Abstract
The effect of aging on functional, biochemical, anatomical and molecular properties of endothelin (ET) receptors in bladder smooth muscle of the 3-week-, 3-month- and 22-month-old rats was examined using isolated muscle bath techniques, radioligand binding on membrane particulates and slide mounted tissue sections, and real-time reverse transcription polymerase chain reaction (RT-PCR). ET-1 induced significantly larger contractile responses in bladder dome muscle strips from 3-week- than from 3-month- and 22-month-old rats. The expression level of total ET receptors, determined by saturation binding experiments with [125I]ET-1, was higher in detrusor from 3-week- than 22-month-old rats. Inhibition studies with BQ123, a selective ETA receptor antagonist, indicated the predominance of the ETA receptor subtype and a similar proportion of ETA to ETB receptor subtypes in the rat detrusor at all ages studied. Autoradiographic data support the age-dependent decrease in the density of ET receptors and also indicate that the ETA receptor subtype is primarily located in the smooth muscle layer, whereas the ETB receptor subtype is located in both the urothelial and smooth muscle layers. Determined by real-time RT-PCR, ET 1, ET-3, ECE-1 and ET receptor subtype (ETA and ETB) mRNAs were shown to be higher in bladders of 3-week- compared to 3-month- or 22-month-old rats. This study indicates age-dependent alterations in the ET receptor system at both gene transcript and protein levels in the Fischer rat detrusor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endothelins (ETs) are a family of peptides consisting of three isoforms, i.e., ET-1, ET-2 and ET-3 (Yanagisawa et al. 1988; Haynes and Webb 1998). ET-1, ET-2 and ET-3, which are encoded by separate genes (Miyauchi and Masaki 1999) mediate a variety of biological actions via two distinct G protein-coupled receptor subtypes, designated ETA and ETB (Haynes and Webb 1998) that have been cloned, sequenced and characterized (Masaki et al. 1992). In addition, an ETC receptor subtype also has been cloned (Karne et al. 1993), although its existence and function remains controversial (Schmitz-Spanke and Schipke 2000). The ETA receptor subtype has a higher affinity for ET-1 and ET-2 than ET-3, while the ETB receptor subtype binds all three ETs with equal affinity (Arai et al. 1990; Sakurai et al. 1990; Masaki et al. 1992). The ETC receptor subtype demonstrates a higher affinity for ET-3 than for ET-1 and ET-2 (Emori et al. 1990; Karne et al. 1993). ETs are generated from a two-step procedure beginning with proteolytic cleavage of a 212 amino acid precursor polypeptide (prepro-ETs) to a relatively inactive 38 amino acid precursor, big ETs. Big ETs are then catalyzed by an ET-converting enzyme (ECE) to their mature 21 amino acid active form (Opgenorth et al. 1992). ET-1 is the only vasoconstrictor known to be primarily produced by human endothelial cells, where it can be stored in secretory vesicles (Russell et al. 1998). The primary target of ET-1 is the vasculature, where it mediates transient vasodilatation via endothelial ETB receptors, followed by a slow onset and sustained contraction mediated by either or both ETA and ETB receptor subtypes located on vascular smooth muscle cells (Rubanyi and Polokoff 1994; Haynes and Webb 1998; Wada et al. 2000). The mitogenic effect of ET-1 on vascular smooth muscle as well as on fibroblasts, osteoblasts and glomerular mesangial cells suggests a diverse growth-promoting role for the peptide (Janakidevi et al. 1992; Rubanyi and Polokoff 1994; Goldie 1999). The functional responses to ET-1 vary in different tissues and are dependent on differences in the distribution and expression of the receptor subtypes. On isolated cardiac muscle, ET-1 induces contraction and exerts a positive inotropic effect (Miyauchi and Masaki 1999). ET-1 also regulates salt and water homeostasis through actions in the kidney, stimulates the renin-angiotensin and sympathetic nervous system, and contracts pulmonary airway smooth muscle (Haynes and Webb 1998). Several agents can regulate the production of ET-1 including insulin, low-density lipoprotein, glucose and calcium ionophores (Rubanyi 1992). The induction of ET-1 mRNA and the rate of peptide release are stimulated by thrombin, angiotensin II, vasopressin, interleukin-1 and hypoxia (Kourembanas et al. 1991; Rubanyi 1992).
The mammalian urinary tract contains significant amounts of ET receptors (Bolger et al. 1990; Traish et al. 1992; Latifpour et al. 1995). ETs induce prolonged contractile responses in isolated rabbit (Garcia-Pascual et al. 1990; Traish et al. 1992), pig (Persson et al. 1992), rat (Donoso et al. 1994a), and human (Maggi et al. 1989) lower urinary tract smooth muscle. Autoradiography and radioligand binding studies have shown that ETA and ETB receptors are distributed in the urothelium and smooth muscle of human and rabbit bladder (Kondo and Morita 1993; Mumtaz et al. 1999; Wada et al. 2000) and their densities vary among different regions of the urinary bladder (Latifpour et al. 1995; Mumtaz et al. 1999). The predominant receptor subtype in the bladder is the ETA subtype while both ETA and ETB receptors are present in similar proportions in the urethra (Latifpour et al. 1995). These findings suggest that the function of ET-1 may vary depending on the region and distribution of its receptors in the bladder. Although the ET-1 induced long lasting contraction of smooth muscle strips of bladder dome has been shown to be mediated via the ETA receptor subtype (Donoso et al. 1994a; Mumtaz et al. 1999; Wada et al. 2000), the exact role of the ETB receptor in the bladder is not known (Sullivan et al. 2000). The ET-1-induced contraction in the rabbit bladder can be antagonized by nifedipine, a calcium channel blocker, indicating the calcium-dependent nature of these contractions (Garcia-Pascual et al. 1990).
In view of the importance of physiological and pathophysiological responses to ETs in the lower urinary tract, and of the presence of age- dependent changes in the properties of ET receptors in several organ systems (Cargnelli et al. 1990; Tomobe et al. 1991; Donoso et al. 1994b; White et al. 1997; Preuss et al. 2000), we examined the influence of age on the responsiveness of detrusor smooth muscle tissue to the ET receptor system.
