Abstract
While bone mineral density (BMD) by dual-energy X-ray absorptiometry is the primary method of determining fracture risk, assessing bone turnover may add valuable information for the management of patients with low bone mass. Bone turnover markers (BTMs) are used in clinical trials where they can provide essential information on the biological efficacy of osteoporosis treatments. In such population-based studies, BTMs can predict fracture risk independent of BMD. When combined with BMD, they improve the fracture risk estimate above and beyond BMD alone in postmenopausal osteoporotic women. Since changes in bone turnover after the initiation of therapy with bone resorption inhibitors occur much more rapidly than changes in BMD, treatment efficacy could, in theory, be determined within weeks of using BTMs. However, such predictive value is limited by the large biological variability of these biochemical markers, even though newer automated methods have reduced their analytical variability. Consequently, widespread adoption as a means of predicting treatment efficacy in fracture prevention for individual patients cannot yet be recommended. BTMs may be useful for monitoring adherence to antiresorptive therapy and may aid in identifying patients for whom antiresorptive therapy is most appropriate. Thus, although BTMs are currently confined to clinical research applications, further improvement in assay precision may extend their diagnostic value in clinical settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The World Health Organization (WHO) employs a functional definition of osteoporosis as a disease in which “low bone mass and microarchitectural deterioration of bone tissue” increases bone fragility, which leads to a higher risk of fracture [1]. Among the several available modalities for diagnosing osteoporosis and its severity, bone mineral density (BMD) of the spine and proximal femur measured by dual-energy X-ray absorptiometry (DEXA) is considered the current standard [2]. The WHO categorizes osteoporosis in postmenopausal women as a BMD of at least 2.5 standard deviations below that of the mean BMD in young adult women (i.e., a T score ≤ −2.5) [1].
The WHO, however, acknowledges the limitations of BMD, noting that it is useful as a diagnostic measure, within the limitations of a fairly arbitrary cutoff point, but less useful as a prognostic measure. In fact, a T score ≤ −2.5 may be regarded simply as a risk factor, much as high blood pressure is a risk factor for stroke [1, 3]. Similar to the relationship between blood pressure and stroke, BMD alone can provide only an estimation of the likelihood of a future fracture. Indeed, BMD represents one important, but not exclusive, dimension of bone strength, a point well illustrated by the fact that a woman aged 75 years is four to seven times more likely than a 45-year-old woman with the same BMD to experience a fracture [4]. The limitations of BMD in fracture prediction are also underscored by a more recent study involving nearly 15,000 postmenopausal women between the ages of 50 and 104 showing that 82% of women with fractures at 1 year had T scores > −2.5, and 67% had T scores > −2.0 [5]. Thus, additional risk factors could be used to increase the predictive value of BMD [3]. To this end, a fracture risk assessment tool (FRAX) has recently been developed that allows for the assessment of fracture risk based on a series of clinical risk factors, such as body mass index, fracture history, parental fracture history, presence of secondary causes of osteoporosis, and use of glucocorticoids, as well as smoking status and alcohol consumption. Although the FRAX tool can be used with or without the inclusion of BMD, BMD of the proximal femur remains the primary component of fracture risk estimation [6].
One important determinant of bone strength that is not assessed by either BMD or clinical risk factors is the rate of bone remodeling. High bone remodeling rates have been associated with more severe forms of osteoporosis [7, 8], and it would seem obvious that a reliable assessment of bone remodeling could be a very helpful tool for predicting fracture risk. Indeed, high levels of bone turnover markers (BTMs) are associated with an increased risk of fracture [8], while reduced bone turnover is associated with therapeutic efficacy of bone resorption inhibitors, as discussed later. Until recently, it was assumed that the efficacy of antiresorptive therapy was entirely due to arresting or reversing the loss of bone mass [9]. However, clinical studies have shown that the efficacy of antiresorptive agents in fracture prevention goes beyond what can be predicted based on improvements in BMD. A meta-analysis of trials on antiresorptive agents calculated that a 20% fracture risk reduction should be expected if BMD changes were the only factor in reducing risk, whereas the actual observed reduction in fracture risk is >40% [10]. These considerations corroborate the notion that factors other than improved bone density contribute to fracture reduction with these medications. Inhibition of bone remodeling itself is certainly another mechanism by which antiresorptive agents can rapidly reduce risk of fracture. No other known mechanism of action could influence bone strength in so short a time as the near-immediate decrease in remodeling loci that occur after antiresorptive therapy [9].
Thus, there is a solid rationale for using measurements of bone turnover as a prognostic tool in the clinical setting. However, while BTMs are used in clinical trials where they can reliably report changes in bone turnover in population-based settings, their large biological variability remains a significant obstacle to broader use in the clinical setting, even though the recent development of automated assays has improved the analytical precision for measurement of several BTMs [11, 12, 13]. Nevertheless, the information provided by clinical research is useful in defining potential diagnostic applications for these markers.
