Abstract
Microbial product lipopolysaccharide has been shown to be involved in the pathogenesis of inflammatory skin diseases. Apocynin has demonstrated to have an anti-inflammatory effect. However, the effect of apocynin on the Toll-like receptor-4-dependent activation of Akt, mammalian target of rapamycin (mTOR), and nuclear factor (NF)-κB pathway, which is involved in productions of inflammatory mediators in keratinocytes, has not been studied. Using human keratinocytes, we investigated the effect of apocynin on the inflammatory mediator production in relation to the Toll-like receptor-4-mediated-Akt/mTOR and NF-κB pathways, which regulates the transcription genes involved in immune and inflammatory responses. Apocynin, Akt inhibitor SH-5, Bay 11-7085 and N-acetylcysteine each attenuated the lipopolysaccharide-induced production of cytokines, PGE2, and chemokines, changes in the levels of Toll-like receptor-4, p-Akt, mTOR, and NF-κB, and production of reactive oxygen species in keratinocytes. The results show that apocynin appears to attenuate the lipopolysaccharide-stimulated production of inflammatory mediators in keratinocytes by suppressing the Toll-like receptor-4-mediated activation of the Akt, mTOR, and NF-κB pathways. The effect of apocynin appears to be attributed to its inhibitory effect on the production of reactive oxygen species. Apocynin appears to attenuate the microbial product-mediated inflammatory skin diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Keratinocytes produce proinflammatory mediators in response to a variety of stimuli, including epidermal barrier perturbation and microbial infections (Pastore et al. 2006; Tsuruta 2009). Keratinocytes cause the amplification and persistence of inflammatory and immune responses in the skin through the production of proinflammatory mediators (Pastore et al. 2006). They respond to microbial products, such as lipopolysaccharide and lipoteichoic acid, through the activation of Toll-like receptors (TLRs), thereby producing various cytokines and chemokines that may evoke the T cell-mediated immune response in inflammatory skin diseases (Baker 2006; Takeda and Akira 2005; Morris et al. 2015). Lipopolysaccharide binding to keratinocyte CD14 and subsequent activation of Toll-like receptor-4 causes the activation of nuclear factor (NF)-κB, leading to the production of proinflammatory cytokines and chemokines (Song et al. 2002; Morris et al. 2015). Lipopolysaccharide induces the activation of the phosphatidylinositol (PI) 3-kinase/Akt (protein kinase B)/mammalian target of rapamycin (mTOR) signaling pathways, which is followed by activation of transcription factors, including activator protein-1 and NF-κB (Thomson et al. 2009; Zhong et al. 2012). NF-κB regulates genes responsible for the innate and adaptive immune responses as well as inflammation (Ghosh and Hayden 2008).
Apocynin has been shown to exhibit antioxidant and anti-inflammatory effects (Kilic et al. 2015; Mouzaoui et al. 2014; Zheng et al. 2013). Apocynin attenuates production of reactive oxygen species and lipid peroxidation in tumor necrosis factor (TNF)-α-induced colitis (Mouzaoui et al. 2014). Apocynin reduces isoproterenol-induced myocardial injury and fibrogenesis by suppressing production of reactive oxygen species in mouse (Liu et al. 2014). Apocynin attenuates interleukin (IL-1)-1β-induced cytosolic phospholipase A2 expression by suppressing production of reactive oxygen species in rheumatoid arthritis synovial fibroblasts (Chi et al. 2012). Apocynin inhibits the advanced oxidation protein product-induced upregulation of the messenger RNA (mRNA) and protein expression of proinflammatory cytokines and production of reactive oxygen species by suppressing activation of NF-κB in fibroblast-like synoviocytes (Zheng et al. 2013). Apocynin reduces the Toll-like receptor-4-mediated expression of proinflammatory cytokines by suppressing intracellular production of reactive oxygen species in vascular smooth muscle cells (Pi et al. 2013). Apocynin blocks lipopolysaccharide-induced phosphorylation of Akt by suppressing NADPH oxidase in human peripheral blood mononuclear cells (Ngkelo et al. 2012). In contrast, despite its inhibitory effect on production of reactive oxygen species and nitric oxide, apocynin does not reduce lipopolysaccharide-induced lung inflammation in mice (Viačková et al. 2011).
Apocynin has been shown to exhibit anti-inflammatory effect. Nevertheless, in keratinocytes, the effect of apocynin on the lipopolysaccharide-stimulated production of inflammatory mediators has not been studied. Furthermore, it is unclear whether the effect of apocynin on the lipopolysaccharide-induced production of inflammatory mediators is mediated by its effect on the Akt, mTOR, and NF-κB pathways. Using human keratinocytes, we examined the effect of apocynin on lipopolysaccharide-induced inflammatory mediator production in relation to activation of the Toll-like receptor-4-mediated Akt, mTOR, and NF-κB pathways.
Materials and methods
Materials
Apocynin, rapamycin (It inhibits mTOR and blocks the subsequent activation of p70 S6 kinase at IC50 = 50 pM), Bay 11-7085 ((2E)-3-[[4-(1,1-Dimethylethyl)phenyl]sulfonyl]-2-propenenitrile. It blocks TNF-α-inducible phosphorylation of IκBα at 10 μM), Akt inhibitor (type II, SH-5; [(2R)-2-methoxy-3-octadecoxypropyl](2,3,4-trihydroxy-6-methoxycyclohexyl hydrogen phosphate), It inhibits Akt activation less than 10 μM), and horseradish peroxidase-conjugated anti-mouse IgG were purchased from EMD-Calbiochem. Co. (La Jolla, CA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for human CXCL1/IL1β, human IL6, human thymus and activation-regulated chemokine (TARC/CCL17), human T cell-attracting chemokine (CTACK/CCL27), and human/mouse/rat phospho-Akt(Pan)), and a parameter assay kit for prostaglandin E2 were purchased from R&D systems, Inc. (Minneapolis, MN, USA). Antibodies (Toll-like receptor (TLR)-4, NF-κB p65, NF-κB p50, phospho-IκB-α, Phospho-Akt1, Akt1, mTOR and β-actin) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The TransAM™ NF-κB assay kit was purchased from Active Motif® (Carlsbad, CA, USA). Lipopolysaccharide (from Escherichia coli), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), N-acetylcysteine, NADPH and other chemicals were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA).
Keratinocyte culture
Human keratinocytes (HEK001, tissue: skin; morphology: epithelial; cell type: human papillomavirus 16 E6/E7 transformed) were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in keratinocyte-serum free media supplemented with bovine pituitary extract, recombinant epidermal growth factor, 100 units/ml penicillin and 100 μg/ml streptomycin (GIBCO®, Invitrogen Co., Grand Island, NY).
Compliance with ethical standards
Normal human keratinocytes were provided by the Department of Urology, Chung-Ang University Hospital (Seoul, South Korea). Keratinocytes were obtained and prepared from neonatal foreskin discarded after circumcision (Kim et al. 2005). Our paper satisfied the ethics. Neonatal foreskin was chopped and split overnight in sucrose-trypsin solution (0.1 % sucrose, 0.25 % trypsin, and 1 mM EDTA) at 4 °C. Keratinocyte suspension was cultured in EpiLife® medium supplemented with growth factor (Cascade Biologics™, Portland, OR, USA).
Cell treatment
HEK001 keratinocytes and primary keratinocytes were treated with 1 μg/ml lipopolysaccharide in combination with 2.5 μM Bay11-7085, 0.5 μM Akt inhibitor (SH-5) or 0.5 μM rapamycin for the designated times at 37 °C. We have determined the concentrations of Bay11-7085, Akt inhibitor (SH-5) and rapamycin based on the concentrations of reagents (5 μM Bay11-7085, 20 μM SH-5 and 2.5 μM rapamycin) described in previous reports (Slomiany and Slomiany 2010; Sanchez et al. 2013; de Oliveira et al. 2016) on inflammatory mediator production, and on the information for reagents provided from EMD-Calbiochem. Co. (see Materials).
Immunoassays for IL-1β, IL-6, PGE2, CCL17, and CCL27
Keratinocytes (1 × 105 cells/300 μl for the cytokines and PGE2, and 5 × 105 cells/400 μl for the chemokine assay) were grown in a 24-well plate and treated with 1 μg/ml lipopolysaccharide for 24 h. After centrifugation at 412g for 10 min, the amounts of IL-1β, IL-6, PGE2, CCL17, and CCL27 in the culture supernatants were analyzed corresponding to the ELISA and parameter assay kits, according to the manufacturer’s instructions. Absorbance was measured at 450 nm using a microplate reader (Magellan, TECAN, Salzburg, Austria).
Preparation of cytosolic and nuclear extracts
Keratinocytes (5 × 106 cells/ml) were treated with lipopolysaccharide for 30 min at 37 °C. Exceptionally, to assay the levels of TLR-4, keratinocytes were treated with lipopolysaccharide for 24 h. Keratinocyte cytosolic and nuclear extracts were prepared, as previously described (Schreiber et al. 1989). Keratinocytes were harvested by centrifugation at 412g for 10 min and washed twice with phosphate-buffered saline (PBS). The cells were suspended in 400 μl lysis buffer (10 mM KCl, 1.5 mM MgCl2, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 0.5 mM dimethylsulfoxide, 1 mM sodium orthovanadate, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 10 mM HEPES-KOH, pH 7.8) and were allowed to swell on ice for 15 min. Next, 25 μl of a 10 % Nonidet NP-40 solution (final concentration approximately 0.6 %) was added, and the tubes were vigorously vortexed for 10 s. The homogenates were centrifuged at 12,000g for 10 min at 4 °C. The supernatants were stored as cytoplasmic extracts and kept at −70 °C. The nuclear pellets were resuspended in 50 μl lysis buffer of an ice-cold hypertonic solution containing 5 % glycerol and 0.4 M NaCl. The tubes were incubated on ice for 30 min and then centrifuged at 12,000g for 15 min at 4 °C. The supernatants were collected as the nuclear extracts and stored at −70 °C. Protein concentrations were determined using the Bradford method according to the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA, USA).
Western blot for phospho-IκB, NF-κB, and TLR-4 levels
The cytosolic and nuclear extracts for the NF-κB assay and the cytosolic fraction for the TLR-4 assay were mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and boiled for 5 min. Samples (30 μg protein/well) were loaded onto each lane of a 10–12 % SDS-polyacrylamide gel and transferred onto polyvinylidene difluoride membranes (GE Healthcare Chalfont St. Giles, Buckinghamshire, UK). Membranes were blocked for 2 h in TBS (50 mM Tris-HCl, pH 7.5 and 150 mM NaCl) containing 0.1 % Tween 20. Each membrane was labeled with the appropriate antibody (TLR-4, NF-κB p65, NF-κB p50, phospho-IκB-α, Akt1, mTOR, or β-actin) overnight at 4 °C with gentle agitation. After four washes in TBS containing 0.1 % Tween 20, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse IgG for 2 h at room temperature. Membranes were treated with SuperSignal® West Pico chemiluminescence substrate and protein bands were visualized by detecting the enhanced chemiluminescence in an appropriate image analyzer (Lite for Las-1000 plus version 1.1; Fuji Photo Film Co., Tokyo, Japan).
The densities of protein bands were determined using TINA 2.10 g software licensed for Seoul National University (SNU and SNUMD, Seoul, South Korea) and were expressed as a fold increase compared to the control density.
Assay for DNA binding activity of NF-κB
The biding of NF-κB p65 to DNA was measured according to the user’s manual for the TransAM™ NF-κB kit. Keratinocytes (2 × 106 cells/ml) were treated with lipopolysaccharide for 30 min. Nuclear extracts were prepared according to the procedure described in the Active Motif® protocol and added to a 96-well plate to which oligonucleotides containing an NF-κB consensus binding site (5′-GGGACTTTCC-3′) were immobilized. The active NF-κB p65 bound to DNA was exposed to a primary antibody specific for NF-κB p65, which was reacted with anti-rabbit horseradish peroxidase-conjugated IgG. Next, the color developer and stop solutions were added to the plate. The absorbance of samples was measured at 450 nm with a reference wavelength of 655 nm in a microplate reader.
Enzyme-linked immunosorbent assays for phosphorylated Akt
Keratinocytes (1 × 106 cells/ml) were treated with lipopolysaccharide for 4 h. Keratinocytes were harvested by centrifugation at 412g for 10 min, washed twice with PBS, and suspended in the lysis buffer provided by R&D systems for whole cell lysates. The homogenates were centrifuged at 2000g for 5 min and the supernatant was used for the ELISA. The amounts of phosphorylated Akt were determined according to the manufacturer’s instructions for the immunoassays. The supernatants were exposed first to the antibodies for the phosphorylated forms of the kinases, next to the biotinylated detection antibodies, and finally to streptavidin-horseradish-peroxidase. The absorbance was measured at 405 nm.
Measurement of intracellular reactive oxygen species production
The dye 2′,7′-dichlorofluorescin-diacetate (DCFH2-DA), which is oxidized to fluorescent dichlorofluorescein (DCF) by hydroperoxides, was used to measure relative levels of cellular peroxides (Fu et al. 1998). Keratinocytes (1 × 105 cells/400 μl in 24-well plate) were treated with lipopolysaccharide for 24 h at 37 °C. The cells were washed, suspended in fetal bovine serum-free RPMI-1640, incubated with 50 μM dye for 30 min at 37 °C, and washed with phosphate buffered saline. The cell suspensions were centrifuged at 412g for 10 min and medium was removed. The pellets were dissolved with 1 % Triton X-100 and fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm using a fluorescence microplate reader (SPECTRAFLUOR, TECAN, Salzburg, Austria).
Measurement of NADPH oxidation
NADPH oxidation was measured according to the method described in previous report (Bruinenberg et al. 1983). HEK001 keratinocytes (2 × 106 cells/ml) were treated with were treated with lipopolysaccharide and apocynin for 24 h at 37 °C. The cell suspensions were sonicated at 55 W for 15 s two times using a sonifier cell disrupter (model W185D; Branson, NY, USA). Cell lysates (approximately 50 μg protein/ml) were added to 1 ml of PRMI medium and reaction was started by the addition of NADPH (final concentration is 0.2 mM) at 25 °C. Changes in the absorbance were measured at 340 nm using a microplate reader.
Measurement of cell viability
Cell viability was measured using an MTT reduction assay, which is based on the conversion of MTT to formazan crystals by mitochondrial dehydrogenases (Mosmann 1983). Cells (3 × 104) were treated with apocynin for 24 h at 37 °C. The medium (200 μl) was incubated with 10 μl of a 10 mg/ml MTT solution for 2 h at 37 °C. After centrifugation at 412×g for 10 min, the culture medium was removed and 100 μl of dimethyl sulfoxide was added to each well to dissolve the formazan. The absorbance was measured at 570 nm using a microplate reader (Magellan, TECAN, Salzburg, Austria). Cell viability was expressed as a percentage of the value measured from the control cultures in which the cells that did not receive any treatments.
Statistical analysis
The data are expressed as the mean ± S.E.M. Statistical analyses were performed by one-way analysis of variance (ANOVA). When significance was detected, the post hoc comparisons between the different groups were made using Duncan’s test for multiple comparisons. A probability value of P < 0.05 was considered statistically significant.
Results
The production of inflammatory mediators
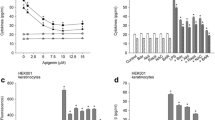
Lipopolysaccharide stimulated productions of cytokines IL-1β and IL-6, proinflammatory mediator PGE2, and chemokines CCL17 and CCL27 in HEK001 keratinocytes and primary keratinocytes. Then, the effect of apocynin on the production of proinflammatory mediators in keratinocytes treated with lipopolysaccharide was examined. Apocynin in a concentration dependent manner reduced lipopolysaccharide-induced production of cytokines and PGE2 in keratinocytes (Fig. 1a, b). It exhibited approximately 61–64 % inhibitory effects at 7.5 μM. Treatment with apocynin alone, up to 10 μM, did not significantly induce cytokine production and PGE2 in cells not treated with lipopolysaccharide. Apocynin at 7.5 μM alone slightly increased the levels of cytokines and PGE2, which was not statistically significant, compared to control values, but attenuated lipopolysaccharide-induced production of cytokines and PGE2.
Effect of apocynin on productions of cytokines and prostaglandin E2. a, b HEK001 keratinocytes (or primary keratinocytes) were pre-treated with 1–10 μM apocynin for 20 min and treated with 1 μg/ml lipopolysaccharide in combination with apocynin for 24 h. The levels of IL-1β, IL-6 and prostaglandin E2 were measured using the ELISA or parameter assay kits. Data represent the mean ± S.E.M. (n = 6). + p < 0.05 compared to the control; *p < 0.05 compared to lipopolysaccharide alone
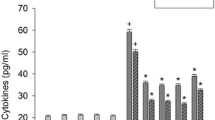
Whether TNF-α-induced production of cytokines was mediated by the Akt, NF-kB, and mTOR pathways, we examined the effect of signaling inhibitors. Treatment with 2.5 μM Bay 11-7085 (an irreversible inhibitor of IkBα phosphorylation), 0.5 μM Akt inhibitor SH-5 or 0.5 μM rapamycin (an mTOR inhibitor) reduced the lipopolysaccharide-induced production of IL-1β, IL-6, and PGE2 in HEK001 keratinocytes and primary keratinocytes (Fig. 2a, b). Signaling inhibitor alone did not significantly induce production of inflammatory mediators. Whether lipopolysaccharide-induced production of cytokines was mediated by reactive oxygen species was examined. Treatment with 1 mM thiol antioxidant N-acetylcysteine reduced the lipopolysaccharide-induced production of cytokines and PGE2 (Fig. 2a). N-acetylcysteine also reduced the lipopolysaccharide-induced production of IL-1 β in primary keratinocytes (Fig. 2b). N-acetylcysteine alone did not induce production of inflammatory mediators.
Effect of signaling inhibitors on productions of cytokines and prostaglandin E2. a, b HEK001 keratinocytes (or primary keratinocytes) were treated with 1 μg/ml lipopolysaccharide in the presence of 2.5 μM Bay 11-7085, 0.5 μM Akt inhibitor SH-5, 0.5 μM rapamycin or 1 mM N-acetylcysteine for 24 h. The levels of IL-1β, IL-6, and prostaglandin E2 were measured using the ELISA or parameter assay kits. Data represent the mean ± S.E.M. (n = 6). + p < 0.05 compared to the control; *p < 0.05 compared to lipopolysaccharide alone
We further examined the effect of apocynin on the lipopolysaccharide-induced production of chemokines. Lipopolysaccharide caused the production of chemokines CCL17 and CCL27 in keratinocytes. Apocynin attenuated the lipopolysaccharide-induced productions of CCL17 and CCL27 with approximately 64–67 % inhibition at 7.5 μM (Fig. 3a). We examined whether lipopolysaccharide-induced production of chemokine was mediated by the Akt, NF-kB, and mTOR pathways. The addition of Bay 11-7085, Akt inhibitor SH-5 or rapamycin attenuated the lipopolysaccharide-induced production of chemokines (Fig. 3b). They alone did not induce the chemokine production. Whether lipopolysaccharide-induced production of chemokine was mediated by reactive oxygen species, we examined the effect of oxidant scavenger. The addition of N-acetylcysteine attenuated the lipopolysaccharide-induced production of chemokines (Fig. 3b). It did not induce the chemokine production.
Effect of apocynin and signaling inhibitors on productions of chemokines. a HEK001 keratinocytes were pre-treated with 1–10 μM apocynin for 20 min and treated with 1 μg/ml lipopolysaccharide in combination with apocynin for 24 h. In B, keratinocytes were treated with lipopolysaccharide in the presence of 2.5 μM Bay 11-7085, 0.5 μM Akt inhibitor SH-5, 0.5 μM rapamycin or 1 mM N-acetylcysteine (NAC) for 24 h. The levels of CCL17 and CCL27 were measured using ELISA kits and parametric assay kit. Data represent the mean ± S.E.M. (n = 6). + p < 0.05 compared to the control; *p < 0.05 compared to lipopolysaccharide alone
Activation of NF-κB
The effect of apocynin on lipopolysaccharide-induced activation of Toll-like receptor 4, which results in the stimulated response of keratinocytes, was examined. Lipopolysaccharide increased the levels of Toll-like receptor 4, which was inhibited by the addition of 5 μM apocynin or 1 mM N-acetylcysteine (Fig. 4a). Next, whether the effect of apocynin on the lipopolysaccharide-induced production of inflammatory mediators in keratinocytes was mediated by its effect on the NF-κB activation was investigated. Lipopolysaccharide increased the cytosolic levels of NF-κB p65, NF-κB p50, and phospho-IκB and the levels of nuclear NF-κB p65 in keratinocytes (Fig. 4b). The addition of apocynin, Bay 11-7085, Akt inhibitor SH-5, or rapamycin inhibited the lipopolysaccharide-induced phosphorylation of IkBα and activation of NF-κB.
Effect of apocynin on Toll-like receptor-4 expression and NF-κB activation. Keratinocytes were pre-treated with compounds (5 μM apocynin, 2.5 μM Bay 11-7085, 0.5 μM Akt inhibitor SH-5, 0.5 μM rapamycin, or 1 mM N-acetylcysteine (NAC)) for 20 min and treated with 1 μg/ml lipopolysaccharide in combination with compounds for 30 min (for NF-κB) or 24 h (for Toll-like receptor-4). a The levels of Toll-like receptor-4 were analyzed by Western blotting with specific antibodies. b The levels of NF-κBp65, NF-κBp50, and phospho-IκB-α were analyzed by Western blotting with specific antibodies. Data are representative of three to four different experiments. The densities of protein bands were determined using TINA 2.10 g software, and data represent a fold increase from the control density. c The NF-κB to DNA binding activity was measured using an assay kit. Data represent the mean ± S.E.M. (n = 5). + p < 0.05 compared to the control; *p < 0.05 compared to lipopolysaccharide alone
We confirmed the inhibitory effect of apocynin on the lipopolysaccharide-induced NF-κB activation by monitoring the effect on the binding of NF-κB to DNA. Non-stimulated cells exhibited a small increase in the NF-κB-DNA binding activity. Lipopolysaccharide produced a marked increase in the NF-κB-DNA binding activity, which was attenuated by the addition of apocynin, Bay 11-7085, Akt inhibitor SH-5, rapamycin or N-acetylcysteine (Fig. 4c).
Akt activation and changes in the levels of mTOR
Whether the lipopolysaccharide-induced NF-κB activation-mediated production of inflammatory mediators was regulated by Akt pathway was examined. To clearly define the inhibitory effect of apocynin, we assessed the effect on changes in the levels of Akt at a 4-h exposure time of lipopolysaccharide. The lipopolysaccharide-induced activation of Akt was confirmed by the suppressive effect of the specific Akt inhibitor SH-5. Treatment with apocynin or N-acetylcysteine inhibited the lipopolysaccharide-induced increase in the levels of phospho-Akt1 (upper part in Fig. 5a). Lipopolysaccharide and stated compounds did not affect the levels of Akt1. We further examined the inhibitory effect of apocynin on phosphorylation of Akt using assay kit. Apocynin, N-acetylcysteine, or Akt inhibitor SH-5 attenuated the lipopolysaccharide-induced phosphorylation of Akt in keratinocytes (lower part in Fig. 5a). Apocynin, N-acetylcysteine, or Akt inhibitor SH-5 alone did not induce Akt phosphorylation.
Effect of apocynin on activation of Akt and levels of mTOR. a Keratinocytes were treated with 1 μg/ml lipopolysaccharide in the presence of compounds (5 μM apocynin, 0.5 μM Akt inhibitor SH-5 or 1 mM N-acetylcysteine (NAC)) for 4 h, and then the level of phospho-Akt was measured by ELISA. Data represent the mean ± S.E.M. (n = 5–6). + p < 0.05 compared to the control; *p < 0.05 compared to lipopolysaccharide treatment alone. The levels of Akt1 and phosphorylated-Akt1 were analyzed by Western blotting and data are representative of three to four different experiments. The densities of protein bands were determined using TINA 2.10 g software, and data represent a fold increase from the control density. b Keratinocytes were treated with 1 μg/ml lipopolysaccharide in the presence of compounds (apocynin, Akt inhibitor SH-5, rapamycin or N-acetylcysteine (NAC)) for 4 h, and the levels of mTOR were analyzed by Western blotting and data are representative of three to four different experiments. The densities of protein bands were determined using TINA 2.10 g software, and data represent a fold increase from the control density
Whether the lipopolysaccharide-induced production of inflammatory mediators was regulated by mTOR signaling, we examined the changes in the levels of mTOR. Lipopolysaccharide increased the levels of mTOR in keratinocytes. The addition of apocynin, Akt inhibitor SH-5, or rapamycin inhibited the lipopolysaccharide-induced increase in the levels of mTOR (Fig. 5b).
The production of reactive oxygen species
The production of reactive oxygen species in keratinocytes treated with lipopolysaccharide was examined as one of the stimulated keratinocyte responses. The production of reactive oxygen species within cells was determined by monitoring a conversion of DCFH2-DA to DCF. Keratinocytes treated with lipopolysaccharide exhibited a significant increase in DCF fluorescence. We examined the effect of oxidant scavengers on the production of reactive oxygen species in keratinocytes treated with lipopolysaccharide. Apocynin, N-acetylcysteine, or 50 μM trolox (a cell-permeable, water-soluble derivative of vitamin E with scavenging effect on hydroxyl radicals and peroxynitrite) inhibited the lipopolysaccharide-induced increase in DCF fluorescence (Fig. 6).
Effect of apocynin on production of reactive oxygen species. Keratinocytes were treated with 1 μg/ml lipopolysaccharide in the presence of compounds (5 μM apocynin, 1 mM N-acetylcysteine (NAC) or 30 μM trolox) for 24 h. a The changes in DCF fluorescence, which indicates production of reactive oxygen species production, were measured and data are expressed as arbitrary units (a.u.) of fluorescence. Data represent the mean ± S.E.M. (n = 6). + p < 0.05 compared to control; *p < 0.05 compared to lipopolysaccharide alone
Effect of apocynin on NADPH oxidation
Whether the preventive effect of apocynin on the lipopolysaccharide-induced production of reactive oxygen species was attributed to its inhibitory effect on NADPH oxidase in keratinocytes, we examined the effect of apocynin on the oxidation of NADPH. When NADPH was added to the cell lysate-containing medium, oxidation of NADPH increased with time, which was greater than that in cell free-medium. Treatment with lipopolysaccharide increased the NADPH oxidation. Apocynin inhibited the oxidation of NADPH in keratinocytes treated with lipopolysaccharide or not (Fig. 7).
The effect of apocynin on NADPH oxidation. HEK001 keratinocytes treated with 1 μg/ml lipopolysaccharide in the presence of 5 μM apocynin or with apocynin alone for 24 h. Cell lysates (approximately 50 μg protein/ml) were added to 1 ml of PRMI medium containing and 5 μM apocynin. Then, the reaction was started by the addition of 0.2 mM NADPH at 25 °C. The changes in the absorbance were measured at 340 nm. The oxidation of NADPH in the absence of cell lysates (None); that in the presence of cell lysates without reagents (Control); that in the presence of lipopolysaccharide and cell lysates (Lipo); that in the presence of apocynin and cell lysates (Apocynin); that in the presence of lipopolysaccharide, apocynin and cell lysates (Lipo + Apocynin). Data indicate mean values of absorbance. Data represent the mean ± S.E.M. (n = 6)
Cell viability
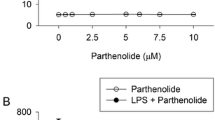
To assess whether the inhibitory effect of apocynin on the stimulated responses in keratinocytes was attributed to its effect on cell viability loss, we examined the cytotoxic effect of apocynin using the MTT assay that provides rapid and precise results for cellular growth and survival. When keratinocytes were treated with 10 μM apocynin for 24 h, approximately 5 % cell death, not statistically significant, was observed (Fig. 8).
Discussion
Cytokines play a critical role in inflammatory and immune responses in the skin (Pastore et al. 2006; Niebuhr et al. 2010). Chemokines are involved in the recruitment of T cells into skin and in the induction of skin inflammation (Schaerli and Moser 2005; Islam and Luster 2012). HEK001 keratinocytes treated with lipopolysaccharide produced a significant amount of cytokines IL-1β and IL-6, inflammatory mediator PGE2, and chemokines CCL17 and CCL27. Apocynin has been demonstrated to exhibit anti-inflammatory and antioxidant effects. Nevertheless, the effect of apocynin on the lipopolysaccharide-induced production of inflammatory mediators in keratinocytes has not been studied. In the present study, apocynin inhibited the lipopolysaccharide-induced production of the proinflammatory cytokines, mediator, and chemokines in keratinocytes. These results suggest that apocynin appears to suppress inflammatory reaction and immune cell function by suppressing production of proinflammatory mediators. In addition, apocynin appears to reduce the infiltration of T cells into skin through the inhibition of chemokine production.
Lipopolysaccharide has been shown to cause the production of cytokines by activating the Toll-like receptor 4-mediated NF-κB pathway (Takeda and Akira 2005; Thomson et al. 2009). The aberrant activation of NF-κB in both keratinocytes and lymphocytes may be involved in the development of inflammatory skin diseases (Rebholz et al. 2007). Lipopolysaccharide causes the production of cytokines in keratinocytes through the activation of NF-κB (Lee et al. 2010; Ge et al. 2012). The phosphorylation and proteolytic degradation of IκB induce the release of NF-κB dimers (Napetschnig and Wu 2013). The translocation of active NF-κB dimers to the nucleus elicits the activation of target genes responsible for the synthesis of cytokines and chemokines (Vestergaard et al. 2005; Ghosh and Hayden 2008). In the present study, lipopolysaccharide increased the levels of Toll-like receptor-4, the levels phospho-IκB and NF-κB p65/50, and the binding of NF-κB to DNA in keratinocytes. These results suggest that the lipopolysaccharide induces inflammatory mediator production in keratinocytes through Toll-like receptor-4-mediated NF-κB pathway activation. Next, we measured whether the suppressive effect of apocynin on the lipopolysaccharide-induced production of inflammatory mediators in keratinocytes was attributed to the inhibitory effect on NF-κB activation. Apocynin inhibited the lipopolysaccharide-induced changes in the levels of Toll-like receptor-4 and activation of NF-κB in keratinocytes. These results suggest that apocynin may inhibit the lipopolysaccharide-induced production of inflammatory mediators by suppressing the Toll-like receptor 4-mediated activation of NF-κB.
Lipopolysaccharide induces NF-κB activation by activating the phosphatidylinositol (PI) 3-kinase/Akt/mTOR pathway (Lee et al. 2008; Thomson et al. 2009; Zhong et al. 2012). We examined whether lipopolysaccharide-induced NF-κB activation and subsequent production of inflammatory mediators in keratinocytes was mediated by Akt/mTOR signaling. Keratinocytes treated with lipopolysaccharide induced activation of (phosphorylated) Akt and increase in the levels of mTOR. Akt inhibitor SH-5 and rapamycin reduced the lipopolysaccharide-induced production of inflammatory mediators and activation of NF-κB. These results suggest that lipopolysaccharide induces activation of NF-κB by activating the Akt and mTOR pathway in keratinocytes. Apocynin, Akt inhibitor SH-5, and rapamycin reduced the lipopolysaccharide-induced production of inflammatory mediators and activation of NF-κB, and reduced the levels of phosphorylated Akt and mTOR. Thus, apocynin appears to attenuate the lipopolysaccharide-induced production of inflammatory mediators by suppressing the Akt/mTOR-regulated NF-κB activation.
Apocynin induced approximately 5 % cell death at 10 μM, not statistically significant, in keratinocytes. Thus, the inhibitory effect of apocynin on the Akt/mTOR and NF-κB pathways involved in the production of inflammatory mediators not appears to be affected by changes in cell viability.
Reactive oxygen species may be involved in the regulation of cell functions and induce NF-κB activation (Gloire et al. 2006; Siomek 2012). In this respect, we examined the production of reactive oxygen species in keratinocytes treated with lipopolysaccharide, which may cause the NF-κB activation. Lipopolysaccharide stimulated production of reactive oxygen species in keratinocytes, which was inhibited by the addition of oxidant scavengers such as N-acetylcysteine and trolox. The N-acetylcysteine is known to inhibit the TNF-α- or lipopolysaccharide-induced cytokine production by suppressing reactive oxygen species production (Young et al. 2008; Wei et al. 2015). In the present study, N-acetylcysteine attenuated the lipopolysaccharide-induced changes in the levels and activity of Toll-like receptor-4, NF-κB, Akt, and mTOR, and the production of reactive oxygen species. Apocynin has been shown to exhibit antioxidant and anti-inflammatory effect by suppressing production of reactive oxygen species (Chi et al. 2012; Liu et al. 2014; Mouzaoui et al. 2014). However, it is unclear whether the inhibitory effect of apocynin on the Akt, mTOR, and NF-κB-mediated inflammatory responses is mediated by its effect on reactive oxygen species. In the present study, treatment with apocynin at the concentration, which did not exhibit toxic effect, inhibited the lipopolysaccharide-induced changes in the levels of signaling proteins and production of reactive oxygen species. These results suggest that apocynin may attenuate the lipopolysaccharide-induced signal transduction-mediated production of proinflammatory mediators by suppressing production of reactive oxygen species.
Whether the suppressive effect of apocynin on the lipopolysaccharide-induced production of reactive oxygen species was attributed to its inhibitory effect on NADPH oxidase in keratinocytes, we examined the effect of apocynin on the oxidation of NADPH. Lipopolysaccharide increased the NADPH oxidation in keratinocytes. Thus, lipopolysaccharide-NADPH oxidation may be partially made up of reactive oxygen species that can be suppressed by apocynin, an NADPH oxidase inhibitor. This result suggests that the suppressive effect of apocynin on the lipopolysaccharide-induced production of reactive oxygen species is partially attributed to its inhibitory effect on NADPH oxidase in keratinocytes. In addition, the suppressive effect of apocynin on the lipopolysaccharide-induced production of inflammatory mediators appears to be partially attributed to its inhibitory effect on NADPH oxidase.
Overall, the results show that apocynin appears to attenuate lipopolysaccharide-stimulated production of inflammatory mediators in keratinocytes by suppressing the Toll-like receptor-4-mediated activation of the Akt, mTOR, and NF-κB pathways. The effect of apocynin appears to be attributed to inhibitory effect on production of reactive oxygen species. Additionally, apocynin appears to attenuate the microbial product-mediated inflammatory skin diseases.
References
Baker BS (2006) The role of microorganisms in atopic dermatitis. Clin Exp Immunol 144:1–9
Bruinenberg PM, van Dijken JP, Scheffers WA (1983) An enzymic analysis of NADPH production and consumption in Candida utilis. J Gen Microbiol 129:965–971
Chi PL, Chen YW, Hsiao LD, Chen YL, Yang CM (2012) Hemeoxygenase 1 attenuates interleukin-1β-induced cytosolic phospholipase A2 expression via a decrease in NADPH oxidase/reactive oxygen species/activator protein 1 activation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 64:2114–2125
de Oliveira AC, Yousif NM, Bhatia HS, Hermanek J, Huell M, Fiebich BL (2016) Poly(I:C) increases the expression of mPGES-1 and COX-2 in rat primary microglia. J Neuroinflammation 13:11
Fu W, Luo H, Parthasarathy S, Mattson MP (1998) Catecholamines potentiate amyloid β-peptide neurotoxicity: involvement of oxidative stress, mitochondrial dysfunction, and perturbed calcium homeostasis. Neurobiol Dis 5:229–243
Ge Y, Xu Y, Sun W, Man Z, Zhu L, Xia X, Zhao L, Zhao Y, Wang X (2012) The molecular mechanisms of the effect of dexamethasone and cyclosporin a on TLR4 /NF-κB signaling pathway activation in oral lichen planus. Gene 508:157–164
Ghosh S, Hayden MS (2008) New regulators of NF-κB in inflammation. Nat Rev Immunol 8:837–848
Gloire G, Legrand-Poels S, Piette J (2006) NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72:1493–1505
Islam SA, Luster AD (2012) T cell homing to epithelial barriers in allergic disease. Nat Med 18:705–715
Kilic T, Parlakpinar H, Taslidere E, Yildiz S, Polat A, Vardi N, Colak C, Ermis H (2015) Protective and therapeutic effect of apocynin on bleomycin-induced lung fibrosis in rats. Inflammation 38:1166–1180
Kim JE, Kim BJ, Jeong MS, Seo SJ, Kim MN, Hong CK, Ro BI (2005) Expression and modulation of LL-37 in normal human keratinocytes, HaCaT cells and inflammatory skin diseases. J Korean Med Sci 20:649–654
Lee SA, Park SH, Kim BC (2008) Raloxifene, a selective estrogen receptor modulator, inhibits lipopolysaccharide-induced nitric oxide production by inhibiting the phosphatidylinositol 3-kinase/Akt/NF-κ B pathway in RAW264.7 macrophage cells. Mol Cells 26:48–52
