Abstract
Di(2-ethylhexyl) terephthalate (DEHTP) is used as a substitute for di(2-ethylhexyl) phthalate (DEHP), an ortho-phthalate-based plasticizer that is classified and labeled due to its toxicity to reproduction. In this study the metabolism and urinary excretion kinetics of DEHTP were investigated by single oral dosage of 50 mg DEHTP to three male volunteers (resulting in individual dosages between 0.55 and 0.59 mg/kg body weight). Separate urine samples were consecutively collected for 48 h. In analogy to DEHP, we quantified specific side-chain-oxidized monoester metabolites of DEHTP (5OH-MEHTP, 5oxo-MEHTP, 5cx-MEPTP and 2cx-MMHTP) by HPLC–MS/MS with online sample clean-up and isotope dilution. All postulated metabolites were detectable in all samples after dosage. The predominant, specific urinary metabolite was 5cx-MEPTP representing about 13.0 % of the applied dose as mean of the three volunteers (range 7.0–20.4 %) in urine, followed by 5OH-MEHTP (mean: 1.8 %; range 1.3–2.4 %) and 5oxo MEHTP (mean: 1.0 %; range 0.6–1.6 %). 2cx-MMHTP was a minor metabolite representing only 0.3 % (range 0.2–0.4 %). In total, about 16.1 % of the dose was recovered in urine as the above investigated specific metabolites within 48 h with the major share (95 %) being excreted within the first 24 h. Investigation of the glucuronidation patterns revealed that the carboxy-metabolites are excreted almost completely in their free form (>90 %), whereas for 5OH-MEHTP and 5oxo-MEHTP, glucuronidation is preferred (>70 %). With this study we provide reliable urinary excretion factors to calculate DEHTP intakes based on metabolite concentrations in environmental and occupational studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics and polymers have become an integral part of today’s everyday life. For some polymers (e.g., polyvinyl chloride; PVC) plasticizers are needed to adjust the polymer´s flexibility depending on its field of application. Many of the classically used plasticizers belong to the substance class of phthalates. Some phthalates however, such as di(2-ethylhexyl) phthalate (DEHP) have been shown to possess reprotoxic properties in rodents (Foster 2006) or are under scrutiny regarding testicular effects and anti-androgenic activity (Boberg et al. 2011). Typical effects, also known as the “phthalate syndrome,” caused by these phthalates are: reduced amount of motile sperms, infertility and influence on the male phenotype. Testicular testosterone reduction during sensitive windows of sexual differentiation has been identified as one relevant mode of action (Furr et al. 2014). Consequently some phthalates, among them DEHP, have been classified as toxic to reproduction category 1B according to the CLP (Regulation (EC) No 1272/2008). DEHP, di-iso-nonyl phthalate (DiNP), and other phthalates like di(iso-decyl) phthalate (DiDP) or di(n-octyl) phthalate (DnOP) have been restricted in sensitive applications such as toys or childcare articles according to Regulation (EC) No 1907/2006, Annex XVII, 51/52. However, the demand for plasticized products is still strong. Therefore, the worldwide plasticizer market is in an ongoing state of flux, and alternative plasticizers with no labelling requirements, no use restrictions, and an advantageous toxicological profile are gaining importance (Bizzari et al. 2013; Bui et al. 2016).
One of these substitute plasticizers is di(2-ethylhexyl) terephthalate (DEHTP), CAS Registry No. 6422-86-2, a structural isomer of DEHP. While the core structure of phthalates like DEHP is 1,2-benzene-dicarboxylic acid, the core structure of DEHTP is 1,4-benzene-dicarboxylic acid. In both DEHTP and DEHP, these isomeric core structures are esterified with 2-ethylhexanol. Toxicological studies with DEHTP have not shown any of the critical effects associated with DEHP toxicity (“phthalate syndrome”) (Earl Grey et al. 2000). A repeated oral dose study derived a no observed effect level (NOEL) of 500 mg DEHTP/kg bw/d based upon increased relative liver weight; peroxisome proliferation was not noted (Barber and Topping 1995). Another study (Topping et al. 1987) reported a very weak peroxisome proliferating potential, albeit only at the highest dietary DEHTP content of 2.5 %. The authors concluded that relative liver weight might have been increased due to reduced feed consumption. In 2008, the European Food Safety Authority (EFSA) evaluated DEHTP (EFSA 2008) and derived a tolerable daily intake (TDI) of 1000 µg/kg bw/day based upon a 2-year combined toxicity/carcinogenicity study (Deyo 2007); the most sensitive end points observed were effects on the retina and nasal turbinates. This TDI is a factor of 20 higher than the TDI for DEHP of 50 µg/kg bw/d (EFSA 2005) and a factor of 50 higher than the reference dose (RfD) of 20 µg/kg bw/d (US EPA 1987).
Data on the Western European production volumes of DEHTP indicate the growing importance of DEHTP as a substitute plasticizer. In 2002 the production volume of DEHTP amounted to a total of 2.000 metric tons. This production volume rose to 45.000 metric tons in 2012, and production volumes are predicted to further rise to 90.000 metric tons in the year 2018 (Bizzari et al. 2013). DEHTP is already used in a wide range of applications from food contact materials, toys, medical devices, and floorings to cable insulations (Eastman 2014; EFSA 2008). Both, the increasing production volumes and the multiplicity of applications close to the consumer suggest exposures of the general population to DEHTP.
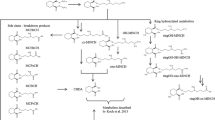
Human biomonitoring studies, through the measurement of specific urinary metabolites, are a proven tool to quantify and evaluate individual and population exposures to phthalates and other plasticizers (Silva et al. 2003, 2004; Koch et al. 2004, 2005, 2006, 2012, 2013; Kasper-Sonnenberg et al. 2014; Schütze et al. 2014). The omnipresent exposure of the general population to phthalates, and some of their novel substitutes like DINCH (=Hexamoll®DINCH®, registered trademarks of BASF SE) and bis(2-propylheptyl) phthalate (DPHP) has already been shown in several studies (Schütze et al. 2014, 2015). Recently, we have published a sensitive analytical method for the determination of oxidized DEHTP metabolites in urine (Lessmann et al. 2016). These metabolites, depicted in Fig. 1, are analogous to the oxidized metabolites of DEHP (Koch et al. 2004, 2005, Kato et al. 2005, Silva et al. 2006) and have already been identified as relevant DEHTP metabolites by in vitro (human) (Silva et al. 2015) and in vivo (rat) (Barber et al.1994) studies. In a pilot biomonitoring study (Lessmann et al. 2016), we have detected one or more of these specific metabolites in the majority (94 %) of spot urine samples from the general German population, and thus proven that these metabolites are promising biomarkers of human DEHTP exposure.
Metabolic pathway of DEHTP to specific, side-chain-oxidized monoesters (modified according to Lessmann et al. 2016). Cleavage to the unspecific metabolite terephthalic acid (TPA), and phase II metabolism (conjugation with, e.g., glucuronic acid) not shown for simplification
Consequently, the aim of this study was the detailed investigation of the human metabolism and renal excretion of DEHTP after oral dosage, and the derivation of metabolic conversion factors for the above mentioned, specific DEHTP metabolites. Metabolic conversion factors enable the back calculation of actual DEHTP intakes in terms of daily intake (in µg/kg bw/day) from urinary metabolite levels. For risk assessment these calculated daily intake levels can be compared with toxicologically derived health benchmarks like the no observed adverse effect level (NOAEL) from animal studies or the derived tolerable daily intake (TDI)/acceptable daily intake (ADI) values, as recently performed for the plasticizer alternatives DINCH and DPHP (Schütze et al. 2014, 2015).
Experimental design
In 2014, three healthy male volunteers (aged 32–45; body weight 85–95 kg) received an oral dose of about 50 mg (weighted exactly) DEHTP dissolved in 1 ml ethanol in a chocolate coated waffle cup containing water. The resulting dosages amounted to 0.55–0.59 mg/kg bw. The volunteers did not have any occupational exposure to DEHTP. The DEHTP dose was about a factor of two below the EFSA evaluated TDI of 1 mg/kg bw/day (EFSA 2008) and considerably lower than the NOEL for chronic toxicity of 79 mg/kg bw/day (Deyo 2007). Furthermore, the dose level is comparable with human metabolism studies on phthalates and alternative plasticizers successfully conducted by our group (Koch et al. 2003a, b; Koch and Angerer 2007; Schütze et al. 2014; Leng et al. 2014). The volunteers donated urine samples right before the oral dosage (t = 0) and consecutively collected their full urine samples for the following 48 h. The time of the urine void was recorded by each volunteer. Sample volume was determined as the mass difference between an empty and a filled sample container. Urine samples were stored in 250 ml polyethylene containers frozen at −20 °C until further use.
