIF. Some studies have shown that the urothelium does not appear to have an inhibitory effect on smooth muscle contractility in the rat bladder (Pinna et al. 1992), while others have used a gentle swabbing technique to remove only the urothelial layer and found an inhibitory effect (Munoz et al. 2010).
Although some early reports demonstrated a non-adrenergic, non-nitrergic, non-prostanoid relaxation factor in the rat bladder, this was shown to be urothelium-independent (Fovaeus et al. 1999; Bozkurt and Sahin-Erdemli 2004). Adding to the complexity, the rat bladder urothelium releases prostaglandins that excite and contract the bladder, rather than inhibit contractions (Nakahara et al. 2003).
Most studies of the inhibitory effect of the bladder urothelium have been performed on the bladder dome, but the urothelium of the bladder trigone has a similar inhibitory effect on smooth muscle contractility, and while release of the inhibitory factor is stimulated by muscarinic, and histamine, receptor activation in this region, it is not seen following stimulation of α1-adrenoceptors with phenylephrine (Templeman et al. 2002). However, complex cross-talk appears to occur between the receptor systems, since an inhibition of phenylephrine-mediated contractions is observed following muscarinic or histamine receptor stimulation.
In addition to the large voiding contractions evoked by motor nerves, which initiate bladder emptying, the bladder also demonstrates spontaneous contractile activity that is non-neuronal in origin. While the role of this spontaneous activity in bladder function and its cellular origin are a focus of ongoing research, the urothelium can modulate spontaneous activity in a dramatic way. Removal of the urothelium from bladder wall preparations significantly reduced spontaneous contractions in the guinea pig (Sui et al. 2008) and rat bladder (Kanai et al. 2007). This is in complete contrast to the inhibitory action of the urothelium on tonic contractions described above. In contrast, in the mouse bladder, the frequency of nifedipine-sensitive spontaneous activity of detrusor increased when the urothelium was removed (Meng et al. 2008) and a similar finding was reported in pig bladder (Buckner et al. 2002). The findings in pig bladder show that removal of the urothelium delays development of spontaneous contractions in the dome, although not in the trigone, but does not influence frequency or amplitude of spontaneous contractions (Akino et al. 2008). Thus, the modulatory effect of the urothelium on bladder spontaneous contractility is still a matter of debate. Either way, while still far from being understood, it has been proposed that the urothelium may have a dual effect on contractility, an inhibitory influence on tonic contractions via a diffusible agent and an excitatory influence on spontaneous contractility via direct cellular interaction with underlying cells, potentially the interstitial cells in the lamina propria (Birder et al. 2010; Fry and Vahabi 2016).
It is easy to see how the inhibitory influence of the urothelium on bladder tonic contractility may be important for normal bladder compliance, since during filling, intravesical pressure remains low until the initiation of tonic contractions and voiding. With respect to the stimulatory action on spontaneous activity, this may reflect a mechanism whereby the bladder is maintained at its smallest volume during filling, to optimize sensation of bladder filling.
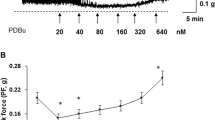
While the urothelium lines the urinary tract from the renal pelvis through to the upper urethra and even the glandular portions of the prostate, there has been limited focus and much less is known about the influence of the urothelium on the smooth muscle of the non-bladder regions of the LUT. In the urethra, the urothelium changes from a transitional epithelium to become stratified or columnar (Romih et al. 2005). However, an inhibitory influence of the urothelium on smooth muscle contractility has been demonstrated in the urethra, similar to that observed in the bladder. The urothelium of the urethra inhibits noradrenaline-induced contractions, but not cholinergic contractions in the hamster (Pinna et al. 1992), contractions to phenylephrine, high KCl and nerve stimulation in the cat (Levin et al.), and bradykinin-induced contractions in the pig bladder neck (Ribeiro et al. 2014). We have recently shown that the pig urethra releases a diffusible inhibitory factor which reduces contractions to carbachol, noradrenaline, and phenylephrine by similar amounts (Fig. 1).
Effects of mucosa denudation on contractile responses of the pig urethra to agonists. Cumulative concentration-response curves to carbachol (upper panel, n = 5), noradrenaline (middle panel, n = 4), and phenylephrine (lower panel, n = 7) on isolated porcine urethral strips prepared either with an intact mucosa (filled circles) or with the mucosa removed (open squares). Data are shown as means ± SD. Differences in Emax between intact and denuded strips were statistically significant (P < 0.05) in unpaired, two-tailed t tests. Adapted with permission from Folasire et al. (2017)
Even less well researched is the urothelium of the ureter, although recent evidence suggests that it can influence the underlying smooth muscle in a manner similar to that seen in the bladder dome, trigone, and urethra (Roedel et al. 2018). The urothelium in the human ureter can inhibit tonic but not spontaneous or KCl-stimulated contractions. In the rat ureter, the urothelium acts to prevent spontaneous contractile activity and also decrease potential excitatory effects of endogenous contractile agents including carbachol, bradykinin, and angiotensin II on ureteral motility (Mastrangelo and Iselin 2007). This effect appears to be via the release of a relaxing agent rather than an inhibitory factor, although responses to NKA and vasopressin were enhanced by urothelium denudation (Mastrangelo and Iselin 2007).
Modulation of relaxant responses
In addition to the inhibitory actions on contractility of LUT smooth muscle described above, the urothelium is also able to modulate relaxation responses. Although fewer studies have investigated this effect, when compared to the number of studies on the inhibitory effects of the urothelium on contraction, the evidence has led to the proposal of the release of a urothelial-derived mediator that is excitatory. Alternatively, the effect may be explained by a direct excitatory cellular interaction of the urothelium with underlying cell types. Regardless of the underlying mechanism, the evidence highlights the multiple roles that the urothelium seems to play in smooth muscle modulation.
Relaxing effects of catecholamines are blunted by the presence of the urothelium in the human bladder (Otsuka et al. 2008; Propping et al. 2013; Propping et al. 2015b) and are mediated via β2-adrenoceptors (Propping et al. 2013). A similar effect was seen in mouse bladder, and the urothelium reduced the sensitivity of KCl precontracted tissues to β-adrenoceptor-induced relaxation to isoprenaline, again via β2-adrenoceptors, although at high concentrations of isoprenaline, there was a minor involvement of β3-adrenoceptors (Propping et al. 2015a). While in human bladder this inhibitory effect on relaxations was seen in tissues precontracted with KCl and carbachol (Propping et al. 2015b), the same authors showed in mouse bladder that the urothelium did not inhibit relaxations if the tissues were precontracted with carbachol (Propping et al. 2015a). The rat bladder urothelium does not appear to influence smooth muscle relaxation to β3-adrenoceptor agonists (Kullmann et al. 2011). Interestingly, in the pig bladder, we have shown that while relaxation responses to isoprenaline are not modulated by the urothelium, the urothelium is involved in mediating inhibitory effects of isoprenaline on cholinergic detrusor contractions, with isoprenaline being more potent at inhibiting carbachol-induced contractions when the urothelium is present (Murakami et al. 2007; Masunaga et al. 2010). This effect was mediated by the β3-adrenoceptor (Masunaga et al. 2010).
Evidence is also conflicting with regard to the role of the urothelium in modulation of non-receptor-mediated relaxations of the bladder smooth muscle. The urothelium does not modulate relaxations to the KATP channel opener rimalkalim in the mouse, pig, or human bladder (Wuest et al. 2005). Although, in contrast, we have also shown that the urothelium does inhibit the relaxant effects of cromakalim on spontaneous contractions of the pig bladder dome, but not in the trigone region (Akino et al. 2008), suggesting that the mechanism for generation of spontaneous contractions in the smooth muscle involves modulation by the urothelium in the dome, though not in the trigone.
There is very limited information on the modulatory effect of the urothelium on relaxations of the non-bladder regions of the lower urinary tract. Prostaglandin (PG) E2 and 2-methyl-thio-ATP-induced relaxations of hamster urethra do not seem to be modulated by the urothelium (Pinna et al. 1996). However, relaxation responses of hamster urethra to electrical field stimulation (EFS) are absent if the urothelium is present, demonstrating an inhibitory modulation of nerve-mediated relaxations, which is absent if L-NAME and suramin was present (Pinna et al. 1996). However, this appears to be species dependent, since EFS-induced relaxations were not affected by the presence of an intact urothelium in the cat urethra (Levin et al. 1995).
In conclusion, the urothelium acts as a sensor for changes in bladder volume, neurotransmitters, pH, temperature, and various chemicals. In turn, it influences smooth muscle activity, inhibiting muscle contraction to many stimuli and also inhibiting some relaxation responses of the detrusor. These effects of the urothelium on smooth muscle occur following the release of a number of mediators, some of which are well documented (e.g., ATP), while others have yet to be identified (e.g., UDIF).
Known mediators released from urothelium
The urothelium releases a wide variety of signaling molecules ranging from the classic neurotransmitter Ach to the more recently recognized gaseous transmitter hydrogen sulfide (H2S). The release of some of these substances is well documented, and their actions on other tissues such as sensory nerves are well established. However, our knowledge of their actions on smooth muscle is limited. The actions of exogenously administered factors can be readily demonstrated in many cases, but very few studies have examined whether factors released from the urothelium reach the detrusor muscle at quantities large enough to modulate smooth muscle tone and contractions. Research in this field has increased enormously in recent years. However, the findings are complex, since many studies have been performed on bladder strips and bladder wall sections, as well as in whole bladders, which invariably contain additional non-urothelial cellular elements within the lamina propria or suburothelium. Thus, it is difficult to rule out mediator release from other cell types, but studies on isolated urothelial cells and cell lines are helping to clarify findings Evidence for urothelial release and smooth muscle action will now be considered individually for each proposed mediator.
Adenosine 5-triphosphate (ATP)
Stretch-induced release
ATP release from the urothelium was first described by Ferguson et al. (1997), and it is now the most researched signaling molecule released by the urothelium. Human urothelial cell lines, both of malignant (RT4 cells) and immortalized but of non-malignant origin (UROtsa and TRT-HU1), spontaneously release ATP, and this is greatly enhanced during cellular stretch most commonly induced by osmotic swelling in hypotonic solution (Sun and Chai 2006; Birder et al. 2013; Mansfield and Hughes 2014a; Mansfield and Hughes 2014b; Kang et al. 2015; Sano et al. 2016; Farr et al. 2017). Similarly, findings have been reported for primary cultures of urothelial cells from the guinea pig (McLatchie and Fry 2015), cat (Birder et al. 2003), and pig (Cheng et al. 2011b).