Materials and methods
Animals and tissue preparation
The animals were handled according to the guidelines of the Institutional Animal Care and Use Committee of Yale University. Three-week- and 3-month-old Fischer 344 rats (31±3 and 284±6 g body weight, respectively) were obtained from Charles River Laboratories (Wilmington, MA, USA), and 22 month old (385±5 g body weight) Fischer 344 rats were obtained from the contract colonies of the National Institute on Aging (Harlan Sprague Dawley, Inc., Contractor, Indianapolis, IN, USA). Rats were sacrificed by decapitation, and their bladder were excised at the level of urethrovesical junction, trimmed of connective tissue and placed in ice cold Krebs-Henseleit (K-H) solution of the following composition: NaCl 118 mM, KCl 4.7 mM, CaCl2 2.5 mM, MgSO4 1.2 mM, NaHCO3 24.9 mM, KH2PO4 1.2 mM, D-glucose 5.6 mM, Na-pyruvate 2.0 mM), pH 7.4 at 37°C. The bladder was opened longitudinally by a posterior incision and the bladder dome was separated from the bladder base at the level of the ureteral orifices. Using razor blades mounted 1.5 mm apart on an aluminum block, uniform longitudinal strips of the anterior wall of bladder dome were cut and trimmed from connective tissues. For light microscopic autoradiography studies, the intact whole bladder was embedded in Histoprep (Fisher Scientific, Fair Lawn, NJ, USA), frozen on dry ice, and stored at −80°C until studied. For biochemical and molecular assays, the dissected bladder domes, were frozen in liquid nitrogen and stored at −80°C until studied.
Functional studies
Functional studies were performed as previously described (Wada et al. 2000). Each tissue strip was suspended in a 3.5 ml organ bath, filled with K-H solution (pH 7.4 at 37°C), which was continuously gassed with a 95% O2 and 5% CO2 mixture. One end of a tissue strip was fastened to the bottom of an organ bath with a 4-0 silk thread, and the other end was connected to an isometric transducer (Radnoti Glass Technology, Inc., Monrovia, CA, USA). The contractile data were recorded and analyzed with a Mac Lab/8e system (AD Instruments, Milford, MA, USA), which is an integrated system of software and hardware designed to monitor, record and analyze experimental data. The optimal resting stress was determined as the resting force at which the maximum force per cross-sectional area was generated in K-H solution containing 80 mM KCl. The 80 mM KCl solution was made by equimolar replacement of NaCl by KCl in the K-H solution. The strips of bladder dome developed maximal contractile forces in the range of 2.0–2.5 g. Each strip was stretched until the length of optimal stress development and then the strip was allowed to equilibrate for 90 min. The responses to 80 mM KCl were assessed two or three times at 60-min intervals until the responses were reproducible. Concentration-response curves to ET-1 were constructed in a cumulative fashion and in a stepwise manner, after the response to the previous concentration had reached a plateau. At the end of the each study, the length and wet weight of the preparations were measured.
Radioligand receptor binding assay
Frozen bladder dome was thawed, minced and homogenized in 40 to 50 volumes of ice-cold homogenization buffer (pH 7.4), containing 20 mM HEPES, 100 mM NaCl, 3 mM EDTA, 1 mM EGTA and the following protease inhibitors: 0.1 mM phenylmethylsulfonyl fluoride (PMSF) and 10 µg/ml each of aprotinine, leupeptine, pepstatin A and soy bean trypsin inhibitor, with a Brinkman Polytron (Westbury, CT, USA) three times for 20 s each with 10-s intervals as previously described (Latifpour et al. 1995; Saito et al. 2000a, 2000b). The homogenate was centrifuged at 49,000×g for 15 min at 4°C. The supernatant was discarded, and the pellet was rehomogenized in the cold homogenization buffer, filtered through a metal sieve with a pore size of 250 μm and centrifuged under the same conditions as above. The final pellet was suspended in 20 volumes of the same buffer. In saturation experiments, the membrane suspensions were diluted in the incubation buffer (50 mM Tris-HCl, pH 7.4 containing 154 mM NaCl, 25 mM MnCl2, 1 mM EDTA, 1 mM N acetyl-DL-methionine, 0.25% BSA and 0.14% bacitracin). Aliquots of membrane preparations of the rat bladder dome were incubated for 2 h with increasing concentrations of [125I]ET-1 (6–240 pM) in a total volume of 100 μl at 23°C in the presence or absence of 100 nM unlabelled ET-1. At the end of the incubation period, the reaction mixtures were filtered rapidly under a vacuum through Whatman GF/B glass fiber filters, which had been previously treated with a 1% solution of BSA to reduce non specific binding to the filter papers, using a Brandel Cell Harvester (Model M-24R, Brandel Instruments, Gaithersburg, MD, USA). Each filter disc was washed intensively with 80 ml of ice-cold 50 mM Tris-HCl buffer (pH 8.0). The radioactivity on glass fiber discs was counted with a gamma counter (Packard Multi-Prias Analyzer) at an efficiency of 70–75%. Nonspecific binding was determined in the presence of 100 nM unlabelled ET-1. Specific binding was calculated by subtracting non-specific binding from total binding of radioligands to the glass fibers.
In inhibition binding studies, aliquots of membrane particulates were incubated with a fixed concentration of [125I]ET-1 (approximately 70 pM) in the presence of increasing concentrations of BQ123 (ETA selective antagonist) (Rubanyi and Polokoff 1994; Goto et al. 1996), in a total volume of 100 μl for 2 h at 23°C. The remainder of the procedure was the same as that for the saturation studies.