Depending upon their origin, BTMs are classified as indices of either bone resorption or formation, even though in most clinical circumstances, when the two arms of the bone remodeling process are coupled, they change in parallel. The most widely used bone resorption markers are products of type I collagen breakdown generated during bone resorption (amino- or carboxyl-terminal cross-linking telopeptides, pyridinium cross-links). Osteoclast activity can be monitored by serum tartrate-resistant acid phosphatase, although the use of this marker is more limited (Table 1). Bone formation markers consist of matrix proteins (osteocalcin), products of posttranslational processing of type I collagen molecules (procollagen type I N- or C-terminal propeptides), or enzymes (alkaline phosphatase) released in the circulation from osteoblasts during their activity of bone matrix synthesis (Table 1) [14].
Markers of bone resorption
Amino-terminal cross-linking telopeptides of type I collagen (NTX)
The result of osteoclastic bone resorption, NTX is a type I collagen breakdown product that can be measured in either serum or urine. Serum NTX decreases significantly after antiresorptive therapies, but it is less sensitive than urinary NTX in detecting changes induced by such therapies [15]. The reason for this is unknown, and it is still unclear whether dietary intake of collagen can interfere with serum NTX levels. Measurement of NTX in 24 h urine has the advantage of overcoming the variability due to circadian changes in bone turnover, and the results are less sensitive to dietary collagen intake [14, 16]. NTX is measured by immunoassays based on antibodies against the α2 cross-linked fragment of type I collagen [14, 17].
Carboxyl-terminal cross-linking telopeptides of type I collagen (CTX)
As with NTX, assays for detecting CTX in both serum and urine have been developed, including enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), and an electrochemiluminescence assay [14, 18, 19]. The development of an automated method for assessment of serum CTX has greatly reduced analytical variability to <5% [20]. The C-terminal telopeptide α1 chain of type I collagen undergoes β-isomerization and racemization, an age-dependent process [21]. Therefore, the ratio of αCTX, representing the breakdown of recently synthesized collagen, to βCTX, representing aged collagen, has been proposed as an index of very rapid bone turnover. For example, the αCTX/βCTX ratio is increased in patients with Paget’s disease, consistent with the breakdown of newly synthesized woven bone, and the ratio decreases dramatically upon bisphosphonate therapy [22]. Interestingly, postmenopausal women with a higher ratio of αCTX to the age-related βCTX at baseline may be at an increased risk of fracture compared to women with lower ratios [23]. However, there is a substantial inter-individual variability of the αCTX/βCTX ratio [14], and further studies are necessary to determine whether this ratio offers an advantage over other total CTX.
PYD and DPD cross-links
Pyridinoline (PYD) and deoxypyridinoline (DPD) are covalent pyridinium cross-links also produced from the breakdown of collagen during bone resorption. They are released into circulation and pass into the urine [24, 25]. Both can be detected by RIA and ELISA, methods that afford clinical application and better sensitivity compared to the earlier high-performance liquid chromatography (HPLC) methods [14, 25, 26]. A new commercially available automated HPLC assay may improve detection with less variability than the older methods [27]. Collagen cross-links are present not only in bone but also in cartilage, vessels, and ligaments. However, since bone is a high turnover tissue relative to other PYD sources, the majority of circulating PYD and urinary PYD originates from bone [26]. On the other hand, DPD originates almost entirely from bone and dentin and is not present in ligaments, cartilage, or tendons [26]. Thus, DPD and PYD offer reliable assessment of bone resorption.
Tartrate-resistant acid phosphatase (TRACP5b)
Acid phosphatases are rather ubiquitous lysosomal enzymes. Most specifically expressed in the osteoclast is the 5b isoform (TRACP5b), which is used as a bone resorption marker. In fact, it is the only marker of osteoclast activity, and as such, it is widely used in animal studies. TRACP5b is typically increased in high bone turnover conditions, such as Paget’s bone disease, bone metastases, and multiple myeloma and after ovariectomy [28]. However, it has not gained widespread acceptance in osteoporosis, perhaps because its sensitivity to report changes in bone turnover following anti-resorptive therapy have not been as consistent as with other markers [28, 29].
Other markers of bone resorption
Other markers have been proposed in the recent past, but because of their lower bone specificity or sensitivity to changes in bone remodeling, or lack of clinically applicable assays, they have been gradually abandoned. An immunoassay that measures a large cross-link of type I collagen C-telopeptides (ICTP) in the serum has been developed, but since the helical part of the α-chains is strongly conserved among different types of collagen, the specificity of this assay in reflecting bone resorption is not the best. In fact, human studies have been disappointing. For example, serum ICTP decreased in patients on estrogen replacement therapy (ERT) but did not return to baseline after ERT as did other markers of bone resorption, suggesting that it is not as sensitive as the other assays [30]. Measurement of hydroxyproline in the urine has been widely used for many years. Since hydroxyproline is mostly present in collagen and it is not reutilized after collagen breakdown, its urine levels are correlated with bone resorption. However, substantial amounts of hydroxyproline are present in the C1q fraction of complement, and its urine concentration is heavily affected by dietary collagen [31]. In fact, correlation with histomorphometric indices of bone turnover is less than optimal [32]. In theory, hydroxylysine and its glycosylated metabolites would be better markers of bone resorption, since posttranslational lysyl hydroxylation is specific for collagen, and β-1-galactosyl-hydroxylysine is not metabolized or influenced by dietary collagen [33]. Unfortunately, there is no analytical method, aside from HPLC, that can be used in clinical settings.
Markers of bone formation
Serum osteocalcin
Osteocalcin is a bone matrix protein synthesized by mature osteoblasts. Also known as bone gla protein, it constitutes approximately 15% of the noncollagenous bone matrix proteins [34, 35]. Characteristic of osteocalcin is its calcium-binding properties, which are mediated by three vitamin K-dependent γ-carboxy glutamic acid residues [36]. Although osteocalcin is involved in the mineralization process, its precise protein function remains poorly known [26].
While most of the osteocalcin synthesized by osteoblasts is incorporated into the bone matrix, a small proportion goes into circulation [37]. Either RIA, ELISA, or a chemiluminesence immunoassay may be used to detect osteocalcin [14]. Osteocalcin in the serum is rapidly degraded, resulting in osteocalcin fragments that are detected by antibody-based assays along with the full-length molecule [37, 38, 39]. Although osteocalcin is a very good marker of bone formation, there is a high biological and circadian variability [26] which, in addition to the presence of osteocalcin fragments in the serum, may negatively affect the reproducibility of repeated measures. Rapid processing of the blood sample for the osteocalcin assay is advisable to avoid the loss of reactivity that can occur in just a few hours at room temperature. Indeed, this is a generally advisable approach for any BTM assay [26].
Serum alkaline phosphatase and bone-specific alkaline phosphatase
Alkaline phosphatase (ALP) is a glycosyl–phosphatidyl–inositol anchored ectoenzyme present on the membrane of osteoblastic cells [40]. Although its exact function is not completely clear, the presence of alkaline phosphatase on the cell membrane is required for bone mineralization [41]. Normally, ALP activity in the circulation is contributed to by bone and liver isoforms in approximately equal amounts [42]. Heat inactivation is the simplest method for distinguishing the two primary isoforms of ALP in serum [14]. However, a much better assessment of bone-specific ALP (bone ALP) is provided by immunoassays for the bone isoform. In general, total ALP assays are suitable in patients with normal liver function, but bone ALP affords greater specificity for osteoblast function [43].
Serum PINP and serum PICP
Procollagen type I N-terminal propeptide (PINP) and procollagen type I C-terminal propeptide (PICP) are peptides derived from posttranslational cleavage of type I procollagen molecules before assembly into fibrils. Some of these cleavage products pass in the circulation and therefore can be used as markers of bone formation [44, 45]. Primarily cleared by liver endothelial cells, PINP and PICP originate primarily from bone, but small contributions come from skin, tendon, dentin, and cartilage [26, 44, 46]. Since skeletal tissues undergo a higher rate of turnover than non-skeletal tissues, they contribute a preponderance of collagen propeptides to circulation [26]. The presence of PINP and PICP in serum can be determined with either RIA or ELISA, and electrochemiluminescence immunoassay analyzers [47], although PINP is preferred to PICP as a marker of bone formation. This is due in part to the fact that PICP, unlike PINP, is cleared by the mannose receptor, which in turn can be regulated by growth hormone and thyroid hormones, thus complicating interpretation in subjects with pituitary or thyroid dysfunction [46, 48, 49]. An automated method for measurement of serum PINP has recently become available, exhibiting very low (<3%) variability [12].
Bone turnover markers as diagnostic tools
A number of clinical applications have been proposed in which BTMs could serve as diagnostic tools for patients with metabolic bone diseases. Some of these remain controversial and are limited by the high biological and individual variability of these markers; others may represent more realistic applications of BTMs.
Estimation of fracture risk
As mentioned above, high bone remodeling rates have been associated with an increased risk of fractures [7, 8]. Large epidemiologic studies demonstrate that bone turnover is an independent contributor of fracture risk. Both the Os des Femmes de Lyon (OFELY) and Epidemiologie de l’Ostéoporose (EPIDOS) studies observed that women with increased levels of CTX and free deoxypyridinium (D-Pyr)—but not bone formation markers—had a twofold relative risk of hip fractures independent of bone density and physical performance [50, 51]. Combining a BTM with bone density showed an additive effect on fracture risk (Fig. 1). The Rotterdam Study similarly found a strong association between elevated pyridinoline levels and risk of hip fracture even after adjustments for BMD and disability [52]. In the OFELY Study, which included early postmenopausal women, only women in the highest quartile of four BTM had twofold higher risk of fractures compared with women with BTM within the normal range, suggesting a nonlinear relationship between excessive bone turnover and fracture risk. Although bone resorption markers are more consistently associated with fracture risk than are markers of bone formation, ALP was also found to be a good predictor of fracture risk in the Hawaii Osteoporosis Study [53]. In the EPIDOS Study, undercarboxylated osteocalcin was a significant predictor of fracture risk independent of bone density [51]. In theory, then, combining one baseline bone mass measurement with BTMs can be seen as a plausible approach to identifying those menopausal women who are at highest risk of bone loss and increased fracture risk. However, whether the additional information provided by biochemical markers can affect clinical decisions that could otherwise be made on the basis of bone density only, or whether additional subjects at risk of fractures would be identified by BTM, will have to be determined before recommending the measurement of BTMs as a part of the diagnostic process for postmenopausal women. It is also unclear whether measurement of a BTM would be helpful in estimating fracture risk in patients who cannot undergo a DEXA test, as other factors, such as body weight, smoking, family history of fractures, and geometry of the hip, may be better predictors of fracture than BTM. Finally, it should be noted that discordant results were obtained in the Study of Osteoporotic Fractures where no evidence of independent relationship between BTMs and fracture risk was found except for a trend for reduced levels of CTX in patients without fractures [54].