In isolated tissue strips, mechanical stretch induces release of ATP from guinea pig (Young et al. 2012; Sui et al. 2014), pig (Kumar et al. 2004; Sadananda et al. 2012; Smith et al. 2014; Kang et al. 2015), and human bladder (Kumar et al. 2007; Kumar et al. 2010; Sui et al. 2014; Sano et al. 2016), while mechanical force induces release in guinea pig urothelial cells (Kullmann et al. 2008b). In vivo release of ATP into the bladder lumen has been documented for the rat and human bladder (Jeremy et al. 1987; Timóteo et al. 2014). In isolated tissue studies, removal of the epithelium from rat bladder strips by swabbing prevents ATP release, indicating that the urothelium is the sole source of ATP in this species (Munoz et al. 2010). In contrast, the release of non-neuronal ATP is not only restricted to the urothelium in the pig bladder but also occurs from interstitial cells in the lamina propria and also from the smooth muscle. However, the majority of ATP release during stretch still occurs from the urothelial cells (Cheng et al. 2011a). Thus, ATP may be released from non-neuronal, non-urothelial sources in the bladders of some species, but the predominant source still appears to be the urothelium.
Chemical-induced release
In addition to stretch, a number of drugs are known to induce ATP release including β-adrenoceptor agonists (Birder et al. 2002b), substance P (Munoz et al. 2010), pituitary adenylate cyclase activating polypeptide (PACAP) (Girard et al. 2008), TRPV1 agonists (Birder et al. 2002a), and also some cytokines such as epidermal growth factor and anti-proliferative factor (Sun et al. 2009). Muscarinic agonists also stimulate release of ATP, the response mediated via the M2 receptor subtype, at least in the guinea pig (Munoz et al. 2010; Sui et al. 2014). Sui et al. (2014) found that ATP itself can exert an autocrine action, enhancing further ATP release via the activation of P2Y6 receptors.
Mechanisms of ATP release
The release of classic transmitters from nerves involves the fusion of storage vesicles with the plasma membrane. However, the release of ATP from the urothelial cells appears to be more complex. A number of toxins that prevent the exocytotic release of transmitters have been used to address this question, and the studies have become more clinically relevant as these toxins increasingly become available to treat the overactive bladder syndrome (OAB) (Chancellor 2017). Rat and human urothelial cells possess the protein target SNAP-23 and SNAP-25 for botulinum toxin, and treatment of urothelial cells with this toxin reduces the release of ATP and surprisingly increases release of NO (Hanna-Mitchell et al. 2015). The contrasting effects of the toxin on ATP and NO release confirmed a previous study in the rat, in which these changes in urothelial mediators were associated with changes in afferent nerve activity (Collins et al. 2013). These reports support the concept of vesicular release of ATP from urothelial cells, but botulinum toxin also exerts other effects such as a reduction in P2X3 and TRPV1 receptors that may alter urothelial function (Apostolidis et al. 2006). Furthermore, evidence is mounting that other mechanisms may be operating in the urothelium. Recently, pannexin channels, which have been demonstrated to have a role in the non-neuronal release of ATP in non-urinary tissues, have been identified in urothelial cells (Negoro et al. 2014). Inhibitors of these channels reduce ATP release in the rat bladder and depress release from human urothelial cells, while mice lacking these channels also exhibit reduced ATP release from the urothelium (Negoro et al. 2014; Beckel et al. 2015; Silva et al. 2015). In anesthetized rats, stimulation of P2Y6 receptors causes release of urothelial ATP and increases voiding frequency, but inhibition of pannexin channels reverses both the change in ATP release and the bladder overactivity (Timóteo et al. 2014). Silva et al. (2015) confirmed that this purinergic autocrine action mediated via P2X6 receptors occurs via pannexin channels rather than vesicular release.
ATP release induced by stretch also involves pannexin channels, which inhibitors of these channels reducing ATP release in rat and human urothelium (Negoro et al. 2014). Other channels have also been linked to ATP release, and urothelial cells from mice lacking P2X7 channel receptors (Negoro et al. 2014) or mechanosensitive Piezo channels (Miyamoto et al. 2014) exhibit reduced ATP release. Thus, ATP release appears to involve a number of mechanisms covering vesicular release and release via channels. It seems likely at this stage that both mechanisms operate to enable ATP release.
Functions of urothelial ATP
In recent years, growing evidence supports the role of urothelial derived ATP in mechanosensation. It is now generally accepted that bladder filling results in stretch of the urothelium stimulating the release of ATP, which acts on suburothelial sensory nerves to signal bladder fullness to the CNS. All aspects of this transduction system have been explored and confirmed: ATP is released during urothelial stretch, and P2X3 receptors are present on sensory nerves (Lee et al. 2000), stimulation of which activates bladder sensory nerves. Furthermore, bladder dysfunction has been observed in P2X2/3 gene knockout animals (Cockayne et al. 2000; Vlaskovska et al. 2001; Cockayne et al. 2005). Another autocrine action has also been reported where ATP via P2X receptors regulates endocytosis and exocytosis to control urothelial surface area as the bladder stretches (Truschel et al. 2002; Wang et al. 2005).
However, it is less clear whether urothelium-derived ATP can directly influence smooth muscle contraction. Detrusor smooth muscle expresses P2X1 purinoceptors, and exogenous ATP induces contraction of the smooth muscle in animals (Lee et al. 2000) and in human bladder (Harvey et al. 2002). However, evidence that endogenous ATP from the urothelium influences smooth muscle contraction is scarce, although in guinea pig bladder strips with an intact urothelium carbachol enhances spontaneous contractile activity at low concentrations that have no effect when applied directly to the muscle (Sui et al. 2014). Since ATP is known to regulate this activity and carbachol induces release of ATP from the urothelium, the results suggest that low-dose carbachol may induce contractile activity indirectly via urothelial ATP release. This conclusion is further supported by the finding that P2X receptor desensitization also prevented the spontaneous activity (Sui et al. 2014).
Another mechanism by which urothelial ATP may influence detrusor activity is via suburothelial interstitial cells, which possess multiple purinergic receptor subtypes. In the human bladder, both P2X1 and P2X3 receptors have been identified on the interstitial cells where they modulate cell spontaneous activity (Cheng et al. 2011b). These cells are coupled to each other and smooth muscle via gap junctions (Ikeda et al. 2007), and thus, the initial signal of ATP release may be transported deep into the smooth muscle following a local effect on interstitial cells.
Thus, in conclusion, the release of ATP from the urothelium has been demonstrated in all species so far studied. The stimuli for release include stretch, shear stress, cholinergic, adrenergic and tachykinin receptor stimulation. There is also a purinergic autocrine feedback mechanism enhancing release. The function of ATP in mechanosensation is well established, but it also influences smooth muscle spontaneous contractile activity. Release of ATP into the urine of patients during bladder filling has been shown, although in this study, ATP release appeared to occur early in filling with quite low bladder volumes (Jeremy et al. 1987; Cheng et al. 2014), suggesting that ATP may have other roles to play in the bladder other than simply mechanotransduction.
Acetylcholine (ACh)
A non-neuronal cholinergic system exists within the urothelium, and Ach release has been widely demonstrated in mouse, pig, guinea pig, and human bladder urothelium preparations (Lips et al. 2007; Nile and Gillespie 2012; Smith et al. 2014), human bladder strips (Yoshida et al. 2006; Bschleipfer et al. 2012; Silva et al. 2015), as well as in isolated rat (Hanna-Mitchell et al. 2007), guinea pig (Kullmann et al. 2008b), and human urothelium cells (Kang et al. 2013; Li et al. 2013; Farr et al. 2017).
While the urothelium can synthesize and release Ach, the mechanisms by which it does so are very different to those seen in cholinergic neurons. The presence of high-affinity choline transporter CHT1 and Ach-synthesizing enzyme choline acetyltransferase is controversial (Yoshida et al. 2006; Hanna-Mitchell et al. 2007; Yoshida et al. 2008; Bschleipfer et al. 2012), and it has been proposed that ACh is synthesized in the urothelium predominantly by the enzyme carnitine acetyltransferase (Lips et al. 2007). In terms of stimulus for release, both mechanical stress (McLatchie et al. 2014) and stretch of the urothelium (Yoshida et al. 2006; Hanna-Mitchell et al. 2007; Nile and Gillespie 2012; Kang et al. 2013; Farr et al. 2017) can evoke Ach release, and, at least in cells, this seems to be an all-or-nothing event, rather than related to degree of stress (McLatchie et al. 2014). ATP is a potent chemical stimulus for Ach release (Hanna-Mitchell et al. 2007; Stenqvist et al. 2017), and this is mediated partly by P2Y6 receptors in the human bladder (Silva et al. 2015). PGE2 also stimulates Ach release (Nile and Gillespie 2012).
The mechanism by which Ach is released from urothelial cells does not involve vesicular exocytosis (Hanna-Mitchell et al. 2007; Lips et al. 2007; Bschleipfer et al. 2012) and is independent of extracellular calcium, connexins, and pannexins. Instead, the anion channel CFTR, intracellular calcium (Bschleipfer et al. 2012), and the organic cation transporters OCT1 and OCT3 play a role (Hanna-Mitchell et al. 2007, Lips et al. 2007, Bschleipfer et al. 2012).
Autocrine and paracrine ACh signaling occurs within the urothelium, although the precise role in normal bladder function is not yet clear. Ach can act via a negative feedback mechanism to inhibit further Ach release from the urothelium (Hanna-Mitchell et al. 2007) while, in contrast, muscarinic receptor agonists evoked Ach release from primary human bladder urothelial cells (Li et al. 2013). Adding to the complexity, Ach causes release of ATP from the urothelium (Kullmann et al. 2008a; McLatchie et al. 2014) and might also indirectly affect the release of NO, tachykinins, and prostanoids (Hanna-Mitchell et al. 2007; Kullmann et al. 2008b; Nile and Gillespie 2012). The question of whether urothelial Ach directly contracts bladder smooth muscle was recently partly addressed by Stenqvist et al. (2017), who showed that ATP stimulates release of urothelial Ach, which contributes to purinergic contractile responses of the rat bladder.
While our understanding of non-neuronal Ach in the urothelium of the bladder has increased, Ach in the urothelium within the remainder of the LUT is a relatively untouched area of research. So far, the only evidence comes from a study of the urothelium of the rat urethra, which can release Ach from specialized polymodal urethral chemosensory cells (urethral brush cells) in response to stimulation of bitter receptors, and a cholinergic negative autocrine feedback mechanism for Ach release appears to exist in this tissue (Deckmann et al. 2018).
In conclusion, Ach is released from urothelial cells and the mechanism of choline uptake, and Ach synthesis and Ach release is different from those involved in neuronal cholinergic systems. There is some evidence for urothelial Ach having actions on smooth muscle, but this is limited and requires further study.