Light microscopic autoradiography
For autoradiographic studies, serial sections (10 µm thick) of frozen whole bladder from each group were cut, using a cryostat (2800 FRIGOCUT N, Leica, Pike Malvern, PA, USA) at −20°C and mounted on aminoalkylsilane-coated microscope slides. The sections initially were preincubated with 0.25 ml of an autoradiography buffer (20 mM HEPES containing 140 mM NaCl, 4 mM KCl, 1 mM KH2PO4, 1 mM MgCl2, 1 mM CaCl2, 10 mM D-glucose, 2.5% BSA and 0.01% bacitracin) for 30 min at 23°C in order to reduce the nonspecific binding of [125I]ET-1 to the rat bladder. After the preincubation period, additional volumes (0.25 ml) of the HEPES buffer containing 120 pM [125I]ET-1 were added to slide-mounted bladder sections to the total volume of 0.50 ml and incubated for 2 h at 23°C. To determine nonspecific binding, adjacent sections were incubated with the same concentration of [125I]ET-1 in the presence of 100 nM unlabelled ET-1. The sections were then washed twice in ice-cold 50 mM Tris-HCl buffer (pH 8.0) for 20 min, quickly dipped in distilled water, and dried in a stream of cold air. The tissue sections were placed in apposition with a high-resolution tritium sensitive film (Hyperfilm-3H, Amersham, Arlington, IL, USA) in standard X-ray cassettes for two days at 4°C to generate autoradiograms. Following the exposure, the film was developed in D19 (Kodak, Rochester, NY, USA), at 23°C for 5 min, fixed for 10 min in the Kodak normal fixer, and washed in tap water for 30 min. The labeled sections were either stained with hematoxylin (10 min) and eosin (2 min), dehydrated and coverslipped with Permount (Fisher Scientific, Fair Lawn, NJ, USA) or used for nuclear (liquid) emulsion autoradiography.
The localization of ETA and ETB receptor subtypes in the rat bladder was accomplished by incubating adjacent tissue sections with fixed [125I]ET-1 and appropriate concentrations of subtype selective compounds, i.e., 300 nM BQ123, and 30 nM Sarafotoxin (STXc). These concentrations of BQ123 and STXc, that are approximately 100 times greater than that of their high affinity constants, KiH (Latifpour et al. 1995; Saito et al. 1996) occupy virtually all of the ETA and ETB receptor subtypes, respectively, in the rat bladder sections.
Liquid emulsion light microscopic autoradiography
After radiolabeling of the slide mounted sections was accomplished, the labeled ligand was irreversibly linked to the receptor by fixation with paraformaldehyde vapor as described by Herkenham and Pert (1982). Briefly, labeled bladder sections were placed in an airtight chamber containing paraformaldehyde crystals and incubated at 80°C for 2 h. The sections then were dehydrated in ascending concentrations of alcohol, stained with eosin (2 min), placed in a series of alcohol and xylene washes, and dried. Under the safe light, the dried sections were dipped into LM-1 Hypercoat emulsion (Amersham Life Science, Inc., Arlington Heights, IL, USA), which had been melted at 43°C, and allowed to uniformly gel the emulsion. After drying, slides were placed in light tight boxes with desiccant and stored at 4°C for 4 days. Development was performed for 5 min in D19 (Kodak, Rochester, NY, USA), followed by a stop bath and fixation in Kodak normal fixer. The sections were then stained with hematoxylin for 10 min, cleared, dehydrated, and coverslipped with Permount (Fisher Scientific, Fair Lawn, NJ, USA). This technique in conjunction with film autoradiography permitted us to localize more accurately the distribution of ET receptor subtypes in the rat bladder of different ages.
Real-time reverse transcription polymerase chain reaction
The expression levels of ET-1, ET-3, ECE-1 and the ET receptor subtypes were quantified by a fluorescence-based real-time relative RT-PCR (real-time reverse transcription polymerase chain reaction) using SYBR Green I, a dsDNA-binding dye, as previously described (Yin et al. 2001; Pfaffl et al. 2002). In this technique all steps subsequent to the RT reaction are carried out by the thermal cycler/detector and quantitative results are processed by computer-assisted programs.
Total RNA was extracted from frozen tissues using TRIZOL (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. The concentration of the RNA was quantified by determination of optical density at 260 nm (OD260).
RT was performed as previously described (Saito et al. 2000a; Ikeda et al. 2001; Takahashi et al. 2002). The RT reaction mixture contained 5 µg of total RNA, 500 ng of oligo(dT) primer, 1× first strand buffer, 10 mM dithiothreitol, 0.5 mM of each deoxynucleotide 5'-triphosphate, and 200 units of a reverse transcriptase (Superscript II, Invitrogen, Carlsbad, CA, USA) in a total volume of 20 µl. The mixture was incubated at 42°C for 50 min, heated to 70°C for 15 min to denature the reverse transcriptase, and then cooled to 4°C. The resulting cDNA was stored at −20°C until assayed by real-time PCR.
The PCR standards were constructed from known numbers of molecules of purified PCR product, prepared as previously described (Bishop et al. 1997; Yin et al. 2001). The PCR product was purified by gel electrophoresis, the band of the correct molecular weight was excised and DNA was separated using QIAquick Gel Extraction Kit (Qiagen, Valencia, CA, USA). The sequence of the purified PCR product was determined by the fluorescent dideoxynucleotide chain termination method with PCR primer and with Taq FS DNA polymerase using an automated sequencer (W.M. Keck Biotechnology Resource Laboratory, Yale University, New Haven, CT, USA). The concentration of the purified PCR product DNA was determined by OD260 and the copy number of standard were calculated using the following equation:
where C=5×10−5 g/ml for DNA and MWt = molecular weight of PCR product (base pairs × 6.58×102 g).
Standards were made to a concentration of 1010 copies/µl and stored at −20°C until used. Standards of ET-1, ET-3, ECE-1, and ETA and ETB receptor mRNAs were made using vas deferens from 3-month-old rats and used for the normalization of all tissues examined.
Sequences of oligonucleotides used as primers for ET-1, ET-3, ECE-1, and ETA and ETB receptor subtypes are summarized in Table 1. Primers for ß-actin with a product size of 294 bp against which the other mRNAs were normalized were purchased from Ambion (Austin, TX, USA).
Real-time PCR was performed in a 96-well 0.2-ml Thin-Wall PCR Plate using the iCycler Thermal Cycler (Bio-Rad, Hercules, CA, USA) and carried out with QuantiTect SYBR Green PCR Master Mix (Qiagen, Valencia, CA, USA), which contained HotStarTaq DNA Polymerase, QuantiTect SYBR Green PCR Buffer and SYBR Green I. The real-time PCR reaction mixture contained 1× QuantiTect SYBR Green PCR Master Mix, 0.3 µM primer pairs and 1 µl cDNA in a total volume of 25 µl. The mixture was heated initially at 95°C for 15 min in order to activate HotStarTaq DNA Polymerase, and then followed by 40 cycles with denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 60 s. Each assay for unknown samples was performed simultaneously with standard samples and negative control samples (non-RT samples) all in duplicate wells in the same plate.