Chemicals
1-Mono-(2-ethyl-5-hydroxy-hexyl) benzene-1,4-dicarboxylate (5OH-MEHTP), 1-Mono-(2-ethyl-5-oxo-hexyl) benzene-1,4-dicarboxylate (5oxo-MEHTP), 1-Mono-(2-ethyl-5-carboxyl-pentyl) benzene-1,4-dicarboxylate (5cx-MEPTP), 1-Mono-(2-carboxyl-methyl-hexyl) benzene-1,4-dicarboxylate (2cx-MMHTP), and their D4-ring labelled analogues were synthesized by Dr. Belov, Max Planck Institute for Biophysical Chemistry, Göttingen, Germany. All synthesized compounds had a purity >95 % determined by 1H-NMR. HPLC-grade water and acetonitrile were purchased from Carl Roth, Karlsruhe, Germany. Ammonium acetate (>98 %) was purchased from Sigma-Aldrich, Steinheim, Germany. β-glucuronidase from E. coli K12 (with no aryl-sulfatase side activity) was purchased from Roche Diagnostics, Mannheim, Germany. Acetic acid and formic acid were purchased from Merck, Darmstadt, Germany.
Analytical procedure
The quantification of DEHTP metabolites was conducted with our previously published HPLC–MS/MS method with isotope dilution and online sample clean-up (Lessmann et al. 2016). In short, to each sample aliquot of 300 µl, 100 µl of ammonium acetate buffer and 20 µl internal standard solution were added. For enzymatic hydrolysis of glucuronidated DEHTP metabolites, to each sample 6 µl of β-glucuronidase from E. coli K12 (diluted 1:1 with ammonium acetate buffer) were added and incubated at 37 °C for 2.5 h. After incubation, the pH was adjusted with 10 µl acetic acid, and samples were frozen over night to precipitate proteins. After thawing, samples were centrifuged and 50 µL of the supernatant were injected into an Agilent Technologies LC 1260 system (Agilent 1260 autosampler, two Agilent 1260 binary pumps coupled to an AB Sciex 4500 triple quadrupole mass spectrometer in negative ionization mode). Online sample clean-up and enrichment of analytes was conducted with a Capcell Pak® C18-MG-II (Waters, 10 × 4 mm, particle size 5 µm) column. Chromatographic separation was performed on an Accucore™ Phenyl X column (Thermo Scientific, 150 × 3 mm, particle size 2.6 µm). The limit of quantification was 0.2 µg/L for 5cx-MEPTP and 5oxo-MEHTP, 0.3 µg/L for 5OH-MEHTP, and 0.4 µg/L for 2cx-MMHTP. Accuracy (relative recovery: 95.8–111 %) and precision (relative standard deviation: <7 %) were highly acceptable. HPLC gradient, column assembly, and MS/MS conditions remain as described in Lessmann et al. (2016). Urinary creatinine concentrations were determined according to Jaffe (1886).
Statistical analysis
Statistical analysis was conducted with Microsoft Excel 2010. Time-dependent functions for decreasing analyte levels (c(t)) after the maximum metabolite concentration (c max) were calculated by exponential regression. k is the metabolite-specific urinary excretion constant. ∆t is the time after c max.
The metabolic half time in urine is given by the natural logarithm of two over k, according to Clark and Smith (Clark and Smith 1986).
Results and discussion
The three volunteers donated 20, 22, and 23 individual urine samples with total 48-h urine volumes of 3620, 4050, and 5590 ml, respectively. We were able to detect and quantify the specific DEHTP metabolites in all donated post-dose samples analyzed. Examples of extracted ion chromatograms of pre- and post-dose samples of one volunteer for each of the four target analytes are shown in Fig. 2.
The left column of Fig. 2 shows extracted ion chromatograms from a representative pre-dose urine sample. The dotted lines represent the labelled internal standards for the respective metabolites, spiked to concentrations of ~12 µg/L. The bold lines show the quantifier and qualifier ion transitions of the four target metabolites. In this pre-dose sample we could detect only 5cx-MEPTP at a level of 1.4 µg/L, with the other three metabolites below the respective LOQs (0.2–0.4 µg/L). Of note, the peaks eluting before the respective DEHTP metabolites represent known background levels of the structurally analogous DEHP metabolites with similar fragmentation characteristics in mass spectrometry. However, these DEHP metabolites were chromatographically separated from the DEHTP metabolites, as described in Lessmann et al. (2016). The right column of Fig. 2 shows extracted ion chromatograms of a urine sample 3 h after dosage. Pronounced peaks of all four DEHTP metabolites are visible in these chromatograms at concentrations of 86.7 µg/L for 5OH-MEHTP, 50.2 µg/L for 5oxo-MEHTP, 923 µg/L for 5cx-MEPTP and 21.5 µg/L for 2cx-MMHTP. These post-dose metabolite levels are several orders of magnitude higher than levels observed in the pre-dose samples or background levels observed in samples from the general population (Lessmann et al. 2016). Thus, the known background exposure to ubiquitous DEHTP of the general population, or the study volunteers respectively, did not interfere with this oral dosage metabolism study.
The urinary excretion kinetics for the four specific DEHTP metabolites are depicted in Fig. 3 on a logarithmic scale in µg/L and creatinine adjusted values in µg/g creatinine.
5cx-MEPTP was the predominant urinary metabolite of the four specific DEHTP metabolites investigated with maximum concentrations between 4900–7790 µg/L and 3300–10,000 µg/g creatinine occurring 2–7 h post-dose. 5OH-MEHTP was the second most important metabolite, though at levels one order of magnitude lower than 5cx-MEPTP, closely followed by 5oxo-MEHTP. 2cx-MMHTP levels were the lowest of all four metabolites investigated. Forty-eight hours post-dose, all metabolites were still detectable in the urine samples of all three individuals with concentrations well above the respective limits of quantification. Metabolite elimination curves were rather similar for all three individuals and the four metabolites and creatinine adjustment lead to somewhat smoother elimination curves. Elimination curves depicted as clearance (in µg/h) are given in supplemental Fig. 1 (Online Resource).
All four metabolites were excreted via urine rather rapidly with the bulk of the dose excreted within 24 h. After absorption and distribution, maximum urinary concentrations occurred at around 4–5 h post-dose for all metabolites. All metabolites were excreted in a single elimination phase with an estimated elimination half time of about 7 h. Basic elimination kinetic data are given in Table 1.
Taking the molar masses of the metabolites and the exact mass of the orally applied DEHTP into account, we calculated the urinary excretion factors (F ue) of the DEHTP metabolites as percentages of the applied dose. Over the sampling time of 48 h, the predominant specific DEHTP metabolite was 5cx-MEPTP with 13.00 % of the applied dose, followed by 5OH-MEHTP with 1.82 % and 5oxo-MEHTP with 1.00 %. 2cx-MMHTP was a minor metabolite with a metabolic conversion factor of 0.29 %. In total, about 16.10 % (mean of the three volunteers) of the orally applied dose of DEHTP was excreted within 48 h as the four specific metabolites. For the simple monoester MEHTP with no oxidative modification, we estimated a metabolic conversion factor of about 0.02 % (data not shown). Within the first 24 h, about 15.20 % was excreted as the four specific metabolites investigated, followed by less than 1 % being excreted 24–48 h after the dose. Thus, about 95 % of the total amount of recovered specific DEHTP metabolites was excreted within 24 h post-dosage. Detailed F ue for all investigated metabolites are given in Table 2.