Nitric oxide (NO)
NO is a gaseous transmitter with inhibitory functions in most systems of the body. It is formed from L-arginine by NO synthases (NOS). These enzymes exist intracellularly as three different isoforms: two calcium-dependent constitutively expressed forms, endothelial NOS (eNOS) and neuronal NOS (nNOS), and one calcium-independent inducible form (iNOS), which is expressed under some conditions such as inflammation (Birder et al. 2005). The main form present in the healthy urothelium has been shown to be nNOS in several animal species including the rat (Birder et al. 2002b; Chuang et al. 2013), guinea pig (Gillespie et al. 2005), and cat (Theobald 2003), while eNOS has been identified in the rat bladder (Giglio et al. 2005). It has been suggested that eNOS is the only form present in the healthy human bladder (Fathian-Sabet et al. 2001), but others have found it in the urothelium and the interstitial cells of the lamina propria of the human bladder (De Ridder et al. 1999). Furthermore, iNOS is upregulated in bladder cancer (Lin et al. 2003) and can be induced during inflammatory conditions such as interstitial cystitis (Birder et al. 2005; Andersson et al. 2012) and following treatment with lipopolysaccharide (Weng et al. 2009). A reduction in nNOS and simultaneous increase in iNOS in urothelial cells has been observed in an obstructed bladder outlet model in the rat (Johansson et al. 2002a). These changes in NOS are likely related to inflammation and the release of cytokines, that have been shown to enhance NOS expression (Johansson et al. 2002b).
A number of stimuli causing non-neuronal NO release have been identified, and these include capsaicin, a TRPV1 receptor agonist that stimulates release from the mucosa and sensory nerves (Birder et al. 1998), β-adrenoceptor agonists (Birder et al. 2002b), and substance P (Munoz et al. 2010). The latter study also showed that the NO originated in the lamina propria rather than the urothelial cells.
The role of NO of neuronal origin in the bladder outlet is well established, where it relaxes the smooth muscle during voiding, thus preventing any large rise in luminal pressure (Andersson and Persson 1994). The role of NO of mucosal origin is difficult to determine due to the multiple sources of NO within the bladder including the urothelium and interstitial cells within the lamina propria (Munoz et al. 2010) and due to its multiple actions that include modulation of afferent nerve function activity (Aizawa et al. 2011) and influences on smooth muscle and interstitial cell function (Gillespie et al. 2004). Surprisingly, detrusor muscle itself is not very sensitive to NO, and NOS inhibitors have little effect on the contractions of the detrusor smooth muscle induced by muscarinic agonists or nerve stimulation (Frazier et al. 2005). Inhibition of NO synthase enhances contraction amplitude in response to EFS (Garcia-Pascual et al. 1991), while NO donors induce detrusor smooth muscle relaxation in precontracted tissues (Hernández et al. 2008). However, the effect on detrusor smooth muscle is small compared to the effects of NO on vascular smooth muscle. Even when comparing the effect on detrusor to its actions on the bladder outlet region, the influence of NO on detrusor contraction is relatively minor (Kedia et al. 2009). Furthermore, in the normal rat bladder, NOS inhibition has little or no effect on detrusor contractile responses to muscarinic stimulation (Andersson et al. 2008; Andersson et al. 2012).
However, NO appears to play a far more significant role during inflammation. In the rat bladder during inflammatory responses induced by cyclophosphamide, the mucosa exerts a considerable (25%) inhibitory effect on detrusor contraction, and muscarinic responses are enhanced when the mucosa is removed. This inhibitory effect in this situation can be reversed in the presence of a NOS inhibitor (Andersson et al. 2008). Thus, it appears that muscarinic agonists induce a direct stimulatory effect on smooth muscle, which is partly reversed by NO release from the mucosa in inflammatory states.
The actions of NO are mediated via cGMP. The targets of NO action have been identified by examining cGMP immunoreactivity after stimulation of the bladder with NO donors and these include detrusor smooth muscle, urothelial, and interstitial cells (Fathian-Sabet et al. 2001; Gillespie et al. 2006). It has been suggested that it is the interstitial cells within the suburothelium that are most sensitive to urothelial NO rather than the smooth muscle (Gillespie et al. 2005). These interstitial cells are thought to relay information from the urothelium to the smooth muscle and thus provide an indirect mechanism by which NO may influence smooth muscle contraction. Another indirect mechanism is via effects on smooth muscle growth, since NO donors have an inhibitory effect on the proliferation of smooth muscle cells (Johansson et al. 2002a).
Thus, NO appears to have only a minor direct role in regulating smooth muscle contraction but may influence muscle tone indirectly by modulating interstitial cells in the suburothelium, which in turn may influence smooth muscle tone. Since the actions of NO are mediated via cGMP and are inhibitory to cells, this indirect action may possibly explain the excitatory effects that are occasionally observed in bladder. Although the vast majority of reports concerning NO have indicated that it exerts inhibitory actions on smooth muscle, there is also some evidence to suggest that NO can exert excitatory effects. In the isolated mouse bladder, NO can increase phasic contractile activity (Gillespie and Drake 2004) and in precontracted human detrusor strips, both relaxation and contraction responses to NO donors have been observed (Moon 2002).
In conclusion, the role of NO in the bladder is complex. It is released from multiple sites and has multiple targets of action. Furthermore, this complex role appears to change in disease states following the induction of iNOS.
Other gaseous transmitters
In addition to NO, there are two other gaseous transmitters, H2S and carbon monoxide. Although there is currently no evidence for the urothelium releasing carbon monoxide, several studies have examined the H2S systems in the bladder. Surprisingly, most of the studies have been performed on human tissues and cells. The human urothelium possesses the H2S synthesizing enzymes, cystathionine-β-synthase (CBS), and cystathionine-γ-lyase (CSE). These enzymes catalyze the conversion of L-cysteine to H2S, and both enzymes have been located to the urothelium of the human bladder dome (Fusco et al. 2012). Also, the H2S precursor L-cysteine relaxes human bladder strips, an effect that is blocked by inhibitors of CBS and CSE (Fusco et al. 2012). Furthermore, in human cultured T24 urothelial cells, stimulation with carbachol causes the release of H2S, and again, this is prevented in the presence of a CBS inhibitor. The release of H2S induced by carbachol in human T24 urothelial cells was mediated via the activation of M1 and M3 muscarinic receptors (d’Emmanuele di Villa Bianca et al. 2016). It is still early days in the study of H2S, but the functions of H2S in the bladder appear twofold: an autocrine effect on the urothelium and an inhibitory effect on the detrusor muscle. H2S appears to exert an autocrine effect by elevating intracellular cGMP levels in urothelial cells, the consequences of which are yet to be established (d’Emmanuele di Villa Bianca et al. 2016). However, sildenafil increases H2S release, suggesting that cGMP is involved in a positive feedback mechanism regulating H2S release. Sildenafil also causes bladder relaxation, and both responses (increased H2S and bladder relaxation) are reduced by inhibitors of CBS and CSE (Fusco et al. 2012). Further evidence for an action on smooth muscle has come from isolated tissue experiments, again on human tissues, where inhibition of H2S synthesis does not impact detrusor contractions to carbachol directly, but does enhance bladder contractions when the urothelium is present (d’Emmanuele di Villa Bianca et al. 2016). Thus, H2S appears to be released from the urothelium and exert at least two effects, enhancement of H2S release and inhibition of detrusor contraction.
Prostaglandins
Prostanoid production in the bladder wall is well established, with prostanoids known to be synthesized locally within both the mucosa and smooth muscle, and thought to play a role in the sensory arm of the micturition reflex. Urothelial production of prostanoids has been demonstrated in the bladder of most species including rat (Pinna et al. 1992; Masunaga et al. 2006; Tanaka et al. 2011), rabbit (Masick et al. 2001; Azadzoi et al. 2004), guinea pig (Saban et al. 1994; Nile et al. 2010; Guan et al. 2014), and human (Jeremy et al. 1987). Cyclo-oxygenase 1 is markedly localized to the urothelium and expressed within the basal and intermediate cells (de Jongh et al. 2009), although not in the umbrella cells (Rahnama’i et al. 2012). Interestingly, production of prostaglandins by the urothelium is far greater than in the suburothelium and smooth muscle (Masick et al. 2001, Azadzoi et al. 2004). The prostaglandins released are those known to be both excitatory and inhibitory in nature within the lower urinary tract and include PGE2, PGF2α, as well as PGI2 and PGD2.
Release of prostaglandins from the bladder urothelium is induced by stretch and bladder distension (Downie and Karmazyn 1984; Jeremy et al. 1987; Tanaka et al. 2011; Farr et al. 2017), although this may vary with pathology since PGE2 release is not stretch-related in urothelial cancer RT4 cells (Kang et al. 2013). ATP, Ach, and NO also regulate PGE2 release in a complex signaling interaction which sees ATP (Nile et al. 2010) and Ach (via M2 receptors) (Nile and Gillespie 2012) stimulate prostaglandin release, while NO has a negative feedback effect on cholinergic release of PGE2 (Nile et al. 2010).
Evidence for a direct influence of urothelially released prostaglandins on the underlying smooth muscle was provided in a study by Nakahara et al., who showed that activation of protease-activated receptor-2 can stimulate release of prostaglandins from the rat bladder mucosa to contract the detrusor smooth muscle (Nakahara et al. 2003).
In summary, a number of prostaglandins are released by the urothelium, with some excitatory and some inhibitory to smooth muscle. Considering the complex pathways, multiple prostaglandins, and many receptors by which these mediate responses, it is surprising how few studies appear in the literature.
Peptides and cytokines
The urothelium is also a source of inflammatory peptides and cytokines, with mediators of inflammation such as substance P stimulating the release of NGF and macrophage migration inhibitory factor (Meyer-Siegler and Vera 2004). The release of interleukins (IL-1β, IL-6, IL-8) has also been observed in several human urothelial cell lines including RT4, T24, and UROtsa cells following treatment with cytotoxic drugs which induce an inflammatory response (Kang et al. 2015; Farr et al. 2017). Also, in the rat, inflammation of the bladder induced by treatment with cyclophosphamide results in the release of the cytokines IL-6 and leukemia inhibitory factor and ciliary neurotrophic factor (Girard et al. 2011). These factors released from the urothelial cells will have localized effects, but their potential effects on smooth muscle are unknown. It is unlikely that NGF will have direct effects on smooth muscle contraction, since overexpression of this peptide in mice does not appear to influence the efferent arm of the micturition reflex (Girard et al. 2012). However, NGF may influence detrusor responses indirectly by altering receptor expression in smooth muscle, since urothelium-specific overexpression of NGF in mice reduces smooth muscle VPAC1 receptor transcripts, the receptor for vasoactive intestinal peptide (Girard et al. 2010). Another indirect effect of NGF on contraction may be to alter muscle mass since NGF increases expression of IGF-1 in isolated detrusor smooth muscle cells. Furthermore, after in vivo cyclophosphamide treatment of mice which causes muscle hypertrophy, the administration of NGF-neutralizing antibody reverses the hypertrophy induced by CPO (Zhang and Qiao 2012). The effects of these inflammatory mediators obviously require further investigation.
In summary, the known mediators released from the urothelium include ATP, Ach, prostaglandins, several peptides, and the gaseous transmitters NO and H2S. Various interactions modify release of these chemicals which complicates studies. A variety of actions have been demonstrated for most of these known mediators, but for each, the evidence to support actions on detrusor smooth muscle is limited.