As SYBR Green I is not specific for the PCR product, an optimum temperature for analysis of specific product, i.e., transcript of interest, was determined in order to avoid nonspecific primer-dimer formation as previously described (Morrison et al. 1998). After adjustments had been made, melt curve protocols designed for increment temperatures of 1°C (starting at 60°C and ending at 90°C) was repeated to ensure that primer-dimers and other non-specific products had been minimized or eliminated.
Data analysis
The contractile data were analyzed as previously described (Latifpour et al. 1992). The contractile responses to ET-1 are expressed in terms of active force in g/ mm2 of cross sectional area, using the equation of cross sectional area = weight/ (length × 1.05), where 1.05 is the assumed density of the muscle, and as percent of tension generated with 80 mM KCl. Emax (the maximum contractile response) and the ED50 value (the concentration of an agonist that produces 50% of the maximal contractile response) were calculated with an iterative non-linear least square curve-fitting program using GraphPad Prism program (GraphPad Software, Inc., San Diego, CA, USA).
Receptor binding data were analyzed as previously reported (Latifpour et al. 1995). Saturation data were analyzed using computer-assisted linear regression of bound/free versus bound in order to calculate the maximum number of binding sites, Bmax values and equilibrium dissociation constants, Kd values. Inhibition data were analyzed by an iterative non-linear least square curve-fitting procedure on the basis of a one or a two binding site model using GraphPad Prism program (GraphPad Software, Inc., San Diego, CA, USA).
The autoradiograms and stained bladder sections were examined and analyzed using computer assisted image processing and analysis programs (NIH Image, Bethesda, MD; Adobe Photoshop, San Jose, CA, USA).
For RT-PCR data, standard curves constructed from serial dilution of a known number of PCR product molecules were analyzed with the iCycler iQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The baseline was set automatically by the software using data collected from cycles 2 to 10, and the threshold cycle (Ct) at which the amplification plot crossed the baseline was calculated. Quantification of the samples was accomplished from the Ct by interpolation from the standard curve to yield a copy number of each cDNA using a computer-assisted program. The raw abundance values for ET-1, ET-3, ECE-1, and ETA and ETB receptor subtypes were divided by the ß-actin values obtained from the same samples to derive a normalized value for each sample. Furthermore, the number of amplified products was identified by melt curve analysis. As the melting temperature (Tm) was reached, the DNA denatured and released SYBR Green I, causing a sharp decline in fluorescence. This decrease in fluorescence was plotted as Fluorescence vs. Temperature. Plotting the negative first derivative of these data versus the temperature change (−dF/dT vs. Temperature) resulted in a melting peak and Tm for each amplified product (Lekanne Deprez et al. 2002).
Statistical analysis
Statistical analyses between groups were performed using analysis of variance and multiple comparison Fisher's test. p<0.05 was regarded as the level of significance.
Chemicals
[125I]ET-1 (2200 Ci/mmol) was purchased from PerkinElmer (Boston, MA, USA). ET-1, BQ123 and sarafotoxin S6c (STXc) were obtained from Peninsula Laboratory Inc. (Belmont, CA, USA). PMSF, aprotinine, leupeptine, pepstatin A, N-acetyl-DL-methionine, soy bean trypsin inhibitor, BSA and bacitracin were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
Results
Functional studies
Contractile responses to ET-1 in the bladder in 3-week-, 3-month- and 22-month-old rat are shown in Fig. 1 and Table 2. ET-1 (10−10–3×10−7 M) produced concentration dependent contractile responses in the rat detrusor muscle strips of all age groups. The contractile responses to exogenous ET-1 decreased with aging, and the maximal contractile responses (Emax values), expressed in terms of active force per cross sectional area in mm2 and/or expressed as percentage of the contractile response to 80 mM KCl, showed a rank order of: 3 weeks >3 months >22 months (Fig. 1, Table 2). Although there were significant differences in the Emax values among the three age groups there were no significant differences in the ED50 values of ET-1 among these groups (Table 2). The maximal contractile responses to 80 mM KCl, expressed in terms of active force per cross sectional area in mm2, were 5.04±0.25, 4.91±0.33 and 4.71±0.25 g/mm2 in the bladder of 3-week-, 3-month- and 22-month-old rats, respectively, indicating that there were no significant differences in the maximum response to KCl among the groups.
Characteristics of [125l]ET-1 binding
The binding assay data revealed the presence of a single class of specific, high affinity binding sites of [125I]ET-1 to membrane preparations from the rat bladder. The maximum number of binding sites (Bmax values) and the equilibrium dissociation constant (Kd) values for [125I]ET-1 binding to the bladder are shown in Table 3. The density of [125I]ET-1 binding sites was significantly higher in 3-week- than 22-month-old rat bladder. Kd values for [125I]ET-1 binding were not significantly different among the three groups studied. In order to study the subtype specificity of ET receptors in the rat detrusor smooth muscle, inhibition binding studies were performed in which [125I]ET-1 binding was inhibited by BQ123 (selective ETA antagonist). Inhibition curves with BQ123 display biphasic patterns for all three age groups studied. According to analysis with a nonlinear curve-fitting program, the data for BQ123 are significantly better fitted to a two-site model than to a one-site model (Table 4). The ETA selective compound inhibited approximately 3/4 of total [125I]ET-1 binding sites in the bladder from the three experimental groups indicating the predominance of the ETA receptor subtype in these tissues. There were no significant differences in inhibition constants, i.e., high and low affinity constants, KiH and KiL values, respectively, and in the proportion of ET receptor subtypes between the groups studied.
Autographic data
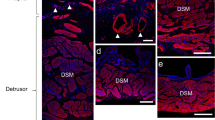
Autoradiograms obtained from the binding of [125I]ET-1 to the bladder smooth muscle demonstrated the specificity, saturability and distribution of ET receptors in the rat bladder of different ages (Figs. 2, 3). The addition of an excess of unlabeled ET-1 completely eliminated the [125I]ET-1 binding to the section indicating that all radioligand binding observed was to specific binding sites. To localize ETA and ETB receptors in the rat detrusor smooth muscle in three different ages, we incubated the tissue sections with a fixed concentration of [125I]ET-1 in the presence of 30 nM STX6c (selective ETB receptor agonist) or 300 nM BQ123 (selective ETA receptor antagonist), respectively. The data indicated that both ET receptor subtypes were present in the rat bladder with a higher density of ETA in 3-week-old rat compared to 22-month-old rat. The subtype specificity and the detailed localization of ET receptor subtypes are shown in images obtained from liquid emulsion autoradiography. The ETA receptor subtype is primarily located only in the smooth muscle layer, whereas the ETB receptor subtype is located in both urothelial and smooth muscle layers (Fig. 3).
Autoradiograms of [125I]ET-1 binding and hematoxylin-eosin staining in adjacent sections of rat bladder in three age groups. Autoradiograms are presented as computer generated pseudocolor images. The color key demonstrates the color coding spectrum from the highest (red) to the lowest (black) concentration of [125I]ET-1 binding to bladder sections. Image A demonstrates the levels of total binding obtained with 120 pM [125I]ET-1. Image B demonstrates the corresponding non-specific binding obtained in the presence of 100 nM unlabelled ET-1. The distribution of ETA and ETB receptors are demonstrated in images C and D obtained with 60 pM [125I]ET-1 binding in the presence of 30 nM Sarafotoxin S6c and 300 nM BQ 123, respectively. Image E shows H&E staining of an adjacent bladder section
Liquid emulsion autoradiographic images of [125I]ET-1 binding and hematoxylin-eosin staining in adjacent sections from rat bladder. A and B show the levels of total binding with 120 pM [125I]ET-1 and the corresponding non-specific binding in the rat bladder, respectively. C and D show the levels of a same amounts of [125I]ET-1 binding in the presence of 30 nM STXc and 300 nM BQ 123, showing the expression of ETA and ETB receptor subtypes, respectively. E shows hematoxylin-eosin staining of an adjacent bladder section (magnification, ×400). EPC and SMC indicate the epithelial and smooth muscle areas of the bladder, respectively
Real-time reverse transcription polymerase chain reaction
Amplification plots of ETA receptor subtype cDNA, made from total RNA, in the detrusor of three age groups of rats shown in Fig. 4A, were constructed from the fluorescence emission data collected during the PCR amplification. In the negative control studies, all samples without RT showed no significant elevation of fluorescence emission during PCR. Similarly, amplification plots for ß-actin, ET-1, ET-3, ECE-1 and the ETB receptor subtype were obtained (data not shown). Specificity of the desired products in the tissues was confirmed by melt curve analysis as well as by purification. A single peak was identified in the melt curve for ETA receptor subtype cDNA at a Tm of 82°C (Fig. 4B). Similarly, a single peak was identified in the melt curve for ß-actin, ET-1, ET-3, ECE-1 and the ETB receptor subtype at a Tm of 88, 86, 87, 88 and 87°C, respectively (data not shown). PCR products were purified, sequenced and confirmed to be the rat ß-actin, ET-1, ET-3, ECE-1, and ETA and ETB receptor subtypes.
Amplification plots, B the melt curve, and C the standard curve, for ETA receptor subtype mRNA in the rat bladder. Unknown samples obtained from three age groups were measured simultaneously with standard and negative control samples. RFU is the relative fluorescent unit. The horizontal orange line in panel A is the base line from which the Ct for each sample (other colors) was calculated. Each color in panels A and B represents a sample either from an unknown or standard. The negative control data points in the panel B are shown with arrows (for detail see the Methods)
Quantification of the unknown samples was achieved from the Ct by interpolation from the standard curve to calculate a copy number for ETA receptor subtype cDNA (Fig. 4C). Copy numbers for ß-actin, ET-1, ET-3, ECE-1 and ETB receptor subtype were obtained from Ct-copy number plots in a similar manner (data not shown). A linear relationship was observed between the initial copy numbers and the Ct values for each standard curve over six log10 dilutions. Regression analysis of the Ct values generated by the log10 dilution series produced R2 values for each standard curve in an excess of >0.99.
The relative expression levels of ET-1 and ET-3, ECE-1, and ETA and ETB receptor subtype mRNAs in the rat bladder are shown in Fig. 5. It is shown that ET-1 and ET-3 mRNA values were significantly higher in the 3-week-old bladder than the other age groups and that the ET-1 mRNA expression level was significantly higher than ET-3 mRNA expression in all tissues studied (Fig. 5A). Similar to ET-1 and ET-3 mRNAs, ECE-1 and ETA and ETB receptor subtype mRNAs were expressed in a significantly higher level in the 3-week-old bladder than in the other age groups (Fig. 5B, C). Furthermore, ETA mRNA level was expressed in a greater level than that of ETB receptor subtype in all three age groups. The mRNAs of various components of the ET system are expressed in the rat bladder of all ages in the following rank order: ECE-1 >ETA >ETB > ET-1>>ET-3.
Expression of A ET-1, ET-3, B ECE-1, and C ETA and ETB receptor mRNAs in the rat bladder. Relative expression levels of ET mRNAs are normalized against ß-actin. Each bar represents the mean ± SEM of the results from five to seven rats. a Significantly different from ET-1 in the same age group; b Significantly different from the value in 3-week-old rat; c Significantly different from ETA in the same age group
Discussion
As a decrease in ET responsiveness with aging has been reported in the cardiovascular systems of man and experimental animals (Cargnelli et al. 1990; Donoso et al. 1994b; White et al. 1997; Preuss et al. 2000), the impact of age in modulating the responses of other tissues to ET-1 has received attention. In perfused rat mesenteric arteries (Dohi and Luscher 1990) and isolated ventricular preparations (Ishikawa et al. 1991) the sensitivity to ET-1 has been shown to be lower in old than in young rats. These decreases in the responsiveness of vasculatures to ET-1 has been attributed to a down-regulation of the ET receptors, alterations in the composition of the ET receptor subtypes and/or impaired ET-1 mediated signal transduction systems in the aging blood vessels. The impact of alteration and/or involvement of different ET receptor subtypes in the age-dependent changes in the response to ET-1 have been more clearly demonstrated in the rat duodenum in which ETA and ETB receptor subtypes were shown to mediate the contractile responses in adult and neonatal tissues, respectively (Irie et al. 1995). Similarly ET-1 was shown to be more potent in mediating contractions, in airway smooth muscle from immature than from adult rabbits (Grunstein et al. 1991). The contractile responses in the airway tissues were blocked to a greater extent by voltage dependent Ca2+ channel blockers in the younger than in the older animals, and this was suggested to be associated with a significantly enhanced ET-1 stimulated accumulation of inositol 1,4,5-trisphosphate in the immature tissues. Kung and Luscher (1995) demonstrated that aging significantly reduced the contraction of rat aorta to ET-1 as well as to KCl, and concluded that as KCl is a receptor-independent agonist, the reduced contractile responsiveness to ET-1 may reflect a decreased effectiveness of the contractile machinery with aging.
