Abstract
Methylisothiazolinone (MI) as well as the mixture of chloromethylisothiazolinone/methyl-iso-thiazolinone (MCI/MI, 3:1) are widespread biocides used in personal care products with potential consumer exposure. Their use is currently under discussion because of rising rates of skin sensitization against these substances in the general population. We have examined the human metabolism of methylisothiazolinone and chloromethylisothiazolinone after oral dosage of stable isotope-labelled analogues. Four human volunteers received 2 mg of labelled MI and MCI separately and at least 2 weeks apart. Consecutive and complete urine samples were collected over 48 h and were examined for the content of N-methylmalonamic acid (NMMA), a previously reported animal metabolite. NMMA represented 23.7 and 13.3% of the dose excreted in urine after dosage of MI and MCI, respectively, with more than 90% excreted within the first 24 h. Excretion of NMMA was rapid with mean half-lives of 6.1 and 7.6 h for MI and MCI, respectively. We have for the first time determined important human toxicokinetic data for the biocides MI and MCI that might be of relevance in future exposure and risk assessments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Methylisothiazolinone (MI) and the mixture of chloromethylisothiazolinone/methylisothiazolinone (3:1, MCI/MI, trade name for example: Kathon CG®, ACTICIDE® SPX) are important biocidal active substances that are used in a variety of commercial products (Scientific Committee of Consumer Safety (SCCS) 2009, 2016). The most important use is the conservation of cosmetic products (only “rinse-off”), where maximum concentrations of 100 and 15 ppm are permitted for MI and the mixture of MCI/MI in the European Union, respectively, (The European Parliament 2009). But both biocidal active substances are also used in many (liquid) household products like dishwashing liquid, cleansers, laundry detergents or wet wipes (Reinhard et al. 2001; Aerts et al. 2015; Garcia-Hidalgo et al. 2017). Both MI and MCI/MI are readily taken up via skin contact (Berthet et al. 2017). In occupational settings, these biocides might be used in cutting fluids, cooling systems or in the paper or cellulose industry (Hartwig 2013). In animal experiments, the acute, chronic and developmental toxicity of MI and MCI/MI was very low with reported NOAELs ranging from 30 mg/kg/d (rabbit developmental toxicity) up to 66–94 mg/kg/day (rat 3-month oral toxicity) (Burnett et al. 2010). However, a possible neurotoxic action of MI from in vitro experiments is still under discussion (Du et al. 2002; He et al. 2006).
The widespread use of these substances in products of everyday life as well as the possible volatilization of MI and MCI to indoor air after application of products containing these biocides (Lundov et al. 2014) makes an exposure of the general population very likely, either by direct skin contact or uptake by inhalation. This potential exposure might raise concerns, as both MI and MCI/MI are known skin allergens and a steep increase in diagnosed skin sensitizations against these substances in the general population has been reported worldwide (Scientific committee of consumer safety 2016; Latheef and Wilkinson 2015).
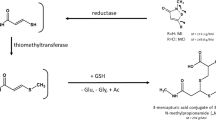
MI and MCI are rapidly metabolized in mammals and are not excreted unchanged. The metabolic pathways of both compounds are nearly identical, making it impossible to distinguish between exposure to MI or MCI from a biomarker perspective. After oral gavage of radiolabelled MI, feces was only a minor route of excretion in Sprague–Dawley rats (21–37%) compared to 53–70% recovered in urine and cage rinse. One of the main urinary metabolites of both MI and MCI in animal experiments in Sprague–Dawley rats was N-methylmalonamic acid (NMMA), representing 21–23% of the dose (Burnett et al. 2010). The structures of the substances in question and the metabolite are shown in Fig. 1.
In our previous works (Schettgen et al. 2017), we have developed a GC/MS/MS-method to specifically quantify NMMA in human urine and found a background excretion of this metabolite in a pilot study in urine samples of the general population (median: app. 3 µg/g creatinine). Currently, there are no indications that NMMA is formed by unspecific physiological processes or from other xenobiotics according to the literature. Thus, we consider NMMA to be a promising biomarker of human exposure to the biocides MI and MCI/MI.