Association of bone mineral density and bone turnover markers with hip fracture risk in postmenopausal women >75 years old. BMD bone mineral density, CTX carboxyterminal telopeptide, DPD deoxypyridinoline. Adapted with permission of the American Society for Bone and Mineral Research [51]
Monitoring therapeutic efficacy of anti-resorptive therapy
Undoubtedly, a determination of bone turnover rate can be used to assess the biologic action, if not the therapeutic efficacy, of anti-resorptive agents. BTMs rapidly decrease during bisphosphonate therapy in postmenopausal women, and these changes are associated with increased bone mass and/or fracture rate reduction. In particular, a 3-year trial of risedronate found a significant association between a reduction in vertebral fracture risk and reductions in NTX and CTX [55], although a subsequent reanalysis of the data revealed that below a certain threshold of bone turnover reduction, such association was no longer present [56]. Nonetheless, the notion that fracture risk reduction is associated with decrease in BTM during therapy is supported by other studies on bisphosphonates. For example, ibandronate at three different doses increased lumbar spine BMD after 1 and 2 years, while CTX levels decreased after only 3 months [57]. Lower doses of intermittently administered intravenous ibandronate showed a similar relationship of increased BMD accompanied by a decrease in CTX [58]. Similar results exist for alendronate [59], and more recent data also demonstrate that zoledronic acid, administered once annually for 3 years, results in a reduction in vertebral fractures accompanied by reduction in CTX, bone ALP, and PINP levels [60]. Likewise, similar correlations have been reported for other anti-resorptive agents. In a 1-year study comparing the effects of raloxifene or hormone replacement therapy with placebo, BMD increased from baseline at lumbar spine and total body in both active treatment groups, and this was accompanied by a significant decrease in serum bone ALP, serum osteocalcin, and urine CTX [61]. More recent data demonstrate that denosumab, a fully human monoclonal antibody against receptor activator of nuclear factor kappa B ligand (RANKL), administered twice yearly for 2 years, increased lumbar spine and proximal femur BMD while decreasing CTX and PINP [62]. Similarly, decreased PINP and CTX have also been observed after treatment with strontium ranelate, without changes in bone formation markers [63]. More importantly, reduction in bone turnover has been demonstrated as an independent predictor of therapeutic efficacy on fracture risk reduction in different studies [55, 57, 59]. A meta-analysis of several randomized clinical trials on osteoporosis treatment estimated that a 70% reduction in resorption markers corresponds to a 40% reduction in the risk of nonvertebral fractures, while a 50% reduction in bone formation markers was associated with a 44% nonvertebral fracture risk reduction [64].
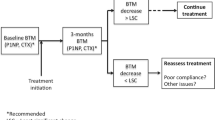
Therefore, the possibility of assessing therapeutic efficacy early using a simple measurement of BTM is very appealing and could have a significant clinical impact, considering that changes in bone turnover occur rapidly after the initiation of antiresorptive therapy, usually within weeks for oral agents [65, 66], whereas changes in BMD can only be detected after 6 months or 1 year of treatment. However, this is an area in which the inherent limitations of these markers become particularly clear and perhaps insurmountable. It is neither appropriate nor adequate to extrapolate correlations between BTMs and therapeutic response from large clinical trials to individual patients, since even the daily oscillations of bone turnover are of a magnitude not dissimilar to the changes that would be anticipated from antiresorptive therapy. For example, daily variation of DPD can be as high as 67% of the average 24-h urine excretion [67], and diurnal variations of lesser magnitude (20–30%) have been observed for serum markers, in particular CTX and NTX [68, 69]. Some measures can be taken to minimize such variability, most importantly by taking serum samples at the same time of day [26]. Fasting is also very important when testing for CTX; in general, patients should be advised not to exercise shortly before having a blood or urine sample taken [26]. While these precautions will not eliminate biological variability in BTM assays, they may help reduce some of the more extreme fluctuations. As noted earlier, the development of automated methods has substantially improved analytical precision of some BTMs [12, 20]. Nonetheless, the concept of least significant differences, commonly applied to DEXA measurements to establish statistical significance of bone density changes in individual patients [70, 71], should be considered for BTMs as well. For example, even with an intra-assay coefficient of variation of 5%, only changes higher than 15% can be considered significant in individual patients. Despite these hurdles, bone turnover assessment may add diagnostic value to BMD in monitoring treatment efficacy, but whether this additional testing and relative cost will improve therapeutic outcome will have to be determined. This conclusion also applies to anabolic agents, which increase BTMs. Although positive correlations have been observed between changes in BTMs and changes in BMD during therapy with teriparatide, it is not known whether this correlation also exists with fracture risk prevention [72]. Because of these limitations, at present, BTMs cannot be recommended for predicting treatment efficacy in individual patients.