Lee CS, Jang ER, Kim YJ, Lee MS, Seo SJ, Lee MW (2010) Hirsutenone inhibits lipopolysaccharide-activated NF-κB-induced inflammatory mediator production by suppressing toll-like receptor 4 and ERK activation. Int Immunopharmacol 10:520–525
Liu L, Cui J, Yang Q, Jia C, Xiong M, Ning B, Du X, Wang P, Yu X, Li L, Wang W, Chen Y, Zhang T (2014) Apocynin attenuates isoproterenol-induced myocardial injury and fibrogenesis. Biochem Biophys Res Commun 449:55–61
Morris MC, Gilliam EA, Li L (2015) Innate immune programing by endotoxin and its pathological consequences. Front Immunol 5:680
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Mouzaoui S, Djerdjouri B, Makhezer N, Kroviarski Y, El-Benna J, Dang PM (2014) Tumor necrosis factor-α-induced colitis increases NADPH oxidase 1 expression, oxidative stress, and neutrophil recruitment in the colon: preventive effect of apocynin. Mediat Inflamm 2014:312484
Napetschnig J, Wu H (2013) Molecular basis of NF-κB signaling. Annu Rev Biophys 42:443–468
Ngkelo A, Meja K, Yeadon M, Adcock I, Kirkham PA (2012) LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Giα dependent PI-3kinase signalling. J Inflamm (Lond) 9:1
Niebuhr M, Baumert K, Werfel T (2010) TLR-2-mediated cytokine and chemokine secretion in human keratinocytes. Exp Dermatol 19:873–877
Pastore S, Mascia F, Girolomoni G (2006) The contribution of keratinocytes to the pathogenesis of atopic dermatitis. Eur J Dermatol 16:125–131
Pi Y, Zhang LL, Li BH, Guo L, Cao XJ, Gao CY, Li JC (2013) Inhibition of reactive oxygen species generation attenuates TLR4-mediated proinflammatory and proliferative phenotype of vascular smooth muscle cells. Lab Investig 93:880–887
Rebholz B, Haase I, Eckelt B, Paxian S, Flaig MJ, Ghoreschi K, Nedospasov SA, Mailhammer R, Debey-Pascher S, Schultze JL, Weindl G, Förster I, Huss R, Stratis A, Ruzicka T, Röcken M, Pfeffer K, Schmid RM, Rupec RA (2007) Crosstalk between keratinocytes and adaptive immune cells in an IκBα protein-mediated inflammatory disease of the skin. Immunity 27:296–307
Sanchez A, Tripathy D, Yin X, Luo J, Martinez JM, Grammas P (2013) Sunitinib enhances neuronal survival in vitro via NF-κB-mediated signaling and expression of cyclooxygenase-2 and inducible nitric oxide synthase. J Neuroinflammation 10:93
Schaerli P, Moser B (2005) Chemokines: control of primary and memory T-cell traffic. Immunol Res 31:57–74
Schreiber E, Matthias P, Müller MM, Schaffner W (1989) Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res 17:6419
Siomek A (2012) NF-κB signaling pathway and free radical impact. Acta Biochim Pol 59:323–331
Slomiany BL1, Slomiany A (2010) Ghrelin protection against lipopolysaccharide-induced gastric mucosal cell apoptosis involves constitutive nitric oxide synthase-mediated caspase-3 S-nitrosylation. Mediat Inflamm 2010:280464
Song PI, Park YM, Abraham T, Harten B, Zivony A, Neparidze N, Armstrong CA, Ansel JC (2002) Human keratinocytes express functional CD14 and toll-like receptor 4. J Invest Dermatol 119:424–432
Takeda K, Akira S (2005) Toll-like receptors in innate immunity. Int Immunol 17:1–14
Thomson AW, Turnquist HR, Raimondi G (2009) Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 9:324–337
Tsuruta D (2009) NF-κB links keratinocytes and lymphocytes in the pathogenesis of psoriasis. Recent Patents Inflamm Allergy Drug Discov 3:40–48
Vestergaard C, Johansen C, Otkjaer K, Deleuran M, Iversen L (2005) Tumor necrosis factor-alpha-induced CTACK/CCL27 (cutaneous T cell-attracting chemokine) production in keratinocytes is controlled by nuclear factor κB. Cytokine 29:49–55
Viačková D, Pekarová M, Crhák T, Búcsaiová M, Matiašovic J, Lojek A, Kubala L (2011) Redox-sensitive regulation of macrophage-inducible nitric oxide synthase expression in vitro does not correlate with the failure of apocynin to prevent lung inflammation induced by endotoxin. Immunobiology 216:457–465
Wei M, Li Z, Xiao L, Yang Z (2015) Effects of ROS-relative NF-κB signaling on high glucose-induced TLR4 and MCP-1 expression in podocyte injury. Mol Immunol 68:261–271
Young CN, Koepke JI, Terlecky LJ, Borkin MS, Boyd SL, Terlecky SR (2008) Reactive oxygen species in tumor necrosis factor-α-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J Invest Dermatol 128:2606–2614
Zheng S, Zhong ZM, Qin S, Chen GX, Wu Q, Zeng JH, Ye WB, Li W, Yuan K, Yao L, Chen JT (2013) Advanced oxidation protein products induce inflammatory response in fibroblast-like synoviocytes through NADPH oxidase -dependent activation of NF-κB. Cell Physiol Biochem 32:972–985
Zhong LM, Zong Y, Sun L, Guo JZ, Zhang W, He Y, Song R, Wang WM, Xiao CJ, Lu D (2012) Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS One 7:e32195
Acknowledgments
This research was supported by the Chung-Ang University Research Scholarship Grants in 2016, Chung-Ang University, Seoul, South Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nam, Y.J., Kim, A., Sohn, D.S. et al. Apocynin inhibits Toll-like receptor-4-mediated activation of NF-κB by suppressing the Akt and mTOR pathways. Naunyn-Schmiedeberg's Arch Pharmacol 389, 1267–1277 (2016). https://doi.org/10.1007/s00210-016-1288-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-016-1288-5