In the present study we demonstrated that there is an age-related decrease in the ET-induced contraction responses of bladder muscle strips. Although the maximum contractile responses (Emax) to ET-1 were significantly higher in 3-week-old rat bladder than in the other age groups, there were no significant differences in the potency of ET-1, i.e., ED50 values, among these groups. As the magnitude of the maximum contractile responses to 80 mM KCl were shown to be similar in bladder muscle strips of all three ages, the age-dependent decrease in contractile responses to ET-1 can be due to a change in characteristics of the ET receptor/effector system in the rat bladder. This is in accord with a study in rat thoracic aorta in which aging attenuated contractile responses to ET-1 but not to KCl and norepinephrine (Ishihata et al. 1991) and with our previous study in the rabbit urinary tract in which the responses to ET-1 were shown to be greater in bladder dome and trigone of 6-week- than 6-month-old rabbits (Wada et al. 2000).
Similar to the functional studies, our saturation experiments with [125I]ET-1 demonstrated that the density of ET receptors (Bmax/g tissue, Bmax/mg protein) decreased with age, although the Kd values for radioligand in the three age groups were not different. This may indicate a quantitative rather than a qualitative change in the binding characteristics of [125 I]ET-1 in the rat detrusor with age. The alterations in the ET receptor binding properties in the rat detrusor are different from those in the cardiac system in which both the affinity of ET-1 in mediating the contractile response and the expression level of [125I]ET-1 binding were decreased in the adult compared with the neonate in the atria and in the ventricle of rats (Ishikawa et al. 1991). Furthermore, unlike the rat heart (Ishikawa et al. 1991) and porcine extra pulmonary and bronchial arteries (Hislop et al. 1995), in which ET receptor subtype specificity, i.e., the proportion of ETA to ETB was shown to be different between neonatal and adult tissues, the proportion of ET receptor subtypes are similar in the detrusor of the three different age groups. Thus, the observed age-dependent decrease in the response to ET-1 can be mainly attributed to the down-regulation of ET receptor density in the rat bladder. However, the impact of other factors such as age related changes in the parameters that may influence the detrusor contraction cannot be ruled out. For example, Pagala et al. 2001 suggested that age-dependent changes in the contractile responses of different regions of detrusor muscle might be due to changes in a variety of parameters including muscle orientation, receptor density, collagen content and depolarization of the muscle preparations.
It also has been shown that that aging significantly increases circulating ET-1 levels in human (Miyauchi et al. 1992; White et aI. 1997), suggesting that the observed decrease in sensitivity to ET-1 with age may be an adaptation to the circulating level of the peptide. Hirata et al. (1988) demonstrated that pretreatment of cultured rat vascular smooth muscle with ET-1 resulted in a marked decrease in ET binding capacity without changing its binding affinity. These studies imply that increasing levels of ET-1 may cause a decrease in the expression of ET receptors. Although we did not assess the production of ET-1 at the protein level in the present study, the increasing serum ET levels with aging may be one of the reasons for the decrease in ET receptor density observed in the present study with aging.
Our autoradiographic studies are in accord with the results obtained in receptor binding experiments using membrane particulates in which an age dependent decrease in the density of ET receptors was demonstrated. The distribution of ET receptor subtypes, i.e., localization of the ETA subtype on the smooth muscle layer, and the ETB receptor subtype on both urothelial and smooth muscle layers in all groups also are similar to our previous data reported for the rabbit bladder (Wada et al. 2000).
Gene expression levels of ETA and ETB receptor subtypes, investigated by real-time relative RT-PCR are in agreement with those of receptor density at the protein level as determined by the receptor binding studies, i.e., the expression levels of ETA and ETB receptors at both protein and mRNA levels in the bladders from 3-week-old rats are higher than those from 22-month-old rats. We have previously reported a similar a positive correlation in the diabetes-induced changes of the ET receptors and their mRNA levels in the rat genitourinary tract (Saito et al. 2000a, 2000b). In these studies the induction of experimental diabetes increased the expression of ET receptors at the protein level, determined by radioligand binding assays, and their mRNAs, as quantified by conventional relative RT-PCR. The upregulation of the ET receptors at the protein and gene transcript levels were reversed and/or normalized by insulin treatment (Saito et al. 2000a, 2000b). As a very small amount of tissue is needed for mRNA quantification, the positive correlation between the ET receptors and their mRNA may prove to be very useful when limited amounts of sample are available. The fact that mRNA levels for ET-1, ET-3 and ECE-1 are lower in 22-month- than in 3-week-old rats may indicate a generalized down regulation in the ET system in the rat detrusor with age. It is to be noted that the development of real-time RT-PCR has offered not only the possibility of quantification of low levels of some species of mRNA, such as ET-3, but has made it possible to determine relative expression levels of ET-1, ET-3, ECE-1, and ETA and ETB receptor mRNA within a tissue.
Conclusion
The present study demonstrates the presence of significant age-related changes in the properties of ET receptors at the gene transcript and protein levels and that these changes correlate with changes in the contractile response to ET-1 in rat isolated detrusor smooth muscle.