Urothelium-derived inhibitor factor (UDIF)
Despite the number of mediators and neurotransmitters known to be released from the urothelium, evidence as to whether any of these may act to directly influence the underlying smooth muscle of the lower urinary tract is limited, as mentioned above for each mediator. In particular, attempts to elucidate the nature of the UDIF modulating smooth muscle contractility in the bladder and urethra have been unsuccessful. In the pig bladder, the inhibitory effect of the urothelium is not due to the release of NO, nor is the UDIF a cyclo-oxygenase product, a catecholamine, adenosine, GABA or an endothelium-released hyperpolarizing factor sensitive to apamin. It is also not affected by antagonists at P1 or P2 purinergic receptors (Hawthorn et al. 2000). Involvement of NO, cyclo-oxygenase products, and β-adrenoceptors was similarly ruled out as candidates for the UDIF in the human bladder (Chaiyaprasithi et al. 2003), and in the guinea bladder, the inhibitory factor is not NO, is not mediated by adenosine receptors, nor is it a cyclo-oxygenase product (Guan et al. 2014). Similarly, in the urethra, the urothelial inhibition is independent of NO (Pinna et al. 1992) and cyclo-oxygenase products (Folasire et al. 2017).
In the rat, exogenous ATP suppresses detrusor contractions in a manner similar to that seen in the presence of the mucosa (Santoso et al. 2010), but there is no direct evidence that endogenous ATP inhibits contraction, and purinergic antagonists such as suramin do not prevent the inhibitory effect of the mucosa in the pig bladder (Hawthorn et al. 2000). In contrast, in the rat ureter the inhibitory action of the urothelium, which prevents spontaneous contractile activity and decreases excitatory effects of carbachol, bradykinin, and angiotensin II on ureteral motility, appears to involve cyclo-oxygenase products, which may activate the release of a relaxing factor (Mastrangelo and Iselin 2007). Additionally, in the hamster urethra, NO- and ATP-dependent inhibitory factors have been proposed (Pinna et al. 1996).
In terms of the putative excitatory factors released from the urothelium, which act to inhibit relaxations of the smooth muscle, these seem to be prostanoid in nature (Nakahara et al. 2003). However, in the human bladder, angiotensin and neurokinin pathways may also be involved (Propping et al. 2015b), and in the pig bladder neck, BK channels may play a role (Ribeiro et al. 2014). Thus, the nature of the urothelium-derived agents that have been shown to modulate smooth muscle in the lower urinary tract appear to be complex and are still far from being understood.
Pathophysiology of the urothelium
Based on the role of the urothelium in sensing the cellular environment and releasing mediators to modify it, several investigators have explored alterations of a wide range of parameters related to the morphology and function of the urothelium (Table 2). Such investigations were reported for various species including mouse (Daly et al. 2014; Pak et al. 2010), rat (Johansson et al. 2002a; Afiatpour et al. 2003; Liu and Daneshgari 2006; Doyle et al. 2018; Eika et al. 1993; Haefliger et al. 2002; Pitre et al. 2002; Murray et al. 2004; Chopra et al. 2005; Tong et al. 2006; Barendrecht et al. 2007; Cheng et al. 2007; Tong and Cheng 2007; Andersson et al. 2008; Hanna-Mitchell et al. 2013; Coelho et al. 2015; Xiao et al. 2015; Andersson et al. 2008), rabbit (Santarosa et al. 1994; Azadzoi et al. 1999; Azadzoi et al. 2010), and human (Lowe et al. 1997; Vaidyanathan et al. 1998; Mansfield et al. 2005; Datta et al. 2010; Kumar et al. 2010; Munoz et al. 2011; Bschleipfer et al. 2012; Kurizaki et al. 2013; Ballouhey et al. 2015), as well as in vitro with cell lines derived from human urothelium (Kang et al. 2015). They involved a range of conditions including aging (Afiatpour et al. 2003; Mansfield et al. 2005; Daly et al. 2014), OAB/idiopathic detrusor overactivity (Datta et al. 2010; Kumar et al. 2010; Munoz et al. 2011), neurogenic voiding dysfunction (Vaidyanathan et al. 1998; Datta et al. 2010; Ballouhey et al. 2015; Doyle et al. 2018), chronic pelvic ischemia (Azadzoi et al. 1999, Azadzoi et al. 2010), bladder outlet obstruction (Santarosa et al. 1994; Haefliger et al. 2002; Johansson et al. 2002a; Barendrecht et al. 2007; Bschleipfer et al. 2012; Kurizaki et al. 2013), diabetes (Eika et al. 1993; Pitre et al. 2002; Liu and Daneshgari 2006; Tong et al. 2006; Cheng et al. 2007; Tong and Cheng 2007; Pak et al. 2010; Hanna-Mitchell et al. 2013; Xiao et al. 2015), and cystitis (Lowe et al. 1997; Sun et al. 2009), the latter mostly in the context of cyclophosphamide treatment (Murray et al. 2004; Chopra et al. 2005; Andersson et al. 2008; Coelho et al. 2015; Andersson et al. 2008). Of note, it is not necessarily the abundance of a given factor but rather its ratio with that of other factors that may be of importance (Yoshida et al. 2004; Munoz et al. 2011; Silva et al. 2015). However, very few combinations of species, conditions and outcome parameters have been studied more than once, which makes it difficult to determine the robustness of any of the reported alterations.
These reports clearly demonstrate that the morphology and function of the urothelium is dynamically regulated in disease. Such alterations could plausibly contribute to the pathophysiology of bladder dysfunction and deserve additional investigation. While it is frequently speculated that such alterations could relate to the pathophysiology of the disease under investigation, it remains uncertain in most cases whether they are a consequence or a cause of alterations of the urothelium in disease. An example for this is the finding of a correlation between urothelial expression of α1-adrenoceptors and symptom severity in men with benign prostatic hyperplasia (Kurizaki et al. 2011).
Therapeutic aspects
A limited number of studies have explored how common treatments of OAB may affect the urothelium. Muscarinic receptor antagonists inhibit ATP release from human bladder strips, which is largely absent after mucosal removal, implying an effect on the mucosa (Yoshida et al. 2009). Studies with isolated rat bladder also reported that muscarinic antagonists reduce ATP release and extended these findings to release of prostaglandin E2 (Yokoyama et al. 2011). Experiments in the human urothelium-derived UROtsa cell line found increased proliferation in response to a muscarinic agonist, which was blocked by a muscarinic antagonist (Arrighi et al. 2011); such findings could provide a mechanistic explanation to increased urothelial thickness in some pathological states (see Table 2).
Botulinum toxin was reported to inhibit ATP release from cultured rat urothelial cells (Hanna-Mitchell et al. 2015) and also from mouse urothelium in vivo (Collins et al. 2013). In contrast, it promoted NO release in the latter model. It attenuated the decrease of urothelial immunoreactivity of M1 muscarinic receptors in patients with detrusor overactivity (Datta et al. 2010). Botulinum toxin also attenuated the increase in urothelial expression of TRPV1 and NGF in rats with bladder outlet obstruction (Ha et al. 2011). In contrast, urothelial NGF overexpression in mice leads to neuronal hyperinnervation and changes in bladder function (Schnegelsberg et al. 2010). Finally, the purinergic P2Y6 receptor agonist UDP inhibited Ach release from rat bladder strips in the presence but not absence of urothelium (Carneiro et al. 2014).
Conclusions
The urothelium plays a central role in the sensing of the filling state of the urinary bladder via the presence of neurotransmitters, paracrine mediators, hormones, and other constituents of the extracellular environment (Fig. 2). In response to such inputs, it can release various mediators that act in an autocrine and paracrine manner to modulate the function of the urothelium itself, but also that of other cells types including smooth muscle and afferent nerves. While the presence of the urothelium has proven a strong modulator of detrusor contractility, such an influence is apparently not mediated by known factors released from the urothelium. The molecular identity of such factors has remained elusive. It may be speculated that it is related, if not identical to, endothelium-derived relaxing factors (other than NO) and endothelium-derived hyperpolarizing factor. The morphology, sensing mechanisms, and mediator release from the urothelium apparently undergo major changes with aging, in pathological conditions and upon treatment, with the influence of gender also unknown. However, the evidence is too scattered to allow robust conclusions on the nature of these alterations and whether they represent causative parts of the pathophysiology or adaptations to an underlying pathology. Robust identification of such alterations may lead to urothelium-directed therapeutics, which allow a more targeted treatment of bladder dysfunction.
Neurotransmitter and hormonal inputs to the urothelium and outputs to the smooth muscle. The urothelium has receptors for a wide variety of transmitters including nerve growth factor (NGF), prostaglandins (PG), acetylcholine (Ach), substance P (SP), neurokinin A (NKA), adenosine triphosphate (ATP), 5-hydroxytryptamine (5HT), noradrenaline (NA), bradykinin (BK), capsaicin (Cap), angiotensin II (AG), cannabinoids (CB), and endothelin (ET). In response to these inputs, the urothelium releases a number of chemical mediators including ciliary neurotrophic factor (CNF), macrophage migration inhibitory factor (MIF), leukocyte inhibitory factor (LIF), hydrogen sulfide (H2S), urothelium-derived inhibitory factor (UDIF), and interleukins (IL). These actions of these mediators on smooth muscle can be excitatory [+], inhibitory [−], or unknown [?]
Abbreviations
- Ach:
-
Acetylcholine
- CBS:
-
Cystathionine-β-synthase
- CSE:
-
Cystathionine-γ-lyase
- EFS:
-
Electrical field stimulation
- H2S:
-
Hydrogen sulfide
- LUT:
-
Lower urinary tract
- NGF:
-
Nerve growth factor
- NO:
-
Nitric oxide
- NOS:
-
NO synthase
- OAB:
-
Overactive bladder syndrome
- ÜG:
-
Prostaglandin
- TRP:
-
Transient receptor potential channel
- UDIF:
-
Urothelium-derived inhibitor factor
References
Afiatpour P, Latifpour J, Takahashi W, Yono M, Foster HE Jr, Ikeda K, Pouresmail M, Weiss RM (2003) Developmental changes in the functional, biochemical and molecular properties of rat bladder endothelin receptors. Naunyn Schmiedeberg's Arch Pharmacol 367:462–472
Aizawa N, Igawa Y, Nishizawa O, Wyndaele J (2011) Effects of nitric oxide on the primary bladder afferent activities of the rat with and without intravesical acrolein treatment. Eur Urol 59:264–271
Akino H, Chapple CR, McKay N, Cross RL, Murakami S, Yokoyama O, Chess-Williams R, Sellers DJ (2008) Spontaneous contractions of the pig urinary bladder: the effect of ATP-sensitive potassium channels and the role of the mucosa. BJU Int 102:1168–1174
Anderson UA, Carson C, McCloskey KD (2009) KCNQ currents and their contribution to resting membrane potential and the excitability of interstitial cells of Cajal from the guinea pig bladder. J Urol 182:330–336
Andersson KE, McCloskey KD (2014) Lamina propria: the functional center of the bladder? Neurourol Urodyn 33:9–16
Andersson KE, Persson K (1994) Nitric oxide synthase and nitric-oxide mediated effects in lower urinary tract smooth muscles. World J Urol 12:274–280
Andersson M, Aronsson P, Doufish D, Lampert A, Tobin G (2012) Muscarinic receptor subtypes involved in urothelium-derived relaxatory effects in the inflamed rat urinary bladder. Auton Neurosci: Basic Clin 170:5–11
Andersson MC, Tobin G, Giglio D (2008) Cholinergic nitric oxide release from the urinary bladder mucosa in cyclophosphamide-induced cystitis of the anaesthetized rat. Br J Pharmacol 153:1438–1444
Anisuzzaman AS, Morishima S, Suzuki F, Tanaka T, Yoshiki H, Sathi ZS, Akino H, Yokoyama O, Muramatsu I (2008) Assessment of muscarinic receptor subtypes in human and rat lower urinary tract by tissue segment binding assay. J Pharmacol Sci 106:271–279
Apodaca G, Balestreire E, Birder LA (2007) The uroepithelial-associated sensory web. Kidney Int 72:1057–1064
Apostolidis A, Dasgupta P, Fowler CJ (2006) Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol 49:644–650
Arrighi N, Bodei S, Lucente A, Michel MC, Zani D, Simeone C, Cunico SC, Spano PF, Sigala S (2011) Muscarinic receptors stimulate cell proliferation in the human urothelium-derived cell line UROtsa. Pharmacol Res 64:420–425
Azadzoi KM, Heim VK, Tarcan T, Siroky MB (2004) Alteration of urothelial-mediated tone in the ischemic bladder: role of eicosanoids. Neurourol Urodyn 23:258–264
Azadzoi KM, Radisavljevic ZM, Golabek T, Yalla SV, Siroky MB (2010) Oxidative modification of mitochondrial integrity and nerve fiber density in the ischemic overactive bladder. J Urol 183:362–369
Azadzoi KM, Tarcan T, Kozlowski R, Krane RJ, Siroky MB (1999) Overactivity and structural changes in the chronically ischemic bladder. J Urol 162:1768–1778
Bahadory F, Moore KH, Liu L, Burcher E (2013) Gene expression of muscarinic, tachykinin, and purinergic receptors in porcine bladder: comparison with cultured cells. Front Pharmacol 4:148
Ballouhey Q, Panicker JN, Mazerolles C, Roumiguié M, Zaidi F, Rischmann P, Malavaud B, Gamé X (2015) Sphingosine kinase 1 urothelial expression is increased in patients with neurogenic detrusor overactivity. Int Braz J Urol 41:1141–1147
Barendrecht MM, Chichester P, Michel MC, Levin RM (2007) Effect of short-term outlet obstruction on rat bladder nerve density and contractility. Auton Autacoid Pharmacol 27:47–54
Beckel JM, Daugherty SL, Tyagi P, Wolf-Johnston AS, Birder LA, Mitchell CH, de Groat WC (2015) Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J Physiol 593:1857–1871
Birder L, Andersson KE (2013) Urothelial signaling. Physiol Rev 93:653–680
Birder LA, Apodaca G, De Groat WC, Kanai AJ (1998) Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Phys 275:F226–F229
Birder LA, Barrick SR, Roppolo JR, Kanai AJ, WCD G, Kiss S, Buffington CA (2003) Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol-Renal Physiol 285:F423–F429
Birder LA, De Groat WC (2007) Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol 4:46–54
Birder LA, Kanai AJ, Cruz F, Moore K, Fry CH (2010) Is the urothelium intelligent? Neurourol Urodyn 29:598–602
Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, de Groat WC, Apodaca G, Watkins S, Caterina MJ (2002a) Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5:856–860
Birder LA, Nealen ML, Kiss S, De Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ (2002b) ß-Adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci 22:8063–8070
Birder LA, Wolf-Johnston A, Buffington CA, Roppolo JR, de Groat WC, Kanai AJ (2005) Altered inducible nitric oxide synthase expression and nitric oxide production in the bladder of cats with feline interstitial cystitis. J Urol 173:625–629
Birder LA, Wolf-Johnston AS, Sun Y, Chai TC (2013) Alteration in TRPV1 and muscarinic M3 receptor expression and function in idiopathic overactive bladder urothelial cells. Acta Physiol (Oxf) 207:123–129
Bozkurt TE, Sahin-Erdemli I (2004) Evaluation of the rat bladder-derived relaxant factor by coaxial bioassay system. Eur J Pharmacol 495:193–199
Braverman AS, Lebed B, Linder M, Ruggieri MR Sr (2007) M2 mediated contractions of human bladder from organ donors is associated with an increase in urothelial muscarinic receptors. Neurourol Urodyn 26:63–70
Bschleipfer T, Schukowski K, Weidner W, Grando SA, Schwantes U, Kummer W, Lips KS (2007) Expression and distribution of cholinergic receptors in the human urothelium. Life Sci 80:2303–2307
Bschleipfer T, Weidner W, Kummer W, Lips KS (2012) Does bladder outlet obstruction alter the non-neuronal cholinergic system of the human urothelium? Life Sci 91:1082–1086
Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M (2002) Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol 135:639–648
Burnstock G (2014) Purinergic signalling in the urinary tract in health and disease. Purinergic Signalling 10:103–155
Canda AE, Chapple CR, Chess-Williams R (2009) Pharmacologic responses of the mouse urinary bladder. Central European Journal of Medicine 4:192
Carneiro I, Timoteo MA, Silva I, Vieira C, Baldaia C, Ferreirinha F, Silva-Ramos M, Correia-de-Sa P (2014) Activation of P2Y6 receptors increases the voiding frequency in anaesthetized rats by releasing ATP from the bladder urothelium. Br J Pharmacol 171:3404–3419
Chaiyaprasithi B, Mang CF, Kilbinger H, Hohenfellner M (2003) Inhibition of human detrusor contraction by a urothelium derived factor. J Urol 170:1897–1890
Chancellor MB (2017) OnabotulinumtoxinA for overactive bladder and urinary incontinence. J Urol 197:S224–S225
Chen MX, Gorman SA, Benson B, Singh K, Hieble JP, Michel MC, Tate SN, Trezise DJ (2004) Small and intermediate conductance Ca2+-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedeberg's Arch Pharmacol 369:602–615
Cheng JT, Yu BC, Tong YC (2007) Changes of M3-muscarinic receptor protein and mRNA expressions in the bladder urothelium and muscle layer of streptozotocin-induced diabetic rats. Neurosci Lett 423:1–5
Cheng S, Scigalla FP, Zhang ZG, Stolzenburg JU, Neuhaus J (2011a) ATP enhances spontaneous calcium activity in cultured suburothelial myofibroblasts of the human bladder. PLoS One 6:e25769
Cheng Y, Mansfield KJ, Allen W, Chess-Williams R, Burcher E, Moore KH (2014) ATP during early bladder stretch is important for urgency in detrusor overactivity patients. Biomed Res Int 2014:6
Cheng Y, Mansfield KJ, Sandow SL, Sadananda P, Burcher E, Moore KH (2011b) Porcine bladder urothelial, myofibroblast, and detrusor muscle cells: characterization and ATP release. Front Pharmacol 2:27
Chopra B, Barrick SR, Meyers S, Beckel JM, Zeidel ML, Ford APDW, De Groat WC, Birder LA (2005) Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol Lond 562:859–871
Chuang S-M, Liu K-M, Li Y-L, Jang M-Y, Lee H-H, Wu W-J, Chang W-C, Levin RM, Juan Y-S (2013) Dual involvements of cyclooxygenase and nitric oxide synthase expressions in ketamine-induced ulcerative cystitis in rat bladder. Neurourol Urodyn 32:1137–1143
Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan H-Z, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford APDW (2005) P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol 567:621–639
Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford APDW (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X 3-deficient mice. Nature 407:1011–1015
Coelho A, Wolf-Johnston AS, Shinde S, Cruz CD, Cruz F, Avelino A, Birder LA (2015) Urinary bladder inflammation induces changes in urothelial nerve growth factor and TRPV1 channels. Br J Pharmacol 172:1691–1699
Collins VM, Daly DM, Liaskos M, McKay NG, Sellers D, Chapple C, Grundy D (2013) OnabotulinumtoxinA significantly attenuates bladder afferent nerve firing and inhibits ATP release from the urothelium. BJU Int 112:1018–1026
d’Emmanuele di Villa Bianca R, Mitidieri E, Fusco F, Russo A, Pagliara V, Tramontano T, Donnarumma E, Mirone V, Cirino G, Russo G, Sorrentino R (2016) Urothelium muscarinic activation phosphorylates CBSSer227 via cGMP/PKG pathway causing human bladder relaxation through H2S production. Sci Rep 6:31491
Daher A, de Boer WI, Le Frere-Belda MA, Kheuang L, Abbou CC, Radvanyi F, Jaurand MC, Thiery JP, Diez de Medina SG, Chopin DK (2004) Growth, differentiation and senescence of normal human urothelium in an organ-like culture. Eur Urol 45:799–805
Daly DM, Nucchi L, Liaskos M, McKay NG, Chapple C, Grundy D (2014) Age-related changes in afferent pathways and urothelial function in the male mouse bladder. J Physiol Lond 592:537–549
Datta SN, Roosen A, Pullen A, Popat R, Rosenbaum TP, Elneil S, Dasgupta P, Fowler CJ, Apostolidis A (2010) Immunohistochemical expression of muscarinic receptors in the urothelium and suburothelium of neurogenic and idiopathic overactive human bladders, and changes with botulinum neurotoxin administration. J Urol 184:2578–2585
