Abstract
Summary
This investigation presents a comparison of calcaneus bone stiffness of endurance runners of different ages and age-matched controls. We found that there was an age-associated decline in calcaneus bone stiffness in the control group while endurance runners prevented this decline, with a higher effect as the participants increased their age.
Purpose
Previous investigations have found that endurance runners have higher bone mineral density and other bone quality variables in mechanically loaded bones. However, it is unknown if endurance running might counteract the decline in bone stiffness that occurs with age. The purpose of this study was to compare calcaneus bone stiffness of endurance runners of different ages to age-matched controls.
Methods
In a descriptive cross-sectional study, 182 endurance-trained male runners and 116 healthy untrained male controls underwent an ultrasonographic assessment of the calcaneus bone in the right and left heels. Calcaneal bone stiffness was calculated from assessments of the broadband ultrasound attenuation and the speed of sound.
Results
The line of best fit for the association between age and calcaneus stiffness was different between marathoners and controls (Z = − 2.1, P = 0.02). A two-way ANCOVA (condition × age) with body mass, and body mass index as covariates, revealed that there were main effects of condition (F = 26.8, P < 0.01) and age (F = 4.2, P < 0.01) for calcaneus stiffness, with a significant interaction between these two factors (F = 2.8, P = 0.03). The post hoc analysis revealed that calcaneus stiffness was significantly higher in marathoners of 40–44 years (121.5 ± 18.2 vs 101.1 ± 21.3 arbitrary units [A.U.], P = 0.01), 45–49 years (121.5 ± 19.7 vs 104.3 ± 13.4 A.U., P = 0.04), and > 50 years (111.2 ± 17.9 vs 92.4 ± 16.0 A.U., P < 0.01) than their untrained counterparts of the same age with no statistically significant differences in the remaining age groups.
Conclusion
Endurance runners of > 40 years had higher values of calcaneus stiffness than controls, providing evidence to support the potential effect of endurance running to reduce the age-related decline on calcaneus bone stiffness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Optimal bone tissue must be stiff, but at the same time, flexible to absorb energy, and light to facilitate locomotion [1]. For this reason, bone health is characterized by different parameters of bone mineral density, geometry, architecture, and strength. Bone tissue is a modifiable type of tissue that might be affected by several environmental factors, mainly dietary patterns, physical activity, sex, and age [2]. Among them, age is the strongest factor affecting bone stiffness and mass, producing a progressive reduction of bone strength and elasticity along with age [3]. Age produces a progressive decline in bone mineral density and stiffness in both women and men which might negatively impact several health variables [4], mainly due to the higher risk of fractures and associated comorbidities. Body weight—particularly at the time of skeletal maturity—dairy calcium intake, smoking, and exercise are other contributing factors for bone stiffness, although their influence is lower than that of age [5].
Bone tissue effectively responds to the demands of mechanical loading by altering its structure and density that depend on the extent of mechanical stresses applied to the bone [6]. To this regard, there is wide evidence supporting the benefits of exercise to improve bone quality. Although increased physical activity may lead to enhanced bone mass, it is suggested that the best way to improve all bone-related variables is participating in structured exercise training programs [4]. Exercise has been reported as one of the best non-pharmacological ways to maintain bone stiffness and strength through age [7]. However, not all exercise programs produce the same benefits on bone health. Weight-bearing exercise seems to be the most effective form of exercise that leads to maintenance or improvement of bone mineral density and stiffness due to the forces exerted on the mechanically loaded bones [8].
One of the simplest and most accessible forms of weight-bearing exercise is endurance running. Long-distance running is gaining popularity among young adults, particularly in the last decades, due to the wide spectrum of benefits over cardiovascular muscle and body composition parameters [9]. Endurance running might also be an optimal exercise to stimulate bone mass gain in ages close to the peak bone mass [10] and then to maintain bone quality throughout life [11]. Although sprinters, gymnasts, or team sports athletes have higher bone mineral density than endurance runners [12, 13], most cross-sectional studies have found a higher bone mineral density in runners compared with inactive controls [14]. In addition, endurance running may have greater osteogenic effects than other forms of endurance exercise such as cycling, triathlon, and swimming [15].
