Abstract
Aniline is an important source material in the chemical industry (e.g., rubber, pesticides, and pharmaceuticals). The general population is known to be ubiquitously exposed to aniline. Thus, assessment of aniline exposure is of both occupational and environmental relevance. Knowledge on human metabolism of aniline is scarce. We orally dosed four healthy male volunteers (two fast and two slow acetylators) with 5 mg isotope-labeled aniline, consecutively collected all urine samples over a period of 2 days, and investigated the renal excretion of aniline and its metabolites by LS-MS/MS and GC–MS. After enzymatic hydrolysis of glucuronide and sulfate conjugates, N-acetyl-4-aminophenol was the predominant urinary aniline metabolite representing 55.7–68.9 % of the oral dose, followed by the mercapturic acid conjugate of N-acetyl-4-aminophenol accounting for 2.5–6.1 %. Acetanilide and free aniline were found only in minor amounts accounting for 0.14–0.36 % of the dose. Overall, these four biomarkers excreted in urine over 48 h post-dose represented 62.4–72.1 % of the oral aniline dose. Elimination half-times were 3.4–4.3 h for N-acetyl-4-aminophenol, 4.1–5.5 h for the mercapturic acid conjugate, and 1.3–1.6 and 0.6–1.2 h for acetanilide and free aniline, respectively. Urinary maximum concentrations of N-acetyl-4-aminophenol were reached after about 4 h and maximum concentrations of the mercapturic acid conjugate after about 6 h, whereas concentrations of acetanilide and free aniline peaked after about 1 h. The present study is one of the first to provide reliable urinary excretion factors for aniline and its metabolites in humans after oral dosage, including data on the predominant urinary metabolite N-acetyl-4-aminophenol, also known as an analgesic under the name paracetamol/acetaminophen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aniline (phenylamine, CAS 65-53-3) is an important and widely used feedstock in chemical industry. The vast majority of aniline (~70 %) is used as a precursor in the production of polyurethane-based polymers. It may also be used in the manufacture of rubber additives (accelerators, antioxidants) and as an intermediate in the production of pesticides, azo dyes, and pharmaceuticals (Deutsche Forschungsgemeinschaft 1993; European Chemicals Bureau 2004; Human Biomonitoring Commission of the German Federal Environment Agency 2011). Aniline has also been detected in indoor and outdoor air (Palmiotto et al. 2001) and in the water of drinking water treatment plants (Palmiotto et al. 2001). Further, aniline is a known constituent of tobacco smoke (Grover 1989; Human Biomonitoring Commission of the German Federal Environment Agency 2011).
Depending on the occupational or environmental setting, exposure to aniline can occur through inhalation and/or oral uptake. In addition, aniline can be readily absorbed through the skin both from the liquid and gaseous phases (MAK Value Documentation in German language 1992; Korinth et al. 2006; American Conference of Governmental Industrial Hygienists 1992), thus making biological monitoring the method of choice for exposure assessment if appropriate biomarkers are at hand. Animal studies conducted in the early 1980s identified N-acetyl-4-aminophenol (NA4AP) as the major metabolite of aniline in urine, representing 75–86 % of the aniline dose. NA4AP is mainly excreted in the form of its glucuronide and sulfate conjugates, whereas free NA4AP reflects about 10 % of the dose. Other metabolites identified were O-conjugates of 2- and 4-aminophenol (5–25 % of dose). Free aniline or acetanilide (ACA) represented only 0.5–3.4 % dose (Kao et al. 1978). Neither NA4AP (and its O-conjugates) nor 4-aminophenol have been used for biological monitoring of aniline exposure in humans. NA4AP (=paracetamol) is the active ingredient of many over-the-counter analgesics. Therefore, NA4AP, NA4AP-derived metabolites, or the amino phenols are not specific to aniline. Consequently, exposure assessments to aniline both in occupational and environmental practices have been carried out by either analyzing aniline in urine or aniline-derived hemoglobin adducts in blood (Riffelmann et al. 1995; Weiss and Angerer 2002; Lewalter and Gries 2000; Lewalter and Korallus 1985). Several human biomonitoring studies have proven that, next to workers in occupational settings (el-Bayoumy et al. 1986; Riffelmann et al. 1995), the general population is ubiquitously exposed to aniline or chemicals which can release aniline during metabolism, e.g., cisanilide, clomeprop, flufenican, mefenacet, and naproanilide (Human Biomonitoring Commission of the German Federal Environment Agency 2011; Kütting et al. 2009; Weiss and Angerer 2002).