de Jongh R, Grol S, van Koeveringe GA, van Kerrebroeck PEV, de Vente J, Gillespie JI (2009) The localization of cyclo-oxygenase immuno-reactivity (COX I-IR) to the urothelium and to interstitial cells in the bladder wall. J Cell Mol Med 13:3069–3081
De Ridder D, Roskams T, van Poppel H, Baert L (1999) Nitric oxide synathase expression in neurogeneic bladder disease: a pilot study. Acta Neurol Belg 99:57–60
Deckmann K, Rafiq A, Erdmann C, Illig C, Durschnabel M, Wess J, Weidner W, Bschleipfer T, Kummer W (2018) Muscarinic receptors 2 and 5 regulate bitter response of urethral brush cells via negative feedback. The FASEB Journal in press: fj.201700582R
Downie JW, Karmazyn M (1984) Mechanical trauma to bladder epithelium liberates prostanoids which modulate neurotransmission in rabbit detrusor muscle. J Pharmacol Exp Ther 230:445–449
Doyle C, Cristofaro V, Sack BS, Mahmood F, Sullivan MP, Adam RM (2018) The role of the mucosa in modulation of evoked responses in the spinal cord injured rat bladder. Neurourol Urodyn in press
Eika B, Levin RM, Monson FC, Murphy M, Longhurst PA (1993) 3H-Thymidine uptake by the rat urinary bladder after induction of diabetes mellitus. J Urol 150:1316–1320
Eryildirim B, Tarhan F, Gül AE, Erbay E, Kuyumcuoglu U (2006) Immunohistochemical analysis of low-affinity nerve growth factor receptor in the human urinary bladder. Urol Int 77:76–80
Farr SE, Chess-Williams R, McDermott CM (2017) Gemcitabine: selective cytotoxicity, induction of inflammation and effects on urothelial function. Toxicol Appl Pharmacol 316:1–9
Fathian-Sabet B, Bloch W, Klotz T, Niggemann S, Jacobs G, Addicks K, Engelmann U (2001) Localization of constitutive nitric oxide synthase isoforms and the nitric oxide target enzyme soluble guanylyl cyclase in the human bladder. J Urol 165:1724–1729
Ferguson DR, Kennedy I, Burton TJ (1997) ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes–possible sensory mechanism? J Physiol 505:503–511
Folasire OS, Chess-Williams R, Sellers DJ (2017) Inhibitory effect of the urothelium/lamina propria on female porcine urethral contractility & effect of age. Clin Exp Pharmacol Physiol 44:954–960
Forner S, Andrade EL, Martini AC, Bento AF, Medeiros R, Koepp J, Calixto JB (2012) Effects of kinin B1 and B2 receptor antagonists on overactive urinary bladder syndrome induced by spinal cord injury in rats. Br J Pharmacol 167:1737–1752
Fovaeus M, Fujiwara M, Högestätt ED, Persson K, Andersson KE (1999) A non-nitrergic smooth muscle relaxant factor released from rat urinary bladder by muscarinic receptor stimulation. J Urol 161:649–653
Frazier EP, Mathy MJ, Peters SLM, Michel MC (2005) Does cyclic AMP mediate rat urinary bladder relaxation by isoproterenol? J Pharmacol Exp Ther 313:260–267
Fry CH, Vahabi B (2016) The role of the mucosa in normal and abnormal bladder function. Basic Clin Pharmacol Toxicol 119(Suppl. 3):57–62
Fusco F, d’Emmanuele di Villa Bianca R, Mitidieri E, Cirino G, Sorrentino R, Mirone V (2012) Sildenafil effect on the human bladder involves the L-cysteine/hydrogen sulfide pathway: a novel mechanism of action of phosphodiesterase type 5 inhibitors. Eur Urol 62:1174–1180
Garcia-Pascual A, Costa G, Garcia-Sacristan A, Andersson KE (1991) Relaxation of sheep urethral muscle induced by electrical stimulation of nerves: involvement of nitric oxide. Acta Physiol Scand 141:531–539
Giglio D, Ryberg AT, To K, Delbro DS, Tobin G (2005) Altered muscarinic receptor subtype expression and functional responses in cyclophosphamide induced cystitis in rats. Autonomic Neuroscience: Basic and Clinical 122:9–20
Gillespie JI, Drake MJ (2004) The acctions of sodium nitroprusside and the phosphodiesterase inhibitor dipyridamole on phasic activity in the isolated guinea pig bladder. BJU Int 93:851–858
Gillespie JI, Markerink-van Ittersum M, De Vente J (2004) cGMP-generating cells in the bladder wall: identification of distinct networks of interstitial cells. BJU Int 94:1114–1124
Gillespie JI, Markerink-van Ittersum M, de Vente J (2005) Expression of neuronal nitric oxide synthase (nNOS) and nitric-oxide-induced changes in cGMP in the urothelial layer of the guinea pig bladder. Cell Tissue Res 321:341–351
Gillespie JI, Markerink-van Ittersum M, De Vente J (2006) Endogenous nitric oxide/cGMP signalling in the guinea pig bladder: evidence for distinct populations of sub-urothelial interstitial cells. Cell Tissue Res 325:325–332
Girard B, Cheppudira B, Malley S, Schutz K, May V, Vizzard M (2011) Increased expression of interleukin-6 family members and receptors in urinary bladder with cyclophosphamide-induced bladder inflammation in female rats. Front Neurosci 5
Girard BM, Malley SE, Braas KM, May V, Vizzard MA (2010) PACAP/VIP and receptor characterization in micturition pathways in mice with overexpression of NGF in urothelium. J Mol Neurosci 42:378–389
Girard BM, Tompkins JD, Parsons RL, May V, Vizzard MA (2012) Effects of CYP-induced cystitis on PACAP/VIP and receptor expression in micturition pathways and bladder function in mice with overexpression of NGF in urothelium. J Mol Neurosci 48:730–743
Girard BM, Wolf-Johnston A, Braas KM, Birder LA, May V, Vizzard MA (2008) PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci 36:310–320
Goepel M, Wittmann A, Rübben H, Michel MC (1997) Comparison of adrenoceptor subtype expression in porcine and human bladder and prostate. Urol Res 25:199–206
Grol S, Essers PBM, van Koeveringe GA, Martinez-Martinez P, de Vente J, Gillespie JI (2009) M3 muscarinic receptor expression on suburothelial interstitial cells. BJU Int 104:398–405
Guan NN, Nilsson KF, Wiklund PN, Gustafsson LE (2014) Release and inhibitory effects of prostaglandin D2 in guinea pig urinary bladder and the role of urothelium. Biochim Biophys Acta Gen Subj 1840:3443–3451
Ha US, Park EY, Kim JC (2011) Effect of botulinum toxin on expression of nerve growth factor and transient receptor potential vanilloid 1 in urothelium and detrusor muscle of rats with bladder outlet obstruction-induced detrusor overactivity. Urology 78:721.e721–721.e726
Haefliger JA, Tissieres P, Tawadros T, Formenton A, Beny JL, Nicod P, Frey P, Meda P (2002) Connexins 43 and 26 are differentially increased after rat bladder outlet obstruction. Exp Cell Res 274:216–225
Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA (2007) Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci 80:2298–2302
Hanna-Mitchell AT, Ruiz GW, Daneshgari F, Liu G, Apodaca G, Birder LA (2013) Impact of diabetes mellitus on bladder uroepithelial cells. Am J Phys 304:R84–R93
Hanna-Mitchell AT, Wolf-Johnston AS, Barrick SR, Kanai AJ, Chancellor MB, De Groat WC, Birder LA (2015) Effect of botulinum toxin A on urothelial-release of ATP and expression of SNARE targets within the urothelium. Neurourol Urodyn 34:79–84
Harmon EB, Porter JM, Porter JE (2005) ß-Adrenergic receptor activation in immortalized human urothelial cells stimulates inflammatory responses by PKD-independent mechanisms. Cell Commun Signal 3:10
Harvey RA, Skennerton DE, Newgreen D, Fry CH (2002) The contractile potency of adenosine triphosphate and ecto-adenosine triphosphatase activity in guinea pig detrusor and detrusor from patients with a stable, unstable or obstructed bladder. J Urol 168:1235–1239
Hawthorn MH, Chapple CR, Cock M, Chess-Williams R (2000) Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol 129:416–419
Hernández M, Barahona MV, Recio P, Navarro-Dorado J, Bustamante S, Benedito S, García-Sacristán A, Prieto D, Orensanz LM (2008) Role of neuronal voltage-gated K+ channels in the modulation of the nitrergic neurotransmission of the pig urinary bladder neck. Br J Pharmacol 153:1251–1258
Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A (2007) Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol-Renal Physiol 293:F1018–F1025
Ishihama H, Momota Y, Yanase H, Wang X, De Groat WC, Kawatani M (2006) Activation of α1D adrenergic receptors in the rat urothelium facilitates the micturition reflex. J Urol 175:358–364
Janssen DAW, Hoenderop JG, Jansen KCFJ, Kemp AW, Heesakkers JPFA, Schalken JA (2011) The mechanoreceptor TRPV4 is localized in adherence junctions of the human bladder urothelium: a morphological study. J Urol 186:1121–1127
Jeremy JY, Tsang V, Mikhailidis DP, Rogers H, Morgan RJ, Dandona P (1987) Eicosanoid synthesis by human urinary bladder mucosa: pathological implications. Br J Urol 59:36–39
Johansson R, Pandita RK, Poljakovic M, Garcia-Pascual A, de Vente J, Persson K (2002a) Activity and expression of nitric oxide synthase in the hypertrophied rat bladder and the effect of nitric oxide on bladder smooth muscle growth. J Urol 168:2689–2694
Johansson RK, Poljakovic M, Andersson KE, Persson K (2002b) Expression of nitric oxide synthase in bladder smooth muscle cells: regulation by cytokines and L-arginine. J Urol 168:2280–2285
Jositsch G, Papadakis T, Haberberger RV, Wolff M, Wess J, Kummer W (2009) Suitability of muscarinic acetylcholine receptor antibodies for immunohistochemistry evaluated on tissue sections of receptor gene-deficient mice. Naunyn Schmiedeberg's Arch Pharmacol 379:389–395
Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, Griffiths D, Wd G, Fry C (2007) Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol-Renal Physiol 292:F1065–F1072
Kang S-H, Chess-Williams R, Anoopkumar-Dukie S, McDermott C (2013) Induction of inflammatory cytokines and alteration of urothelial ATP, acetylcholine and prostaglandin E2 release by doxorubicin. Eur J Pharmacol 700:102–109
Kang SH, Chess-Williams R, Anoopkumar-Dukie S, McDermott C (2015) Recovery of urothelial mediated release but prolonged elevations in interleukin-8 and nitric oxide section following mitomycin C treatment. Naunyn Schmiedeberg's Arch Pharmacol 388:781–791
Kedia GT, Neumayer E, Scheller F, Kuczyk MA, Uckert S (2009) In vitro effects of a novel class of nitric oxide donating compounds on isolated human urinary bladder. Georgian Med News 167:7–16
Krege S, Kinzig-Schppers M, Sörgel F, Baschek R, Michel MC, Rübben H (2004) Absorption of intravesically applied drugs: comparison of normal and ileal-augmented rabbit bladder. J Urol 172:2045–2050
Kullmann FA, Artim D, Beckel J, Barrick S, WCd G, Birder LA (2008a) Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol-Renal Physiol 294:F971–F981