We can compare the share of the excreted DEHTP metabolites with the share of the analogous DEHP metabolites previously determined by Koch et al. (2005), Anderson et al. (2011) and Kessler et al. (2012): The share of oxidized DEHTP metabolites excreted in urine (~16 %) is considerably smaller than the share reported for the analogous DEHP metabolites 5cx-MEPP, 5OH-MEHP and 5oxo-MEHP (41–57 %). Looking at the oxidized metabolites individually, the renal excreted shares of 5OH-MEHTP (~2 %) and 5oxo-MEHTP (~1 %) are about one order of magnitude lower than the renal excreted shares of 5OH-MEHP (15–25 %) and 5oxo-MEHP (11–15 %). Only the carboxylated DEHTP metabolite 5cx-MEPTP (13 %) is excreted in shares comparable to the carboxylated DEHP metabolite 5cx-MEPP (14–22 %). For the simple monoester MEHTP we determined a negligible share of 0.02 %, while for the DEHP monoester MEHP shares of ~6 % have been reported. We assume that one reason for this lower share of DEHTP monoester metabolites might be that DEHTP is cleaved more rapidly to terephthalic acid (TPA) than DEHP to phthalic acid (PA). The structural differences between the two isomers might make DEHTP more accessible to (enzymatic) ester cleavage than DEHP. This would be in accordance with a study describing the kinetics of enzymatic cleavage of aromatic ethers, where the authors observed that para-substituted aromatic ethers were cleaved to a bigger extent than the corresponding ortho-substituted ethers (Axelrod 1956). Our findings in regard to the share of side-chain-oxidized metabolites of DEHTP are also in good accordance with the results from Silva et al. (2015) and Barber et al. (1994). They reported that TPA was the main metabolite of DEHTP in vitro and in vivo (rat), respectively. Since TPA is an unspecific metabolite of DEHTP and thus not applicable as a biomarker of human DEHTP exposure, we did not quantify TPA in the samples of our metabolism study. However, based upon the rather low share of the monoester metabolites excreted in urine in our study, we also can assume that TPA would have shown up as the major metabolite, if analyzed.
One common characteristics of DEHTP and DEHP metabolism is that the carboxylated monoester metabolites 5cx-MEPTP and 5cx-MEPP are the major oxidized metabolites excreted in urine. In DEHTP metabolism the share of 5cx-MEPTP is even more dominant than the share of 5cx-MEPP in DEHP metabolism. This finding is in some contrast to the results obtained from in vitro experiments using human liver microsomes (Silva et al. 2015). In their study, the hydroxylated metabolite 5OH-MEHTP seemed to be the most dominant oxidized DEHTP metabolite. However, the authors already speculated that the fraction of DEHTP excreted as 5cx-MEPTP may be higher in vivo than in vitro, because in vitro metabolism of DEHP also produced 5cx-MEPP only as a minor metabolite (Silva et al. 2007) in contrast to being the major urinary DEHP metabolite in humans (Koch et al. 2005).
In addition to the metabolic conversion factors, we investigated the glucuronidation patterns of the specific DEHTP metabolites. To do so, representative 48-h pooled urine samples were prepared separately for each of the three volunteers. By processing these pooled samples as described above once with and once without addition of the β-glucuronidase (replaced with high purity water), we were able to determine the shares of DEHTP metabolites in unconjugated form (sample preparation without enzyme) and glucuronidated form (difference between total amount determined after enzymatic deconjugation and unconjugated amount determined without enzymatic deconjugation). Detailed percentages of unconjugated metabolites (in relation to total amounts) are given in Table 3.
The distribution of free and glucuronidated metabolites was similar over the three volunteers investigated. Whereas the hydroxy- and oxo-metabolites were excreted with about 70 % predominantly in their glucuronidated form, the more polar carboxy-metabolites were excreted almost completely (>90 %) in their free (unconjugated) form. These findings are in good accordance with studies on human DINCH metabolism (Koch et al. 2013), who reported that conjugation with glucuronic acid is preferred for the hydroxy- and oxo-metabolite compared to the carboxy metabolite. Silva et al. (2003) investigated the glucuronidation patterns of different monoester phthalate metabolites and also observed that the more polar metabolites are excreted preferably in their free form compared to the more lipophilic metabolites. It must be noted, that the conjugation patterns of the metabolites investigated might change over the time course of excretion. The values presented provide information on the conjugation pattern of a 48-h pooled urine for each study volunteer.