Endurance running is a multifaceted and competitive sports discipline that can be performed over a wide range of distances. In recent years, the number of amateur runners that train and compete in marathons has greatly increased [16] because amateur runners perceive completing the distance as a personal challenge. Interestingly, marathoners have higher calcaneus stiffness than untrained controls and this difference was greater to that found when comparing controls to half-marathoners and 10 K runners [11]. This effect was likely produced by the higher running distances covered during training for a marathon as running mileage has been previously deemed as an essential factor for enhancements in bone mineral density [17]. In marathoners, Drysdale et al. [18] evidenced that runners of 20–93 years of age had higher broadband ultrasound attenuation (an indirect measurement of bone stiffness) in the calcaneus than untrained controls, suggesting a beneficial role of endurance running on stiffness throughout a life span. However, there was not a specific analysis on the influence of the marathoners’ age on calcaneus stiffness. Because of this, it is difficult to determine if endurance running produces different magnitudes of effect depending on participant age.
Therefore, the purpose of this study was to identify the effect of endurance running on bone stiffness in runners of different ages by comparing calcaneus stiffness of endurance runners of various ages with age-matched controls. We hypothesized that the magnitude of the effect found when comparing marathoners to the age-matched controls would increase with age.
Methods
Participants
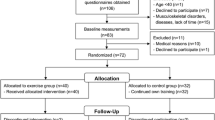
A total of 182 endurance-trained runners and 116 healthy untrained controls volunteered to participate in this investigation. All participants of both groups were Caucasian males. The sample of endurance runners was recruited from the competitors in the 2018 Edition Rock’n’Roll Madrid Marathon. The inclusion criteria for the marathon group were the following: being an active endurance runner with more than 3 years of endurance running experience, having more than 3 sessions of training per week, and running with a mileage higher than 30 km per week. The exclusion criteria for the marathoners were the following: having a musculoskeletal injury in the previous month, a positive smoking status, medication or dietary supplement usage within the previous month, previous history of cardiopulmonary or musculoskeletal diseases, previous history of fracture in any bone of the upper leg, lower leg, or foot, and intolerance to milk or dairy products.
Control participants were recruited among students and staff of Spanish universities by using recruiting emails and posters. As the measurement of the controls was carried out after the measurement of marathoners, we especially chose control participants to match the age of the marathoners. The inclusion criteria for the control group required participants to be mentally active—as evidenced by occupation, educational participation, or sedentary behavior (< 1 h of exercise per week)—and be free of any musculoskeletal and metabolic disorders known to affect the bones. The exclusion criteria for controls required participants to have not been enrolled in any kind of exercise training activity within the previous 2 years, in addition to the same exclusion criteria applied to marathoners. Participants’ information was obtained by using a personal interview and questionnaire. Detailed information was collected, including date of birth, training habits (if any), previous medical conditions, and injuries. All participants were fully informed of any risks and discomforts associated with the experiments before giving their informed written consent to participate. The study protocol was reviewed and approved by the Camilo Jose Cela Ethics Committee (code:13/2017) in accordance with the latest version of the Declaration of Helsinki.
Experimental design
This is a descriptive cross-sectional study designed to determine the effect of endurance running on calcaneus bone stiffness in runners with increasing age, while this effect was assessed by comparing densitometry variables in runners versus healthy sedentary controls. For this reason, the sample of marathoners and the sample of untrained controls were clustered in the following groups: < 35 years (35 marathoners and 34 controls); between 35 and 39 years (36 marathoners and 27 controls); between 40 and 44 years (39 marathoners and 14 controls); between 45 and 49 years (31 marathoners and 24 controls); and > 50 years (41 marathoners and 17 controls). Categorization into the age groups was determined by the participant’s age at the time of measurement. Information about participants’ physical characteristics and running experience is included in Table 1.