Monitoring compliance and adherence to therapy
Monitoring compliance and adherence to treatment represents a potentially useful application of BTMs, particularly in the case of bone resorption inhibitors. Adherence to oral medications is notoriously poor and represents one of the major challenges to reducing the incidence of fractures in the elderly [73–75]. This problem is particularly acute for bisphosphonates, which must be taken following a strict dosing procedure, and the introduction of weekly and monthly oral formulations has only slightly improved adherence or persistence [76–78]. Thus, BTM could be useful in identifying a less-than-expected suppression of bone turnover, which may suggest either persistence failure (i.e., the patient has discontinued treatment) or that the patient has not been fully compliant with the dosing regimen [79]. Such findings could then be discussed with the patient and corrective measures implemented. Alternatively, inadequate suppression of BTMs might indicate poor intestinal absorption of oral bisphosphonates. Unfortunately, these highly polar compounds are poorly absorbed by the intestine [80], and low bioavailability may be more frequent than generally thought, especially in elderly patients. This problem might well be a frequent cause of “treatment failure” of oral bisphosphonates. In such cases, a change in treatment modality, e.g., switching from oral to parenteral delivery or changing to a different agent or class of medication, should be considered.
A few studies corroborate, at least in part, such premises. In postmenopausal women treated with raloxifene, NTX measurement and providing the patient with feedback during treatment increased treatment adherence and persistence [81]. However, discussion of NTX results had an impact on adherence equivalent to interaction with a nurse, suggesting that just having monitoring visits encouraged patients to continue taking their medication [81]. On the other hand, in the multinational Improving Measurements of Persistence on Actonel Treatment Trial of vertebral fractures in postmenopausal women, verbal feedback regarding changing NTX levels had an effect on persistence: Those who experienced a beneficial NTX response became more persistent in their treatment, while those with a poor BTM response became less persistent [82]. Therefore, BTMs could be used to monitor biologic efficacy or adherence to treatment with antiresorptive agents, although this of course implies that BTMs be determined before initiation of treatment, an action that cannot be recommended in every patient evaluated for osteoporosis.
Selection of pharmacologic therapy
Patients with accelerated bone turnover tend to lose bone at a faster rate than those with normal turnover; therefore, they should be the best candidates for anti-resorptive therapy. Earlier work demonstrated that postmenopausal women with high-turnover osteoporosis responded to subcutaneous salmon calcitonin therapy, with significant gains in vertebral bone density, as opposed to no changes observed in individuals with normal bone remodeling [83]. Similar results for estrogen replacement therapy were reported later [84, 85]. Greater reduction in non-vertebral fractures was observed in subjects with high PINP levels after alendronate treatment [59]. However, no such differences were detected after stratification by other markers, i.e., bone ALP or CTX [59]; nor were they reproduced in other randomized clinical trials of raloxifene or residronate [86, 87]. Despite these somewhat contradictory results, the concept of tailoring therapeutic strategies to bone turnover state remains valid, especially after the introduction of anabolic agents, which can uncouple the remodeling cycle by stimulating bone formation. Ideally, an anabolic agent would be more appropriate than an antiresorptive in cases of low-bone-turnover osteoporosis, as for example in chronic oral corticosteroid users. With the caveat of a high degree of biological variability, BTMs can certainly be considered in specific cases when determination of bone turnover status is felt to be important for therapeutic decisions.
Some concerns have been raised regarding the effect of long-term treatment with bisphosphonates and the possible effect of sustained suppression of bone remodeling. One particular concern is microcracks, which normally accumulate with age, increasing bone fragility [88]. In dogs, microdamage accumulation has been observed after 12-month treatment with alendronate, and this could increase skeletal fragility, even though in that study, bone strength and stiffness were not compromised [89]. That said, the relevance of microcracks to fracture risk has been questioned, and no compelling data support the notion that microcracks cause fractures [90]. A few reports of unusual fractures of long bones after prolonged therapy with bisphosphonates have recently come to light [91, 92]. Although in many of these cases “severe” suppression of bone turnover has been reported on bone biopsy, the pathogenesis of these unexpected fractures and their link to prolonged bisphosphonate treatment and low bone turnover remain obscure. Nonetheless, it is quite possible that some of these patients may have been in a low turnover state before starting bisphosphonate therapy and thus might not have been the most appropriate candidates for such agents. While a bone biopsy remains the best diagnostic procedure to unambiguously determine the turnover state at the tissue and cell level, as we learn more about the pathogenesis of these atypical fractures, BTMs may be a potentially useful aid in identifying subjects at risk of such adverse events.
Conclusions
At present, BTMs are not routinely used in the clinical setting. BMD is considered the method of choice for diagnosing and measuring the severity of osteoporosis. However, BTMs have a variety of potential clinical applications based on their rapid response to treatment, their value in monitoring compliance to medications, and in guiding therapeutic decisions in certain cases. While BTMs have not yet been universally accepted as diagnostic tools for prediction of fracture risk and therapeutic efficacy in clinical practice, methodological improvements may ultimately make their use more common beyond the clinical trial environment.
