Abstract
Nitric oxide (NO) is synthesized by the enzyme family of nitric oxide synthases (NOS) and plays an important role in tumor growth and angiogenesis. The expression of two of the NOS isoforms, the endothelial and inducible isoforms (eNOS and iNOS, respectively), were evaluated in bladder tissue from patients with transitional cell carcinoma (TCC). The specimens were procured from 58 patients with TCC and 14 cases of normal bladder mucosa were used as a control group. NOS immunohistochemistry was performed and microvessal density (MVD) was determined. iNOS specific proteins were found in 47 of 58 bladder cancer specimens but not in control bladder tissue. The endothelial cells in both normal urothelium and tumor tissue showed a highly positive eNOS immunostaining. The MVD was 39.3±19.5 and 29.3±10.5 in TCC positive and negative for iNOS, respectively (P<0.01). A correlation between iNOS immunoreactivity and tumor grade in bladder carcinoma could not be verified. These results indicate that NO generation from iNOS in the malignant epithelium and from eNOS in tumor stroma play a important role in tumor angiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitric oxide (NO) is a short-lived, pleiotropic biomolecule with a multitude of biological functions which plays a vital role as a mediator in the vascular, nervous and immune systems [5, 17, 19]. It is a product of the conversion of L-arginine to L-citrulline by nitric oxide synthase (NOS), which exists as three enzyme classes: the calcium-dependent endothelial (eNOS), the neuronal or brain isoforms (nNOS) and a calcium-independent inducible or immunologic NOS (iNOS). eNOS and nNOS are constitutively expressed and have a physiological role. They are also called constitutive NOS (cNOS). In contrast, iNOS is induced during disease processes, such as infection or tumor growth, and is involved in the cytotoxicity of macrophages and tumor-induced immune reaction. Although NOS expression occurs in various tumor cell lines and solid tumors, the role of NO in tumor biology is still unclear. Previous reports have seldom demonstrated the expression of NOS in human bladder cancer, so the aim of this study was to investigate the expression of NOS in transitional cell carcinoma (TCC) and its relationship to angiogenesis.
Materials and methods
Tissue collection
Specimens of human bladder cancer tissue were obtained from 58 patients (39 males, 19 females, mean age 44.3 years ) and from 14 individuals with normal adult bladder mucosa which acted as a control group. All patients underwent cystectomy or transurethral resection for bladder cancer between January 1996 and September 1999. No patients had signs of a bladder infection before surgery and none of the patients had received preoperative radiation therapy or chemotherapy. There were 21 patients with G1, 23 with G2, 14 with G3 and 28 with superficial bladder cancer (Ta-T1) as well as 2 with tumor in situ (Tis), while 28 had invasive bladder cancer (T2-T4).
Immunohistochemistry
Sections of formalin-fixed, paraffin embedded specimens were cut 4.0 μm thick. These were deparaffinised and blocked for endogenous activity by 1% hydrogen peroxide in methanol for 30 min. Non-specific binding was blocked by incubating the sections with normal goat serum for 30 min. The sections were then incubated with the polyclonal rabbit antibody against iNOS, eNOS or factor VIII (Boster, China) overnight at 4°C, then the slides were sequentially rinsed in PBS for 30 min before being incubated with goat anti-rabbit biotinylated conjugate for 30 min. The specimens were stained with diaminobenzidine (DAB) or 3-amino-9-ethylcarbozole (AEC). Negative controls were treated with 1% normal goal serum in PBS instead of primary antibodies. At least one control was done in each experiment.
Microscopic evaluation and statistical analysis
The slides were examined under a Nikon fluorescence microscope. The amount and cell type exhibiting eNOS and iNOS immunoreactivity were recorded for each case. The mean overall intensity of the immunostaining cells was evaluated as positive for immunostaining in more than 10% of the cells within malignant tissue or normal bladder mucosa. Microvessal density (MVD) was qualified using the method described by Weidner et al. [24]. The sections of tumor were scanned at low magnification (×40) to identify areas of greatest intratumor vascularity. At a higher magnification (×200), counts were made of all distinct brown-staining endothelial cells and cell clumps within a medium-power field area of 0.94 mm2. The average field count of five random selections was designated the MVD for each specimen.
The statistical significance of intergroup differences was determined using the two-tailed, unpaired t-test and the χ2 test. Significance was defined as P<0.05. The analyses were carried out using SPSS for Windows (version 8.0).
Results
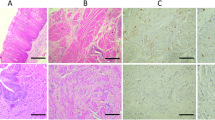
There was either no staining or occasional weak background staining of the negative controls. iNOS specific proteins were found in 47 of 58 bladder carcinoma specimens but not in control bladder tissue. The malignant cells and inflammatory cells within the carcinomas were highly iNOS positive, specimens of bladder mucosa outside of the malignant regions showed no or occasionally a weak positive iNOS immunostaining (Figs. 1, 2). Positive rates of iNOS immunostaining were 76.2%, 82.6% and 85.7% in bladder carcinoma of G1, G2 and G3, respectively, and 78.6%, 82.1% and 100% in the superficial, invasive and Tis groups. A correlation between iNOS immunoreactivity and tumor grade or tumor stage could not be verified (Table 1). MVD was 39.3±19.5(SD) and 29.3±10.5 in TCC positive and negative for iNOS, respectively (P<0.05), the expression of iNOS in bladder carcinoma was positively correlated to the tumor MVD (Table 2). The endothelial cells in both normal urothelium and tumor tissue showed a distinct positive eNOS immunostaining, and an immunoreaction with eNOS was not detected in either malignant or benign epithelium (Figs. 3, 4).