Interestingly, knowledge of the quantitative metabolism of aniline in humans is scarce despite the current specific use of aniline in urine or aniline-derived Hb adducts for exposure assessment. For example, no valid data concerning excretion kinetics and urinary conversion of aniline in humans are available. Furthermore, the limited data from animal experiments found large variations in metabolism between the surveyed animal species (Kao et al. 1978), thus making it difficult directly transferring results from animal studies to those in humans. In addition, the lack of basic toxicokinetic data of aniline in humans makes it difficult to recalculate the absolute amount of aniline taken up by humans based on biomonitoring results. This lack of data makes it difficult to establish a reference dose (RfD) or a tolerable daily intake (TDI) for aniline exposure from the environment. A recalculation is also necessary in risk assessment and risk communication, i.e., to compare the absolute amount of aniline taken up in humans to those applied in rodent toxicity studies.
Irrespective of the aniline-specific biomarkers aniline and ACA in urine, NA4AP (either as the active ingredient in analgesics or the major metabolite of aniline) recently came into the focus of scientific interest. Epidemiological and experimental (animal, ex vivo and in vitro) studies suggest intrauterine exposure to NA4AP as a possible risk factor for male reproduction disorders and possible antiandrogenic effects (Rebordosa et al. 2008, 2009; Jensen et al. 2010; Philippat et al. 2011; Christiansen et al. 2012; Albert et al. 2013). NA4AP is furthermore suspected to have detrimental effects on the neurodevelopment of the unborn child, resulting in hyperkinetic disorders in early childhood and school age (Liew et al. 2014; Thompson et al. 2014; Brandlistuen et al. 2014). NA4AP as the major metabolite of aniline was found in the mg/L range in urine samples of employees exposed to aniline air concentrations below 2 ppm (Lewalter and Korallus 1985; Deutsche Forschungsgemeinschaft 1993). In recent studies from our group with volunteers exposed to (airborne) aniline, we also quantified urinary NA4AP in the mg/L range (Dierkes et al. 2014; Modick et al. 2014). However, we detected NA4AP not only in urine samples after occupational exposure to aniline, but also in each sample from individuals without occupational exposure to aniline. While for some individuals the use of analgesics (acetaminophen/paracetamol) could explain high urinary NA4AP levels, for many other individuals with high NA4AP levels, we could rule out recent intake of the pharmaceutical by questionnaire (Modick et al. 2013, 2014; Nielsen et al. 2015). We suggested exposure to aniline to be one possible origin of the internal burden to NA4AP. In this context, not only the quantitative investigation of human aniline metabolism resulting in its “specific” biomarkers aniline and acetanilide in urine seems of utmost interest, but also its metabolism resulting in its major metabolite NA4AP and NA4AP downstream metabolites.
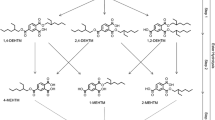
Here, we present the elimination kinetics and urinary conversion factors for aniline and acetanilide (ACA) but also basic toxicokinetic data for NA4AP and its mercapturic acid N-acetyl-4-aminophenol-3-mercapturate (NA4AP-MA) (Fig. 1) after a single oral dose of isotope-labeled aniline-d5 in four volunteers.
Experimental
Study design
Four healthy Caucasian male volunteers (30–32 years old, 71–95 kg, non-smokers) were dosed orally with 4.6 mg aniline-d5 (ring deuterated) reflecting a total intake between 48 and 64 µg/kg body weight. Isotope-labeled aniline-d5 was dosed to avoid interferences occurring from the known background exposure to aniline. For this purpose, 1 mL of a stock solution prepared from 46 mg aniline-d5 dissolved in 10 mL ethanol was spiked into decaffeinated coffee or tea and ingested in a single step as part of a breakfast. The administered doses were approximately four hundred times lower than the measured dose at 20 mg/kg bw (single oral dose) which leads to no significant increase in blood methemoglobin in rats (Jenkins et al. 1972). All volunteers had no known occupational exposure to aniline. Two of the volunteers were slow and two were fast acetylators. The acetylator phenotype (NAT2) was determined according to a previously published method (Grant et al. 1983; Bolt et al. 2005) at the Leibniz Research Centre for Working Environment and Human Factors (IfADo) in Dortmund, Germany.
