Abstract
Summary
Men experience declining bone mineral density (BMD) after hip fracture; however, changes attributable to fracture are unknown. This study evaluated the excess BMD decline attributable to hip fracture among older men. Older men with hip fracture experienced accelerated BMD declines and are at an increased risk of secondary fractures.
Introduction
The objective was to determine whether bone mineral density (BMD) changes in men after hip fracture exceed that expected with aging.
Methods
Two cohorts were used: Baltimore Hip Studies 7th cohort (BHS-7) and Baltimore Men’s Osteoporosis Study (MOST). BHS-7 recruited older adults (N = 339) hospitalized for hip fracture; assessments occurred within 22 days of admission and at 2, 6, and 12 months follow-up. MOST enrolled age-eligible men (N = 694) from population-based listings; data were collected at a baseline visit and a second visit that occurred between 10 and 31 months later. The combined sample (n = 452) consisted of Caucasian men from BHS-7 (n = 89) and MOST (n = 363) with ≥ 2 dual-energy X-ray absorptiometry scans and overlapping ranges of age, height, and weight. Mixed-effect models estimated rates of BMD change, and generalized linear models evaluated differences in annual bone loss at the total hip and femoral neck between cohorts.
Results
Adjusted changes in total hip and femoral neck BMD were − 4.16% (95% CI, − 4.87 to − 3.46%) and − 4.90% (95% CI, − 5.88 to − 3.92%) in BHS-7 participants; − 1.57% (95% CI, − 2.19 to − 0.96%) and − 0.99% (95% CI, − 1.88 to − 0.10%) in MOST participants; and statistically significant (P < 0.001) between-group differences in change were − 2.59% (95% CI, − 3.26 to − 1.91%) and − 3.91% (95% CI, − 4.83 to − 2.98%), respectively.
Conclusion
Hip fracture in older men is associated with accelerated BMD declines at the non-fractured hip that are greater than those expected during aging, and pharmacological interventions in this population to prevent secondary fractures may be warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic musculoskeletal disorder that is characterized by abnormalities in bone structure and strength [1, 2]. Epidemiologic studies have demonstrated the condition to be an increasingly under-recognized problem among older men that does not receive adequate clinical attention [3]. Osteoporosis often leads to hip fracture, the most significant sequela of the condition [4, 5]. Approximately 72,000 older men fracture a hip each year in the USA, and this rate is projected to increase 51.8% among men to 109,000 annual hip fractures by 2030 [6]. Decreased bone mineral density (BMD) results in greater bone fragility and is a strong predictor of hip fracture risk [7]. Men generally have stable BMD and largely maintain bone structure and strength throughout the life course [8]. Although, in older age, men experience accelerated bone loss, resulting in BMD declines and deficits to the structural integrity of the proximal femur, which are associated with an increased risk of hip fracture [9,10,11,12]. Thus, changes to BMD among older men have a critical role in the experience of osteoporotic hip fractures, and findings from prior studies in women cannot be generalized to men.
Also of clinical importance are BMD declines after hip fracture, leading to an increased risk for new fractures [13]. Prior studies demonstrate that men experience profound bone loss after hip fracture that is accompanied by increases in bone fragility [14, 15]. Specifically, recent evidence indicates that men sustain clinically significant declines in total hip and femoral neck BMD (2.4 and 4.6%, respectively) during the 1-year post-fracture recovery period [14]. Furthermore, decreases in bone tissue among men after hip fracture occur in conjunction with declines in proximal femur bending strength and cortical stability [15]. However, the decrement in BMD among men that is attributable to hip fracture remains unclear and is an important clinical management concern. Therefore, the objective was to compare BMD changes between older men after hip fracture and community-dwelling older men, and it was hypothesized that men after hip fracture would experience greater declines than community-dwelling older men.
Methods
Data and sample
The current study leveraged data from two existing cohorts: Baltimore Hip Studies 7th cohort (BHS-7) and Baltimore Men’s Osteoporosis Study (MOST) [14, 16]. Recruitment methods and inclusion and exclusion criteria for both studies have been published previously [14, 16]. Study protocols were approved by the University of Maryland Baltimore IRB and review boards of participating hospitals [14, 16].
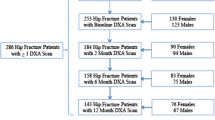
BHS-7 was a prospective observational study examining sex differences in the sequelae of hip fracture. Older adults (N = 362) hospitalized for hip fracture were recruited from 2006 to 2011 and enrolled from participating BHS hospitals in the Baltimore metropolitan area (180 males and 182 females). Five participants did not provide data at the baseline or 2-month follow-up visit, and another 18 participants were removed as a result of an IRB-requested post-procedure audit (six participants were subsequently found to be ineligible because they did not meet the study inclusion criteria, and 12 participants were determined to be ineligible secondary to failures of the informed consent process), leaving a sample of 339 participants [14]. BHS-7 participants or their proxies consented to enroll, and data were collected within 3 weeks of hospital admission and at 2-, 6-, and 12-month follow-up visits.
MOST was an observational cohort study evaluating racial differences in BMD among older men (N = 694). Volunteers were recruited from July 2000 through July 2001 from population-based listings of age-eligible men in the Baltimore metropolitan area and surrounding counties [16]. Study participants provided written informed consent, and from July 2001 to July 2003, surviving participants (N = 542) attended a second follow-up visit between 10 and 31 months (mean = 18 months) after baseline [16].
Eligible participants drawn from the BHS-7 (N = 339) and MOST (N = 694) cohorts were older Caucasian men with (n = 146) and without (n = 503) hip fracture (Fig. 1) because between-group comparisons for other racial and ethnic groups were not possible due to insufficient numbers of non-white men (n = 17) with hip fracture participating in BHS-7 that included participants who are black, Asian, and from other backgrounds (e.g., American Indian/Native Alaskan). Among eligible participants, the analytic sample was restricted to those from BHS-7 (n = 98) and MOST (n = 384) with ≥ 2 dual-energy X-ray absorptiometry (DXA) scans to estimate changes in BMD. The sample was further limited to 89 BHS-7 participants and 363 MOST participants to truncate the age, height, and weight distributions so they overlapped between cohorts. The final sample included older Caucasian men (n = 452) used to evaluate differences in annual BMD decline between those with and without hip fracture. Two secondary samples were used in sensitivity analyses: (1) a sample without covariate truncation; and (2) a sample that additionally restricted based on baseline BMD (total hip or femoral neck).
DXA
Total hip DXA scans were performed on the contralateral (i.e., non-fractured) hip in BHS-7 participants using either a Lunar Prodigy (Madison, WI, USA) or Hologic densitometer (Waltham, MA, USA) at baseline (within 22 days of hospital admission) and at 2, 6, and 12 months follow-up [14]. Participants had their BMD scans performed at one of seven different DXA facilities; four sites used Lunar Prodigy machines, and three sites used Hologic machines. The coefficient of variation for these measurements on Lunar Prodigy and Hologic machines ranged from 0.17 to 0.19% and 0.18 to 0.23%, respectively. Standardized methods were used for quality control, certification of DXA operators, and scanning procedures to guarantee the reproducibility of results [14]. Reproducibility measurements of every DXA machine were conducted separately at each clinical site and not provided by the manufacturer. While the DXA site may have changed for BHS-7 participants between visits, all scans were performed on a machine of the same manufacturer; thus, within-person BMD change was independent of inter-individual differences in DXA machine manufacturer [14].