Kullmann FA, Artim DE, Birder LA, de Groat WC (2008b) Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci 28:1977–1987
Kullmann FA, Downs TR, Artim DE, Limberg BJ, Shah M, Contract D, De Groat WC, Rosenbaum JS (2011) Urothelial beta-3 adrenergic receptors in the rat bladder. Neurourol Urodyn 30:144–150
Kumar V, Chapple C, Chess-Williams R (2004) Characteristics of adenosine triphosphate [corrected] release from porcine and human normal bladder. J Urol 172:744–747
Kumar V, Chapple CR, Rosario D, Tophill PR, Chess-Williams R (2010) In vitro release of adenosine triphosphate from the urothelium of human bladders with detrusor overactivity, both neurogenic and idiopathic. Eur Urol 57:1087–1092
Kumar V, Chapple CR, Surprenant AM, Chess-Williams R (2007) Enhanced adenosine triphosphate release from the urothelium of patients with painful bladder syndrome: a possible pathophysiological explanation. J Urol 178:1533–1536
Kurizaki Y, Ishizuka O, Imamura T, Ichino M, Ogawa T, Igawa Y, Nishizawa O, Andersson KE (2011) Relation between expression of α1-adrenoceptor mRNAs in bladder mucosa and urodynamic findings in men with lower urinary tract symptoms. Scand J Urol Nephrol 45:15–19
Kurizaki Y, Ishizuka O, Imamura T, Ishikawa M, Ichino M, Ogawa T, Nishizawa O, Andersson KE (2013) Relationship between expression of ß3-adrenoceptor mRNA in bladder mucosa and urodynamic findings in men with lower urinary tract symptoms. Neurourol Urodyn 32:88–91
Kurzrock EA, Lieu DK, de Graffenried LA, Isseroff RR (2005) Rat urothelium: improved techniques for serial cultivation, expansion, freezing and reconstitution onto acellular matrix. J Urol 173:281–285
Lazzeri M, Vannucchi MG, Spinelli M, Bizzoco E, Beneforti P, Turini D, Faussone-Pellegrini MS (2005) Transient receptor potential vanilloid type 1 (TRPV1) expression changes from normal urothelium to transitional cell carcinoma of human bladder. Eur Urol 48:691–698
Lee HY, Bardini M, Burnstock G (2000) Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol 163:2002–2007
Levin RM, Wein AJ, Krasnopolsky L, Atta MA, Ghoniem GM (1995) Effect of mucosal removal on the response of the feline bladder to pharmacological stimulation. J Urol 153:1291–1294
Li M, Sun Y, Tomiya N, Hsu Y, Chai TC (2013) Elevated polyamines in urothelial cells from OAB subjects mediate oxotremorine-evoked rapid intracellular calcium rise and delayed acetylcholine release. Am J Physiol-Renal Physiol 305:F445–F450
Lin Z, Chen S, Ye C, Zhu S (2003) Nitric oxide synthase expression in human bladder cancer and its relation to angiogenesis. Urol Res 31:232–235
Lips KS, Wunsch J, Sarghooni S, Bschleipfer T, Schukowski K, Weidner W, Wessler I, Schwantes U, Koepsell H, Kummer W (2007) Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol 51:1042–1053
Liu G, Daneshgari F (2006) Temporal diabetes- and diuresis-induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol 291:R837–R843
Lowe EM, Anand P, Terengh G, Williams-Chestnut RE, Sinicropi DV, Osborne JL (1997) Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 79:572–577
Maggi CA, Santicioli P, Parlani M, Astolfi M, Patacchini R, Meli A (1987) The presence of mucosa reduces the contractile response of the guinea-pig urinary bladder to substance P. J Pharm Pharmacol 39:653–655
Mansfield KJ, Chandran JJ, Vaux KJ, Millard RJ, Christopoulos A, Mitchelson FJ, Burcher E (2009) Comparison of receptor binding characteristics of commonly used muscarinic antagonists in human bladder detrusor and mucosa. J Pharmacol Exp Ther 328:893–899
Mansfield KJ, Hughes JR (2014a) Effect of inflammatory mediators on ATP release of human urothelial RT4 cells. Biomed Res Int 2014:6
Mansfield KJ, Hughes JR (2014b) P2Y receptor modulation of ATP release in the urothelium. Biomed Res Int 2014:8
Mansfield KJ, Liu L, Mitchelson FJ, Moore KH, Millard RJ, Burcher E (2005) Muscarinic receptor subtypes in human bladder detrusor and mucosa, studied by radioligand binding and quantitative competitive RT-PCR: changes in ageing. Br J Pharmacol 144:1089–1099
Masick JM, Levin RM, Hass MA (2001) The effect of partial outlet obstruction on prostaglandin generation in the rabbit urinary bladder. Prostaglandins Other Lipid Mediat 66:211–219
Mastrangelo D, Iselin CE (2007) Urothelium dependent inhibition of rat ureter contractile activity. J Urol 178:702–709
Masunaga K, Chapple CR, McKay NG, Yoshida M, Sellers DJ (2010) The ß3-adrenoceptor mediates the inhibitory effects of ß-adrenoceptor agonists via the urothelium in pig bladder dome. Neurourol Urodyn 29:1320–1325
Masunaga K, Yoshida M, Inadome A, Iwashita H, Miyamae K, Ueda S (2006) Prostaglandin E2 release from isolated bladder strips in rats with spinal cord injury. Int J Urol 13:271–276
McLatchie LM, Fry CH (2015) ATP release from freshly isolated guinea-pig bladder urothelial cells: a quantification and study of the mechanisms involved. BJU Int 115:987–993
McLatchie LM, Young JS, Fry CH (2014) Regulation of ACh release from guinea pig bladder urothelial cells: potential role in bladder filling sensations. Br J Pharmacol 171:3394–3403
Meng E, Young JS, Brading AF (2008) Spontaneous activity of mouse detrusor smooth muscle and the effects of the urothelium. Neurourol Urodyn 27:79–87
Meyer-Siegler KL, Vera PL (2004) Substance P induced release of macrophage migration inhibitor factor from rat bladder epithelium. J Urol 171:1698–1703
Michel MC, Seifert R (2015) Selectivity of pharmacological tools: implications for use in cell physiology. Am J Phys 308:C505–C520
Michel MC, Wieland T, Tsujimoto G (2009) How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedeberg's Arch Pharmacol 377:385–388
Miyamoto T, Mochizuki T, Nakagomi H, Kira S, Watanabe M, Takayama Y, Suzuki Y, Koizumi S, Takeda M, Tominaga M (2014) Functional role for Piezo1 in stretch-evoked Ca2+ influx and ATP release in urothelial cell cultures. J Biol Chem 289:16565–16575
Moon A (2002) Influence of nitric oxide signalling pathways on pre-contracted human detrusor smooth muscle in vitro. BJU Int 89:942–949
Munoz A, Gangitano DA, Smith CP, Boone TB, Somogyi GT (2010) Removal of urothelium affects bladder contractility and release of ATP but not release of NO in rat urinary bladder. BMC Urol 10:10
Munoz A, Smith CP, Boone TB, Somogyi GT (2011) Overactive and underactive bladder dysfunction is reflected by alterations in urothelial ATP and NO release. Neurochem Int 58:295–300
Murakami S, Chapple CR, Akino H, Sellers DJ, Chess-Williams R (2007) The role of the urothelium in mediating bladder responses to isoprenaline. BJU Int 99:669–673
Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA (2004) Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol 172:2434–2439
Nakahara T, Kubota Y, Mitani A, Maruko T, Sakamoto K, Ishii K (2003) Protease-activated receptor-2-mediated contraction in the rat urinary bladder: the role of urinary bladder mucosa. Naunyn Schmiedeberg's Arch Pharmacol 367:211–213
Negoro H, Urban-Maldonado M, Liou LS, Spray DC, Thi MM, Suadicani SO (2014) Pannexin 1 channels play essential roles in urothelial mechanotransduction and intercellular signaling. PLoS One 9:e106269
Nile CJ, De Vente J, Gillespie JI (2010) Stretch independent regulation of prostaglandin E2 production within the isolated guinea-pig lamina propria. BJU Int 105:540–548
Nile CJ, Gillespie JI (2012) Interactions between cholinergic and prostaglandin signaling elements in the urothelium: role for muscarinic type 2 receptors. Urology 79:240.e217–240.e223
Nilius B, Szallasi A (2014) Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev 66:676–814
Ochodnicky P, Cruz CD, Yoshimura N, Michel MC (2011) Nerve growth factor in bladder dysfunction: contributing factor, biomarker and therapeutic target. Neurourol Urodyn 30:1227–1241
Ochodnicky P, Humphreys S, Eccles R, Poljakovic M, Wiklund P, Michel MC (2012) Expression profiling of G-protein-coupled receptors in human urothelium and related cell lines. BJU Int 110:e293–e300
Ochodnicky P, Michel-Reher MB, Butter JJ, Seth J, Panicker JN, Michel MC (2013) Bradykinin modulates spontaneous nerve growth factor production and stretch-induced ATP release in human urothelium. Pharmacol Res 70:147–154
Otsuka A, Kawasaki H, Matsumoto R, Shinbo H, Kurita Y, Iwashita T, Ozono S (2013) Expression of ß-adrenoceptor subtypes in urothelium, interstitial cells and detrusor of the human urinary bladder. Lower Urinary Tract Symptoms 5:173–180
Otsuka A, Shinbo H, Matsumoto R, Kurita Y, Ozono S (2008) Expression and functional role of ß-adrenoceptors in the human urinary bladder. Naunyn Schmiedeberg's Arch Pharmacol 377:473–481
Pak KJ, Ostrom RS, Matsui M, Ehlert FJ (2010) Impaired M3 and enhanced M2 muscarinic receptor contractile function in a streptozotocin model of mouse diabetic urinary bladder. Naunyn Schmiedeberg's Arch Pharmacol 381:441–454
Parsons CL (2011) The role of a leaky epithelium and potassium in the generation of bladder symptoms in interstitial cystitis/overactive bladder, urethral syndrome, prostatitis and gynaecological chronic pelvic pain. BJU Int 107:370–375
Pinna C, Caratozzolo O, Puglisi L (1992) A possible role of urinary bladder epithelium in bradykinin-induced contraction in diabetic rats. Eur J Pharmacol 214:143–148
Pinna C, Ventura S, Puglisi L, Burnstock G (1996) A pharmacological and histochemical study of hamster urethra and the role of urothelium. Br J Pharmacol 119:655–662
Pitre DA, Ma T, Wallace LJ, Bauer JA (2002) Time-dependent urinary bladder remodeling in the streptozotocin-induced diabetic rat model. Acta Diabetol 39:23–27
Propping S, Newe M, Lorenz K, Wirth MP, Ravens U (2015a) ß-Adrenoceptor-mediated relaxation of carbachol-pre-contracted mouse detrusor. Urol Int 95:92–98
Propping S, Roedel M, Wirth MP, Ravens U (2015b) Pharmacological modulation of mucosa-related impairment of ß-adrenoceptor-mediated relaxation in human detrusor. Urol Int 95:300–308
Propping S, Wuest M, Eichhorn B, Wirth MP, Kaumann AJ, Ravens U (2013) Mucosa of human detrusor impairs contraction and ß-adrenoceptor-mediated relaxation. BJU Int 112:1215–1222
Rahnama'i MS, van Koeveringe GA, Essers PB, de Wachter SGG, de Vente J, van Kerrebroeck PE, Gillespie JI (2010) Prostaglandin receptor EP1 and EP2 site in Guinea pig bladder urothelium and lamina propria. J Urol 183:1241–1247