Each volunteer provided one urine sample before dosage. After dosage, urine samples were collected continuously for 48 h in 250-mL polyethylene containers. Volunteers recorded the time of each urine void. Volumes of each urine void were determined as the mass difference between the empty and the filled sample container. When urine had to be collected in more than one container at a time, volumes of the containers were added, and samples were combined and mixed before further processing. Aliquots of 15 mL of each sample were stored in 15-mL polypropylene containers and frozen at −18 °C within 12 h after collection.
The study was carried out according to the Declaration of Helsinki and was approved by the ethical board of the Ruhr University Bochum (Approval No. 4730-13).
In order to investigate the human metabolism of aniline, we focused on metabolites known from former aniline metabolism studies in experimental studies (Kao et al. 1978; Lewalter and Korallus 1985). Given a cross-connection between the metabolism of aniline and the one of paracetamol, we also took into account experimental studies on paracetamol metabolism (An et al. 2012; Andrews et al. 1976; Ladds et al. 1987). Thus, we determined unconjugated aniline (AN), N-acetylaniline (acetanilide, ACA), the sum of N-acetyl-4-aminophenol (NA4AP) and its O-conjugates in terms of total NA4AP after enzymatic hydrolyses, and N-acetyl-4-aminophenol-3-mercapturate (NA4AP-MA), a mercapturic acid metabolite of NA4AP.
Because ring-labeled aniline-d5 was administered, the corresponding ring-labeled metabolites were formed, i.e., NA4AP-d4, NA4AP-MA-d3, and ACA-d5. Isotope-labeled target analytes based on the metabolism of aniline-d5 and substances used for internal standardization and quantitation (with isotope labels at different positions: NA4AP-d3, NA4AP-MA-d5, ACA-d3) are shown in Fig. 1. Aniline was determined applying a GC–MS method, while NA4AP, NA4AP-MA, and ACA were determined by HPLC–MS/MS. All analytes (except free aniline) were determined after enzymatic hydrolysis using ß-glucuronidase/arylsulfatase.
Chemicals
Aniline-d5 (CAS 4165-61-1, purity > 99.0 %), acetanilide (CAS 103-84-4, purity > 99 %), acetic acid-d4 (CAS 1186-52-3), ammonium acetate p.a., HP2 β-glucuronidase (≥100,000 U/mL), arylsulfatase activity (~7500 U/mL), 4-aminophenol, pyridine, dichloromethane, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride, 2-(N-morpholino) ethanesulfonic acid (MES), and heptafluorobutyric anhydride (HFBA) were all purchased from Sigma-Aldrich (Steinheim, Germany). N-acetyl-4-aminophenol-d4 (NA4AP-d4, CAS 64315-36-2, purity 99 %) was obtained from LGC Standards (Wesel, Germany). Acetanilide-d5 (ACA-d5, CAS 15826-91-2) was purchased from CDN Isotopes (Quebec, Canada). Paracetamol-3-mercapturate sodium salt (NA4AP-MA, CAS 52372-86-8, purity 95 %) and paracetamol-3-mercapturate-d5 sodium salt (NA4AP-MA-d5, purity 97 %) were purchased from Toronto Research Chemicals Inc. (North York, Canada). o-Toluidine-d7 was purchased from Santa Cruz Biotechnology Inc. (Heidelberg, Germany). Deionized water was obtained using a Millipore Advantage A10 with a Quantum® cartridge. Acetonitrile and methanol (LC–MS grade) were purchased from Roth (Darmstadt, Germany). Formic acid was obtained from MERCK (Darmstadt, Germany). Finally, N-acetyl-4-aminophenol-d3 and acetanilide-d3 were synthesized by selective acetylation of 4-aminophenol at the amino group and by acetylation of aniline, both with activated acetic acid-d4 as previously described by (Dierkes et al. 2014).
Analysis of total NA4AP-d4, NA4AP-MA-d3, and ACA-d5
Quantitation of total NA4AP-d4, NA4AP-MA-d3, and ACA-d5 was carried out by HPLC–MS/MS. For this purpose, stock solutions of the standards and the corresponding internal standards were prepared (see suppl. File). Internal standard solutions of NA4AP-d3 and ACA-d3 were prepared as previously described by Dierkes et al. (2014). The internal standard solution of NA4AP-MA-d5 was prepared by dissolving 1.5 mg of NA4AP-MA-d5 sodium salt in 10 mL methanol. An internal standard stock solution was prepared by mixing the three standard solutions and further dilution in water, leading to final concentrations of 800 µg/L for NA4AP-d3, 500 µg/L for ACA-d3, and 500 µg/L for NA4AP-MA-d5 (calculated paracetamol-3-mercapturic acid-d5).
Frozen urine samples were thawed and equilibrated to room temperature (RT) before analysis. All samples were vortex mixed, and aliquots of 300 µL were transferred into a 1.8-mL screw cap vial. After adding 300 µL ammonium acetate buffer (0.5 M, pH 5.5–6.0), 6 µL glucuronidase/arylsulfatase solution, and 30 µL of the internal standard solution, the samples were incubated for 3.5 h at 37 °C in a water bath. After incubation, 160 µL 3 M formic acid was added. All samples were frozen overnight at −18 °C for protein precipitation. After thawing, the samples were centrifuged at 4000 g for 10 min, the supernatant was transferred into a second 1.8-mL screw cap vial, and 25 µL was analyzed by 2D-HPLC–MS/MS. The limits of quantification (LOQ, S/N 9) were 0.5 µg/L for total NA4AP, 1 µg/L for NA4AP-MA, and 0.02 µg/L for ACA. Relative standard deviations for intraday imprecision and for inter-day imprecision were <15 % for all analytes at two different concentrations (Q low, Q high), whereas mean relative recoveries were in the range between 98.8 and 109.4 % depending on the analyte (see supplementary material Tables 5 and 6). Urinary creatinine concentrations were determined using a Beckman Coulter AU 5822 analyzer, which determines creatinine based on its color formation reaction with picric acid (Jaffé-method) (Jaffe 1886).
Analyses of unconjugated aniline-d5 by GC–MS/MS
Unconjugated aniline-d5 was determined using a previously described method (Weiss et al. 2002) with minor modifications. In short, 5 mL urine was combined with 3 mL MES buffer (0,5 M; pH 6), and 50 µL internal standard solution (o-toluidine-d7; 50 µg/L) was added. 6 mL n-hexane was added, and the mixture was shaken for 20 min on a laboratory shaker. The n-hexane was removed and concentrated to 1 mL in a vacuum centrifuge (Christ RVC 2-33 IR; 1000 g; 50 °C; 5 min). 30 µL HFBA was added, and samples were incubated for 1 h at 80 °C. Afterward, the solution was washed with 1 mL phosphate buffer (0.02 M, pH 8). The organic phase was then combined with 100 µL toluene and evaporated to 20 µL by vacuum centrifugation. From this solution, 1 µL was injected into the GC–MS/MS for quantitative analysis (see suppl. File). The LOQ for free aniline estimated by a signal-to-noise ratio of 9 was 0.1 µg/L. Method validation was performed in analogy to the HPLC method validation. Intraday and inter-day imprecisions of the method were below 10 % for both quality control concentrations (Q low and Q high). Mean relative recoveries ranged between 97 and 107 % for both spiked concentration levels (see supplementary material Table 7).
Pharmacokinetic parameters and statistical analysis
Maximum concentrations (c max) of all metabolites in urine and the corresponding time points (t max) were determined on the basis of volume-related (mg/L) and creatinine-adjusted (mg/g) values. The same is true for the determination of the elimination kinetics, which were also additionally determined using the elimination rate in µg/h. The elimination half-times were calculated from the rate constant κ (t ½ = ln(2)/κ) obtained from a first-order elimination, beginning at c max to the end of sample collection or to the time point where the metabolite level in question decreased below LOQ. The amounts of metabolites excreted (in %) were calculated based on the metabolite concentrations and the urinary volumes and are reflecting molecular mass-corrected aniline dose equivalents.
Results and discussion
The four volunteers provided 81 consecutive and complete urine voids within 48 h. HPLC–MS/MS chromatograms of the target analytes NA4AP-d4, NA4AP-MA-d3, and ACA-d5 in a representative pre-dose urine sample and a representative post-dose sample from one volunteer are shown in the supplementary material. In none of the pre-dose urine samples, any of the (labeled) target analytes was detected, whereas all analytes were found post-dose in the first urine samples of all volunteers. Similar results were obtained for unconjugated aniline-d5 by GC–MS (see supplementary material Figs. 2 and 3).
The elimination curves of all target analytes for all four volunteers are shown in Fig. 2. Elimination curves are plotted on semilogarithmic scale and represent creatinine-adjusted concentration values in µg/g creatinine. The metabolite concentrations rose rapidly within just a few hours after the dosage in all four volunteers, followed by a first-order decline during the entire time of the study (48 h). NA4AP-d4 and NA4AP-MA-d3 were detected in all post-dose urine samples of the volunteers throughout the study. In contrast, ACA-d5 and unconjugated aniline-d5 were excreted rather fast and at considerably lower concentrations. The concentrations were falling below the LOQ about 8–12 h after dosage.
Urinary excretion of aniline and aniline metabolites after single oral dosage for all four volunteers. Black lines (V1 and V2) represent the two slow and gray lines (V3 and V4) the two fast acetylators. All graphs are plotted on a semilogarithmic scale and represent aniline excretion in µg/g creatinine
Elimination characteristics, maximum urinary concentrations (c max), time of maximum concentrations (t max), and calculated elimination half-times (t ½) each based on both µg/L and creatinine-adjusted µg/g creatinine values over all four volunteers are shown in Table 1. Elimination characteristics and elimination half-times based on the elimination rate approach in µg/h are shown in the supplementary material (Fig. 4, Table 8). Maximum concentrations of NA4AP-d4 and NA4AP-MA-d3 occurred ~4–6 h after the dosage (based upon µg/L values) and ~3 h after the dosage (based upon µg/g creatinine values). Concentrations of ACA-d5 and unconjugated aniline-d5 peaked much faster at about 1 h after dosage for both the µg/L and µg/g creatinine values. The most abundant urinary target analyte was NA4AP-d4 with maximum urinary concentrations well in the mg/L range and a mean maximum concentration of 6 mg/L (range 1490–8670 µg/L). With these numbers, it has to be kept in mind that NA4AP-d4 as determined with the approach described above represents the total of unconjugated NA4AP-d4 and its O-conjugates (due to an enzymatic hydrolysis step during sample preparation). The maximum concentration of NA4AP-MA-d3 was about 0.5 mg/L (460–586 µg/L) and approximately one order of magnitude below that of total NA4AP-d4. Maximum urinary concentrations of ACA-d5 and unconjugated aniline-d5 were considerably lower and with maximum concentrations around 15–30 µg/L, each.
Elimination half-times differed considerably between the analytes but were in good agreement between the four volunteers (Table 1). Unconjugated aniline-d5 and ACA-d5 were rapidly excreted with mean elimination half-times of 1.7 and 2.2 h, respectively (based on µg/L values), and 0.9 and 1.4 h, respectively (based on µg/g creatinine values). Mean elimination half-times for NA4AP-d4 were 4.2 and 3.9 h. NA4AP-MA-d3 exhibited the longest elimination half-times of around 5 h.
Urinary excretion factors (F ue) of unconjugated aniline and its metabolites (calculated as molar equivalents of the aniline dose in %) are shown in Table 2. In total, during the 48-h course of the study, a mean of 65.2 % (55.7–68.9 %) of the aniline-d5 dose was excreted as total NA4AP-d4 in urine in terms of unconjugated NA4AP-d4 and its O-conjugates. The second most abundant target analyte was NA4AP-MA-d3 representing a mean of 4.0 % (2.5–6.1 %) of the dose. ACA-d5 and unconjugated aniline-d5 represented only a minor share of the aniline-d5 dose (~0.25 % each). The major fraction of all four target analytes (representing 68–75 % of the total dose) was excreted during the first 24 h. From 24–48 h, only a small amount of the dose (0.5–0.9 %) was excreted as total NA4AP-d4 and NA4AP-MA-d3. Neither ACA-d5 nor unconjugated aniline-d5 was detectable any more in the 24–48 h post-dose samples. However, urinary concentrations of total NA4AP-d4 and NA4AP-MA-d3 were still measurable 48 h post-dose.
As shown in our study, NA4AP and its O-conjugates are the major urinary metabolites of aniline and can be analyzed in terms of total NA4AP after using an enzymatic hydrolysis step during sample preparation. This is in accordance with Lewalter and Korallus (1985) who identified NA4AP (after acid hydrolysis) as the major metabolite after occupational exposure to aniline. However, their study design did not allow deriving urinary metabolite conversion factors. Moreover, our study refers to oral exposure and continuous urine sampling. There are still uncertainties remaining when extrapolating to other exposure and sampling types due to first-pass effect, or other absorption or elimination rates.
Investigating several animal species (pig, rat, and sheep) Kao et al. (1978) recovered a total (sum of conjugated and unconjugated) of 75–86 % of an oral aniline dose as NA4AP in urine, which is close to the conversion of 55.7–68.9 % observed in this study in humans. About 0.5 % (0.4–1.0 %) of an oral aniline dose was excreted as ACA in the urines of rats (Kao et al. 1978), which is also in good accordance with the recoveries of ACA in our study. Conversion factors for ACA in sheep and pigs, however, were approximately 10-fold higher than those observed in humans and rats. Until now, NA4AP-MA has not been subject of quantitative investigations as a metabolite of aniline in biomonitoring studies. In human metabolism studies after dosage of paracetamol (chemically identical to NA4AP), the NA4AP-MA metabolite reflected 0.5–6.1 % of the paracetamol dose (Ladds et al. 1987), which is very similar to the conversion we found from aniline (2.4–5.9 %).
Overall, our study shows a total recovery of 64.4–72.7 % of the aniline dose in terms of total NA4AP, NA4AP-MA, unconjugated aniline, and ACA. We can only speculate about the remaining 27–34 %. Certainly, excretion of additional aniline-related metabolites is highly likely, e.g., ortho- and para-aminophenol and its conjugates. Aminophenols (para-aminophenol) have been reported in animal studies to contribute to about 5.5–28.5 % of the metabolite spectrum in urine, with large interspecies variations (sheep 10.8–18.7 %; pigs 5.5–9 %; rats 20.2–28.5 % (Kao et al. 1978). Further, NA4AP-derived metabolites contributing to the aniline metabolite spectrum may be NA4AP-3-cysteine or 3-hydroxy-NA4AP-4-sulfate, which have been reported to represent 0.6–13.7 % and 0.4–4.8 % of an administered paracetamol dose, respectively (Ladds et al. 1987).
Regarding the acetylator status of the volunteers, we observed no influence on urinary excretion factors (F ue) and maximum urinary concentrations (c max) for total NA4AP and NA4AP-MA (Table 3). However, the acetylator status might have some influence on the excretion of unconjugated aniline and ACA. ACA levels in terms of F ue of the two fast acetylators (0.31 and 0.36 %) were about twofold higher than those of the two slow acetylators (0.16 and 0.19 %). In reverse, excretion of unconjugated aniline in the slow acetylators (0.28 and 0.33 %) was about twice as high compared to the fast acetylators (0.14 and 0.23 %). Notably, a shift from NA4AP to NA4AP-MA could be observed for volunteer 4 (see Table 3). This volunteer excreted the smallest amount of NA4AP-d4 and the highest amount of NA4AP-MA-d3. This may be a result of higher activity of glutathione S-transferase (GST) in this volunteer. However, we did no assessment of the GST phenotype of the volunteers in this study.
For none of the analytes, elimination half-times seemed to be influenced by the acetylator phenotype.
Summary and conclusion
In summary, the present study determined the urinary excretion kinetics and metabolic parameters for aniline and its major metabolites in four male volunteers after oral dosage of deuterium-labeled aniline. By dosing labeled aniline, we were able to avoid interferences arising from omnipresent urinary background levels of aniline, paracetamol, and their metabolites.
NA4AP and NA4AP-MA were present in the urines of the volunteers during the whole 48 h of sampling and exhibited elimination half-times which were long enough to capture aniline exposure that occurred up to 48 h in the past. However, NA4AP and NA4AP-MA are no specific biomarkers of aniline but are excreted in a comparable manner after exposure to paracetamol. This overlap of aniline and paracetamol metabolic pathways is of importance when interpreting urinary NA4AP and NA4AP-MA levels with the goal to specifically assess exposure either to aniline or to paracetamol. However, both ACA and unconjugated aniline possess short elimination half-times (0.6–1.2 h) and small excretion factors. They were also only detectable within the first 8–12 h after a relatively high dosage of 5 mg. In this study and in the occupational setting with aniline exposure, we were able to detect both unconjugated aniline and ACA in post-exposure urine samples. However, we could not detect ACA and unconjugated aniline in any urine sample from the general population, in any urine samples prior to dosage or in urine samples prior to known exposure (Modick et al. 2014; Dierkes et al. 2014). These findings are in contrast to former studies (Human Biomonitoring Commission of the German Federal Environment Agency 2011; Kütting et al. 2009; Weiss and Angerer 2002), reporting omnipresent aniline in urine, but only after hydrolysis. Our study suggests that the urinary aniline levels reported in these studies seem unlikely to be caused by either free aniline or acetanilide. Future studies will have to reveal the sources leading to aniline in urine after chemical hydrolysis.
