Abstract
High impact loading is known to prevent some of the age-related bone loss but its effects on the density distribution of cortical bone are relatively unknown. This study examined the effects of age and habitual sprinting on tibial and fibular mid-shaft bone traits (structural, cortical radial and polar bone mineral density distributions). Data from 67 habitual male sprinters aged 19–39 and 65–84 years, and 60 non-athletic men (referents) aged 21–39 and 65–80 years are reported. Tibial and fibular mid-shaft bone traits (strength strain index SSI, cortical density CoD, and polar and radial cortical density distributions) were assessed with peripheral quantitative computed tomography. Analysis of covariance (ANCOVA) adjusted for height and body mass indicated that the sprinters had 21 % greater tibial SSI (P < 0.001) compared to the referents, with no group × age-group interaction (P = 0.54). At the fibula no group difference or group × age-group interaction was identified (P = 0.12–0.81). For tibial radial density distribution ANCOVA indicated no group × radial division (P = 0.50) or group × age-group × division interaction (P = 0.63), whereas an age × radial division interaction was observed (P < 0.001). For polar density distribution, no age-group × polar sector (P = 0.21), group × polar sector (P = 0.46), or group × age-group × polar sector interactions were detected (P = 0.15). Habitual sprint training appears to maintain tibial bone strength, but not radial cortical density distribution into older age. Fibular bone strength appeared unaffected by habitual sprinting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age-related bone loss, especially in the form of osteoporosis is associated with increased fracture risk [1]. Estimates of femoral neck strength based on three-dimensional data acquired with computed tomography have indicated that increased fracture risk is partially caused by insufficient structural strength [2]. Exercise during growth and into older age has been suggested to be a promising non-pharmacological means to prevent fractures by preventing falls and improving bone integrity [3]. Although, exercise may increase or prevent loss of mineral mass [4, 5], less is known, however, whether bone gains associated with lifelong exercise are related to long-term adaptations in bone structure and strength. This evidence is essential to inform future prevention of osteoporosis and reduction in fracture incidence.
Investigations in athletes offer some evidence of long-term natural loading experiments, which may shed some light on the potential of exercise in modulating bone traits. Cross-sectional studies comparing athletes (of all ages) to non-athletes have shown >20 % greater bone strength favouring athletes [6–8]. It is noteworthy, that the differences between athletes and non-athletes have been more marked in the weight-bearing tibia than the fibula [8, 9], indicating that the differences may be related to the loading environment rather than genetic predisposition and consequent selection bias from cross-sectional examination [10, 11]. Moreover, evidence of the effects of loading independent of genes is provided by a study of monozygotic twins discordant for physical activity, which indicated that habitual exercise may increase bone strength indices by ~20 % [12], thus the effects on bone attainable exercise alone seem markedly higher than that achieved through drug therapy (increases of ~10 %) and may be of sufficient magnitude to prevent fracture if maintained over long-term.
The bone gains associated with physical activity appear to be direction specific and are deposited in mechanically advantageous sites [8, 12–15]. Moreover, the adaptation seems to be primarily in geometry instead of material properties (i.e., volumetric bone mineral density, vBMD) [16]. However, bone loss and bone gain occur with differing mechanisms. Bone is deposited at the periosteal surface with exercise [14] and during aging [17, 18], while bone loss is more pronounced at the endocortical surface [19, 20]. Much of the age-related bone loss occurs through increasing cortical porosity [20], which has been shown to be increased in femoral neck fracture cases compared to non-fractured age-peers [19]. Age-related bone loss may thus be expected to modify apparent (i.e., including both the mineralization of the tissue and the porosity of the tissue) volumetric bone density more than the geometry of the bone. However, it is currently unknown, whether exercise may modulate this age-related cortical porosity increase.
It is well established that cortical bone density varies around (polar density distribution) and through (radial density distribution) the cortex, as well as along the length of the shaft of the lower limb [7, 16, 17, 19, 21, 22]. Studies on age-related changes on cortical density distribution have indicated that radial and polar distributions are changed with aging [17, 20–22]. For radial distribution, more density is lost from the endocortical border with age [17, 20], whereas for polar distribution, more volumetric density is lost from sections which bear less mechanical load during locomotion [21, 22]. However, it is currently unclear whether increased long-term mechanical loading prevents some of this age-related localized density loss (~porosity increase [23, 24] ). In one of the few related studies, Bailey et al. (2010) reported in 280 men aged 50–79 years characterized by tertiles based on lifelong physical activity that greater lifetime loading was associated with region-specific adaptations in cortical bone density [25].
While both the tibia and fibula can be examined from a single computed tomography measurement, the latter remains relatively sparsely explored. In untrained people, age-related bone loss has been reported to be more marked in the tibia than in the fibula [26] and as mentioned, the marked differences have been found in the tibia rather than the fibula between young adult athletes (men and women) and referents [8, 9]. However, age-loading interaction on the fibula in the athletes and referents remains unexplored. Due to the paucity of literature and the important role cortical bone plays in bone strength [27, 28], the primary purpose of the present study was to examine the effects of age and habitual sprinting on tibial and fibular mid-shaft bone traits (cortical radial and polar bone mineral density distributions), with a secondary aim to examine the effects of age and habitual sprinting on fibular bone traits.
Materials and Methods
For the present analysis, a convenience sample of males aged 19–84 years, 67 habitual athletes and 60 age-matched referents were acquired from the data collected at the University of Jyväskylä (Table 1) [17, 29–32]. The sprinters were recruited by letter from Finnish track and field organizations and the referents from the Jyväskylä city area. To qualify for the study, the athletes had to have a long-term sprint training background, and currently competing at a national or international level in events from 100 to 400-m (selection was based on the official results over the previous few years). The exclusion criteria included; no current/chronic major cardiovascular, endocrinological, musculoskeletal, neurological or psychiatric illnesses, no use of oral corticosteroids, and no use of medications for osteoporosis. The referent data were pooled from three convenient studies previously carried out in our lab conducted with healthy male participants [30–32]. The inclusion criteria for the referent subjects varied slightly between the studies, the age range (between 18 and 35 [30], 31 to 45 [31], and ≥65 years-of-age [32] ) in particular, but all studies included physically active healthy volunteers with regular participation in recreational physical activities such as ball games and jogging/cross-country skiing. The health-related exclusion criteria for the referents were the same as for the athletes in all of the three studies included. Moreover, the referents were not allowed to have a history of sprinting training. In addition, one of the studies excluded participants with a history of competitive sport participation (referents between 31 and 40 years-of-age) [31]. All participants provided written informed consent. Ethical approval was received from the local ethical committee and the studies were conducted in agreement to the Helsinki declaration.
The dominant leg (the leg used for the take-off in a one-footed jump) of the sprinters, whereas either the dominant or right leg of the referents group was scanned using peripheral quantitative computed tomography (pQCT, XCT 2000, Stratec Medizintechnik GmbH, Pforzheim, Germany). Scout scans were performed over the ankle joint, with the scanner reference line positioned at the post proximal aspect of the endplate of the distal tibia. Cross-sectional pQCT images were acquired proximal to the reference point at 50 % of the tibial length and included a 0.8 mm in-plane pixel size, a 2.5-mm slice thickness acquired using a maximum tube voltage of 30 kV with a 20 mm/s scanning speed. The slice was visually inspected for any possible motion artefact and re-measured if necessary.
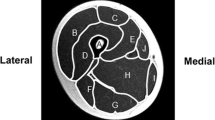
Total cross-sectional area (ToA, mm2), cortical bone area (CoA, mm2) and cortical vBMD (CoD, mg/cm3) were derived with a threshold of 710 mg/cm3. Density-weighted polar section modulus strength strain index (SSI, mm3)) was also derived to estimate diaphyseal bone resistance to torsional load [33]. In addition, polar cortical vBMD distribution and endo- and pericortical radii were calculated for the tibia (Fig. 1) as previously described [17] using the BoneJ [34] ImageJ (rsbweb.nih.gov/ij) plug-in. The coefficient of variation for the bone variables reported in the present study range from 0.6 to 6.0 % [17, 30].
Mid-tibial subcutaneous fat (Fat CSA, mm2) tissue was defined as voxels with a density <40 mg/cm3. Muscle tissue (muscle CSA, mm2) was defined as voxels with a density >40 mg/cm3 (differentiating muscle from subcutaneous fat) and <200 mg/cm3 (differentiating muscle from bone). To determine fat percentage (Fat %), density-weighted limb area was calculated from the data by weighing each of the pixels with its pQCT-measured density. Similarly, density-weighted fat area was calculated by weighing the fat pixels with their density. Dividing the density-weighted fat area with the density-weighted limb area and multiplying by 100 subsequently derived fat %. The aforementioned BoneJ [34] ImageJ (rsbweb.nih.gov/ij) plug-in was used for soft tissue analyses.
Anthropometric measures height (cm) and body mass (kg) were assessed using standardized protocols, via a stadiometer and electronic scales, respectively.
Statistical Analysis
All results are reported as means and standard deviations (SD) (unless otherwise notes). The data were split into two age groups (age-group): <40, and >65 years-of age. Sprinters and referents were compared to each other in terms of age, height and weight with analysis of variance (ANOVA) using group (sprinter or referent) and age-group (<40, and >65) as between subject factors. To assess differences in bone densitometric and structural parameters between athletes and referents Analysis of Covariance (ANCOVA) with group (sprinter or referent) and age-group (<40, and >65) as between subjects factors was performed with height and weight included as covariates were performed. A group × age-group interaction was tested using ANCOVA. In addition, the differences between tibia and fibula were evaluated by adding bone (tibia or fibula) as a within subject factor. Similarly, polar and radial density distributions were evaluated by including sector or division (endo-, mid- or pericortical/sectors 1 through 36) as a within subject factor. Where applicable, percentage group differences were calculated from estimated marginal means based on the ANCOVA with the referent group as the denominator. Statistical analyses were conducted with IBM SPSS version 20 (IBM corporation.) software and the significance level was set at P ≤ 0.05.
Results
Descriptive characteristics for sprinters and referents in different age groups are given in Table 1. Univariate ANOVA comparisons indicated that sprinters were 4.2 % lighter than the referents (P = 0.03), with height and body mass significantly differing between the age-groups (P < 0.001). Both the younger sprinters and referents were 5.3 % taller and 5.6 % heavier than the older sprinters and referent groups (P = 0.006–P < 0.001). No group × age-group interaction was observed (P = 0.21–0.54) (Table 1).
Univariate ANCOVA comparisons adjusted for height and body mass indicated that sprinters had 7.6 % larger muscle CSA (P = 0.003), 38 % smaller fat CSA and 30 % lower fat percentage (P < 0.001) when compared to the referents group. Only muscle CSA differed between the age-groups, with the younger age-group having 16 % larger muscle CSA than the older age-group (P < 0.001). No group × age-group interaction was observed (P = 0.13–0.72) (Table 2).
At the tibia, athletes had significantly greater adjusted (height and weight) ToA, CoA, and SSI and lower CoD (P < 0.001–P = 0.01) when compared to the referent groups, but these variables did not differ between age-groups (P = 0.05–0.60). Sprinters had 12 % larger ToA (P < 0.001), 15 % larger CoA (P < 0.001), 1 % lower adjusted CoD (P = 0.01) and 21 % higher SSI (P < 0.001) than the referents. All findings persisted when muscle CSA was additionally added as a covariate. No group × age-group interaction was observed (P = 0.19–0.82) (Fig. 2; Table 2).
At the Fibula, the younger males after adjustment had 7.8 % smaller ToA and 4.1 % higher CoD (P < 0.001–P = 0.01) when compared to the older males, however, no differences were observed between the groups (P = 0.07–0.29). All findings persisted when muscle CSA was additionally added as a covariate. No group × age-group interaction was observed (P = 0.68–0.94) (Fig. 3; Table 2).
A bone × group (P < 0.001) and bone × age-group (P < 0.001) interaction was observed in the comparison between the adjusted tibial and fibular bone traits, whereas no bone × group × age-group interaction was observed (P = 0.55).
Repeated measures ANCOVA adjusted for height and body mass indicated that there was no group × sector (P = 0.46), age-group × sector (P = 0.21), or group × age-group × sector (P = 0.15) interactions in the tibial mid-shaft polar density distribution. In pairwise comparisons, the sprinters had 1.5 % lower mean cortical density than the referents (P < 0.001) (Fig. 4). The same test for radial density distribution indicated no group × division (P = 0.50) or group × age-group × division interaction (P = 0.63), but a significant age-group × division interaction (P < 0.001) (Fig. 5).
Tibial mid-shaft polar cortical density distributions (95 % confidence interval) from ANCOVA estimated marginal means adjusted for height and body mass for the athletes (solid line -) and referents (dashed line - -). Sector 1 opens towards fibula, sector 2 lateral, sector 12 anterior, sector 22 medial, sector 31 posterior. a younger group. b older group
Discussion
The results from this study add to the increasing evidence that there are positive benefits to the skeleton though lifelong exercise, moreover, these benefits seem to still be evident in older adulthood. While bone strength index of the tibial mid-shaft was superior in the young and older sprint-trained athletes (21 % higher), when compared to the age-matched referents, the generic pattern of either polar or radial density distribution did not differ between groups. Indicating that although benefits in bone strength may be maintained with exercise, it is unlikely that age-related cortical bone 'quality' deterioration can be prevented with exercise into old age. Furthermore, as previously indicated [8, 9], the current findings show that the differences in the bone densitometry and structural properties between groups (sprinters versus referent) are larger at the tibia (12–21 % difference) than those seen at the fibula (5–8 % difference, n.s.).
As no group × age-group × division interaction was detected in the current study in radial density distribution of mid-tibia it would seem that habitual lifelong sprint training does not prevent the age-related bone 'quality' deterioration manifested with increasing cortical porosity particularly at the endocortical border [19, 20]. The sprinters in the current study who maintained high levels of physical activity through aging, exhibited markedly higher bone strength indices (up to 21 %) than their age-matched referents, consequently, the implication is that any possible decrease in endocortical vBMD (indicating increased porosity [23, 24] ) in older adults compared to younger individuals may be part of the normal aging process. In this context, however, it needs to be pointed out that in the present study, even though the age-group × division interaction indicated that the pattern of radial density distribution differs between the age-groups, the older group did not have significantly lower endocortical density compared to the younger group (adjusted for height and weight, younger group had 0.8 % higher endocortical vBMD [P = 0.17] than the older group) and no bone loss was thus actually observed in the present study. These findings are in contrast to previous studies, Cheng et al. (2002) reported that 12-months of high impact exercise (jumping and bounding activities) can increase pQCT-measured vBMD at the vicinity of the endocortical border suggesting that an increase in skeletal loading with respect to the pre-existing levels may [15] be able to ameliorate the porosity increase associated with aging.
As previously reported for young adult men [7] and women [16], our findings indicated that there was no group × sector interaction in polar density distribution. This result supports our previous suggestion that bone strength in the shaft of long bone is primarily modulated by increasing mineral mass and changing the bones geometric cross-section, as opposed to changing the mineralization of the bone [16]. Knowing that many bones are loaded in bending, it may be argued that the exercise-induced gains in bone are more advantageous compared to the changes associated with drug therapy which do not induce gains in bone geometric parameters [35]. Exercise interventions, however, need to be specifically tailored to increase exercise-induced gains at skeletal sites prone to fracture such as the femoral neck [36].
In regards to the age-related findings on polar density distribution, no difference was observed between age-groups. This is in contrast to previous reports from the femur [21, 22]. The ambiguity may have several plausible explanations, with the most obvious being the difference in the method used to analyse polar distributions. In the present study, a layer of endo- and pericortical pixels was peeled away to minimize the partial volume effect prior to analysing the polar density distribution, whereas no such exclusion of endo- and pericortical surface was applied in the aforementioned studies on the femur [21, 22]. It is known that much of the age-related bone loss occurs at the vicinity of endosteal surface [19, 20]. Consequently, the elimination of partial volume effect pixels could have masked possible aging effects on the polar density distribution.
Consistent with previous studies comparing athletes to non-athletic referents [6, 7, 16, 37, 38] or even within an individual for dominant versus non-dominant limb [39–41] the mean CoD within the cortical envelope in the tibia was found to be 1.5 % lower in the sprinters compared to referents. Jepsen et al. [42, 43] identified different skeletal phenotypes in terms of the combination of bone morphology and material properties. Where slender bones typically have high apparent bone volumetric density and robust bones lower apparent bone volumetric density. The slender phenotype is known to be associated with stress fractures, as it is not able to adapt the geometry of the bone to a sufficient amount [43–45]. Studies in athletes [39–41] seem to indicate that the slender bone phenotype with high mineralization may be at least partially attributable to the lack of loading. As we and others [6, 7, 16, 37] have speculated, it could be that the high apparent volumetric density is secondary to a less active remodelling of a smaller bone compared to a larger bone caused by older bone having higher mineralization than newer, as well as secondary bone having higher porosity than primary [46]. Therefore interventions examining the skeletal adaptation in response to a loading, stratified by bone phenotype, would provide the evidence to explain the extent of the adaptability of the skeleton in the slender phenotype, but unfortunately to our knowledge no such studies have been reported in the literature to date.
The secondary aim of the present study was to examine the effects of age and habitual sprinting on fibular bone traits. In line with previous studies in young adult men [9] and women [8], we found that the difference in bone traits in the fibula when comparing athletes with the referents group was markedly less (and not statistically significant) than those observed at the tibia. Since the fibula possesses the ability to dramatically increase its bone mass with dramatic changes in the loading environment [47, 48], the result lends further support to our hypothesis that the tibia and fibula experience substantially different loading environments during locomotion [8]. Similarly, site- and bone-specific differences between gymnasts and referents have been observed at radius and ulna highlighting that even spatially related bones may have rather dis-similar loading environments [49, 50]. Also, as previously reported by McNeil et al. (2009), the observed bone × age-group interaction suggests that the tibia may be susceptible to more marked age-related changes than the fibula [26]. Considering the findings together, we postulate that the loading environment of the fibula changes less than that of tibia with aging, which then causes the observed difference in age-related results between the tibia and fibula.
This research has specific limitations related to the study design and the analysis methods. First, the cross-sectional design of the present study is neither free from potential selection bias nor able to show causal effects and consequently the results can only be hypothesis generated. In addition, no attempt was made to adjust for any possible generation related cohort effects, such as training duration, and age at first participation. It could be argued that individuals with well-developed skeletal structure are those who are naturally more active in young adulthood and later in life. Second, since pQCT at a relatively coarse resolution was used to assess the density distribution, it is impossible to tell whether the density findings are related to porosity or mineralization of the bone tissue. While it has been shown previously that a large proportion (~70 %) of the variation in the density values measured with quantitative computed tomography is explained by porosity [24], some of the variation may also be explained by mineralization of compact bone material [46]. Furthermore, due to the lack of standardized measurement locations, data acquisition and analysis methods for pQCT, a comparison of data with other studies is challenging. However, excluding the inner and outer cortical pixels strengthened the pQCT analysis in this study, and the bone geometries were carefully aligned minimizing the partial volume effect of the pQCT measurement. Despite these limitations, the current study provides evidence that lifelong exercise (in this case high impact sprinting) induces structurally stronger bone rather than causing volumetric bone density gains in both the tibia and fibula. Moreover, these gains seem to be maintained at similar magnitudes in both younger and older exercising adults.
In conclusion, this study indicates that although sprinters had exercise-induced bone gains at the tibia the density distribution of bone did not differ between sprinters and referents nor was there an age interaction with habitual sprinting observed. While bone strength benefit may be maintained with exercise, it is unlikely that age-related cortical bone 'quality' deterioration can be prevented with exercise.
References
Cummings SR, Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. The Lancet 359(9319):1761–1767. doi:10.1016/S0140-6736(02)08657-9
Keaveny TM, Kopperdahl DL, Melton LJ et al (2010) Age-dependence of femoral strength in white women and men. J Bone Miner Res 25(5):994–1001
Karinkanta S, Piirtola M, Sievänen H et al (2010) Physical therapy approaches to reduce fall and fracture risk among older adults. Nat Rev Endocrinol 6(7):396–407. doi:10.1038/nrendo.2010.70
Asikainen T-M, Kukkonen-Harjula K, Miilunpalo S (2004) Exercise for health for early postmenopausal women: a systematic review of randomised controlled trials. Sports Med Auckl NZ 34(11):753–778
Nikander R, Sievanen H, Heinonen A et al (2010) Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med 8(1):47. doi:10.1186/1741-7015-8-47
Wilks DC, Winwood K, Gilliver SF et al (2009) Bone mass and geometry of the tibia and the radius of master sprinters, middle and long distance runners, race-walkers and sedentary control participants: a pQCT study. Bone 45(1):91–97. doi:10.1016/j.bone.2009.03.660
Weidauer LA, Eilers MM, Binkley TL et al (2012) Effect of different collegiate sports on cortical bone in the tibia. J Musculoskelet Neuronal Interact 12(2):68–73
Rantalainen T, Nikander R, Heinonen A et al (2010) Direction-specific diaphyseal geometry and mineral mass distribution of tibia and fibula: a pQCT study of female athletes representing different exercise loading types. Calcif Tissue Int 86(6):447–454. doi:10.1007/s00223-010-9358-z
Marchi D, Shaw CN (2011) Variation in fibular robusticity reflects variation in mobility patterns. J Hum Evol 61(5):609–616. doi:10.1016/j.jhevol.2011.08.005
Mikkola TM, Sipilä S, Rantanen T et al (2008) Genetic and environmental influence on structural strength of weight-bearing and non–weight-bearing bone: a twin study. J Bone Miner Res 23(4):492–498. doi:10.1359/jbmr.071205
Mikkola TM, Sipilä S, Rantanen T et al (2009) Muscle cross-sectional area and structural bone strength share genetic and environmental effects in older women. J Bone Miner Res 24(2):338–345. doi:10.1359/jbmr.081008
Ma H, Leskinen T, Alen M et al (2009) Long-term leisure time physical activity and properties of bone: a twin study. J Bone Miner Res 24(8):1427–1433. doi:10.1359/jbmr.090309
Macdonald HM, Cooper DML, McKay HA (2008) Anterior–posterior bending strength at the tibial shaft increases with physical activity in boys: evidence for non-uniform geometric adaptation. Osteoporos Int 20(1):61–70. doi:10.1007/s00198-008-0636-9
Leppanen OV, Sievanen H, Jokihaara J et al (2010) The effects of loading and estrogen on rat bone growth. J Appl Physiol 108(6):1737–1744. doi:10.1152/japplphysiol.00989.2009
Cheng S, Sipilä S, Taaffe DR et al (2002) Change in bone mass distribution induced by hormone replacement therapy and high-impact physical exercise in post-menopausal women. Bone 31(1):126–135
Rantalainen T, Nikander R, Daly RM et al (2011) Exercise loading and cortical bone distribution at the tibial shaft. Bone 48(4):786–791. doi:10.1016/j.bone.2010.11.013
Rantalainen T, Nikander R, Heinonen A et al (2011) An open source approach for regional cortical bone mineral density analysis. J Musculoskelet Neuronal Interact 11(3):243–248
Feik SA, Thomas CDL, Bruns R, Clement JG (2000) Regional variations in cortical modeling in the femoral mid-shaft: sex and age differences. Am J Phys Anthropol 112(2):191–205
Bell KL, Loveridge N, Power J et al (1999) Regional differences in cortical porosity in the fractured femoral neck. Bone 24(1):57–64
Zebaze R, Ghasem-Zadeh A, Bohte A et al (2010) Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. The Lancet 375(9727):1729–1736
Atkinson PJ, Weatherell JA (1967) Variation in the density of the femoral diaphysis with age. J Bone Joint Surg Br 49:781–788
Carballido-Gamio J, Harnish R, Saeed I et al (2013) Proximal femoral density distribution and structure in relation to age and hip fracture risk in women. J Bone Miner Res 28(3):537–546
Seeman E (1998) Growth in bone mass and size—are racial and gender differences in bone mineral density more apparent than real? J Clin Endocrinol Metab 83(5):1414–1419. doi:10.1210/jc.83.5.1414
Bousson V, Bergot C, Meunier A et al (2000) CT of the middiaphyseal femur: cortical bone mineral density and relation to porosity1. Radiology 217(1):179–187
Bailey CA, Kukuljan S, Daly RM (2010) Effects of lifetime loading history on cortical bone density and its distribution in middle-aged and older men. Bone 47(3):673–680. doi:10.1016/j.bone.2010.06.027
McNeil CJ, Raymer GH, Doherty TJ et al (2009) Geometry of a weight-bearing and non-weight-bearing bone in the legs of young, old, and very old men. Calcif Tissue Int 85(1):22–30. doi:10.1007/s00223-009-9261-7
Holzer G, von Skrbensky G, Holzer LA, Pichl W (2009) Hip fractures and the contribution of cortical versus trabecular bone to femoral neck strength. J Bone Miner Res 24(3):468–474. doi:10.1359/jbmr.081108
Koivumäki JEM, Thevenot J, Pulkkinen P et al (2012) Cortical bone finite element models in the estimation of experimentally measured failure loads in the proximal femur. Bone 51(4):737–740. doi:10.1016/j.bone.2012.06.026
Rantalainen T, Sievänen H, Linnamo V et al (2009) Bone rigidity to neuromuscular performance ratio in young and elderly men. Bone 45(5):956–963. doi:10.1016/j.bone.2009.07.014
Rantalainen T, Heinonen A, Komi PV, Linnamo V (2008) Neuromuscular performance and bone structural characteristics in young healthy men and women. Eur J Appl Physiol 102(2):215–222. doi:10.1007/s00421-007-0575-8
Korhonen MT, Heinonen A, Siekkinen J et al (2012) Bone density, structure and strength, and their determinants in aging sprint athletes. Med Sci Sports Exerc 44(12):2340–2349. doi:10.1249/MSS.0b013e318267c954
Rantalainen T, Hoffrén M, Linnamo V et al (2011) Three-month bilateral hopping intervention is ineffective in initiating bone biomarker response in healthy elderly men. Eur J Appl Physiol 111(9):2155–2162. doi:10.1007/s00421-011-1849-8
Ashe MC, Khan KM, Kontulainen SA et al (2006) Accuracy of pQCT for evaluating the aged human radius: an ashing, histomorphometry and failure load investigation. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 17(8):1241–1251. doi:10.1007/s00198-006-0110-5
Doube M, Kłosowski MM, Arganda-Carreras I et al (2010) BoneJ: free and extensible bone image analysis in ImageJ. Bone 47(6):1076–1079. doi:10.1016/j.bone.2010.08.023
Genant HK, Engelke K, Hanley DA et al (2010) Denosumab improves density and strength parameters as measured by QCT of the radius in postmenopausal women with low bone mineral density. Bone 47(1):131–139. doi:10.1016/j.bone.2010.04.594
Nikander R, Kannus P, Dastidar P et al (2009) Targeted exercises against hip fragility. Osteoporos Int 20(8):1321–1328. doi:10.1007/s00198-008-0785-x
Weidauer LA, Binkley TL, Berry R, Specker BL (2013) Variation in cortical density within the cortical shell of individuals across a range in densities and ages. J Musculoskelet Neuronal Interact 13(1):89–96
Smock AJ, Hughes JM, Popp KL et al (2009) Bone volumetric density, geometry, and strength in female and male collegiate runners. Med Sci Sports Exerc 41(11):2026–2032. doi:10.1249/MSS.0b013e3181a7a5a2
Kontulainen S, Sievänen H, Kannus P et al (2003) Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res 18(2):352–359. doi:10.1359/jbmr.2003.18.2.352
Neil JMB, Schweitzer ME (2008) Humeral cortical and trabecular changes in the throwing athlete: a quantitative computed tomography study of male college baseball players. J Comput Assist Tomogr 32(3):492–496. doi:10.1097/RCT.0b013e31811ec72d
Ireland A, Maden-Wilkinson T, Mcphee J et al (2013) Upper limb muscle-bone asymmetries and bone adaptation in elite youth tennis players. Med Sci Sports Exerc 45:1749–1758. doi:10.1249/MSS.0b013e31828f882f
Jepsen KJ, Centi A, Duarte GF et al (2011) Biological constraints that limit compensation of a common skeletal trait variant lead to inequivalence of tibial function among healthy young adults. J Bone Miner Res 26(12):2872–2885. doi:10.1002/jbmr.497
Jepsen KJ, Evans R, Negus CH et al (2013) Variation in tibial functionality and fracture susceptibility among healthy, young adults arises from the acquisition of biologically distinct sets of traits. J Bone Miner Res 28(6):1290–1300. doi:10.1002/jbmr.1879
Giladi M, Milgrom C, Simkin A et al (1987) Stress fractures and tibial bone width. A risk factor. J Bone Joint Surg Br 69(2):326–329
Popp KL, Hughes JM, Smock AJ et al (2009) Bone geometry, strength, and muscle size in runners with a history of stress fracture. Med Sci Sports Exerc 41(12):2145–2150. doi:10.1249/MSS.0b013e3181a9e772
Roschger P, Paschalis EP, Fratzl P, Klaushofer K (2008) Bone mineralization density distribution in health and disease. Bone 42(3):456–466. doi:10.1016/j.bone.2007.10.021
Gan AWT, