Abstract
Summary
This randomized, controlled, high-intensity strength and sprint training trial in middle-aged and older male sprint athletes showed significant improvements in mid-tibial structure and strength. The study reveals the adaptability of aging bone, suggesting that through a novel, intensive training stimulus it is possible to strengthen bones during aging.
Introduction
High-load, high-speed and impact-type exercise may be an efficient way of improving bone strength even in old age. We evaluated the effects of combined strength and sprint training on indices of bone health in competitive masters athletes, who serve as a group of older people who are likely to be able to participate in vigorous exercise of this kind.
Methods
Seventy-two men (age 40–85) were randomized into an experimental (EX, n = 40) and a control (CTRL, n = 32) group. EX participated in a 20-week program combining heavy and explosive strength exercises with sprint training. CTRL maintained their usual, run-based sprint training schedules. Bone structural, strength and densitometric parameters were assessed by peripheral QCT at the distal tibia and tibial midshaft.
Results
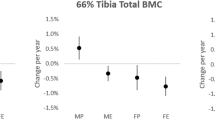
The intervention had no effects on distal tibia bone traits. At the mid-tibia, the mean difference in the change in cortical thickness (ThCO) in EX compared to CTRL was 2.0% (p = 0.007). The changes in structure and strength were more pronounced in the most compliant athletes (training adherence >75%). Compared to CTRL, total and cortical cross-sectional area, ThCO, and the area and density-weighted moments of inertia for the direction of the smallest flexural rigidity (I minA , I minD ) increased in EX by 1.6–3.2% (p = 0.023–0.006). Polar mass distribution analysis revealed increased BMC at the anteromedial site, whereas vBMD decreased (p = 0.035–0.043).
Conclusions
Intensive strength and sprint training improves mid-tibia structure and strength in middle-aged and older male sprint athletes, suggesting that in the presence of high-intensity loading exercise, the adaptability of the bone structure is maintained during aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise has shown good potential to strengthen bones by increasing bone mass, structure and strength at loaded sites across the age spectrum. Previous studies have indicated that the most osteogenic exercise includes high-magnitude loads that are unusual, dynamic, rapid, multidirectional and applied at intervals [1,2,3,4,5]. Older people with a low level of physical functioning and reduced bone and muscle strength may not, however, tolerate or be willing or able to participate in exercise at the intensity required to stimulate osteogenic adaptation. Consequently, the adaptability of aging bone to intensive exercise remains unclear.
Observational studies have shown that middle-aged and older masters athletes with a high-impact training background have greater bone strength than non-active counterparts [6, 7], or even a younger physically active reference group [8]. According to these studies, the adaptation of bone strength in adult bone shafts appears to be more evident in bone geometry than in volumetric bone mineral density (vBMD) [8, 9]. However, in distal parts of the bone, compressive strength is also related to higher trabecular vBMD [10]. Structural adaptation in strength and sprint trained athletes manifests as greater cortical area and thickness at the loaded sites [6, 8, 9, 11]. In addition, bone mass distribution analyses have revealed higher site-specific cortical bone mass at the tibial and femoral mid-shaft, which may contribute to higher direction-specific bending strength [8,9,10, 12].
The experimental evidence on the effects of exercise on bone structure and strength in older people is scarce and somewhat conflicting. A previous meta-analysis [13] found no significant exercise effects on bone strength, which may partly be explained by the short duration and inadequate power of the few published trials, along with the use of non-athletic study populations and less intensive training programs. Since then, studies on middle-aged and older people have found positive, site-specific effects on proximal femoral bone mass after impact training [14] but no effects on mid-femoral or mid-tibial structure and strength after strength training or combined strength and impact training [15, 16]. To date, no attempts have been made to investigate the effects of intensive strength, sprint and plyometric training on bone in older athletes. Using an experimental design with masters athletes who are likely to be able to participate in vigorous exercise of this kind, our study can provide valuable insight into the osteogenic potential of specific types of training among older people. We hypothesized that a 20-week training program combining heavy and explosive strength exercises with sprint training would increase bone strength in middle-aged and older masters athletes by improving the geometrical properties of the tibial shaft and by adding density in the distal tibia. In light of the evidence from cross-sectional athlete studies, the main focus was on the structural adaptation of bone.