Discussion
The present study demonstrates abundant iNOS immunoreactivity in human bladder tumor but not in normal bladder tissue. Our results support other findings which have been published [9, 20, 21, 25]. So far, iNOS has been detected in many lines of malignant cells as well as in solid tumors such as human breast, lung, prostate, and gynaecological cancer etc. There is a growing body of evidence to suggest that the output of NO generated by iNOS, is higher in malignant tissue and results in an increase in tumor growth rate, vascular density and invasiveness [6, 11, 22, 23]. Although the biological role of NO in malignancies is still unclear, more and more investigations suggest that it may have a dual pro- and antitumor action depending on local concentration. On the one hand, high concentrations can inhibit cell growth and induce apoptosis in tumor cells [4, 26]. On the other hand, NO can stimulate cell growth, dilate the malignant vessels so as to maintain the blood supply to the tumor and be important for tumor angiogenesis in low concentrations [2, 7, 13, 26]. Further studies show that NO controls angiogenesis by modulating the activity of angiogenic factors released by tumor cells, such as vascular endothelial growth factor , which requires a functioning NO/cyclic guanosine monophsophate pathway within the endothelial compartment to promote neovascular growth, and plays a central role in the angiogenic cascade [7, 10]. Thomsen and co-workers analyzed the activity of nitric oxide synthase in human gynaecological cancer and found that the NOS activity in vivo was at least 1–2 orders of magnitude lower than the enzyme activity associated with phenomena such as cytotoxicity and apoptosis [22]. That is to say, the concentration of NO in vivo is much lower than the level of anti-tumor effects and contributes to pro-tumor activity. Jenkins et al. drew the same conclusion by evaluating the NOS activity in genetically engineered, xenografted tumors in nude mice. They transfected murine macrophage iNOS cDNA into a human adenocarcinoma cell line and these cells, which were engineered to continuously produce NO, grew more slowly than wild-type parental cells in vitro while the tumors in nude mice from genetically engineered NO-producing cells grew faster and were more vascularized than the tumors derived from wild-type cells [10]. There are several other reports in which a positive correlation between NOS activity and tumor grade was observed. The fact that the administration of a highly selective inhibitor of iNOS limited the invasion and growth rate of iNOS-transfected tumor cell lines, as well as of other tumors expressing this isoform, is also consistent with this finding [1, 14, 15, 19, 22, 23]. Nevertheless, neither the actual local concentration in the tissue of bladder carcinoma nor the correlation between iNOS immunoreactivity and tumor grade in bladder carcinoma could be verified from our study. However, we found a significant correlation between the iNOS expression and MVD in bladder carcinoma tissue, suggesting that the NOS positive tissue was markedly more vascularized. In addition, the findings of Jansson et al. suggest that the mean NO concentration in the tissue of bladder cancer in BCG-treated patients was 30 times higher than in control subjects, which resulted in significant anti-tumor effects [9]. All of these results suggest that the concentration of NO in bladder carcinoma is low and has a pro-tumor effect.
In spite of our encouraging findings, there were several different or conflicting results obtained in other studies in which a pronounced reduction in NOS activity was found in several tumor cells relative to normal tissue [3, 18]. Moreover, in another investigation of bladder cancer, Mitropoulos et al. noticed an absence of iNOS staining in 32 of the 36 bladder tumors studied [16]. We postulate that one reason for this is due to the limitations of the current experimental techniques. In most cases, assays for iNOS were preformed by means of immunocytochemistry and the results were determined by histopathological criteria, the type of antibody and other factors. Most importantly, the high inhomogeneity of iNOS expression in different tumor cells may be the result of a selection process, which reflects the diverse biological behavior of tumors. In this regard, the extent of iNOS expression in carcinoma may be viewed as a key characteristic of the type of tumor present.
In addition, we suggest that not only iNOS but also eNOS is relevant for tumor neovasculature, because we found a distinct immunoreactivity for eNOS in the endothelial cells of bladder tumor vessels. The study of Klotz et al. also showed a highly positive eNOS immunostaining in the endothelium of vessels in bladder carcinomas [12]. These findings support the point of view put forward by Geller and Billar that eNOS may be induced in pathological conditions even though it is usually regarded as being constitutively expressed [8].
In conclusion, we demonstrated the presence of NOS expression in human bladder cancer and showed that it markedly favors the angiogenesis of TCC. The results of the present study suggest a lower concentration of NO in bladder tumor, which may have a pro-tumor effect. This opens the possibility of increasing or decreasing the NOS activities in human carcinoma as a possible anti-tumor therapy. BCG intravesical therapy provides a excellent example, which could markedly enhance the activity of NOS with large amounts of NO being formed in the bladder in during treatment, resulting in strong inhibitory effects on the growth of bladder cancer cells [9]. Furthermore, the selective iNOS inhibitor may also be used to block the generation of NO, with therapeutic effects, because of its specific expression in bladder cancer.
References
Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Oser SM, Klein S, Shields PG, Billiar TR, Harris CC (1998) Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res 58: 334
Andrade SP, Hart ID, Piper PJ (1992) Inhibitors of nitric oxide synthase selectively reduce flow in tumor associated neovasculature. Br J Pharmacol 107: 1092
Chhatwal VJ, Ngoi SS, Chan ST, Chia YW, Moochhala SM (1994) Aberrant expression of nitric oxide synthase in human polyps, neoplastic colonic mucosa and surrounding peritumoral normal mucosa. Carcinogenesis 15: 2081
Edwards P, Cendan JC, Topping DB, Moldawer LL, MacKay S, Copeland EMIII, Lind DS (1996) Tumor cell nitric oxide inhibits cell growth in vitro, but stimulates tumorigenesis and experimental lung metastasis in vivo. J Surg Res 63: 49
Forstermann U, Kleinert H (1995) Nitric oxide synthase expression and expressional control of three isoforms. Arch Pharmacol 352: 351
Fujimoto H, Ando Y, Yamashita T (1997) Nitric oxide synthase activity in human lung cancer. Jpn J Cancer Res 88: 1190
Gallo O, Masini E, Morbidelli L, Franchi A, Fini-Storchi I, Vergari WA, Ziche M (1998) Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J Natl Cancer Inst 90: 587
Geller DA, Billiar TR (1998) Molecular biology of nitric oxide synthases Cancer Metastasis Rev 17: 7
Jansson OT, Morcos E, Brundin L, Lundberg JON, Adolfsson J, Soderhall M, Wiklund NP (1998) The role of nitric oxide in bacillus Calmette-Guerin mediated anti-tumor effects in human bladder cancer. Br J Cancer 78: 588
Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S (1995) Roles of nitric in tumor growth. Proc Natl Acad Sci U S A 92: 4392
Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K (1998) Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer 82: 1897
Klotz T, Bloch W, Jacobs G, Niggemann S, Engelmann U, Addicks K (1999) Immunolocalization of inducible and constitutive nitric oxide synthases in human bladder cancer. Urology 54: 416
Leibovich SJ, Polverini PJ, Fong TW, Harbow LA, Koch AE (1994) Production of angiogenetic activity by human monocytes requires an L-arginine/nitric oxide-synthase-dependent effector mechanism. Proc Natl Acad Sci U S A 91: 4190
Macdonald JE (1996) Nitric oxide synthase inhibitors. Ann Rep Med Chem 23: 221
Mayer B, Andrew P (1998) Nitric oxide synthases catalytic function and progress towards selective inhibition. Arch Pharmacol 358: 127
Mitropoulis D, Petris D, Kiroudi A, Kyriakou G, Rasdan A, Zervas A, Dimopoulos C. (2000) The effect of intravesical Bacillus Calmette-Guerin (BCG) instillations on the expression of inducible nitric oxide synthase (iNOS) in humans. Eur Urol 2000 38: 516
Moncada S, Higgs A (1993) The L-arginine nitric oxide pathway. N Engl J Med 329: 2002
Moochala S, Chhatwal VJS, Ngoi SS, Chia YW, Rauff A (1996) Nitric synthase activity and expression in human colorectal cancer. Carcinogenesis 17: 1171
Pitzele BS (1997) The modulation of the action of isoforms of nitric oxide synthase. Expert Opin Ther Patients 7: 717
Shochina M, Fellig Y, Sughayer M, Pizov G, Vitner K, Podeh D, Hochberg A, Ariel I (2001) Nitric oxide synthase immunoreactivity in human bladder carcinoma. J Clin Pathol 54: 248
Swana HS, Smith SD, Perrotta PL, Saito N, Wheeler MA, Weiss RM (1999) Inducible nitric oxide synthase with transitional cell carcinoma of the bladder. J Urol 161: 630
Thomsen LL, Lawton FG, Knowles RG, Beesley JE, Riverros-Moreno V, Moncada S (1994) Nitric oxide synthase activity in human gynecological cancer. Cancer Res 54: 1352
Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S (1995) Nitric oxide synthase activity in human breast cancer. Br J Cancer 72: 41
Weidner N, Semple J, Welch W (1991) Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med 324: 1
Wolf H, Haeckel C, Roessner A (2000) Inducible nitric oxide synthase expression in human urinary bladder cancer. Virchows Arch 437: 662
Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R (1997) Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 99: 2625
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, Z., Chen, S., Ye, C. et al. Nitric oxide synthase expression in human bladder cancer and its relation to angiogenesis. Urol Res 31, 232–235 (2003). https://doi.org/10.1007/s00240-003-0302-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-003-0302-9