Total hip DXA scans in MOST participants were performed by Hologic-certified technicians using a QDR-2000 (Hologic, Waltham, MA, USA) at baseline and on a QDR-4500 (Hologic) at the follow-up visit [16]. The DXA systems were calibrated daily to provide accurate BMD measurements in vivo using an anthropomorphic phantom, and precision error rates for both the QDR 2000 and QDR 4500 were 1% or less [16]. To address potential variability between QDR 2000 DXA measures at baseline and QDR 4500 DXA measures at follow-up, densitometers were cross-calibrated using data from repeated measures of hip and spine phantoms obtained on both devices. The hip and spine phantoms were measured 20 times on each QDR machine without repositioning, and t tests evaluated whether phantom measurements were significantly different between the QDR-2000 and QDR 4500. Constants were applied to baseline QDR 2000 measures to adjust for inter-machine measurement differences, and all analyses were performed using the corrected values [16].
Covariates
Covariates were selected a priori based on literature review of factors significantly (P < 0.05) associated with BMD change among participants in the Osteoporotic Fractures in Men Study and are similar to those used in prior BHS research [14, 15, 17]. Variables considered risk factors (e.g., cognitive function) for the occurrence of hip fracture, which are not predictive of BMD changes in men, were not included as potential confounders because the outcome measures were indictors of bone loss and not secondary fracture. Variables measured at study baseline were assessed via medical record abstraction and interview by study staff in BHS-7 and a modified self-administered questionnaire and interview by trained examiner in MOST [14, 16]. Measures included age (years), height (meters), weight (kilograms), smoking (ever smoked 100 cigarettes), alcohol consumption (any in the previous 12 months), comorbidity, concomitant medications, and baseline BMD (total hip and femoral neck). Comorbidity was assessed as a single continuous score using available data within both cohorts based on having a history of the following conditions: chronic obstructive pulmonary disease, depression, diabetes, kidney disease, liver disease, osteoporosis, Parkinson’s disease, rheumatoid arthritis, and peptic ulcer disease. Medication utilization was defined as using the medications within the 6 months preceding enrollment in BHS-7 and concurrent with entry in MOST [14, 16]. Bone-active drugs included etidronate, alendronate, risedronate, ibandronate, teriparatide, calcitonin, zoledronic acid, and pamidronate for BHS-7 participants and etidronate, alendronate, risedronate, and calcitonin for MOST participants. Additional concomitant medications measured in both BHS-7 and MOST included glucocorticoids (prednisone), hormone therapy (testosterone), and calcium supplements.
Statistical analysis
Frequencies and percentages were computed for categorical variables, and means and standard deviations were calculated for continuous variables. Participants’ baseline characteristics were compared between the cohorts using chi-square tests for categorical variables and Student’s t tests for continuous variables. Also, participant characteristics were compared between those with and without ≥ 2 DXA scans. To account for missing baseline covariate data (n = 22) on smoking, comorbidity, bone-active drugs, glucocorticoids, and calcium supplements, inverse probability of observation weights were used to reweight participants (n = 430) included in multivariable models to be representative of the original analytic sample (n = 452) [18, 19]. Probabilities for missing covariates were calculated using logistic regression conditioning on fully observed variables: age, height, weight, annual femoral neck BMD decline, alcohol consumption, hormone therapy, and cohort. The inverse of the conditional probability for observation was stabilized with the observed sampling fraction and used to reweight participants with complete covariate data. Weighted generalized linear models were used, where the weight was the inverse probability of observation given predictors of missing data [18, 19].
The comparison of BMD declines among men after hip fracture to those in community-dwelling older men was conducted in two stages. First, linear mixed-effect models were used to estimate individual rates of change in total hip and femoral neck BMD separately among men in BHS-7 and MOST by regressing BMD on time (years) as a continuous covariate [20]. Locally weighted scatter plot smoothing curves were used to evaluate longitudinal functional forms; there were no departures from linearity. Mixed-effect models incorporated random intercepts and slopes to address the correlation of observations within subjects. The random slope for time was used to obtain individual slopes, 1-year changes in BMD (total hip or femoral neck). Second, weighted generalized linear regression was used to model annual percent BMD decline ((slope/baseline BMD) × 100) [18, 19]. Prior BHS-7 analyses have shown this outcome to be robust to different types of sensitivity analysis, with adjustment for machine manufacturer in statistical models, conversion to a standardized BMD, and use of intra-individual percent change all yielding similar results [14]. Given that within-person BMD change is independent of inter-individual differences in DXA machine manufacturer, lack of a method to standardize values from the four different DXA machines used between cohorts, and inability to adjust for machine type in statistical models after applying constants to MOST DXA scans, operationalizing the outcome variables as annual percent change provides a reasonable and valid way to conduct between-cohort comparisons that has been used in prior studies [21, 22].