Rahnama’i MS, Biallosterski BT, de Wachter SGG, Van Kerrebroeck PEV, van Koeveringe GA (2012) The distribution of the prostaglandin E receptor type 2 (EP2) in the detrusor of the Guinea pig. Prostaglandins Other Lipid Mediat 99:107–115
Ribeiro ASF, Fernandes VS, Martinez MP, Martinez-Saenz A, Pazos MR, Orensanz LM, Recio P, Bustamente S, Carballido J, Garcia-Sacristan A, Prieto D, Hernandez M (2014) Neuronal and non-neuronal bradykinin receptors are involved in the contraction and/or relaxation to the pig bladder neck smooth muscle. Neurourol Urodyn this issue
Roedel M, Ravens U, Kasper M, Wirth MP, Jepps TA, Propping S (2018) Contractile responses in intact and mucosa-denuded human ureter—a comparison with urinary bladder detrusor preparations. Naunyn Schmiedebergs Arch Pharmacol this issue
Romih R, Korošec P, de Mello W, Jezernik K (2005) Differentiation of epithelial cells in the urinary tract. Cell Tissue Res 320:259–268
Saban R, Keith IM, Nielsen KT, Christensen MM, Rhodes PR, Bruskewitz RC (1992) In vitro effects of bladder mucosa and an enkephalinase inhibitor on tachykinin induced contractility of the dog bladder. J Urol 147:750–755
Saban R, Undem BJ, Keith IM, Saban MR, Tengowski MW, Graziano FM, Bjorling DE (1994) Differential release of prostaglandins and leukotrienes by sensitized Guinea pig urinary bladder layers upon antigen challenge. J Urol 152:544–549
Sadananda P, Chess-Williams R, Burcher E (2008) Contractile properties of the pig bladder mucosa in response to neurokinin A: a role for myofibroblasts? Br J Pharmacol 153:1465–1473
Sadananda P, Kao FCL, Liu L, Mansfield KJ, Burcher E (2012) Acid and stretch, but not capsaicin, are effective stimuli for ATP release in the porcine bladder mucosa: are ASIC and TRPV1 receptors involved? Eur J Pharmacol 683:252–259
Sano T, Kobayashi T, Negoro H, Sengiku A, Hiratsuka T, Kamioka Y, Liou LS, Ogawa O, Matsuda M (2016) Intravital imaging of mouse urothelium reveals activation of extracellular signal-regulated kinase by stretch-induced intravesical release of ATP. Physiological Reports 4
Santarosa R, Colombel MC, Kaplan S, Monson F, Levin RM, Buttyan R (1994) Hyperplasia and apoptosis. Opposing cellular processes that regulate the response of the rabbit bladder to transient outlet obstruction. Laboratory Investigations 70:503–510
Santoso AGH, Sonarno IAB, Arsad NAB, Liang W (2010) The role of the urothelium and ATP in mediating detrusor smooth muscle contractility. Urology 76:1267.e1267–1267.e1212
Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford APDW, Vizzard A, Cockayne DA (2010) Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Phys 298:R534–R547
Silva I, Ferreirinha F, Magalhães-Cardoso MT, Silva-Ramos M, Correia-de-Sá P (2015) Activation of P2Y6 receptors facilitates nonneuronal adenosine triphosphate and acetylcholine release from urothelium with the lamina propria of men with bladder outlet obstruction. J Urol 194:1146–1154
Smith KJ, Chess-Williams R, McDermott C (2014) Luminal DMSO: effects on detrusor and urothelial/lamina propria function. Biomed Res Int 2014:8
Stenqvist J, Winder M, Carlsson T, Aronsson P, Tobin G (2017) Urothelial acetylcholine involvement in ATP-induced contractile responses of the rat urinary bladder. Eur J Pharmacol 809:253–260
Sui G-P, Wu C, Roosen A, Ikeda Y, Kanai AJ, Fry CH (2008) Modulation of bladder myofibroblast activity: implications for bladder function. Am J Physiol-Renal Physiol 295:F688–F697
Sui G, Fry CH, Montgomery B, Roberts M, Wu R, Wu C (2014) Purinergic and muscarinic modulation of ATP release from the urothelium and its paracrine actions. Am J Physiol-Renal Physiol 306:F286–F298
Sun Y, Chai TC (2006) Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Phys Cell Phys 290:C27–C34
Sun Y, Keay S, Lehrfeld TJ, Chai TC (2009) Changes in adenosine triphosphate-stimulated ATP release suggest association between cytokine and purinergic signaling in bladder urothelial cells. Urology 74:1163–1168
Tanaka I, Nagase K, Tanase K, Aoki Y, Akino H, Yokoyama O (2011) Modulation of stretch evoked adenosine triphosphate release from bladder epithelium by prostaglandin E2. J Urol 185:341–346
Templeman L, Chapple CR, Chess-Williams R (2002) Urothelium derived inhibitory factor and cross-talk among receptors in the trigone of the bladder of the pig. J Urol 167:742–745
Theobald RJ (2003) Differing effects of NG-monomethyl L-arginine and 7-nitroindazole on detrusor activity. Neurourol Urodyn 22:62–69
Thorneloe KS, Knorn AM, Doetsch PE, Lashinger ESR, Liu AX, Bond CT, Adelman JP, Nelson MT (2008) Small-conductance, Ca2+-activated K+ channel 2 is the key functional component of SK channels in mouse urinary bladder. Am J Phys 294:R1737–R1743
Timóteo MA, Carneiro I, Silva I, Noronha-Matos JB, Ferreirinha F, Silva-Ramos M, Correia-de-Sá P (2014) ATP released via pannexin-1 hemichannels mediates bladder overactivity triggered by urothelial P2Y6 receptors. Biochem Pharmacol 87:371–379
Tong Y-C, Cheng J-T, Hsu C-T (2006) Alterations of M2-muscarinic receptor protein and mRNA expression in the urothelium and muscle layer of the streptozotocin-induced diabetic rat urinary bladder. Neurosci Lett 406:216–221
Tong YC, Cheng JT (2007) Alterations of M 2.3-muscarinic receptor protein and mRNA expression in the bladder of the fructose fed obese rat. J Urol 178:1537–1542
Truschel ST, Wang E, Ruiz WG, Leung S-M, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G (2002) Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell 13:830–846
Tyagi P, Thomas CA, Yoshimura N, Chancellor MB (2009a) Investigations into the presence of functional ß1, ß2 and ß3-adrenoceptors in urothelium and detrusor of human bladder. Int Braz J Urol 35:76–83
Tyagi S, Tyagi P, Van-le S, Yoshimura N, Chancellor MB, de Miguel F (2006) Qualitative and quantitative expression profile of muscarinic receptors in human urothelium and detrusor. J Urol 176:1673–1678
Tyagi V, Philips BJ, Su R, Smaldone MC, Erickson VL, Chancellor MB, Yoshimura N, Tyagi P (2009b) Differential expression of functional cannabinoid receptors in human bladder detrusor and urothelium. J Urol 181:1932–1938
Vaidyanathan S, Krishnan KR, Mansour P, Soni BM, McDicken I (1998) p75 nerve growth factor receptor in the vesical urothelium of patients with neuropatic bladder: an immunohistochemical study. Spinal Cord 36:541–547
Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford APDW, Burnstock G (2001) P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21:5670–5677
Walden PD, Durkin MM, Lepor H, Wetzel JM, Gluchowski C, Gustafson EL (1997) Localization of mRNA and receptor binding sites for the α1A-adrenoceptor subtype in the rat, monkey and human urinary bladder and prostate. J Urol 157:1032–1038
Wang ECY, Lee J-M, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G (2005) ATP and purinergic receptor–dependent membrane traffic in bladder umbrella cells. J Clin Invest 115:2412–2422
Weng TI, Wu HY, Lin PY, Liu SH (2009) Uropathogenic Escherichia coli-induced inflammation alters mouse urinary bladder contraction via an interleukin-6-activated inducible nitric oxide synthase-related pathway. Infect Immun 77:3312–3319
Wuest M, Kaden S, Hakenberg OW, Wirth MP, Ravens U (2005) Effect of rilmakalim on detrusor contraction in the presence and absence of urothelium. Naunyn Schmiedeberg's Arch Pharmacol 372:203–212
Xiao N, Huang Y, Kavran M, Elrashidy RA, Liu G (2015) Short-term diabetes- and diuresis-induced alterations of the bladder are mostly reversible in rats. Int J Urol 22:410–415
Yokoyama O, Tanaka I, Kusukawa N, Yamauchi H, Ito H, Aoki Y, Oyama N, Miwa Y, Akino H (2011) Antimuscarinics suppress adenosine triphosphate and prostaglandin E2 release from urothelium with potential improvement in detrusor overactivity in rats with cerebral infarction. J Urol 185:2392–2397
Yoshida M, Inadome A, Maeda Y, Satoji Y, Masunaga K, Sugiyama Y, Murakami S (2006) Non-neuronal cholinergic system in human bladder urothelium. Urology 67:425–430
Yoshida M, Masunaga K, Nagata T, Maeda Y, Miyamoto Y, Kudoh J, Homma Y (2009) Attenuation of non-neuronal adenosine triphosphate release from human bladder mucosa by antimuscarinic agents. LUTS 1:88–92
Yoshida M, Masunaga K, Satoji Y, Maeda Y, Nagata T, Inadome A (2008) Basic and clinical aspects of non-neuronal acetylcholine: expression of non-neuronal acetylcholine in urothelium and its clinical significance. J Pharmacol Sci 106:193–198
Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A (2004) Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology 63(Suppl. 1):17–23
Young JS, Matharu R, Carew MA, Fry CH (2012) Inhibition of stretching-evoked ATP release from bladder mucosa by anticholinergic agents. BJU Int 110:E397–E401
Zarghooni S, Wunsch J, Bodenbenner M, Brüggmann D, Grando SA, Schwantes U, Wess J, Kummer W, Lips KS (2007) Expression of muscarinic and nicotinic acetylcholine receptors in the mouse urothelium. Life Sci 80:2308–2313
Zhang QL, Qiao L-Y (2012) Regulation of IGF-1 but not TGF-β1 by NGF in the smooth muscle of the inflamed urinary bladder. Regul Pept 177:73–78
Acknowledgements
Work in the authors’ labs is being or has been funded by the Australian National Health and Medical Research Council, Cancer Council Queensland, Australian Bladder Foundation and Deutsche Forschungsgemeinschaft (Mi 294/8-1).
Author information
Authors and Affiliations
Contributions
DS, RCW, and MCM conceived the outline of this review, retrieved and extracted the references, and contributed to write sections of the manuscript. All authors read, critically revised for content, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
DS and RCW do not declare a conflict of interest. In the field of urology, MCM is a consultant to and shareholder of Velicept Therapeutics, a former employee (2011–2016) and present consultant of Boehringer Ingelheim, and a consultant of Apogepha and Dr. Wilmar Schwabe.
Rights and permissions
About this article
Cite this article
Sellers, D., Chess-Williams, R. & Michel, M.C. Modulation of lower urinary tract smooth muscle contraction and relaxation by the urothelium. Naunyn-Schmiedeberg's Arch Pharmacol 391, 675–694 (2018). https://doi.org/10.1007/s00210-018-1510-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-018-1510-8