References
Albert O, Desdoits-Lethimonier C, Lesne L, Legrand A, Guille F, Bensalah K, Dejucq-Rainsford N, Jegou B (2013) Paracetamol, aspirin and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod 28(7):1890–1898. doi:10.1093/humrep/det112
American Conference of Governmental Industrial Hygienists (1992) Occupational Safety and Health Guideline for Aniline. http://www.cdc.gov/niosh/docs/81-123/pdfs/0033-rev.pdf. Accessed 24 Mar 2015
An JH, Lee HJ, Jung BH (2012) Quantitative analysis of acetaminophen and its six metabolites in rat plasma using liquid chromatography/tandem mass spectrometry. Biomed Chromatogr. doi:10.1002/bmc.2737
Andrews RS, Bond CC, Burnett J, Saunders A, Watson K (1976) Isolation and identification of paracetamol metabolites. J Int Med Res 4(4 Suppl):34–39
Bolt HM, Selinski S, Dannappel D, Blaszkewicz M, Golka K (2005) Re-investigation of the concordance of human NAT2 phenotypes and genotypes. Arch Toxicol 79(4):196–200. doi:10.1007/s00204-004-0622-8
Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H (2014) Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int J Epidemiol 42(6):1702–1713. doi:10.1093/ije/dyt183
Christiansen S, Kortenkamp A, Axelstad M, Boberg J, Scholze M, Jacobsen PR, Faust M, Lichtensteiger W, Schlumpf M, Burdorf A, Hass U (2012) Mixtures of endocrine disrupting contaminants modelled on human high end exposures: an exploratory study in rats. Int J of Androl 35(3):303–316. doi:10.1111/j.1365-2605.2011.01242.x
Deutsche Forschungsgemeinschaft (1993) Aniline (MAK value documentation). The MAK collection for occupational health and safety. Occup Toxic VCH 6:17–36. doi:10.1002/3527600418.mb6253e0006
Dierkes G, Weiss T, Modick H, Käfferlein HU, Brüning T, Koch HM (2014) N-Acetyl-4-aminophenol (paracetamol), N-acetyl-2-aminophenol and acetanilide in urine samples from the general population, individuals exposed to aniline and paracetamol users. Int J Hyg Environ Health 217(4–5):592–599. doi:10.1016/j.ijheh.2013.11.005
el-Bayoumy K, Donahue JM, Hecht SS, Hoffmann D (1986) Identification and quantitative determination of aniline and toluidines in human urine. Cancer Res 46(12 Pt 1):6064–6067
European Chemicals Bureau (2004) EU Summary Risk Assessment Report Aniline. http://echa.europa.eu/documents/10162/0abd36ad-53de-4b0f-b258-10cf90f90493. Accessed 24 March 2015
Grant DM, Tang BK, Kalow W (1983) Polymorphic N-acetylation of a caffeine metabolite. Clin Pharmacol Ther 33(3):355–359
Grover P (1989) Chemical carcinogenesis and mutagenesis: advances in tobacco carcinogesis. 2. Cigarette smoke. Springer, London
Human Biomonitoring Commission of the German Federal Environment Agency (2011) Stoffmonographie und -Referenzwerte für -monocyklische Amino-aromaten im Urin. Stellungnahme der Kommission Human--Biomonitoring des Umweltbundesamtes. Bundesgesundheitsbl 54(5):650–663. doi:10.1007/s00103-011-1256-7
Jaffe M (1886) Über den Niederschlag, welchen Pikrinsäre in normalem Harn erzeugt und über eine neue Reaction des Kreatinins. Physiol Chem 10:391
Jenkins FP, Robinson JA, Gellatly JB, Salmond GW (1972) The no-effect dose of aniline in human subjects and a comparison of aniline toxicity in man and the rat. Food Cosmet Toxicol 10(5):671–679
Jensen MS, Rebordosa C, Thulstrup AM, Toft G, Sørensen HT, Bonde JP, Henriksen TB, Olsen J (2010) Maternal use of acetaminophen, ibuprofen, and acetylsalicylic acid during pregnancy and risk of cryptorchidism. Epidemiology 21(6):779–785. doi:10.1097/EDE.0b013e3181f20bed
Kao J, Faulkner J, Bridges JW (1978) Metabolism of aniline in rats, pigs and sheep. Drug Metab Dispos 6(5):549–555
Korinth G, Weiss T, Penkert S, Schaller KH, Angerer J, Drexler H (2006) Percutaneous absorption of aromatic amines in rubber industry workers: impact of impaired skin and skin barrier creams. Occup Environ Med 64(6):366–372. doi:10.1136/oem.2006.027755
Kütting B, Göen T, Schwegler U, Fromme H, Uter W, Angerer J, Drexler H (2009) Monoarylamines in the general population—a cross-sectional population-based study including 1004 Bavarian subjects. Int J Hyg Environ Heal 212(3):298–309. doi:10.1016/j.ijheh.2008.07.004
Ladds G, Wilson K, Burnett D (1987) Automated liquid chromatographic method for the determination of paracetamol and six metabolites in human urine. J Chromatogr 414(2):355–364
Lewalter J, Gries W (2000) Haemoglobin adducts of aromatic amines: aniline, o-, m- and p-toluidine, o-anisidine, p-chloroaniline, α-and β-naphthylamine, 4-aminodiphenyl, benzidine, 4,4′-diaminodiphenylmethane, 3,3′-dichlorobenzidine. Anal Hazard Subst Biol Mater. doi:10.1002/3527600418.biha_aame0007
Lewalter J, Korallus U (1985) Blood protein conjugates and acetylation of aromatic amines. New findings on biological monitoring. Int Arch Occup Environ Health 56(3):179–196
Liew Z, Ritz B, Rebordosa C, Lee P, Olsen J (2014) Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr 168(4):313–320. doi:10.1001/jamapediatrics.2013.4914
MAK value documentation in German language (1992) Anilin [MAK Value Documentation in German language, 1992]
Modick H, Schütze A, Pälmke C, Weiss T, Brüning T, Koch HM (2013) Rapid determination of N-acetyl-4-aminophenol (paracetamol) in urine by tandem mass spectrometry coupled with on-line clean-up by two dimensional turbulent flow/reversed phase liquid chromatography. J Chromtogr B 925:33–39. doi:10.1016/j.jchromb.2013.02.023
Modick H, Weiss T, Dierkes G, Brüning T, Koch HM (2014) Ubiquitous presence of paracetamol in human urine: sources and implications. Reproduction 147(4):R105–R117. doi:10.1530/REP-13-0527
Nielsen JK, Modick H, Mørck TA, Jensen JF, Nielsen F, Koch HM, Knudsen LE (2015) N-acetyl-4-aminophenol (paracetamol) in urine samples of 6–11-year-old Danish school children and their mothers. Int J Hyg Environ Health 218:28–33. doi:10.1016/j.ijheh.2014.07.001
Palmiotto G, Pieraccini G, Moneti G, Dolara P (2001) Determination of the levels of aromatic amines in indoor and outdoor air in Italy. Chemosphere 43(3):355–361
Philippat C, Giorgis-Allemand L, Chevrier C, Cordier S, Jégou B, Charles M, Slama R (2011) Analgesics during pregnancy and undescended testis. Epidemiology 22(5):747–749. doi:10.1097/EDE.0b013e318225bf33
Rebordosa C, Kogevinas M, HorváthPuhó E, Nørgård B, Morales M, Czeizel AE, Vilstrup H, Sørensen HT, Olsen J (2008) Acetaminophen use during pregnancy: effects on risk for congenital abnormalities. Am J Obstet Gynecol 198(2):178.e1–178.e7. doi:10.1016/j.ajog.2007.08.040
Rebordosa C, Kogevinas M, Bech BH, Sorensen HT, Olsen J (2009) Use of acetaminophen during pregnancy and risk of adverse pregnancy outcomes. Int J Epidemiol 38(3):706–714. doi:10.1093/ije/dyp151
Riffelmann M, Müller G, Schmieding W, Popp W, Norpoth K (1995) Biomonitoring of urinary aromatic amines and arylamine hemoglobin adducts in exposed workers and nonexposed control persons. Int Arch Occup Environ Health 68(1):36–43
Thompson JM, Waldie KE, Wall CR, Murphy R, Mitchell EA (2014) Associations between acetaminophen use during pregnancy and ADHD symptoms measured at Ages 7 and 11 years. PLoS One 9(9):e108210. doi:10.1371/journal.pone.0108210
Weiss T, Angerer J (2002) Simultaneous determination of various aromatic amines and metabolites of aromatic nitro compounds in urine for low level exposure using gas chromatography-mass spectrometry. J Chromatogr B 778(1–2):179–192
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The present work was completely financed by the Institute for Prevention and Occupational Medicine of the German Social Accident Insurance Institute of the Ruhr-Universität Bochum (IPA).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Modick, H., Weiss, T., Dierkes, G. et al. Human metabolism and excretion kinetics of aniline after a single oral dose. Arch Toxicol 90, 1325–1333 (2016). https://doi.org/10.1007/s00204-015-1566-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1566-x