Methods
Subjects and study design
This study was a 20-week randomized controlled trial (ISRCTN17271498; Fig. 1). The study was part of a larger research program on the effects of age and long-term sprint training on musculoskeletal characteristics and neuromuscular function among male masters athletes [8, 17]. Athletes with a long-term training background and success in international or national masters sprint events (n = 111) were contacted by a personal letter. A detailed questionnaire on current and former training, competition performance, and injuries or diseases hindering physical training was mailed along with the recruitment letter. After assessment of eligibility, based on the questionnaire responses, a total of 83 voluntary athletes were invited to participate in the baseline measurements. The inclusion criteria were age ≥40, and ongoing systematic training and competing. Exclusion criteria included uncontrolled medical conditions or musculoskeletal disorders contraindicating exercise, medical conditions which would limit training program participation, and medications affecting bone metabolism. The health history and current health of those invited to the baseline measurements was assessed in more detail by means of a mailed questionnaire and, along with training status, confirmed in a short interview and clinical examination including resting electrocardiogram (ECG, athletes aged ≥55) and blood pressure measurements. For those under age 55, resting ECG was obtained at the athlete’s own request or based on a physician’s assessment (n = 9).
Eleven athletes were excluded for medical reasons contraindicating intensive training (cardiovascular disease, hip osteoarthritis, rheumatoid arthritis of the spine, Parkinson’s disease, prostate cancer; n = 10) or unwillingness to participate (n = 1). Among the participants accepted for the trial, 14 presented with chronic conditions (asthma, n = 3; celiac disease, n = 1; type 1 diabetes, n = 1; hypertension, n = 7; hypothyroidism, n = 2); however, all conditions had been adequately diagnosed by their own physician and thereafter treated with good response.
After the baseline measurements, the athletes were randomized into an experimental (EX, n = 40) and a control (CTRL, n = 32) group. First, a list of participants (sampling frame) was constructed, with subjects stratified by age. Next, lots were drawn manually, separately for each 10-year age group. Numbered and folded pieces of paper were well-shaken and blindly drawn one by one from a box for each subject in the sampling frame. To compensate for the possibility of a higher drop-out rate and larger variance in the results, in each age group a higher number of subjects was assigned to EX than to CTRL. Three subjects entered the trial after the initial randomization had been performed and thus were separately randomized by simple randomization. The randomization was performed by HS and MK. All the bone outcome assessors were blinded to the treatment-group assignment.
The experimental group participated in a 20-week program combining heavy and explosive strength exercises with sprint training. Controls were asked to maintain their usual, mostly run-based, sprint training schedules. Follow-up measurements were completed immediately after the training period. Written informed consent was obtained from all subjects prior to participation in the study. The study was approved by the University of Jyväskylä Ethical Committee and conformed with the principles of the Declaration of Helsinki.
Periodized training program
The combined strength and sprint training program, which has been described in detail in our previous article [18], was collaboratively designed by researchers and coaches, and utilized knowledge obtained from earlier studies in young adult athletes [19,20,21]. Although the main initial focus of the training program was to improve sprint performance and muscle strength, the osteogenic effect was also considered. The 20-week program was designed to fit into the training and competitive seasons of the athletes, and aimed at maximizing their performance at major championships. To reduce the potential for overtraining and to optimize adaptation to training, attention was paid to the proper periodization of training (Supplementary Fig. 1). The program consisted of two 11- and 9-week periods that were further divided into three phases of 3–4 weeks with a different intensity, volume and type of training. The first 4 weeks of strength training consisted of strength endurance and hypertrophy exercises. In the second and third phases, maximal strength and explosive strength exercises (weightlifting and plyometrics) alternated. During the latter half of the training program, the three-phase protocol was repeated with a progressive increase in training intensity. The strength training sessions focused on the leg extensor and hamstring muscle groups, with a limited number of exercises for the upper body and trunk. Plyometric exercises progressed from lower-intensity vertical jumps to higher-intensity horizontal bounding exercises. The sprint training program was similar during both training periods, progressing from speed-endurance to maximum speed exercises. Both the strength and sprint training were performed twice weekly on non-consecutive days. This was expected to provide adequate overload without overtraining or injuries, as well as to provide a sufficient quantity of strength training, which was a novel training stimulus for these athletes. The plyometric exercises were performed at the beginning of the speed training session, 1–2 times per week.
Because of the wide age range of the subjects, and the fact that most of them were not accustomed to heavy strength training, the subjects were divided into two age groups (40–64 and 65–85 years) receiving slightly different strength training programs. The intensity of the strength training was, in part, slightly lower in the older age group (more repetitions, lower resistance).
Training programs, along with written, pictorial and videotaped instructions for the different exercises, were mailed to EX. Both EX and CTRL filled out detailed training logs (describing sets, repetitions, loads, distances and times) to monitor progress and to enhance motivation for maximal effort. Based on the logs (collected every 5th week), total and weekly numbers of training sessions in the different training modes were calculated for each participant. The training adherence rate of the EX group members was calculated as the percentage of the training session successfully completed. Field tests for running performance and muscle power (data not shown) were organized in weeks 5, 10 and 15 to obtain feedback on the athlete’s training status and degree of progress.
Peripheral quantitative computed tomography (pQCT)
Properties of the distal tibia and tibial shaft of the dominant leg (the leg used for take-off in a one-footed jump) were determined by pQCT (XCT-2000, Stratec Medizintechnik, Pforzheim, Germany) according to the methods described earlier [8, 10]. The distal tibia was defined as 5% and tibial shaft as 50% of the measured tibial length proximal to the distal end plate. Tibial length was defined as the distance between the lateral malleolus and the condyle of tibia. Cross-sectional pQCT images included a single (2-mm) axial slice with pixel size of 0.8 × 0.8 mm. The images were analyzed with software designed for analyzing cross-sectional CT images (Geanie 2.1; Commit Ltd., Espoo, Finland). A threshold of 169 mg/cm3 for the distal tibia and 280 mg/cm3 for the midshaft site was used to determine the outer bone border. Separation of trabecular and cortical bone was performed using an automatic contour detection algorithm (K-mode). At the distal site, bone marrow was included in the analyses, whereas at the midshaft site bone marrow was excluded by applying a threshold of 100 mg/cm3.
The main parameters for the midshaft site were total cross-sectional area (CSATOT, mm2), cortical CSA (CSACO, mm2), mean cortical wall thickness (ThCO, mm) and area moments of inertia (I minA , I maxA and I polarA , mm4). I minA and I maxA reflect the bone’s resistance to bending in the direction of the smallest and greatest flexural rigidity, while I polarA refers to bending and torsional rigidity around the neutral axis of the bone. Secondary parameters for the midshaft site were total bone mineral content (BMCTOT, mg/mm), total volumetric bone mineral density (vBMDTOT, mg/cm3), cortical vBMD (vBMDCO) and density-weighted moments of inertia (I minD , I maxD and I polarD , mg*cm). In addition, BMC was further analyzed as the polar distribution of bone mineral mass around its center, using 5° steps that were subsequently averaged into eight 45° sectors: anterior (A), anteromedial (A-M), medial (M), posteromedial (P-M), posterior (P), posterolateral (P-L), lateral (L) and anterolateral (A-L) (Supplementary Fig. 2). For the distal tibia, BMCTOT, vBMDTOT, trabecular vBMD (vBMDTRAB), CSATOT, trabecular CSA (CSATRAB) and a compressive bone strength index (BSI, g2/cm4 = vBMDTOT 2 × CSATOT) [22, 23] were determined. The root mean square coefficient of variation (CVRMS) for the BMD, structure and strength index measurements in our laboratory ranges from 0.4 to 1.6% [24]. In terms of least significant change (LSC = 2.77 × CVRMS) [25], which refers to a change greater than the precision error for a single individual, this corresponds to a range of 1.2–4.4%.
Anthropometry, calcium and vitamin D intake, hormone measurements and physical performance
Body height and weight were measured using standard procedures. Lean body mass (LBM, kg) was assessed with a bioimpedance device using the manufacturer’s equations (Spectrum II, RJL Systems, Detroit, MI, USA). Before the measurements, the subjects had fasted for at least 3 h. Calcium and vitamin D intakes were obtained from 5-day food diaries kept in week 15. The diaries were analyzed by Micro Nutrica 3.0 software (Social Insurance Institution of Finland). Blood samples were drawn from the antecubital vein after an overnight fast. Specimens were centrifuged (3500 rpm, 4 °C for 10 min) and frozen at −75 °C until assayed. Serum concentration of total testosterone (total T, nmol/L) was analyzed by applying the Immulite chemiluminescent method (Diagnostic Products Corporation, Los Angeles, CA). The intra-assay CV for total T was 5.5%. Maximal 60-m running times on an indoor synthetic track with spiked shoes were obtained using double-beam photocell gates (starting line 0.7 m behind the first photocell gates). Own standing start without commands was used.
Statistical analysis
Mean values and standard deviations (SD) were calculated using standard procedures. The main outcome variables were analyzed according to the intention-to-treat principle. Baseline characteristics and training characteristics of the EX and CTRL groups during the experimental period were compared by independent samples t test. For variables that were not normally distributed, the Mann-Whitney U test was applied instead of the t test. The effect of the intervention was examined by means of repeated measures ANOVA. If the significance of the interaction of the effects of group and time was p < 0.1, an independent samples t test was used to test the differences in percentage changes between EX and CTRL, and separately in the two age groups [40–64 (EX, n = 21; CTRL, n = 14) and 65–85 (n = 17 and 16, respectively) years]. The effect of the intervention was also examined by per protocol analysis, in which case only subjects who had completed over 75% of the assigned 75 strength and speed exercises were chosen from the experimental group. Data were analyzed using SPSS 22.0 software (IBM, NY, USA) with the significance level set at p < 0.05.
Power analysis was conducted by setting the significance level at 0.05 and power at 80%. We used a sequential method of sample size calculation that permits having more than one primary outcome [26, 27]. The six primary outcomes were expected to be highly dependent and hence, based on the assumed effectiveness of the intervention on the variables, the dependency measure was set to range between 0.90 and 0.95. The follow-up correlations were likely to be high and we set these to be approximately within the range 0.975–0.977. Sample size was then calculated by allocating the significance level sequentially for the outcome variables, starting from the outcome likely to require the largest sample size (CSATOT, 2% difference, pilot data standard deviation: 56) and proceeding to CSACO (difference: 2%, pilot data SD: 50), ThCO (difference: 2.5%, pilot data SD 0.69), I polarA (difference: 3.5%, pilot data SD: 14,652), I minA (difference: 3.5%, pilot data SD: 4053) and, finally, I maxA (difference: 3.7, pilot data SD: 11,636). These settings, with the allocated significance level of 0.03 for each outcome variable, led to sample sizes varying between 25 and 38 for the intervention and control groups.
Results
No differences were observed at baseline between EX and CTRL in physical characteristics or training background (Table 1). Training programs during the year preceding the intervention mainly consisted of sprint training, speed-endurance training and plyometric exercises. Strength training was performed by 80% of the athletes. No age-group differences were observed in the training programs of the preceding year, except in the amount of strength training, which was significantly higher in the group aged 40–64 than in the group aged 65–85 [1.6 (1.5) vs. 0.8 (0.9) h/week, p = 0.018]. The 5-day food-intake diaries collected during the experimental period showed no differences between EX and CTRL in calcium or vitamin D intake. In the per protocol analysis, no between-group differences were observed in baseline characteristics. The intervention had no effect on body weight, LBM or total T. The 60-m trial times of the EX (n = 30) group improved from 8.54 (0.76) to 8.50 (0.91) s, whereas in CTRL (n = 29) the corresponding times were 8.40 (0.61) and 8.50 (0.61) s (group × time interaction p = 0.025).
The intervention did not cause major injuries or health problems. Minor musculoskeletal discomfort (transient muscle strains and joint sprains) were reported in both the EX (n = 16) and CTRL (n = 9) groups during testing, training and competitions. Two EX participants withdrew from the study due to persistent musculoskeletal disorder (knee pain, ankle pain due to pre-existing injury; unrelated to the exercise intervention). Two controls dropped out for personal reasons.
Training adherence
In EX, the overall training adherence rate was 68 (26) % [51 (20) strength and speed training sessions completed out of the 75 prescribed, n = 37]. For strength training, it was 64 (30) % [23 (11) sessions], for sprint training 69 (30) % [27 (12) sessions] and for the plyometric exercises 52 (32) % [17 (10) sessions]. The CTRL group maintained their own habitual training programs, which included 46 (18) strength and speed training sessions [19 (10) strength training sessions, 30 (14) sprint training sessions and 11 (12) plyometric exercise sessions, n = 29]. The strength training of the controls consisted mainly of strength endurance and hypertrophy exercises.
The number of active training weeks varied across the EX and CTRL groups [17 (4.6) and 18 (2.8), respectively]. During the active weeks, the EX participants reported a significantly higher average number of strength [1.3 (0.5) vs. 1.0 (0.5), p = 0.034] and plyometric training sessions [0.9 (0.5) vs. 0.6 (0.6), p = 0.035] than controls.
Bone traits
At baseline, no differences were found in bone traits between EX and CTRL, except in vBMDTOT and vBMDCO of the tibial midshaft, which were 2–3% higher in EX (p = 0.002 and 0.001, respectively). The intervention had no effect on distal tibia bone traits (Supplementary Table 1). The effect of the training on the tibial midshaft is shown in Table 2, Supplementary Table 2 and in Fig. 2. The average difference in change in ThCO in EX compared to CTRL was 2.0% (p = 0.007) across the whole EX group and 2.8% (p = 0.008) in the group aged 65–85 (EX, n = 17; CTRL, n = 16). The corresponding differences in I minA were 1.9% (p = 0.034) across the whole EX group and 2.8% (p = 0.031) in the group aged 40–64 (n = 21 and 14, respectively).
Per protocol analysis
As in intention-to-treat analysis, no changes were detected in the distal tibia bone traits. The effect of the training on the tibial midshaft bone traits is shown in Tables 3 and 4 and in Fig. 2. In the EX compared to CTRL group, CSATOT increased by 1.6% (p = 0.013), CSACO by 1.8% (p = 0.007), and ThCO by 2.6% (p = 0.012). In the area and density-weighted moments of inertia, the increase in favor of EX was 3.2% for I minA (p = 0.006), 1.8% for I minD (p = 0.023), and 2.0% for I polarA (p = 0.035). In the EX compared to CTRL group, BMCTOT increased by 0.7% (p = 0.017), whereas the changes in density favored CTRL (vBMDTOT 0.9%, p = 0.035 and vBMDCO 0.8%, p = 0.043). The polar mass distribution analysis revealed a significant group × time interaction at the A-M site. In EX compared to CTRL, BMCA-M increased by 2.2% (p = 0.051).
Discussion
As hypothesized, this 20-week randomized, controlled, high-intensity strength and sprint training trial in middle-aged and older male sprint athletes showed significant, albeit modest changes in tibial midshaft structure and strength. The changes were more pronounced in the most compliant athletes, which indicates that novel, intensive training, even of short duration, can strengthen aging bones, even in subjects with a long-term high-impact training background. The intervention had no effect on distal tibia bone traits.
The adaptation in this exercise training program, as in some previous studies [28,29,30], occurred in bone structure and, theoretically, in bending strength without increases in BMD. The greatest structural changes were observed in the cortical thickness of the tibial shaft, which accords with findings from previous observational athlete and twin studies [6,7,8, 10]. ThCO increased slightly in the EX group, while in the CTRL group ThCO decreased, which indicates that the training maintained rather than improved ThCO. In addition, there was a tendency towards increased total and cortical CSA in the EX group; in the per protocol analysis, the increase was significant in both parameters.
Parallel results have also been observed in some intervention studies on premenopausal [31] and postmenopausal [28, 32, 33] women after 6 to 12 months’ strength and/or impact training. Interventions targeting bone structure among older people are, however, scarce and comparison of the results is challenging due to variation in study populations, training programs, intervention duration and the bone sites measured. Ashe et al. [15] found no effect on mid-tibia vBMD, structure or strength in pretrained, postmenopausal women after resistance training for 1 year. Similarly, in the study by Kukuljan et al. [16] 18 months’ combined high-intensity strength training and weight-bearing impact exercises had no effect on mid-femur or mid-tibia vBMD, structure or strength in 50- to 79-year-old men, despite an increase in femoral neck aBMD. In the present study, we detected changes in bone in subjects with prior exposure to vigorous exercise. In previous studies, conducted mainly on average older populations, the intensity of the strength and/or impact training might have been too low or the training might not have been progressive or specific for bone adaptation. The exercises performed in this study were characterized by high magnitude and/or a high strain rate, both of which are determinants of bone adaptation [5, 34]. The initial muscle structural and functional characteristics of the athletes were already clearly above average, and were further improved by the training program [18], probably reaching a level high enough to trigger adaptive response in bone.
The increased cortical thickness and cortical CSA observed in the present study was located by mass distribution analysis. According to the per protocol analysis, the increase in bone mass occurred in the A-M region. Cheng et al. [1] found that 12 months’ hormone replacement therapy (HRT) and HRT combined with high-impact training mostly increased proximal tibia BMC in the A-P direction, resulting in increased bending resistance at the maximum axis (I max ). Similarly, in previous observational studies comparing athletes and reference subjects [8, 9], active and inactive monozygotic and dizygotic twins [10] or the jump and lead leg of jumping athletes [35], bone mass was greater in the A-P direction, as indicated by higher I max . In the present study, adaptation in that direction might have already reached its maximum owing to the long-term training history of the athletes, as the intervention-related increase in bending resistance was observed largely at the minimum axis (I minA ).
The increase in the moments of inertia (I minA , I minD and I polarA ) was more pronounced in the per protocol analysis. It appears that the increase in bone strength did not occur unambiguously in the direction of the greatest or the smallest flexural rigidity, but in the A-P direction between the maximal and minimal moments (Supplementary Fig. 2), where, according to the bone mass distribution analyses, bone mass also increased. The increase in the moments of inertia suggests that the geometrical changes that occurred improved bending strength without accompanying improvements in BMD. Because, in the per protocol analysis, BMD decreased, the increase in BMC likely occurred due to the increase in bone area. These observations may indicate corticalization of the subcortical trabecular bone, which shows as thickened cortical bone without significant external expansion (p = 0.135, data not shown), that is, increased bone mass in an enlarged area (cortical wall thickness and cortical area), resulting in lower bone density. This result is in line with our earlier findings in premenopausal women [36].
As in some previous studies [15, 37,38,39,40] the lack of training-induced improvements in BMD could be attributable to normal or high pretraining BMD values. Another possible reason for the unchanged BMD in this study is the short duration of the intervention (20 weeks). Changes in bone geometry occur faster than changes in BMD. The bone mineralization cycle takes 3–4 months to complete, and therefore at least 6–8 months is needed to observe a new, measurable balance in BMD [41]. In the present study, bone mineralization might have occurred later, and hence, theoretically, the reason for our results could be earlier growth in bone size than in bone density, a phenomenon observed during adolescence [42]. As a result of this process, bone density decreases momentarily, as was also observed in the efficacy analysis of the present study. The decrease in cortical vBMD could also be related to exercise-induced microdamage that leads to targeted remodeling and thus increased intracortical porosity, as suggested by previous observational studies [6, 43].
In a study conducted on sedentary older people [32], impact-loading increased bone mass and estimated strength of the distal tibia. In the present study, adaptation occurred in the cross-sectional geometry of the tibial shaft without changes in the distal tibia or mid-tibia vBMD. This indicates that the adaptation occurred in response to bending strain derived from the increased and intensified strength and plyometric training rather than vertical compression from impact-loading, a phenomenon to which the athletes’ bones might already have adapted. Most of the athletes were not accustomed to heavy strength exercises in their normal training routines. Previous strength training, especially among the older age group, might have focused more on light-resistance and high-repetition strength endurance exercises.
The amount of previous strength training was significantly lower in the older age group. Therefore, the overall intervention-induced increase in the amount and quality of the training might have been greater in the older age group than in the younger group. The latter group, in turn, was probably able to train harder than the older group, and the intensity of their intervention-related strength training, in particular, was higher. This could be related to their higher muscular capacity to produce bending strains. These possible differences in our athletes’ previous training and the intervention-related training might account for the differences observed in the training response between the age groups. Compared to the CTRL group, ThCO increased in the 65- to 85-year-olds but not in the 40- to 64-year-olds, whereas I minA tended to increase in the younger but not in the older age group. Older age did not prevent adaptation, as changes were observed in both age groups. The testosterone values of our athletes were normal. No differences in total T values (baseline or follow-up) were observed between the age groups and the changes in bone variables were not related to the changes in total T levels (data not shown).
The strengths of this study include a randomized controlled trial design and the use of pQCT, which enables detection of changes in bone cross-sectional geometry and different bone tissue types. PQCT is precise and reproducible, and it can detect even the smallest changes in bone properties. Further strengths of our study include the unique focus on middle-aged and older male athletes as well as the examination of a novel training program combining high-intensity strength and sprint training with plyometric exercises. This study addresses a knowledge gap in the research regarding bone-targeted exercise interventions for middle-aged and older men, and yields wholly novel information, as no corresponding studies conducted with a similar group have thus far been reported. Only few studies have demonstrated structural adaptation of the older skeleton. Most of the previous studies have utilized older populations with a low level of physical activity and reduced BMD. The competitive masters athletes studied here were both able and highly motivated to participate in vigorous training of a kind which could affect their bones, and the target intensities were likely to be achieved. Despite the minor musculoskeletal discomforts typical in competitive older athletes, all subjects were able to continue their training after a few days or weeks of modified or discontinued training. The intervention adherence was relatively high given the highly intensive, independently performed training program, and dropouts were few. The detailed bone mass distribution analyses can also be considered a strength, as only relatively few of these have been performed earlier.
The adaptations observed in this study were modest, which may have been attributable to the relatively short intervention period (may not have seen full mineralization in 20 weeks). In addition, the subjects had long-term training backgrounds and bones that were already strong, and thus major changes in bone properties during the relatively short study period were not expected. The study had multiple endpoints, which again means that the results have to be viewed with caution. The potential partial volume effect must also be considered, especially in relation to the area of trabecular bone. At the mid-shaft site, however, where the cortices are thick, this should not be an issue. The pQCT-related beam hardening may also have had some impact on our results, but probably not on the effect of the intervention. More detailed BMD analyses would have required a higher imaging resolution. We chose to use highly selected subjects and an active control group, who may have increased or intensified their training. The training practices of the intervention and control groups were rather similar, which may also have accounted for the modest adaptations observed. However, because an intervention-induced training effect was observed, it is likely that the quality and the intensity of the training of the intervention group changed more than that of the control group.
Our 20-week intervention challenges the idea that physical exercise is unlikely to enhance bone properties among older people who already have a strong bone structure. On the contrary, this study suggests that through physically active lifestyle the adaptability of the bone structure is maintained during aging. More research is needed on the effects of similar training programs of longer duration on aging people in general. Longer interventions would enable the examination of the maximal adaptive capacity of aging bone. Longer follow-ups would also allow examination of the possible interactions of strength and sprint training with the susceptibility to fractures. Although the intensive training program of athletes cannot, as such, be recommended for ordinary aging people, masters athletes serve as good examples of the upper limits of physical performance and the adaptability of musculoskeletal health.
References
Cheng S, Sipila S, Taaffe DR, Puolakka J, Suominen H (2002) Change in bone mass distribution induced by hormone replacement therapy and high-impact physical exercise in post-menopausal women. Bone 31:126–135
Heinonen A, Oja P, Kannus P, Sievanen H, Haapasalo H, Manttari A, Vuori I (1995) Bone mineral density in female athletes representing sports with different loading characteristics of the skeleton. Bone 17:197–203
Lanyon LE (1996) Using functional loading to influence bone mass and architecture: objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone 18:37S–43S
Nikander R, Sievanen H, Heinonen A, Kannus P (2005) Femoral neck structure in adult female athletes subjected to different loading modalities. J Bone Miner Res 20:520–528
Heinonen A, Kannus P, Sievanen H, Oja P, Pasanen M, Rinne M, Uusi-Rasi K, Vuori I (1996) Randomised controlled trial of effect of high-impact exercise on selected risk factors for osteoporotic fractures. Lancet 348:1343–1347
Wilks DC, Winwood K, Gilliver SF, Kwiet A, Chatfield M, Michaelis I, Sun LW, Ferretti JL, Sargeant AJ, Felsenberg D, Rittweger J (2009) Bone mass and geometry of the tibia and the radius of master sprinters, middle and long distance runners, race-walkers and sedentary control participants: a pQCT study. Bone 45:91–97
Rantalainen T, Duckham RL, Suominen H, Heinonen A, Alen M, Korhonen MT (2014) Tibial and fibular mid-shaft bone traits in young and older sprinters and non-athletic men. Calcif Tissue Int 95:132–140
Korhonen MT, Heinonen A, Siekkinen J, Isolehto J, Alen M, Kiviranta I, Suominen H (2012) Bone density, structure and strength, and their determinants in aging sprint athletes. Med Sci Sports Exerc 44:2340–2349
Rantalainen T, Nikander R, Heinonen A, Suominen H, Sievanen H (2010) Direction-specific diaphyseal geometry and mineral mass distribution of tibia and fibula: a pQCT study of female athletes representing different exercise loading types. Calcif Tissue Int 86:447–454
Ma H, Leskinen T, Alen M, Cheng S, Sipila S, Heinonen A, Kaprio J, Suominen H, Kujala UM (2009) Long-term leisure time physical activity and properties of bone: a twin study. J Bone Miner Res 24:1427–1433
Suominen H (2006) Muscle training for bone strength. Aging Clin Exp Res 18:85–93
Bailey CA, Kukuljan S, Daly RM (2010) Effects of lifetime loading history on cortical bone density and its distribution in middle-aged and older men. Bone 47:673–680
Nikander R, Sievanen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P (2010) Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med 8:47
Allison SJ, Poole KE, Treece GM, Gee AH, Tonkin C, Rennie WJ, Folland JP, Summers GD, Brooke-Wavell K (2015) The influence of high-impact exercise on cortical and trabecular bone mineral content and 3D distribution across the proximal femur in older men: a randomized controlled unilateral intervention. J Bone Miner Res 30:1709–1716
Ashe MC, Gorman E, Khan KM, Brasher PM, Cooper DM, McKay HA, Liu-Ambrose T (2013) Does frequency of resistance training affect tibial cortical bone density in older women? A randomized controlled trial. Osteoporos Int 24:623–632
Kukuljan S, Nowson CA, Sanders KM, Nicholson GC, Seibel MJ, Salmon J, Daly RM (2011) Independent and combined effects of calcium-vitamin D3 and exercise on bone structure and strength in older men: an 18-month factorial design randomized controlled trial. J Clin Endocrinol Metab 96:955–963
Korhonen MT, Cristea A, Alen M, Hakkinen K, Sipila S, Mero A, Viitasalo JT, Larsson L, Suominen H (2006) Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol (1985) 101:906–917
Cristea A, Korhonen MT, Hakkinen K, Mero A, Alen M, Sipila S, Viitasalo JT, Koljonen MJ, Suominen H, Larsson L (2008) Effects of combined strength and sprint training on regulation of muscle contraction at the whole-muscle and single-fibre levels in elite master sprinters. Acta Physiol (Oxf) 193:275–289
Delecluse C, Van Coppenolle H, Willems E, Van Leemputte M, Diels R, Goris M (1995) Influence of high-resistance and high-velocity training on sprint performance. Med Sci Sports Exerc 27:1203–1209
Joch W (1992) Rahmentrainingsplan für das Aufbautraining Sprint. Meyer & Meyer Verlag, Aachen
Kraemer WJ, Häkkinen K (2002) Strength training for sport. Blackwell Science, Oxford
Carter DR, Hayes WC (1976) Bone compressive strength: the influence of density and strain rate. Science 194:1174–1176
Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I (2003) Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res 18:352–359
Rantalainen T, Heinonen A, Komi PV, Linnamo V (2008) Neuromuscular performance and bone structural characteristics in young healthy men and women. Eur J Appl Physiol 102:215–222
Wong AK, Beattie KA, Min KK, Merali Z, Webber CE, Gordon CL, Papaioannou A, Cheung AM, Adachi JD (2015) A trimodality comparison of volumetric bone imaging technologies. Part II: 1-yr change, long-term precision, and least significant change. J Clin Densitom 18:260–269
O'Brien PC (1984) Procedures for comparing samples with multiple endpoints. Biometrics 40:1079–1087
Dubey SD (1985) Adjustment of p-values for multiplicities of intercorrelating symptoms. Proceedings of the VIth International Society for Clinical Biostatisticians, Düsseldorf
Adami S, Gatti D, Braga V, Bianchini D, Rossini M (1999) Site-specific effects of strength training on bone structure and geometry of ultradistal radius in postmenopausal women. J Bone Miner Res 14:120–124
Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I (2000) Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone 27:351–357
Liu L, Maruno R, Mashimo T, Sanka K, Higuchi T, Hayashi K, Shirasaki Y, Mukai N, Saitoh S, Tokuyama K (2003) Effects of physical training on cortical bone at midtibia assessed by peripheral QCT. J Appl Physiol (1985) 95:219–224
Vainionpaa A, Korpelainen R, Sievanen H, Vihriala E, Leppaluoto J, Jamsa T (2007) Effect of impact exercise and its intensity on bone geometry at weight-bearing tibia and femur. Bone 40:604–611
Uusi-Rasi K, Kannus P, Cheng S, Sievanen H, Pasanen M, Heinonen A, Nenonen A, Halleen J, Fuerst T, Genant H, Vuori I (2003) Effect of alendronate and exercise on bone and physical performance of postmenopausal women: a randomized controlled trial. Bone 33:132–143
Karinkanta S, Heinonen A, Sievanen H, Uusi-Rasi K, Pasanen M, Ojala K, Fogelholm M, Kannus P (2007) A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporos Int 18:453–462
Vainionpaa A, Korpelainen R, Vihriala E, Rinta-Paavola A, Leppaluoto J, Jamsa T (2006) Intensity of exercise is associated with bone density change in premenopausal women. Osteoporos Int 17:455–463
Weatherholt AM, Warden SJ (2016) Tibial bone strength is enhanced in the jump leg of collegiate-level jumping athletes: a within-subject controlled cross-sectional study. Calcif Tissue Int 98:129–139
Heinonen A, Mantynen J, Kannus P, Uusi-Rasi K, Nikander R, Kontulainen S, Sievanen H (2012) Effects of high-impact training and detraining on femoral neck structure in premenopausal women: a hip structural analysis of an 18-month randomized controlled exercise intervention with 3.5-year follow-up. Physiother Can 64:98–105
Bolam KA, Skinner TL, Jenkins DG, Galvao DA, Taaffe DR (2015) The osteogenic effect of impact-loading and resistance exercise on bone mineral density in middle-aged and older men: a pilot study. Gerontology 62:22–32
Nichols J, Nelson K, Peterson K, Sartoris D (1995) Bone mineral density responses to high-intensity strength training in active older women. J Aging Phys Act 3:26–38
Pruitt LA, Taaffe DR, Marcus R (1995) Effects of a one-year high-intensity versus low-intensity resistance training program on bone mineral density in older women. J Bone Miner Res 10:1788–1795
Vincent K, Braith R (2002) Resistance exercise and bone turnover in elderly men and women. Med Sci Sports Exerc 34:17–23
Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR, American College of Sports Medicine (2004) American College of Sports Medicine position stand: physical activity and bone health. Med Sci Sports Exerc 36:1985–1996
Bass S, Delmas PD, Pearce G, Hendrich E, Tabensky A, Seeman E (1999) The differing tempo of growth in bone size, mass, and density in girls is region-specific. J Clin Invest 104:795–804
Rantalainen T, Nikander R, Daly RM, Heinonen A, Sievanen H (2011) Exercise loading and cortical bone distribution at the tibial shaft. Bone 48:786–791
Acknowledgments
The authors gratefully acknowledge the assistance of Martti J. Koljonen in designing the training program. We would also like to thank Timo Annala, Milan Sedliak, Leena Hakola and Risto Puurtinen for valuable technical assistance with the data collection and analysis, and all the athletes participating in this study. The study was supported by grants from the Academy of Finland (250683), Ministry of Education and Culture, Peurunka-Medical Rehabilitation Foundation, Ellen and Artturi Nyyssönen Foundation, Juho Vainio Foundation and Finnish Cultural Foundation to MK and HS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Written informed consent was obtained from all subjects prior to participation in the study. The study was approved by the University of Jyväskylä Ethical Committee and conformed with the principles of the Declaration of Helsinki.
Conflicts of interest
None.
Electronic supplementary material
ESM 1
(PDF 1939 kb)
Rights and permissions
About this article
Cite this article
Suominen, T.H., Korhonen, M.T., Alén, M. et al. Effects of a 20-week high-intensity strength and sprint training program on tibial bone structure and strength in middle-aged and older male sprint athletes: a randomized controlled trial. Osteoporos Int 28, 2663–2673 (2017). https://doi.org/10.1007/s00198-017-4107-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4107-z