Conclusion
With this study we provide the first human in vivo data investigating the metabolism and urinary excretion kinetics of DEHTP, a direct substitute for high molecular weight plasticizers like DEHP. As the most dominant and promising specific urinary biomarker of DEHTP exposure, we identified 5cx-MEPTP with 13 % of an orally applied dose excreted via urine. This share is roughly comparable to the analogous DEHP metabolite 5cx-MEPP that represents between 14 and 22 % of an orally applied dose. The other oxidized DEHTP metabolites 5OH-MEHTP and 5oxo-MEHTP are clearly of lesser importance representing only 2 and 1 % of the dose, respectively. This finding is in some contrast to DEHP metabolism where the analogous metabolites represented much bigger shares of the renal excreted dose. This has to be kept in mind when directly comparing urinary DEHTP and DEHP metabolite levels with each other in human biomonitoring studies. The simple monoester MEHTP, representing only 0.02 % of the orally applied dose in urine cannot be regarded as an applicable biomarker at all.
As a note of caution we have to point out that already established analytical methodologies to quantify DEHP exposure have to be checked for sufficient chromatographic separation of DEHP from DEHTP metabolites since metabolites from both plasticizers share similar mass fragments and therefore cannot be reliably distinguished my means of mass spectrometry. Furthermore, similar restrictions as for DEHP metabolite analysis apply here for the use of the right enzyme: Since enzymes with aryl-sulfatase activity (such as HP2 from Helix pomatia) rapidly cleave the ester bonds of the monester metabolites of both DEHP and DEHTP such enzymes must not be used.
We expect rising exposures to DEHTP, because DEHTP is increasingly used as a substitute for DEHP and other high molecular weight plasticizers such as DiNP. We have already proven that the oxidized DEHTP metabolites can be detected and quantified in urine samples of the general population. In a pilot biomonitoring study with 34 spot urine samples (Lessmann et al. 2016), 5cx-MEPTP was quantifiable in 94 % of the samples analyzed. The median in these samples was 0.9 µg/L and the maximum level 38.7 µg/L. The other metabolites 5OH-MEHTP and 5oxo-MEHTP were quantifiable in only 18 and 21 %, respectively, and at considerably lower levels. This dominance of the carboxylated metabolite 5cx-MEPTP over 5OH-MEHTP and 5oxo-MEHTP can now be explained by the metabolic conversion factors derived in this study. With increasing DEHTP usage and the resulting exposures, we also expect these metabolites to be detectable in more and more urinary samples in the years to come. However, for now, 5cx-MEPTP is clearly the most sensitive urinary metabolite to detect the omnipresent exposure to DEHTP. With this metabolite we will be able to follow trends in internal exposures to DEHTP as we have previously done for other novel plasticizer substitutes such as DINCH or DPHP (Schütze et al. 2014, 2015). In conjunction with the now derived metabolic conversion factors, we enable the estimation of daily DEHTP intakes and thus contribute to a sound exposure and risk assessment for this alternative plasticizer.
References
Anderson WAC, Castle L, Hird S, Jeffery J, Scotter MJ (2011) A twenty-volunteer study using deuterium labelling to determine the kinetics and fractional axcretion of primary and secondary urinary metabolites of di-2-ethylhexylphthtalate and di-iso-nonylphthalate. Food Chem Toxicol 49(9):2022–2029
Axelrod J (1956) The enzymatic cleavage of aromatic ethers. Biochem J 63(4):634–639
Barber ED, Topping DC (1995) Subchronic 90-day oral toxicology of di(2-ethylhexyl) terephthalate in the rat. Fd Chem Toxic 33(11):971–978
Barber ED, Fox JA, Giordano CJ (1994) Hydrolysis, absorption and metabolism of di(2-ethylhexy1) terephthalate in the rat. Xenobiotica 24(5):441–450
Bizzari SN, Blagoev M, Kishi A (2013) Chemical economics handbook plasticizers. IHS Global Inc., Douglas County
Boberg J, Christiansen S, Axelstad M, Seidler Kledal T, Vinggaard AM, Dalgaard M, Nellemann C, Hass U (2011) Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol 31(2):200–209
Bui TT, Giovanoulis G, Cousins AP, Magnér J, Cousins IT, de Wit CA (2016) Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci Total Environ 541:451–467
Clark B, Smith DA (eds) (1986) An introduction to pharmacokinetics, 2nd edn. Blackwell Scientific, Oxford
Deyo JA (2007) Carcinogenicity and chronic toxicity of di-2-ethylheyl terephthalate (DEHT) following a 2-year dietary exposure in Fischer 344 rats. Food Chem Toxicol 46(3):990–1005
Eastman Chemical Company (2014) Product Datasheet Eastmann 168TM non- phthalate plasticizers; http://ws.eastman.com/ProductCatalogApps/PageControllers/ProdDatasheet_PC.aspx?Product=71072819&sCategoryName=Generic. Accessed 25 June 2015
European Food Safety Authority (2005) Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to Bis(2-ethylhexyl)phthalate (DEHP) for use in food contact materials Question No EFSA-Q-2003-191, Adopted on 23 June 2005 by written procedure. EFSA J 243:1–20
European Food Safety Authority (2008) Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 18th list of substances for food contact materials. Question No EFSA-Q-2007-167, EFSA-Q-2006-177, EFSA-Q-2005-152, EFSA-Q-2007-022, EFSA-Q-2007-004, EFSA-Q-2007-024. EFSA J 628–633:1–19
Foster PMD (2006) Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl 29(1):140–147
Furr J, Lambright C, Wilson VS, Foster PM, Gray LE Jr (2014) A short-term in vivo screen using fetal testosterone production, a key event in the phthalate adverse outcome pathway, to predict disruption of sexual differentiation. Toxicol Sci 140(2):403–424
Gray LE Jr, Ostby J, Furr J, Price M, Rao Veeramachaneni DN, Parks L (2000) Perinatal exposure to the phthalates DEHP, BBP, and DINP but not DEP, DMP or DOTP, alters sexual differentiation of the male rat. Toxicol Sci 58(2):350–365
Jaffe MZ (1886) About the precipitation caused by pikrinic acid in normal urine and about a new reaction of creatinine. Physiol Chem 10:391–400
Kasper-Sonnenberg M, Koch HM, Wittsiepe J, Brüning T, Wilhelm M (2014) Phthalate metabolites and bisphenol A in urines from German school-aged children: results of the Duisburg Birth Cohort and Bochum Cohort Studies. Int J Hyg Environ Health 217(8):830–838
Kato K, Silva MJ, Needham LL, Calafat AM (2005) Determination of 16 pthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem 77(9):2985–2991
Kessler W, Numtip W, Völkel W, Seckin E, Csanády GA, Pütz C, Klein D, Fromme H, Filser JG (2012) Kinetics of di(2-ethylhexyl) phthalate (DEHP) and mono(2-ethylhexyl) phthalate in blood and of DEHP metabolites in urine of male volunteers after single ingestion of ring-deuterated DEHP. Toxicol Appl Pharmacol 264(2):284–291
Koch HM, Angerer J (2007) Di-iso-nonylphthalate (DINP) metabolites in human urine after single oral dose of deuterium-labelled DINP. Int J Hyg Environ-Health 210(1):9–19
Koch HM, Drexler H, Angerer J (2003a) An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int J Hyg Environ Health 206(2):77–83
Koch HM, Gonzalez-Reche LM, Angerer J (2003b) On-line clean-up by multidimensional liquid chromatography electrospray ionization tandem mass spectrometry for high throughput quantification of primary and secondary pththalate metabolites in human urine. J Chromatogr B 784(1):169–182
Koch HM, Preuss R, Drexler H, Angerer J (2004) Exposure of nursery school children and their parents and teachers to di-n-butylphthalate and butylbenzylphthalate. Int Arch Occup Environ Health 78(3):223–229
Koch HM, Angerer J, Drexler H, Eckstein R, Weisbach V (2005) Di(2-ethylhexyl)phthalate (DEHP) exposure of voluntary plasma and platelet donors. Int J Hyg Environ Health 208(6):489–498
Koch HM, Preuss R, Angerer J (2006) Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure—an update and latest results. Int J Androl 29(1):155–165
Koch HM, Haller A, Weiß T, Käfferlein HU, Stork J, Brüning T (2012) Phthalate exposure during cold plastisol application—a human biomonitoring study. Toxicol Lett 213(1):100–106
Koch HM, Schütze A, Pälmke C, Angerer J, Brüning T (2013) Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH) in humans after single oral doses. Arch Toxicol 87(5):799–806