Annual percent BMD change was regressed on cohort membership (BHS-7 vs. MOST) to estimate between-group differences in decline. Multivariable models adjusted for covariates were used to calculate cohort-specific covariate-adjusted mean annual percent BMD declines and their corresponding 95% confidence intervals (95% CI). This analytical process was replicated for sensitivity analyses in secondary samples described previously to test the robustness of the study findings. Sensitivity analyses were also conducted to assess for potential non-linear effects of baseline age on BMD changes in multivariable outcome models by modeling participants’ age using different polynomial functions (e.g., quadratic, cubic) and restricted cubic smoothing splines with varying degrees of freedom (e.g., k = 3, 4, 5, 6). Statistical tests were two-sided, a Bonferroni correction (0.05/2) was applied to account for the two primary outcome variables, and significance was set at an alpha level of 0.025 [23]. All analyses were conducted using R statistical software (version 3.4.1).
Results
Sample characteristics
BHS-7 participants were older and taller and had more comorbid conditions compared to MOST participants (Table 1). Also, there was a higher frequency of bone-active drug utilization in BHS-7 than in MOST, but the proportion of use among older men in both cohorts was low. MOST participants had a significantly higher likelihood of alcohol consumption in the previous year and greater total hip BMD. Participant characteristics in BHS-7 (Online Resource 1) and MOST (Online Resource 2) were generally similar between those with and without ≥ 2 DXA scans.
Total hip BMD
Mean absolute 1-year change in total hip BMD was − 0.0233 g/cm2 per year (95% CI, − 0.0352 to − 0.0115) in BHS-7 participants (Fig. 2a) and − 0.0150 g/cm2 per year (95% CI, − 0.0273 to − 0.0026) among MOST participants (Fig. 2c). The unadjusted difference in change in total hip BMD between BHS-7 participants and MOST participants was − 0.0083 g/cm2 (95% CI, − 0.0137 to − 0.0029; P value = 0.002). Unadjusted annual percent decline in total hip BMD was greater among men in BHS-7 than in MOST (Fig. 3a). Estimated adjusted mean annual percent change in total hip BMD was − 4.16% (95% CI, − 4.87 to − 3.46%) among BHS-7 participants and − 1.57% (95% CI, − 2.19 to − 0.96%) in MOST participants. The estimated between-group difference (Table 2) in total hip BMD decline of − 2.59% (95% CI, − 3.26 to − 1.91%) was statistically significant (P value = <0.001).
Femoral neck BMD
Mean absolute 1-year change in femoral neck BMD was − 0.0270 g/cm2 per year (95% CI, − 0.0375 to − 0.0165) among BHS-7 participants (Fig. 2b) and − 0.0052 g/cm2 per year (95% CI, − 0.0174 to 0.0068) in MOST participants (Fig. 2d). The unadjusted difference in femoral neck BMD change between BHS-7 participants and MOST participants was − 0.0218 g/cm2 (95% CI, − 0.0301 to − 0.0133; P value = <0.001). Unadjusted annual percent decline in femoral neck BMD was also greater among men in BHS-7 than in MOST (Fig. 3b). Estimated adjusted mean annual percent change in femoral neck BMD was − 4.90% (95% CI, − 5.88 to − 3.92%) in BHS-7 participants and − 0.99% (95% CI, − 1.88 to − 0.10%) among MOST participants. The statistically significant difference in change (Table 2) in femoral neck BMD decline between BHS-7 participants and MOST participants was − 3.91% (95% CI, − 4.83 to − 2.98%; P value = <0.001).
Sensitivity analyses
Results from sensitivity analyses using sample inclusion criteria that were (1) less rigorous (Online Resource 3) or (2) more stringent (Online Resource 4) were similar to the primary findings. Adjustment for baseline age using different approaches that allow for non-linear functional relationships yielded findings that were identical to results from primary multivariable outcome models (data now shown).
Discussion
Results demonstrated significantly greater BMD declines in men with hip fracture than non-fractured comparators selected from community-dwelling older men. Participants from BHS-7 had adjusted annual declines in total hip and femoral neck BMD that were approximately 2.7 times and 5.0 times greater than changes in MOST participants (4.16 vs. 1.57% and 4.90 vs. 0.99%, respectively) who represent a group of men with similar characteristics and were aging without hip fracture. In context, men generally experience a small loss of bone tissue and maintain structural bone strength as they age, but these findings describe the extent to which bone homeostasis is altered in those who have a hip fracture. The results highlight the clinically significant impact of hip fracture on the already compromised and aging musculoskeletal system among older men.
Although men experience more post-fracture complications and greater mortality than women, studies evaluating the excess consequences to health attributable to hip fracture have primarily examined women, resulting in little research focused on men [21, 22, 24,25,26]. Prior studies have shown that after hip fracture, women experience greater declines in hip bone structure and strength, more functional limitations, and higher mortality rate compared to age-matched controls [21, 22, 25, 26]. Of particular importance, post-fracture decrements in BMD are responsible, in part, for the large increase in risk of secondary fractures [13, 21]. Extant research indicates that bone loss in women starts after menopause and increases until older age, when total bone mineral content (BMC) has diminished considerably [27,28,29]. These patterns of BMD change among women could be due to accelerating endocortical reabsorption and decelerating periosteal apposition, resulting in more bone loss after menopause but smaller declines as age advances because there is less bone tissue to lose [30]. By contrast, higher BMC in men is protective and inversely associated with subsequent bone loss, and BMD declines accumulate in a non-linear manner and increase exponentially [8, 10]. Trends of rapid bone loss during aging among men who are older and have lower BMD may be a consequence of increased endocortical reabsorption that is not offset by stable periosteal apposition [31]. Nonetheless, findings of the current study provide empirical data quantifying the magnitude that hip fracture alters and/or exacerbates the physiological processes that are responsible for hip bone loss among older men. Unlike research using earlier cohorts [21, 32,33,34], recent studies have demonstrated improved post-fracture outcomes in women, specifically, minimal and statistically insignificant declines in bone structure and strength [14, 15]. Collectively, current evidence demonstrates high rates of bone loss among older men, suggesting this group experiences poor post-fracture recovery, and improvements in clinical management might mitigate these changes.
The exact mechanisms underlying excess bone loss among men after hip fracture have not been definitively identified. Hip fracture is associated with significantly more activities of daily living disability than that explained by aging, likely due to the sudden reductions in physical function that occur during the post-fracture recovery period [25]. The ensuing period of prolonged bed rest and immobility after hip fracture may cause greater post-fracture bone loss among older men compared to the expected BMD declines that occur in the context of normal aging [15]. Alternatively, the strongest predictor of bone loss after hip fracture is BMD at the time of fracture, explaining 70 to 90% of the subsequent variation in change [33]. Given that higher BMC in men is protective against bone loss and fragility fractures of the hip primarily affect older adults with low BMD, men could experience accelerated decreases in bone tissue after hip fracture because of how their BMD declines increase exponentially during aging [14]. Altered physiological processes, including heightened inflammatory activity and hormonal fluctuations, may further exacerbate the alterations to bone homeostasis that are attributable to hip fracture [15]. However, osteoporosis is also under-recognized and under-treated in men, and women are more likely to undergo DXA scan and initiate oral bisphosphonates after hip fracture [3, 35]. When combined with decreased post-fracture physical function and skeletal loading, greater susceptibility to bone loss due to aging, and physiological changes after hip fracture that increase bone turnover, a lack of appropriate osteoporosis management among older men during the subsequent recovery period may lead to significant declines in bone structure and strength that ultimately elevate risk for secondary fractures and mortality [14, 15, 26].
The results have strong internal validity due to the careful selection of comparators with similar demographic and anthropometric characteristics, multivariable adjustment for many potential confounders, and statistical methods to address missing covariate data and selection bias. Also, both cohorts were recruited from the same metropolitan area during similar time periods; thus, geographic and secular trends regarding the diagnosis and treatment of osteoporosis in men were similar. In addition, the results were extremely robust to sensitivity analyses assessing the strongest potential confounders, and the magnitude of the estimated rates of BMD decline in BHS-7 and MOST participants is similar to findings in previous studies [14, 15]. Consequently, the results are likely analogous as to what would be obtained from a study of bone loss in men using a larger population-based sample. This is the first study to examine decrements in BMD among older men that occur as a consequence of hip fracture as compared to changes associated with normal aging.
Nonetheless, the findings need to be interpreted within the context of the study’s limitations. First, BHS-7 participants comprised older men who experienced a hip fracture, a group that is inherently different and less healthy than the community-dwelling older men enrolled in MOST, and both samples may lack generalizability to more diverse cohorts. However, extensive efforts were made to assure that the comparisons took into consideration as many between-cohort differences as possible, thereby leaving a reasonable estimate of the excess BMD loss attributable to hip fracture. Second, the exclusion of participants without ≥ 2 DXA scans due to attrition could induce bias, but analytical methods were used to mitigate potential selection effects, and excluded individuals were not different from included participants. Third, BHS-7 participants were followed for only 1 year, while MOST participants were assessed over a variable follow-up period, and BMD change was assumed to be constant. Different DXA machines were also used between the two cohorts, and it is well known that they yield estimates of BMD that differ in magnitude. This inter-machine measurement variability could have influenced the magnitude of the estimated annual percent changes and thus the between-cohort differences. Last, there is potential for confounding by unmeasured factors, such as pre-fracture physical activity and concomitant medications (e.g., proton pump inhibitors, selective serotonin reuptake inhibitors, and diuretics), which were either not measured or could not be harmonized. However, there would need to be (1) an unmeasured confounder strongly associated with both exposure and outcome or (2) many weak unmeasured confounders to bias the findings [36]. Given that BMD at the time of fracture is the strongest predictor of subsequent changes [33], and post-fracture bone loss is largely independent of other pre-fracture clinical characteristics [37], it is unlikely confounding by unmeasured factors substantively influenced the results. Moreover, both cohorts were designed specifically to study bone changes in older adults, and relevant and comprehensive osteoporosis-related factors were measured.
In summary, significant decrements to total hip and femoral neck BMD after hip fracture were found in older men, and these declines were several times greater than the bone loss that occurred in men who did not sustain a fracture. Deteriorations in bone structure and strength in men after hip fracture may put them at higher risk for secondary fractures, and intervention strategies that are effective in women cannot be assumed to work as well among men [24]. Men may benefit from integrated clinical management after hip fracture, and treatments to prevent bone loss during the post-fracture recovery period may be an essential part of broader intervention strategies [38]. For example, parenterally administered bisphosphonates significantly decrease the loss of BMD in older men after hip fracture [34, 39]. Thus, treatment strategies that are tailored to men and combine bone-active drugs with other types of interventions should be evaluated in future studies.
References
Beck TJ, Ruff CB, Scott WW Jr, Plato CC, Tobin JD, Quan CA (1992) Sex differences in geometry of the femoral neck with aging: a structural analysis of bone mineral data. Calcif Tissue Int 50(1):24–29
Seeman E (2002) Pathogenesis of bone fragility in women and men. Lancet 359(9320):1841–1850
Lambert JK, Zaidi M, Mechanick JI (2011) Male osteoporosis: epidemiology and the pathogenesis of aging bones. Curr Osteoporos Rep 9(4):229–236
Melton L (1993) Hip fractures: a worldwide problem today and tomorrow. Bone 14:1–8
Curtis EM, Moon RJ, Harvey NC, Cooper C (2017) The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone
Stevens JA, Rudd RA (2013) The impact of decreasing US hip fracture rates on future hip fracture estimates. Osteoporos Int 24(10):2725–2728
Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P et al (2005) Predictive value of BMD for hip and other fractures. J Bone Miner Res 20(7):1185–1194
Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW (2000) Structural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. J Bone Miner Res 15(12):2297–2304
Cawthon PM, Ewing SK, Mackey DC, Fink HA, Cummings SR, Ensrud KE et al (2012) Change in hip bone mineral density and risk of subsequent fractures in older men. J Bone Miner Res 27(10):2179–2188
Cawthon PM, Ewing SK, McCulloch CE, Ensrud KE, Cauley JA, Cummings SR et al (2009) Loss of hip BMD in older men: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res 24(10):1728–1735
Esenyel M, Ozen A, Esenyel CZ, Rezvani A, Sariyildiz MA, Ergin O (2011) Hip structural changes and fracture risk in osteopenia and osteoporosis. Eurasian J Med 43(2):73
Yates LB, Karasik D, Beck TJ, Cupples LA, Kiel DP (2007) Hip structural geometry in old and old-old age: similarities and differences between men and women. Bone 41(4):722–732
Colón-Emeric C, Kuchibhatla M, Pieper C, Hawkes W, Fredman L, Magaziner J et al (2003) The contribution of hip fracture to risk of subsequent fractures: data from two longitudinal studies. Osteoporos Int 14(11):879–883
Rathbun AM, Shardell M, Orwig D, Hebel JR, Hicks GE, Beck T et al (2016) Differences in the trajectory of bone mineral density change measured at the total hip and femoral neck between men and women following hip fracture. Arch Osteoporos 11(1):1–9
Rathbun AM, Shardell M, Orwig D, Hebel JR, Hicks GE, Beck TJ et al (2016) Difference in the trajectory of change in bone geometry as measured by hip structural analysis in the narrow neck, intertrochanteric region, and femoral shaft between men and women following hip fracture. Bone 92:124–131
Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC (2005) Racial differences in rate of decline in bone mass in older men: the Baltimore men’s osteoporosis study. J Bone Miner Res 20(7):1228–1234
Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K et al (2005) Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26(5):569–585
Shardell M, Hicks GE, Ferrucci L (2015) Doubly robust estimation and causal inference in longitudinal studies with dropout and truncation by death. Biostatistics (Oxford, England) 16(1):155–168
Shardell M, Hicks GE, Miller RR, Magaziner J (2010) Semiparametric regression models for repeated measures of mortal cohorts with non-monotone missing outcomes and time-dependent covariates. Stat Med 29(22):2282–2296
Bliese P. (2006) Multilevel modeling in R (2.2)—a brief introduction to R, the multilevel package and the nlme package
Magaziner J, Wehren L, Hawkes WG, Orwig D, Hebel JR, Fredman L et al (2006) Women with hip fracture have a greater rate of decline in bone mineral density than expected: another significant consequence of a common geriatric problem. Osteoporos Int 17(7):971–977
Reider L, Beck TJ, Hochberg MC, Hawkes WG, Orwig D, YuYahiro JA et al (2010) Women with hip fracture experience greater loss of geometric strength in the contralateral hip during the year following fracture than age-matched controls. Osteoporos Int 21(5):741–750
Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B (Methodological) 289–300
Orwig DL, Chan J, Magaziner J (2006) Hip fracture and its consequences: differences between men and women. Orthop Clin N Am 37(4):611–622