Experimental protocol
In the group of marathoners, measurements were carried out in the facility used to collect race bibs one to two days before the marathon (21 ± 1 °C), in April 2018. Controls were measured in a laboratory room simulating the conditions of the aforementioned area (21 ± 1 °C). The measurements in the control group were performed between April and May 2018. All measurement methods were identical in both groups. Upon arrival, each participant filled out an ad hoc questionnaire about age, medical history, training habits (if any, including running experience and mean weekly mileage in the last three months). Body height was then measured with a stadiometer to the nearest 0.1 cm and body mass was measured with a weight scale with a precision of ± 0.05 kg (Radwag, Poland). Body mass index (BMI) was calculated from body height and mass. Then, participants underwent an ultrasonographic assessment of the calcaneus bone in the right and left heels using a bone scanner (Achilles, General Electric Health Care-Lunar, WI, USA). For this measurement, participants remained comfortably seated and their bare foot was placed in a water bath while minimal foot movement was ensured during the measurement by an experimenter that kept participant’s calf in position. This scanner consisted of a control box, a heel water bath, and two transducers placed at each side of the water bath. During the assessment, a known ultrasound signal was sent from one transducer to the other while the heel was placed in the bath. Calcaneus bone stiffness was calculated from the magnitude of the ultrasound signal’s attenuation, as previously indicated [11]. Signal parameters obtained by the bone scanner were digitized and sent to a computer for automated analysis. Scanner’s expandable membranes were filled with warm water, and isopropyl alcohol was used to provide coupling between the heel and the membranes to eliminate spaces with air. During each ultrasonographic assessment, the speed of sound, in m/s, and the broadband ultrasound attenuation, in dB/MHz, were directly measured. Calcaneal bone stiffness was calculated in arbitrary units as (0.67 × broadband ultrasound attenuation + 0.28 × speed of sound) − 420. In previous investigations, the coefficient of variation for the speed of sound was 0.47%, 2.6% for broadband ultrasound attenuation, and 1.6% for calcaneal stiffness [19]. In the case of a right-to-left difference in calcaneus stiffness greater than 10%, the measurement was repeated in each heel. If the difference persisted, participants were excluded from the analysis due to an asymmetry from a speculated previous injury.
Statistical analysis
Initially, all bone densitometry variables measured in the calcaneus (speed of sound, broadband ultrasound attenuation, and stiffness) were compared between the right and left foot by using a Student’s t test for paired samples. Densitometry variables were very comparable in both feet (less than 1% of variation), and thus, mean values for both feet were used for statistical analysis. Afterwards, T scores and Z scores were calculated for calcaneus stiffness in each individual using the reference population included in the manufacturer’s software. The individual T score represents the difference between the participant’s value for calcaneus stiffness and the mean value for a population of young adults of the same sex with peak bone mass. The individual Z score represents the difference between the participant’s value for calcaneus stiffness and the value of a sex- and age-adjusted population [20]. Differences in age, height, body mass, and body mass index were made using Student’s t test for unpaired samples. Differences in densitometry variables were analyzed by using a two-way ANCOVA (condition × age) with body mass, and body mass index as covariates. For this analysis, there were two clusters for condition (marathoner vs controls) and five clusters for age (< 35 years, 35–39 years, 40–44 years, 45–49 years, and > 50 years of age). In the case of a significant F test, Tukey’s post hoc was used to detect difference in pairwise comparisons within the individuals of the same age group. Data are expressed as mean values ± standard deviation (SD) for each age group. In the scatterplots, the association between densitometry variables and age is presented, including the line of best fit for the two conditions. The Fisher’s r to z transformation was used to verify if the slope of the line of best fit was different between marathoners and controls. The significance level was set at P < 0.05 for all statistical analysis. In addition, to determine the magnitude of differences, Cohen’s effect size (d) and 95% confidence intervals (CI) were calculated and interpreted as follows: < 0.2 trivial; 0.2–0.6 small; 0.6–1.2 moderate; or > 1.2 large [21]. All these statistical analyses were performed using the SPSS v.20 software package (SPSS Inc., USA).
Results
Marathoners and controls had similar age and body height in all age groups. However, controls were heavier (from > 40 years of age) and had a higher body mass index (from > 45 years of age) than the age-matched marathoners.