Consequently, the aim of this study was the thorough investigation of human metabolism and renal excretion of NMMA after separate oral dosage of MI and MCI to volunteers. With the data obtained, we can calculate metabolic conversion factors for NMMA for both compounds and other important toxicokinetic parameters. To be able to specifically distinguish between background excretion of NMMA and the metabolites from oral dosage, we have dosed isotopically labelled isothiazolinones (13C3-MI and D3-MCI) and quantified the corresponding labelled urinary NMMA. The derived metabolic conversion factors allow the backcalculation of the actual daily intake of MI and MCI/MI from the NMMA-levels found in the general population. Within risk assessment, these levels can then be compared with the toxicologically derived NOAELs from animal experiments or other health based guidance values, if available for MI and MCI/MI. To our knowledge, there has been no previous investigation of the human metabolism of MI and/or MCI.
Experimental design
In January 2016, four healthy volunteers (2 m/2 f, age 20–44 years, body weight 75–100 kg) received an oral dose of 2 mg of either labelled 13C3-MI or D3-MCI (16.3 and 13 µMol, respectively) in 200 µL of ethanol in a glass of water separately and at least 2 weeks apart. An overview on the biometric and anamnestic data of the volunteers is given in Table 1.
The resulting dosages of the isothiazolinones amounted to 20–26.7 µg/kg body weight and are three orders of magnitude lower than the lowest NOAEL from animal experiments. Furthermore, this dose level is even comparatively lower than human metabolism studies on other sensitizing substances such as lysmeral previously conducted (Scherer et al. 2017). Due to the risk of possible skin sensitization, we have omitted a skin dosage experiment for ethical reasons.
The volunteers provided urine samples before the dosage (t 0) and consecutively collected their full urine over the following 48 h. The time of each urination was noted by the volunteer. Urine volume for each void was determined by weighing difference of the filled and empty urine containers. The urine samples provided were aliquoted in 10-ml aliquots and stored frozen at −20 °C until analysis. Creatinine content of the urine samples was determined photometrically using the Jaffé method (Larsen 1972).
Chemicals
13C3-2-methylisothiazolinone and 5-chloro-2-(methyl-D3)-isothiazolinone and 13C3-N-methylmalonamic acid was synthesized by Dr. Belov, Max Planck Institute for Biophysical Chemistry, Göttingen with a purity >95% as determined by 1H-NMR and mass spectrometry. N-acetyl-cysteine (>99%) was purchased from Merck (Darmstadt, Germany) and 2,3,4,5,6-pentafluorobenzylbromide (PFBBr, 99%) was obtained from Aldrich (Steinheim, Germany). Potassium carbonate (p.a.) and dried acetonitrile (SeccoSolv, max. 0.005% water) was also obtained from Merck (Darmstadt, Germany). Toluene, n-hexane and ethanol (abs.) were of the highest analytical grade available and supplied by Merck (Darmstadt, Germany).
Analytical procedure
The quantification of urinary labelled NMMA was performed according to our previously published GC/MS/MS-method (Schettgen et al. 2017). In short, 100 µL of urine was freeze dried and then redissolved in 1 mL of dry acetonitrile (SeccoSolv) by short vortexing and subsequent placing in an ultrasonic bath for 2 min. 100 µL of the derivatization solution of PFBBr (5 g in 6 ml acetonitrile) were added and after that, app. 10–20 mg of potassium carbonate were added to the sample. The vials were sealed tightly and placed in an oven at 60 °C overnight for 16 h. After the addition of 3 ml of an aqueous solution of N-acetyl-cysteine (10 g/L) and subsequent extraction of the PFB-derivative of NMMA, the combined organic phases were evaporated under a gentle stream of nitrogen to dryness and redissolved in toluene, transferred to microvials and sealed tightly with vial caps. A 1 µl volume of this sample was then analysed by GC–MS/MS in EI-mode. Gas chromatographic separation was performed using a HP-5-MS capillary column (crosslinked 5%-Phenyl-95%-dimethylpolysiloxane, 60 m × 0.25 mm I.D., 0.25 µm film thickness) purchased from Agilent (Waldbronn, Germany). We used Helium 5.0 as carrier gas at a constant flow of 1.2 ml/min. The simultaneous determination of two specific ion transitions in the tandem mass spectrometer for the PFB-derivative provides high sensitivity and specificity. Different from the original method, quantification of labelled NMMA in the native samples was performed using a calibration in pooled urine with 13C3-NMMA (D3-NMMA showing identical mass transitions) and adding unlabelled NMMA as internal standard to the urine samples at a high concentration (750 µg/L), outnumbering possible background excretions of NMMA in these urine samples. The precision (relative standard deviation %) of the original method ranged from 3.7 to 10.9% and accuracy was between 89.1 and 113.9%. The limit of quantification of the method was 0.5 µg/L urine for NMMA.