Magaziner J, Fredman L, Hawkes W, Hebel JR, Zimmerman S, Orwig DL et al (2003) Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol 157(11):1023–1031
Magaziner J, Lydick E, Hawkes W, Fox KM, Zimmerman SI, Epstein RS et al (1997) Excess mortality attributable to hip fracture in white women aged 70 years and older. Am J Public Health 87(10):1630–1636
Hansen MA, Overgaard K, Christiansen C (1995) Spontaneous postmenopausal bone loss in different skeletal areas—followed up for 15 years. J Bone Miner Res 10(2):205–210
Ensrud KE, Palermo L, Black DM, Cauley J, Jergas M, Orwoll ES et al (1995) Hip and calcaneal bone loss increase with advancing age: longitudinal results from the study of osteoporotic fractures. J Bone Miner Res 10(11):1778–1787
Hansen MA, Overgaard K, Riis BJ, Christiansen C (1991) Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ 303(6808):961–964
Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD (2006) Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res 21(12):1856–1863
Szulc P, Delmas PD (2007) Bone loss in elderly men: increased endosteal bone loss and stable periosteal apposition. The prospective MINOS study. Osteoporos Int 18(4):495–503
Fox KM, Magaziner J, Hawkes WG, Yu-Yahiro J, Hebel JR, Zimmerman SI et al (2000) Loss of bone density and lean body mass after hip fracture. Osteoporos Int 11(1):31–35
Wehren LE, Hawkes WG, Hebel JR, Orwig D, Zimmerman SI, Fox KM et al (2004) Predictors of bone loss after hip fracture. Osteoporos Int 15(2):125–131
Boonen S, Orwoll E, Magaziner J, Colón-Emeric CS, Adachi JD, Bucci-Rechtweg C et al (2011) Once-yearly zoledronic acid in older men compared with women with recent hip fracture. J Am Geriatr Soc 59(11):2084–2090
Antonelli M, Einstadter D, Magrey M (2014) Screening and treatment of osteoporosis after hip fracture: comparison of sex and race. J Clin Densitom 17(4):479–483
Groenwold RHH, Sterne JAC, Lawlor DA, Moons KGM, Hoes AW, Tilling K (2016) Sensitivity analysis for the effects of multiple unmeasured confounders. Ann Epidemiol 26(9):605–611
Wehren LE, Hawkes WG, Hebel JR, Orwig DL, Magaziner J (2005) Bone mineral density, soft tissue body composition, strength, and functioning after hip fracture. J Gerontol A Biol Sci Med Sci 60(1):80–84
Beaupre LA, Binder EF, Cameron ID, Jones CA, Orwig D, Sherrington C et al (2013) Maximising functional recovery following hip fracture in frail seniors. Best Pract Res Clin Rheumatol 27(6):771–788
Magaziner JS, Orwig DL, Lyles KW, Nordsletten L, Boonen S, Adachi JD et al (2014) Subgroup variations in bone mineral density response to zoledronic acid after hip fracture. J Bone Miner Res 29(12):2545–2551
Acknowledgments
This material is based on upon work supported (or supported in part) by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, VA Maryland Health Care System, and Baltimore VA Medical Center. The authors would like to thank the facilities, orthopedic surgeons, and hospital personnel; Baltimore Hip Studies research staff; and participants for volunteering their time and information for this work.
Funding
This work was supported by grants from the National Institute on Aging (R37 AG009901, R01 AG029315, P30 AG028747, and T32 AG000262). The funding sponsor had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jay Magaziner has consulting agreements with Ammonett, Novartis, and Pluristem. Denise Orwig has consulting agreements with Viking Therapeutics, Inc. Laura M. Yerges-Armstrong is a statistical geneticist at GlaxoSmithKline. Drs. Alan M. Rathbun, Michelle D. Shardell, Gregory E. Hicks, and Marc C. Hochberg have no disclosures to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individuals participants included in the study.
Electronic supplementary material
Supplemental Table 1
(DOCX 22 kb)
Supplemental Table 2
(DOCX 22 kb)
Supplemental Table 3
(DOCX 21 kb)
Supplemental Table 4
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Rathbun, A.M., Magaziner, J., Shardell, M.D. et al. Older men who sustain a hip fracture experience greater declines in bone mineral density at the contralateral hip than non-fractured comparators. Osteoporos Int 29, 365–373 (2018). https://doi.org/10.1007/s00198-017-4280-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4280-0