References
World Health Organization (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group.. World Health Organ Tech Rep Ser 843:1–129
ACOG practice bulletin (2004) Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol 103:203–216 (Number 50, January 2003)
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936
Burr DB (2003) Introduction—bone turnover and fracture risk. J Musculoskelet Neuronal Interact 3:408–409
Siris ES, Chen YT, Abbott TA et al (2004) Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 164:1108–1112
Kanis JA, Johnell O, Oden A et al (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397
Garnero P, Sornay-Rendu E, Chapuy MC et al (1996) Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11:337–349
Riggs BL, Melton LJ 3rd (2002) Bone turnover matters: the raloxifene treatment paradox of dramatic decreases in vertebral fractures without commensurate increases in bone density. J Bone Miner Res 17:11–14
Heaney RP (2003) Remodeling and skeletal fragility. Osteoporos Int 14(suppl 5):S12–S15
Cummings SR, Karpf DB, Harris F et al (2002) Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 112:281–289
Claudon A, Vergnaud P, Valverde C et al (2008) New automated multiplex assay for bone turnover markers in osteoporosis. Clin Chem 54:1554–1563
Garnero P, Vergnaud P, Hoyle N (2008) Evaluation of a fully automated serum assay for total N-terminal propeptide of type I collagen in postmenopausal osteoporosis. Clin Chem 54:188–196
Schmidt-Gayk H, Spanuth E, Kötting J et al (2004) Performance evaluation of automated assays for beta-CrossLaps, N-MID-Osteocalcin and intact parathyroid hormone (BIOROSE Multicenter Study). Clin Chem Lab Med 42:90–95
Cremers S, Garnero P (2006) Biochemical markers of bone turnover in the clinical development of drugs for osteoporosis and metastatic bone disease: potential uses and pitfalls. Drugs 66:2031–2058
Abe Y, Ishikawa H, Fukao A (2008) Higher efficacy of urinary bone resorption marker measurements in assessing response to treatment for osteoporosis in postmenopausal women. Tohoku J Exp Med 214:51–59
Clemens JD, Herrick MV, Singer FR et al (1997) Evidence that serum NTx (collagen-type I N-telopeptides) can act as an immunochemical marker of bone resorption. Clin Chem 43:2058–2063
Hanson DA, Weis MA, Bollen AM et al (1992) A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res 7:1251–1258
Risteli J, Risteli L (1999) Products of bone collagen metabolism. In: Seibel M, Robins S, Bilezikian J (eds) Dynamics of bone and cartilage metabolism. Academic, San Diego, California, pp 275–288
Risteli J, Elomaa I, Niemi S et al (1993) Radioimmunoassay for the pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen: a new serum marker of bone collagen degradation. Clin Chem 39:635–640
Garnero P, Borel O, Delmas PD (2001) Evaluation of a fully automated serum assay for C-terminal cross-linking telopeptide of type I collagen in osteoporosis. Clin Chem 47:694–702
Fledelius C, Johnsen AH, Cloos PA et al (1997) Characterization of urinary degradation products derived from type I collagen. Identification of a beta-isomerized Asp-Gly sequence within the C-terminal telopeptide (alpha1) region. J Biol Chem 272:9755–9763
Garnero P, Gineyts E, Schaffer AV et al (1998) Measurement of urinary excretion of nonisomerized and beta-isomerized forms of type I collagen breakdown products to monitor the effects of the bisphosphonate zoledronate in Paget’s disease. Arthritis Rheum 41:354–360
Garnero P, Cloos P, Sornay-Rendu E et al (2002) Type I collagen racemization and isomerization and the risk of fracture in postmenopausal women: the OFELY prospective study. J Bone Miner Res 17:826–833
Srivastava AK, Vliet EL, Lewiecki EM et al (2005) Clinical use of serum and urine bone markers in the management of osteoporosis. Curr Med Res Opin 21:1015–1026
Gunja-Smith Z, Boucek RJ (1981) Collagen cross-linking compounds in human urine. Biochem J 197:759–762
Seibel MJ (2005) Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev 26:97–122
Kraenzlin ME, Kraenzlin CA, Meier C et al (2008) Automated HPLC assay for urinary collagen cross-links: effect of age, menopause, and metabolic bone diseases. Clin Chem 54:1546–1553
Halleen JM, Alatalo SL, Janckila AJ et al (2001) Serum tartrate-resistant acid phosphatase 5b is a specific and sensitive marker of bone resorption. Clin Chem 47:597–600
Hannon R, Blumsohn A, Naylor K et al (1998) Response of biochemical markers of bone turnover to hormone replacement therapy: impact of biological variability. J Bone Miner Res 13:1124–1133
Prestwood KM, Pilbeam CC, Burleson JA et al (1994) The short-term effects of conjugated estrogen on bone turnover in older women. J Clin Endocrinol Metab 79:366–371
Eyre D (1992) New biomarkers of bone resorption. J Clin Endocrinol Metab 74:470A–470C
Delmas PD (1990) Biochemical markers of bone turnover for the clinical assessment of metabolic bone disease. Endocrinol Metab Clin North Am 19:1–18
Moro L, Pozzi Mucelli RS et al (1988) Urinary beta-1-galactosyl-0-hydroxylysine (GH) as a marker of collagen turnover of bone. Calcif Tissue Int 42:87–90
Price PA (1987) Vitamin K-dependent proteins. In: Cohn DV (ed) Calcium regulation and bone metabolism basic and clinical aspects. Amsterdam, Elsevier Science, The Netherlands, pp 419–425
Dickson IR (1993) Bone. In: Royce PM, Steinmann B (eds) Connective tissue and its heritable disorders. Wiley-Liss, New York, pp 249–285
Price PA (1985) Vitamin K-dependent formation of bone Gla protein (osteocalcin) and its function. Vitam Horm 42:65–108
Riggs BL, Tsai KS, Mann KG (1986) Effect of acute increases in bone matrix degradation on circulating levels of bone-Gla protein. J Bone Miner Res 1:539–542
Garnero P, Grimaux M, Seguin P et al (1994) Characterization of immunoreactive forms of human osteocalcin generated in vivo and in vitro. J Bone Miner Res 9:255–264
Taylor AK, Linkhart S, Mohan S et al (1990) Multiple osteocalcin fragments in human urine and serum as detected by a midmolecule osteocalcin radioimmunoassay. J Clin Endocrinol Metab 7:467–472
Low MG (1987) Biochemistry of the glycosyl–phosphatidylinositol membrane protein anchors. Biochem J 244:1–13
Harris H (1990) The human alkaline phosphatases: what we know and what we don’t know. Clin Chim Acta 186:133–150
Green S, Anstiss CL, Fishman WH (1971) Automated differential isoenzyme analysis. II. The fractionation of serum alkaline phosphatases into “liver”, “intestinal” and “other” components. Enzymologia 41:9–26
Farley JR, Chesnut CH 3rd, Baylink DJ (1981) Improved method for quantitative determination in serum of alkaline phosphatase of skeletal origin. Clin Chem 27:2002–2007
Liu SH, Yang RS, al-Shaikh R et al (1995) Collagen in tendon, ligament, and bone healing. A current review. Clin Orthop Relat Res 318:265–278
Woo SLY, An KN, Arnoczky SP et al (1994) Anatomy, biology and biomechanics of tendon, ligament and meniscus. In: Simon SR (ed) Orthopaedic basic science. American Academy of Orthopaedic Surgeons, Chicago, pp 45–88
Smedsrod B, Melkko J, Risteli L et al (1990) Circulating C-terminal propeptide of type I procollagen is cleared mainly via the mannose receptor in liver endothelial cells. Biochem J 271:345–350
Lüftner D, Jozereau D, Schildhauer S et al (2005) PINP as serum marker of metastatic spread to the bone in breast cancer patients. Anticancer Res 25:1491–1499
Toivonen J, Tähtelä R, Laitinen K et al (1998) Markers of bone turnover in patients with differentiated thyroid cancer with and following withdrawal of thyroxine suppressive therapy. Eur J Endocrinol 138:667–673
Crofton PM, Wade JC, Taylor MR et al (1997) Serum concentrations of carboxyl-terminal propeptide of type I procollagen, amino-terminal propeptide of type III procollagen, cross-linked carboxyl-terminal telopeptide of type I collagen, and their interrelationships in schoolchildren. Clin Chem 43:1577–1581
Garnero P, Sornay-Rendu E, Claustrat B et al (2000) Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY Study. J Bone Miner Res 15:1526–1536
Garnero P, Hausherr E, Chapuy MC et al (1996) Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res 11:1531–1538
van Daele PL, Seibel MJ, Burger H et al (1996) Case-control analysis of bone resorption markers, disability, and hip fracture risk: the Rotterdam Study. BMJ 312:482–483
Ross PD, Kress BC, Parson RE et al (2000) Serum bone alkaline phosphatase and calcaneus bone density predict fractures: a prospective study. Osteoporos Int 11:76–82
Bauer DC, Sklarin PM, Stone KL et al (1999) Biochemical markers of bone turnover and prediction of hip bone loss in older women: the study of osteoporotic fractures. J Bone Miner Res 14:1404–1410
Eastell R, Barton I, Hannon RA et al (2003) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 18:1051–1056
Eastell R, Hannon RA, Garnero P et al (2007) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate: review of statistical analysis. J Bone Miner Res 22:1656–1660
Reginster JY, Adami S, Lakatos P et al (2006) Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2-year results from the MOBILE Study. Ann Rheum Dis 65:654–661
Recker R, Stakkestad JA, Chesnut CH 3rd et al (2004) Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosis. Bone 34:890–899
Bauer DC, Garnero P, Hochberg MC, for the Fracture Intervention Research Group et al (2006) Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res 21:292–299
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Weinstein RS, Parfitt AM, Marcus R et al (2003) Effects of raloxifene, hormone replacement therapy, and placebo on bone turnover in postmenopausal women. Osteoporos Int 14:814–822
Bone HG, Bolognese MA, Yuen CK et al (2008) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 93:2149–2157
Anastasilakis AD, Goulis DG, Polyzos SA, et al (2009) No difference between strontium ranelate and calcium/vitamin D on bone turnover markers in women with established osteoporosis previously treated with teriparatide: a randomized controlled trial. Clin Endocrinol (Oxf). doi:10.1111/j.1365-2265.2008.03342.x
Hochberg MC, Greenspan S, Wasnich RD et al (2002) Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab 87:1586–1592
Raisz L, Smith JA, Trahiotis M et al (2000) Short-term risedronate treatment in postmenopausal women: effects on biochemical markers of bone turnover. Osteoporos Int 11:615–620
Braga de Castro Machado A, Hannon R, Eastell R (1999) Monitoring alendronate therapy for osteoporosis. J Bone Miner Res 14:602–608
Aoshima H, Kushida K, Takahashi M et al (1998) Circadian variation of urinary type I collagen crosslinked C-telopeptide and free and peptide-bound forms of pyridinium crosslinks. Bone 22:73–78
Borderie D, Roux C, Toussaint B et al (2001) Variability in urinary excretion of bone resorption markers: limitations of a single determination in clinical practice. Clin Biochem 34:571–577
Ju HS, Leung S, Brown B et al (1997) Comparison of analytical performance and biological variability of three bone resorption assays. Clin Chem 43:1570–1576
Gluer C (1999) Monitoring skeletal changes by radiologic techniques. J Bone Miner Res 14:1952–1962
Shepherd JA, Morgan SL, Lu Y (2008) Comparing BMD results between two similar DXA systems using the generalized least significant changes. J Clin Densitom 11:237–242
Bauer DC, Garnero P, Bilezikian JP et al (2006) Short-term changes in bone turnover markers and bone mineral density response to parathyroid hormone in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 91:1370–1375
Raehl CL, Bond CA, Woods TJ et al (2006) Screening tests for intended medication adherence among the elderly. Ann Pharmacother 40:888–893
McHorney CA, Schousboe JT, Cline RR et al (2007) The impact of osteoporosis medication beliefs and side-effect experiences on non-adherence to oral bisphosphonates.. Curr Med Res Opin 23:3137–3152 (Erratum in: Curr Med Res Opin. 2008;24:707)
Caro JJ, Ishak KJ, Huybrechts KF et al (2004) The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int 15:1003–1008
Cramer JA, Amonkar MM, Hebborn A et al (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21:1453–1460
Solomon DH, Avorn J, Katz JN et al (2005) Compliance with osteoporosis medications. Arch Intern Med 165:2414–2419
Cooper A, Drake J, Brankin E (2006) Treatment persistence with once monthly ibandronate and patient support vs once-weekly alendronate: results from the PERSIST Study. Int J Clin Pract 60:896–905
Seibel MJ (2006) Biochemical markers of bone turnover part II: clinical applications in the management of osteoporosis. Clin Biochem Rev 27:123–138
Russell RG, Rogers MJ (1999) Bisphosphonates: from the laboratory to the clinic and back again. Bone 25:97–106
Clowes JA, Peel NF, Eastell R (2004) The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 89:1117–1123
Delmas PD, Vrijens B, Roux C et al (2003) A reinforcement message based on bone turnover markers response influences long-term persistence with risedronate in osteoporosis: the IMPACT Study. J Bone Miner Res 18(suppl 2):S374
Civitelli R, Gonnelli S, Zacchei F et al (1988) Bone turnover in postmenopausal osteoporosis. Effect of calcitonin treatment. J Clin Invest 82:1268–1274
Chesnut CH III, Bell NH, Clark GS et al (1997) Hormone replacement therapy in postmenopausal women: urinary N-telopeptide of type I collagen monitors therapeutic effect and predicts response of bone mineral density. Am J Med 102:29–37
Uebelhart D, Schlemmer A, Johansen JS et al (1991) Effect of menopause and hormone replacement therapy on the urinary excretion of pyridinium cross-links. J Clin Endocrinol Metab 72:367–373
Ettinger B, Black DM, Mitlak BH et al (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282:637–645
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
Schaffler MB, Choi K, Milgrom C (1995) Aging and matrix microdamage accumulation in human compact bone. Bone 17:521–525
Mashiba T, Hirano T, Turner CH et al (2000) Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 15:613–620
Bouxsein ML (2003) Bone quality: where do we go from here? Osteoporos Int 14(suppl 5):118–127
Visekruna M, Wilson D, McKiernan FE (2008) Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab 93:2948–2952
Neviaser AS, Lane JM, Lenart BA et al (2008) Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma 22:346–350
Acknowledgments
Editing assistance was provided by Insight Medical Communications, which was financially supported by Roche Laboratories. Roche Laboratories did not participate in the preparation or writing of the manuscript nor did they provide direct financial support to the authors for the purpose of writing this manuscript.
Conflicts of interest
Roberto Civitelli, MD, Honoraria/Speaker Bureau: Novartis, Roche, GSK, Amgen, Research Grant Support: Eli-Lilly, Hoffman-La Roche, Stock ownership: Eli-Lilly, Wyeth, Merck, Amgen, Reina Armamento-Villareal, MD, none, Nicola Napoli, MD, none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Civitelli, R., Armamento-Villareal, R. & Napoli, N. Bone turnover markers: understanding their value in clinical trials and clinical practice. Osteoporos Int 20, 843–851 (2009). https://doi.org/10.1007/s00198-009-0838-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-0838-9