Figure 1 contains the association between age and calcaneus stiffness, BUA, and speed of sound in marathoners and controls. The line of best fit was different between marathoners and controls for calcaneus stiffness (Z = − 2.1, P = 0.02) and for the speed of sound (Z = − 2.0, P = 0.02), but it was similar between conditions for BUA (Z = − 0.1, P = 0.02). The ANCOVA revealed that there were main effects of the condition (F = 26.8, P < 0.01) and age (F = 4.2, P < 0.01) for calcaneus stiffness, with a significant interaction between these two factors (F = 2.8, P = 0.03). The post hoc analysis revealed that calcaneus stiffness was significantly higher in 40–44, 45–49, and > 50-year-old marathoners than their untrained counterparts of the same age (all P < 0.05; Table 2) with no differences in the remaining age groups. The magnitude of the differences in runners older than 40 years of age was moderate (from 0.99 to 1.10). There was a main effect of the condition (F = 16.9, P < 0.01) for BUA, while the main effect of age (F = 2.1, P = 0.07) and the interaction between variables did not reach statistical significance (F = 1.6, P = 0.17). Marathoners of 40–44 years of age and of > 50 years had higher BUA than their age-matched controls (P < 0.05, effect size from small-to-moderate) with no other significant differences in the remaining age groups. There were main effects of the condition (F = 23.1, P < 0.01) and age (F = 4.2, P < 0.01) for the speed of sound (F = 3.0, P = 0.01) with a significant interaction between these two factors (F = 2.7, P = 0.03). The speed of sound was higher in endurance runners of 40–44 years, 45–49 years, and > 50 years of age (P < 0.05) with moderate effect sizes.
Association between age and stiffness, broadband ultrasound attenuation (BUA), and speed of sound measured in the calcaneus bone of marathoners and untrained controls. Each black dot represents data for one marathoner (n = 182) and each white square represents an age-matched untrained control (n = 116). The black line represents the line of best fit for marathoners and the dashed line represents the line of best fit for controls. (*) The line of best fit for marathoners was different from the line of best fit for controls (P < 0.05)
Figure 2 contains the association between age and T and Z scores in marathoners and controls. The line of best fit was different between marathoners and controls for both T score (Z = − 2.5, P < 0.01) and Z score (Z = − 2.1, P = 0.02). The ANCOVA revealed that there were main effects of the condition (F = 26.0, P < 0.01) and age (F = 3.5, P < 0.01), and an interaction between these conditions (F = 3.3, P = 0.01) for T score. The T score of marathoners was higher than those of untrained controls in the 40–44, 45–49, and > 50-year-old age groups (P < 0.05). However, for Z score, there was a main effect of the condition (F = 23.2, P < 0.01) and a condition × age interaction (F = 2.6, P = 0.05) while the main effect of age did not reach statistical significance (F = 1.8, P = 0.11). The Z score of marathoners was higher than those of untrained controls in the 40–44, 45–49, and > 50-year-old age groups (P < 0.05). The magnitude of the difference in T and Z scores between marathoners and controls increased with the age (Table 2).
Association between age and calcaneus stiffness T score and Z score of marathoners and untrained controls. Each black dot represents data for one marathoner (n = 182) and each white square represents an age-matched untrained control (n = 116). The black line represents the line of best fit for marathoners and the dashed line represents the line of best fit for controls. (*) The line of best fit for marathoners was different from the line of best fit for controls (P < 0.05)
Discussion
The aim of this study was to investigate the effect of endurance running on calcaneus bone stiffness in runners of different ages by comparing calcaneus stiffness of endurance runners of various ages with untrained controls. By using a cross-sectional study, male marathoners and age-matched controls underwent an ultrasonographic assessment of the calcaneus bone to determine stiffness. Overall, the association between age and calcaneus stiffness was different between marathoners and controls for calcaneus stiffness. Specifically, the decline with age in calcaneus bone stiffness was more pronounced in controls than in marathoners as it was represented by the statistically significant differences in the lines of best fit between these two conditions (Fig. 1). This produced that the calcaneus stiffness of marathoners was higher than the value of the control group only in runners older than 40 years of age (Table 2), with no differences in younger participants. Last, T scores and Z scores were also higher in marathoners older than 40 years of age than in age-matched controls, with a tendency for a higher effect size of endurance running on bone stiffness with age (Table 2). Collectively, all this information suggests that being involved in endurance running was effective to reduce the age-related decline on calcaneal bone stiffness. This might indicate a positive effect of endurance running on bone stiffness in loaded bone areas, which would be of higher magnitude along with participant’s age.
Mechanical loading through external impact forces and internal muscle forces plays an important role in the regulation of bone strength and geometry. For this reason, it has been found that bone mineral content and the cortical area of the tibia of master athletes are the largest in sprinters, followed by (in descending order) middle-distance runners, long-distance runners, and race-walkers [22]. This indicates that endurance running may induce lower effect on bone quality than other shorter and more intense sport activities because it is a discipline with lower external impact forces and lower values of force. However, endurance running is an excellent choice due to its cardiovascular benefits, while lifelong participation in endurance running slows the inevitable age-related decline in aerobic function and muscular strength [23]. Additionally, previous investigations have deemed that weekly running mileage is an essential factor to obtain the benefits of endurance running on bone quality [17], and for this reason, training for and participating in marathons may produce a greater effect on bone stiffness than training and competing in shorter running distances [11]. This might be because, during training for a marathon, a runner generates repetitive forces of low peak intensity that must be maintained for long periods in order to obtain the cardiovascular and muscle adaptations to complete 42.2 km in 3 to 6 h. Hence, endurance running may be an exercise activity with positive effects on bone quality while the running volume per week seems to be the main factor to determine the magnitude of the benefits.
It has been previously suggested that the effect of exercise on bone tissue may depend on age because bone modeling can become less active and responsive to mechanical loading with age [24]. The current investigation adds new and important information to the effects of endurance running on bone quality because it indicates that the effect of training/competing in marathon events is greater with individual age. Specifically, the values obtained in the densitometry variables measured in the calcaneus were not different between controls and marathoners younger than 40 years of age. However, after this age, marathoners presented calcaneus with higher values of stiffness than their age-matched controls. Furthermore, the association between age and calcaneus bone stiffness in the group of untrained controls presented a clear age-induced decline which was notably reduced in marathoners. These data suggest that endurance running may be an excellent method of preventing age-related declines in calcaneus bone stiffness. The continuation of endurance running training through life appears to be an important contributor to maintain bone condition, as found in other types of exercise [6].
Table 2 contains the comparison of calcaneus stiffness against reference values of young and healthy individuals (T score) and age-matched healthy individuals (Z score). Interestingly, marathoners of > 50 years of age had the lowest T score despite having the longest running experience (with more than 20 years of experience on average). This indicates that even in marathoners that had been training and competing for several years, there is a progressive age-related decline in calcaneal bone stiffness. To this respect, it seems that the accumulation of years of running experience does not produce a progressive increase in calcaneus stiffness but a lower rate of decline on this densitometry variable. Thus, the higher magnitude of the effect of endurance running on calcaneus stiffness in runners > 50 years of age is the result of the highly pronounced reduction in calcaneus stiffness with age in the population of controls, as it is presented in Figs. 1 and 2. As previously mentioned, marathon runners younger than 40 years had no differences in calcaneus stiffness with respect to age-matched controls, although this lack of effect was mainly due to controls still had T scores close to 0. This indicates that they had not yet started their decline in bone stiffness. The results of this investigation indicate that endurance runners might prevent the decline in calcaneus bone stiffness by maintaining an active lifestyle that includes endurance running. This positive effect of running might be due to the deceleration of bone stiffness loss rather than to a progressive enhancement of bone quality induced by the accumulation of running years, as previously suggested [25].
The current investigation presents some limitations. First, this is a cross-sectional study including marathoners with no standardized training programs in terms of volume, running pace, or frequency of training sessions. Although the current investigation is able to define the overall effect of endurance running on calcaneus bone stiffness, it does not define the most appropriate characteristics of a training program that maintains bone condition throughout a life-span [2]. In this investigation, we selected runners with > 3 years of endurance experience to assess the effect of endurance running on calcaneus bone stiffness. However, running experience increased along participant’s age (Table 1). This might affect the understanding of research outcomes since it is unfeasible to separate the effect of age from that of accumulated running experience in this study. Third, this investigation was carried out with only male runners and controls. The results might not be applicable to female endurance runners, particularly because of the effect of menopause on bone condition [5]. Thus, further investigations in female marathoners, considering the time after menopause, should be carried out to confirm the positive effect of endurance running to prevent the age-related decline on bone stiffness in women. Fourth, we merged participants older than 50-year-old because we were unable to recruit a significant number of marathoners to maintain our analysis by 5-year intervals. Thus, further investigations should investigate the effect of running in samples > 60 years of age to see if the running-induced prevention of the age-decline in bone stiffness persists at this age [12]. Fifth, there were differences between controls and marathoners in body mass and body mass index in participants older than 40–45 years (Table 1). It is possible that these variables affected the differences in calcaneus bone stiffness between groups [26] and further experiments are needed to confirm that calcaneus bone stiffness is better preserved in runners in absence of differences of body mass. Finally, we assessed the effect of endurance running on just one bone site. Although the assessment of calcaneus stiffness is a valid [27] and reliable [28] manner to estimate bone mineral density in healthy and clinical populations, it is also necessary to examine other bones to fully determine the effect of endurance running at preventing age-related bone stiffness declines. The measurement of the effect of endurance running on bone mineral density (BMD) measured by dual-energy X-ray absorptiometry (DXA) along lifespan also warrants further experimentations. To the authors’ opinion, these limitations do not hinder the main outcomes and applicability of the investigation.
In summary, it was possible to evidence an age-associated decline in calcaneus bone stiffness in a control group of sedentary individuals while long-distance runners (i.e., marathoners) presented a reduced age-related decline. Therefore, regular running, particularly at the training level required for completing a marathon, may be a good strategy to maintain bone stiffness through lifespan. As a novel outcome, we found that endurance runners older than 40 years of age had higher values of calcaneus stiffness than age-matched untrained controls. The difference between runners and controls did not reach statistical significance in participants younger than 40 years of age because at this age, the reduction on bone stiffness induced by age was still minor. This investigation provides evidence to support the potential effect of endurance running to reduce the age-related decline on calcaneal bone stiffness. As a practical application, healthy individuals seeking to maintain bone health through endurance running should engage in endurance training programs at a young age and continue training later in life to effectively reduce the effect of age on bone stiffness.
Data availability
The data of the participants have been encrypted and are archived according to data protection guidelines.
References
Seeman E, Delmas PD (2006) Bone quality - the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261
Weaver CM, Gordon CM, Janz KF et al (2016) The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 27:1281–1386. https://doi.org/10.1007/s00198-015-3440-3
Zioupos P, Currey JD (1998) Changes in the stiffness, strength, and toughness of human cortical bone with age. Bone 22:57–66. https://doi.org/10.1016/S8756-3282(97)00228-7
Gómez-Cabello A, Ara I, González-Agüero A et al (2012) Effects of training on bone mass in older adults: a systematic review. Sport Med 42:301–325
Adami S, Giannini S, Giorgino R et al (2003) The effect of age, weight, and lifestyle factors on calcaneal quantitative ultrasound: the ESOPO study. Osteoporos Int 14:198–207. https://doi.org/10.1007/s00198-002-1352-5
Nowak A, Straburzyńska-Lupa A, Kusy K et al (2010) Bone mineral density and bone turnover in male masters athletes aged 4064. Aging Male 13:133–141. https://doi.org/10.3109/13685531003657776
Body JJ, Bergmann P, Boonen S et al (2011) Non-pharmacological management of osteoporosis: a consensus of the Belgian Bone Club. Osteoporos Int 22:2769–2788. https://doi.org/10.1007/s00198-011-1545-x
Karlsson MK, Rosengren BE (2012) Training and bone - from health to injury. Scand J Med Sci Sports 22:e15–23
Mitchell UH, Bailey B, Owen PJ (2020) Examining bone, muscle and fat in middle-aged long-term endurance runners: a cross-sectional study. J Clin Med 9:522. https://doi.org/10.3390/jcm9020522
Heaney RP, Abrams S, Dawson-Hughes B et al (2000) Peak bone mass. Osteoporos Int 11:985–1009
Lara B, Salinero JJ, Gutiérrez J et al (2016) Influence of endurance running on calcaneal bone stiffness in male and female runners. Eur J Appl Physiol 116:327–333. https://doi.org/10.1007/s00421-015-3285-7
Leigey D, Irrgang J, Francis K et al (2009) Participation in high-impact sports predicts bone mineral density in senior olympic athletes. Sports Health 1:508–513. https://doi.org/10.1177/1941738109347979
Fredericson M, Chew K, Ngo J et al (2007) Regional bone mineral density in male athletes: a comparison of soccer players, runners and controls. Br J Sports Med 41:664–668. https://doi.org/10.1136/bjsm.2006.030783
Feldman S, Capozza RF, Mortarino PA et al (2012) Site and sex effects on tibia structure in distance runners and untrained people. Med Sci Sports Exerc 44:1580–1588. https://doi.org/10.1249/MSS.0b013e31824e10b6
Scofield KL, Hecht S (2012) Bone health in endurance athletes: runners, cyclists, and swimmers. Curr Sports Med Rep 11:328–334
Del Coso J, Salinero JJ, Lara B et al (2017) A comparison of the physiological demands imposed by competing in a half-marathon vs. a marathon. J Sports Med Phys Fitness 57. https://doi.org/10.23736/S0022-4707.17.07056-6
MacKelvie KJ, Taunton JE, McKay HA, Khan KM (2000) Bone mineral density and serum testosterone in chronically trained, high mileage 40–55 year old male runners. Br J Sports Med 34:273–278. https://doi.org/10.1136/bjsm.34.4.273
Drysdale IP, Collins AL, Walters NJ et al (2007) Potential benefits of marathon training on bone health as assessed by calcaneal broadband ultrasound attenuation. J Clin Densitom 10:179–183. https://doi.org/10.1016/j.jocd.2007.02.001
Cepollaro C, Agnusdei D, Gonnelli S et al (1995) Ultrasonographic assessment of bone in normal Italian males and females. Br J Radiol 68:910–914. https://doi.org/10.1259/0007-1285-68-812-910
Gast U, Belavý DL, Armbrecht G et al (2013) Bone density and neuromuscular function in older competitive athletes depend on running distance. Osteoporos Int 24:2033–2042. https://doi.org/10.1007/s00198-012-2234-0
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale
Wilks DC, Winwood K, Gilliver SF et al (2009) Bone mass and geometry of the tibia and the radius of master sprinters, middle and long distance runners, race-walkers and sedentary control participants: a pQCT study. Bone 45:91–97. https://doi.org/10.1016/j.bone.2009.03.660
Willy RW, Paquette MR (2019) The physiology and biomechanics of the master runner. Sports Med Arthrosc 27:15–21
Frost HM (1999) Why do bone strength and “mass” in aging adults become unresponsive to vigorous exercise? Insights of the Utah paradigm. J Bone Miner Metab 17:90–97
Wiswell RA, Hawkins SA, Dreyer HC, Jaque SV (2002) Maintenance of BMD in older male runners is independent of changes in training volume or VO(2)peak. J Gerontol A Biol Sci Med Sci 57:M203–M208. https://doi.org/10.1093/gerona/57.4.m203
Ali K, Said SMSE, Adly NN, Abdul-Rahman SA (2019) The relation between calcaneus stiffness index as a measure of bone density and body mass index in an Egyptian cohort. J Multidiscip Healthc 12:1085–1090. https://doi.org/10.2147/JMDH.S230730
Moayyeri A, Adams JE, Adler RA et al (2012) Quantitative ultrasound of the heel and fracture risk assessment: an updated meta-analysis. Osteoporos Int 23:143–153. https://doi.org/10.1007/s00198-011-1817-5
Economos C, Sacheck J, Wacker W et al (2007) Precision of Lunar Achilles+ bone quality measurements: time dependency and multiple machine use in field studies. Br J Radiol 80:919–925
Acknowledgements
The authors gratefully acknowledge the participation of the subjects for their invaluable contributions to this study. In addition, we are very grateful for the Organization of the Rock’n’Roll Madrid Marathon & Half-Marathon for their contribution to the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study protocol was reviewed and approved by the Camilo Jose Cela Ethics Committee in accordance with the latest version of the Declaration of Helsinki.
Consent to participate
All participants were fully informed of any risks and discomforts associated with the experiments before giving their informed written consent to participate.
Consent for publication
The authors give their consent for the publication of the article.
Conflicts of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ruiz-Vicente, D., García-Pastor, T., Lara, B. et al. Endurance running prevents the age-related decline of calcaneal bone stiffness. Arch Osteoporos 16, 83 (2021). https://doi.org/10.1007/s11657-021-00942-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-021-00942-5