Leng G, Koch HM, Gries W, Schütze A, Langsch A, Brüning T, Otter R (2014) Urinary metabolite excretion after oral dosage of bis(2-propylheptyl) phthalate (DPHP) to five male volunteers—characterization of suitable biomarkers for human biomonitoring. Toxicol Lett 231(2):282–288
Lessmann F, Schütze A, Weiss T, Brüning T, Koch HM (2016) Determination of metabolites of Di(2-ethylhexyl) terephthalate (DEHTP) in human urine by HPLC-MS/MS with on-line clean-up. J Chromatogr B 1011:196–203
Schütze A, Kolossa-Gehring M, Apel P, Brüning T, Koch HM (2014) Entering markets and bodies: increasing levels of the novel plasticizer Hexamoll® DINCH® in 24 h urine samples from the German Environmental Specimen Bank. Int J Hyg Environ Health 217(2–3):421–426
Schütze A, Gries W, Kolossa-Gehring M, Apel P, Schröter-Kermani C, Fiddicke U, Leng G, Brüning T, Koch HM (2015) Bis-(2-propylheptyl)phthalate (DPHP) metabolites emerging in 24 h urine samples from the German Environmental Specimen Bank (1999–2012). Int J Hyg Environ Health 218(6):559–563
Silva MJ, Barr DB, Reidy JA, Kato K, Malek NA, Hodge CC, Hurtz D III, Calafat AM, Needham LL, Brock JW (2003) Glucuronidation patterns of common urinary and serum monoester pththalate metabolites. Arch Tox 77(10):561–567
Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM (2004) Urinary Levels of Seven Phthalate Metabolites in the U.S. Population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect 112(3):331–338
Silva MJ, Samandar E, Preau JL Jr, Needham LL, Calafat AM (2006) Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology 219(1–3):22–32
Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM (2007) Quantification of 22 phthalate metabolites in human urine. J Chromatogr B 860(1):106–112
Silva MJ, Samandar E, Calafat AM, Ye X (2015) Identification of di-2-ethylhexyl terephthalate (DEHTP) metabolites using human liver microsomes for biomonitoring applications. Toxicol In Vitro 29(4):716–721
The European Parliament (2006) Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC., Official Journal L 396 30/12/2006, p. 1, Consolidated text of 5 September 2015
The European Parliament (2008) Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Official Journal L 353, 31/12/2008 P. 0001-1355, Consolidated text of 06 December 2014
Topping DC, Ford GP, Evans JG, Lake BG, O´Donoghue JL, Lockhart HB (1987) Peroxisome induction studies on di(2-ethylhexyl)terephthalate. Toxicol Ind Health 3(2):63–78
United States Environmental Protection Agency (1987) Integrated Risk Information System, Chemical Assessment Summary Di(2-ethylhexyl)phthalate; CASRN 117-81-7. Last Revised 31 January 1987. http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0014_summary.pdf. Accessed 03 Feb 2016
Acknowledgments
The development of the analytical method and its first application in a human metabolism and population study are part of a large-scale 10-year project on the advancement of human biomonitoring in Germany. This project is a cooperation agreed in 2010 between the German Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety (BMUB) and the Verband der Chemischen Industrie e.V. (German Chemical Industry Association—VCI) and is managed by the German Environment Agency (UBA). In this cooperation project the analytical method development and the human metabolism study are financed by the Chemie Wirtschaftsförderungsgesellschaft mbH while the first application of the novel methodology in a population study is financed by the German Environment Agency. Experts from governmental scientific authorities, industry and science closely accompany and advise the project in selecting substances and developing methods.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study design was in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Approval for the study protocol was obtained from the Ethics Commission of the Faculty of Medicine of the Ruhr-Universität Bochum, Germany (IRB Reg. No.: 4757-13). Written informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lessmann, F., Schütze, A., Weiss, T. et al. Metabolism and urinary excretion kinetics of di(2-ethylhexyl) terephthalate (DEHTP) in three male volunteers after oral dosage. Arch Toxicol 90, 1659–1667 (2016). https://doi.org/10.1007/s00204-016-1715-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1715-x