Statistical analysis
The statistical analysis was performed using Microsoft Excel 2010. The function for decreasing analyte levels after the maximum concentration were calculated by exponential regression as previously reported by Lessmann et al. for the phthalate substitute DEHTP (Lessmann et al. 2016). The regression equation was as follows:
with k as urinary excretion constant, and Δt as time after maximum concentration in hours. The individual urinary half-life for each volunteer and each dosage was then calculated as natural logarithm of 2, divided by k (Fichtl 2001).
Results and discussion
The four volunteers donated between 11 and 17 individual urine samples over 48 h with a total volume ranging from 3.91 to 6.39 L. The corresponding labelled NMMA as metabolite of 13C3-MI and D3-MCI was determined in all urine samples post-dosage. Figure 2 shows exemplarily extracted ion chromatograms of one volunteer pre- and post-dosage of 13C3-MI. In the pre-dose sample (A, creatinine: 1.63 g/L), the level of 13C3-NMMA was below LOQ (<0.5 µg/L), while in the first post-dose sample (B, creatinine: 1.87 g/L), the level of 13C3-NMMA was determined to be 1073 µg/L.
The urinary excretion kinetics of 13C3-NMMA after dosage of 2 mg 13C3-MI in all four volunteers are depicted in Fig. 3a, b on a logarithmic scale in µg/L and creatinine-adjusted values, respectively. Figure 4a, b show the kinetics of D3-NMMA after dosage of 2 mg D3-MCI in the same persons.
The maximum concentrations for NMMA varied from 174 to 1375 µg/L (574 to 1048 µg/g creatinine) and were obtained 2–3 h post-dosage for 13C3-MI. For dosage of D3-MCI, maximum concentrations of D3-NMMA were considerably lower with levels ranging from 115 to 579 µg/L (192–333 µg/g creatinine) and also with a slightly later time of maximum. The elimination curves for NMMA were very similar for all four volunteers, both for dosage of 13C3-MI as well as for D3-MCI. A significant difference in kinetics could not be observed between the male and female volunteers. Also BMI showed no significant influence on kinetics. As visible from Figs. 3 and 4, creatinine correction considerably smoothed the elimination curves for NMMA, as creatinine values were highly variable within these 48 h for all volunteers, ranging from 0.12 to 2.70 g/L. Notably, labelled NMMA was still quantifiable in all urine samples 48 h post-dosage with values well above LOQ. The whole dataset for all volunteers and all dosages is given in the supplemental information to this article (Supplement Tables 1, 2). The renal clearance of NMMA for all dosages and volunteers is also displayed in the Supplement (Figure S1).
After a rapid absorption and distribution phase, NMMA was excreted via urine rather fast with maximum concentrations appearing 2–4 h post-dosage. The elimination phase of NMMA occurred via first order kinetics for both dosages. We have calculated the individual urinary half-life for NMMA as described under Statistical analysis using the creatinine-corrected excretions and found a half-life ranging from 5.0 to 7.4 h (mean 6.1 h) after dosage of 13C3-MI and a slightly slower half-life ranging from 6.3 to 10.3 h (mean 7.6 h) after dosage of D3-MCI. Consequently, with more than three half-lives, excretion of NMMA was more than 90% complete within 24 h post-dosage for both compounds.