References
Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S (1990) Cloning and expression of cDNA encoding receptor. Nature 348:730–732
Bishop GA, Rokahr KL, Lowes M, McGuinness PH, Napoli J, DeCruz DJ, Wong WY, McCaughan GW (1997) Quantitative reverse transcriptase-PCR amplification of cytokine mRNA in liver biopsy specimens using a non-competitive method. Immunol Cell Biol 75:142–147
Bolger GT, Liard F, Krogsrud R, Thibeault D, Jaramillo J (1990) Tissue specificity of endothelin binding sites. J Cardiovasc Pharmacol 16:367–375
Cargnelli G, Rossi G, Bova S, Pessina AC (1990) In vitro vascular reactivity to endothelin: a comparison between young and old normotensive and hypertensive rats. Clin Exp Hypertens 12:1437–1451
Dohi Y, Luscher TF (1990) Aging differentially affects direct and indirect actions of endothelin-1 in perfused mesenteric arteries of the rat. Br J Pharmacol 100:889–893
Donoso MV, Salas G, Sepulveda J, Fournier LA, Huidobro-Toro JP (1994a) Involvement of ETA receptors in the facilitation by endothelin-1 of non-adrenergic, non-cholinergic transmission in the rat urinary bladder. Br J Pharmacol 111:473–482
Donoso MV, Fournier A, Peschke H, Faundez H, Domenech R, Huidobro-Toro JP (1994b) Aging differentially modifies arterial sensitivity to endothelin-1 and 5-hydroxytryptamine: studies in dog coronary arteries and rat arterial mesenteric bed. Peptides 15:1489–1495
Emori T, Hirata Y, Marumo F (1990) Specific receptors for endothelin-3 in cultured bovine endothelial cells and its cellular mechanism of action. FEBS Lett 263:261–264
Garcia-Pascual A, Larsson B, Andersson K-E (1990) Contractile effects of endothelin-1 and localization of endothelin binding sites in rabbit lower urinary tract smooth muscle. Acta Physiol Scand 140:545–555
Goldie RG (1999) Endothelins in health and disease: an overview. Clin Exp Pharmacol Physiol 26:145–148
Goto K, Hama H, Kasoya Y (1996) Molecular and pathophysiological significance of endothelin. Jpn J Pharmacol 72:261–290
Grunstein MM, Rosenberg SM, Schramm CM, Pawlowski NA (1991) Mechanisms of action of endothelin 1 in maturing rabbit airway smooth muscle. Am Physiol 260:L434–L443
Haynes WG, Webb DJ (1998) Endothelin as a regulator of cardiovascular function in health and disease. J Hypertens 16:1081–1098
Herkenham M, Pert CB (1982) Light microscopic localization of brain opiate receptors: a general autoradiographic method, which preserves tissue quality. J Neurosci 2:1129–1149
Hirata Y, Yoshimi H, Takaichi S, Yanagisawa M, Masaki T (1988) Binding and receptor down-regulation of a novel vasoconstrictor endothelin in cultured rat vascular smooth muscle cells. FEBS Lett 239:13–17
Hislop AA, Zhao YD, Springall DR, Polak JM, Haworth SG (1995) Postnatal changes in endothelin-1 binding in porcine pulmonary vessels and airways. Am J Respir Cell Mol Biol 12:557–566
Ikeda K, Wada Y, Sanematsu H, Foster HE Jr, Shin D, Weiss RM, Latifpour J (2001) Regulatory effect of experimental diabetes on the expression of endothelin receptor subtypes and their gene transcripts in the rat adrenal gland. J Endocrinol 168:163–175
Irie K, Uchida Y, Fujii E, Muraki T (1995) Developmental changes in response to endothelins and receptor subtypes of the isolated rat duodenum. Eur J Pharmacol 275:45–51
Ishihata A, Katano Y, Morinobu S, Endoh M (1991) Influence of aging on the contractile response to endothelin of rat thoracic aorta. Eur J Pharmacol 200:199–201
Ishikawa T, Li L, Shinmi O, Kimura S, Yanagisawa M, Goto K, Masaki T (1991) Characteristic of binding of endothelin-1 and endothelin-3 to rat hearts. Circ Res 69:918–926
Janakidevi K, Fisher MA, de Vacchio PJ, Tiruppathi C, Figge J, Malik AB (1992) Endothelin-1 stimulates DNA synthesis and proliferation of pulmonary artery smooth muscle. Am J Physiol 263:C1295–C1301
Karne S, Jayawickreme CK, Lerner MR (1993) Cloning and characterization of an endothelin-3 specific receptor (ET C receptor) from Xenopus laevis dermal melanophores. J Biol Chem 268:19126–19133
Kondo S, Morita T (1993) Effect of benign prostatic hypertrophy on endothelin-1 receptor density in human urinary bladder and prostate. Jpn J Urol 84:1821–1827
Kourembanas S, Marsden PA, McQuillan LP, Faller DV (1991) Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest 88:1054–1057
Kung C, Luscher TF (1995) Different mechanisms of endothelial dysfunction with aging and hypertension in rat aorta. Hypertension 25:194–200
Latifpour J, Yoshida M, Nishimoto T, Weiss RM (1992) Evidence for the presence of regional differences in the calcium antagonist receptors in lower urinary tract smooth muscle. Naunyn-Schmiedebergs Arch Pharmacol 354:679–687
Latifpour J, Fukumoto Y, Weiss RM (1995) Regional differences in the density and subtype specificity of endothelin receptors in rabbit urinary tract. Naunyn-Schmiedebergs Arch Pharmacol 352:459–468
Lekanne Deprez RH, Fijnvandraat AC, Ruijter JM, Moorman, AFM (2002) Sensitivity and accuracy of quantitative real-time polymerase chain reaction using SYBR green I depends on cDNA synthesis conditions. Anal Biochem 307:63–69
Lin HY, Kaji EH, Winkel GK, Ives HE, Lodish HF (1991) Cloning and functional expression of a vascular smooth muscle endothelin 1 receptor. Proc Natl Acad Sci USA 88:3185–3189
Maggi CA, Giuliani S, Patacchini R, Santicoli P, Turini D, Barbanti G, Meli A (1989) A potent contractile activity of endothelin on the human isolated urinary bladder. Br J Pharmacol 96:755–757
Masaki T, Yanagisawa M, Goto K (1992) Physiology and pharmacology of endothelin. Med Res Rev 12:391–421
Miyauchi T, Masaki T (1999) Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol 61:391–415
Miyauchi T, Yanagisawa M, Iida K, Ajisaka R, Suzuki N, Fujino M, Goto K, Masaki T, Sugishita Y (1992) Age- and sex-related variation of plasma endothelin-1 concentration in normal and hypertensive subjects. Am Heart J 123:1092–1093
Morrison TB, Weis JJ, Wittwer CT (1998) Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques 24:954–962
Mumtaz FH, Dashwood MR, Thompson CS, Sullivan ME, Mikhailidis DP, Morgan RJ (1999) Increased expression of endothelin-B receptors in the diabetic urinary bladder: functional relevance. BJU Int 83:113–122
Opgenorth T, Wu Wong JR, Shiosaki K (1992) Endothelin-converting enzymes. FASEB J 6:2653–2659
Pagala MK, Tetsoti L, Nagpal D, Wise GJ (2001) Aging effects on contractility of longitudinal and circular detrusor and trigone of rat bladder. J Urol 166:721–727
Persson K, Garcia-Pascual A, Holmquist F, Andersson KE (1992) Endothelin-1-induced contractions of isolated pig detrusor and vesical arterial smooth muscle: calcium dependence and phosphoinositide hydrolysis. Gen Pharmacol 23:445–453
Pfaffl MW, Georgieva TM, Georgiev IP, Ontsouka E, Hageleit M, Blum JW (2002) Real-time RT-PCR quantification of insulin-like growth factor (IGF)-1, IGF-1 receptor, IGF-2, IGF-2 receptor, insulin receptor, growth hormone receptor, IGF-binding proteins 1, 2 and 3 in the bovine species. Domest Anim Endocrinol 22:91–102
Preuss JM, Rigby PJ, Goldie RG (2000) Aging and epithelial integrity as modulators of airway smooth muscle responsiveness to endothelin-1. Naunyn-Schmiedebergs Arch Pharmacol 361:391–396
Rubanyi G (1992) Potential physiological and pathological significance of endothelin. Drugs Future 17:915–936
Rubanyi GM, Polokoff MA (1994) Endothelins: molecular biology, biochemistry, pharmacology, physiology and pathophysiology. Pharmacol Rev 46:325–415
Russell FD, Skepper JN, Davenport AP (1998) Endothelin peptide and converting enzymes in human endothelium. J Cardiovasc Pharmacol 31 [Suppl 1]:S19–S21
Saito M, Nishi K, Fukumoto Y, Weiss RM, Latifpour J (1996) Effect of experimental diabetes on rat prostate endothelin receptors. Eur J Pharmacol 310:197–200
Saito M, Wada Y, Ikeda K, Wang Z-J, Foster HE Jr, Smith SD, Weiss RM, Latifpour J (2000a) Expression of endothelin receptor subtypes and their messenger RNAs in diabetic rat prostate: effect of insulin treatment. Mol Cell Biochem 210:1–12
Saito M, Wada Y, Ikeda K, Wang Z, Smith SD, Foster HE Jr, Nishi K, Weiss RM, Latifpour J (2000b) Gene expression, localization, and pharmacological characterization of endothelin receptors in diabetic rat bladder dome. Eur J Pharmacol 387:253–263
Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T (1990) Cloning of a cDNA encoding a non-iso-peptide selective subtype of the endothelin receptor. Nature 348:732–735
Sakurai T, Yanagisawa M, Inoue A, Ryan US, Kimura S, Mitsui Y, Goto K, Masaki T (1991) cDNA cloning, sequence analysis and tissue distribution of rat preproendothelin-1 mRNA. Biochem Biophys Res Comm 175:44–47
Schmitz-Spanke S, Schipke JD (2000) Potential role of endothelin-1 and endothelin antagonists in cardiovascular diseases. Basic Res Cardiol 75:290–298
Shiba R, Sakurai T, Yamada G, Morimoto H, Saito A, Masaki T, Goto K (1992) Cloning and expression of rat preproendothelin-3 cDNA. Biochem Biophys Res Commun 186:588–594
Shimada K, Takahashi M, Tanzawa K (1994) Cloning and functional expression of endothelin-converting enzyme from rat endothelial cells. J Biol Chem 269:18275–18278
Sullivan ME, Mumtaz FH, Khan MA, Dashwood MR, Thompson CS, Mikhailidis DP, Morgan RJ (2000) Endothelins in the urinary tract. BJU Int 86:97–106
Takahashi W, Afiatpour P, Foster HE Jr, Ikeda K, Wada Y, Weiss RM, Latifpour J (2002) The effect of castration on endothelins, their receptors and endothelin converting enzyme in rat prostate. Naunyn-Schmiedebergs Arch Pharmacol 366:166–176
Tomobe Y, Ishikawa T, Yanagisawa M, Kimura S, Masaki T, Goto K (1991) Mechanisms of altered sensitivity to endothelin-1 between aortic smooth muscles of spontaneously hypertensive and Wistar-Kyoto rats. J Pharmacol Exp Ther 257:255–261
Traish AM, Morgan E, Krane RJ, Saenz de Tejada I (1992) Endothelin in the urinary bladder. II. Characterization of endothelin receptor subtypes. J Urol 148:1299–1306
Wada Y, Latifpour J, Sanematsu H, Afiatpour P, Wang Z, Saito M, Nishi K, Weiss RM (2000) Age-related changes in contractile responses of rabbit lower urinary tract to endothelin. J Urol 164:806–813
White M, Courtemanche M, Stewart DJ, Talajic M, Mikes E, Cernacek P, Vantrimpont P, Leclerc D, Bussieres L, Rouleau JL (1997) Age- and gender-related changes in endothelin and catecholamine release, and in autonomic balance in response to head-up tilt. Clin Sci 93:309–316
Yanagisawa M, Kurihara H, Kimura S (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332:411–415
Yin JL, Shackel NA, Zekry A, McGuinness PH, Richards C, Van Der Putten K, McCaughan GW, Eris JM, Bishop GA (2001) Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for measurement of cytokine and growth factor mRNA expression with fluorogenic probes or SYBR Green I. Immunol Cell Biol 79:213–221
Acknowledgements
This study was supported in part by NIH grants, DK 42530 and DK 38311. The experiments comply with the current laws of the USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afiatpour, P., Latifpour, J., Takahashi, W. et al. Developmental changes in the functional, biochemical and molecular properties of rat bladder endothelin receptors. Naunyn-Schmiedeberg's Arch Pharmacol 367, 462–472 (2003). https://doi.org/10.1007/s00210-003-0715-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-003-0715-6