The cumulative excreted doses of labelled NMMA for all volunteers and both dosages are displayed in Fig. 5a, b. Under consideration of the molar masses, the mean total excreted amount of labelled NMMA (urinary excretion factor, FUE) within 48 h post-dosage amounts to 23.7% (range 18.0–30.9%) after dosage of MI and 13.3% (range 10.9–15.9%) after dosage of MCI. This is comparable to the percentage excretion of NMMA reported for Sprague–Dawley rats after application of radiolabelled MI (21–23%). Consequently, NMMA can be regarded as a major human metabolite of both MI and MCI. It is worth noticing that the amount of NMMA after oral dosage of MI showed higher variability than for MCI with two volunteers excreting app. 18%, while two others excreted nearly 30%. It is known that MCI shows higher reactivity (and sensitizing potency) compared to MI and the initial ring opening is faster, probably not involving enzymatic processes. Thus, we might speculate that enzymatic polymorphisms might be responsible for the variability in dose excretion of NMMA after dosage of MI. Table 2 summarizes the pharmacokinetic data obtained for all volunteers and both dosages.
Conclusion
With this study, we provide the first data on human metabolism of the biocidal active substances MI and MCI. The use of isotopically labelled substances allowed the unequivocal quantification of urinary labelled NMMA without being influenced by background levels. NMMA has previously been reported as main metabolite of these biocides in rats and with our determined mean metabolic conversion factors of 23.7 and 13.3% for MI and MCI in humans, we confirm NMMA to be also a main human metabolite and potential biomarker of exposure. Currently, there are no indications on other sources for the excretion of urinary NMMA than exposure to MI and/or MCI according to an intensive literature search. Further major urinary metabolites of MI described in animal experiments represent a mercapturic acid conjugate of 3-thiomethyl-N-methylpropionamide as well as N-methyl-3-hydroxypropionamide (Burnett et al. 2010). The investigation of other metabolites of MI and MCI in future studies might help to complete the picture on the human metabolism of MI and/or MCI.
References
Aerts O, Meert H, Goossens A, Janssens S, Lambert J, Apers S (2015) Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermat 73:142–149. doi:10.1684/ejd.2015.2608
Berthet A, Spring P, Vernez D, Plateel G, Hopf NB (2017) Ex vivo human skin permeation of methylchloroisothiazolinone (MCI) and methylisothiazolinone (MI). Arch Toxicol. doi:10.1007/s00204-017-1978-x (in press)
Burnett CL, Bergfeld WF, Belsito DV, Klaassen CD, Marks JG, Shank RC, Slaga TJ, Snyder PW, Alan Andersen F (2010) Final report of the safety assessment of methyl-iso-thia-zo-linone. Int J Toxicol 29(4 Suppl):187S–213S. doi:10.1177/1091581810374651
Du S, McLaughlin B, Pal S, Aizenman E (2002) In vitro neurotoxicity of methylisothiazolinone, a commonly used industrial and household biocide, proceeds via a zinc and extracellular signal-regulated kinase mitogen-activated protein kinase-dependent pathway. J Neurosci 22(17):7408–7416
Fichtl B, Starke K (2001) Arzneistoffkonzentration im organismus in abhängigkeit von der zeit: pharmakokinetik im engeren sinn. In: Forth W, Henschler D, Rummel W, Förstermann U (eds) Allgemeine und spezielle pharmakologie und toxikologie, 8. Auflage. Urban und Fischer Verlag, München, pp 56–77
Garcia-Hidalgo E, Sottas V, von Götz N, Hauri U, Bogdal C, Hungerbühler K (2017) Occurence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermat 76(2):96–106. doi:10.1111/cod.12700
Giménez-Arnau AM, Scientific Committee of Consumer Safety (SCCS) (2016) Opinion of the Scientific Committee on Consumer safety (SCCS)—Opinion on the safety of the use of Methylisothiazolinone (MI) (P94), in cosmetic products (sensitisation only). Regul Toxicol Pharmacol l76:211–212. doi:10.1016/j.yrtph.2016.01.001
Hartwig A (ed) (2013) The MAK Collection for Occupational Health and Safety: 2-Methyl-4-isothiazolin-3-one (MAK value documentation)., pp 1–26. doi:10.1002/3527600418.mb268220kske5515
He K, Huang J, Lagenaur CF, Aizenman E (2006) Methylisothiazolinone, a neurotoxic biocide, disrupts the association of SRC family tyrosine kinases with focal adhesion kinase in developing cortical neurons. J Pharmacol Exp Ther 317(3):1320–1329
Larsen K (1972) Creatinine assay by a reaction-kinetic principle. Clin Chim Acta 41:209–217
Latheef F, Wilkinson SM (2015) Methylisothiazolinone outbreak in the European Union. Curr Opin Allergy Clin Immunol 15:461–466. doi:10.1097/ACI.0000000000000208
Lessmann F, Schütze A, Weiss T, Langsch A, Otter R, Brüning T, Koch HM (2016) Metabolism and urinary excretion kinetics of di(2-ethylhexyl)terephthalate (DEHTP) in three male volunteers after oral dosage. Arch Toxicol 90(7):1659–1667
Lundov MD, Kolarik B, Bossi R, Gunnarsen L, Johansen JD (2014) Emission of isothiazolinones from water-based paints. Environ Sci Technol 48:6989–6994. doi:10.1021/es500236m
Reinhard E, Waeber R, Niederer M, Maurer T, Maly P, Scherer S (2001) Preservation of products with MCI/MI in Switzerland. Contact Dermat 45(5):257–264
Scherer M, Koch HM, Schütze A, Pluym N, Krnac D, Gilch G, Leibold E, Scherer G (2017) Human metabolism and excretion kinetics of the fragrance lysmeral after a single oral dosage. Int J Hyg Environ Health 220(2):123–129. doi:10.1016/j.ijheh.2016.09.005
Schettgen T, Bertram J, Kraus T (2017) Quantification of N-methylmalonamic acid in urine as metabolite of the biocides methylisothiazolinone and chloromethylisothiazolinone using gas chromatography-tandem mass spectrometry. J Chromatogr B 1044–1045:185–193
Scientific Committee of Consumer Safety (SCCS) (2009) Opinion on the mixture of 5-chloro-2-methylisothiazolin-3(2H)-one and 2-methylisothiazolin-3(2H)-one (P56). SCCS/1238/09, Adopted at 08 December 2009. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_009.pdf. Accessed: 08 June 2016
The European Parliament (2009) Regulation (EC) no. 1223/2009 of the European parliament and of the council of 30 November 2009 on cosmetic products. Official Journal of the European Union, L 342/59ff
Acknowledgements
The development of the analytical method and its first application in a human metabolism study are part of a large-scale 10-year project on the advancement of human biomonitoring in Germany. This project is a cooperation agreed in 2010 between the German Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety (BMUB) and the Verband der Chemischen Industrie e.V. (German Chemical Industry Association—VCI) and is managed by the German Environmental Agency (UBA). In this cooperation project, the analytical method development and the human metabolism study are financed by the Chemie Wirtschaftsförderungsgesellschaft mbH by a grant to the University hospital of RWTH Aachen University. Experts from governmental scientific authorities, industry and science closely accompany and advise the project in selecting substances and developing methods.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study design was in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Approval for the study protocol was obtained from the Ethics Commission of the Faculty of Medicine of the RWTH Aachen University, Germany (Reg. no. EK 336/14). Written informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schettgen, T., Kraus, T. Urinary excretion kinetics of the metabolite N-methylmalonamic acid (NMMA) after oral dosage of chloromethylisothiazolinone and methylisothiazolinone in human volunteers. Arch Toxicol 91, 3835–3841 (2017). https://doi.org/10.1007/s00204-017-2051-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-017-2051-5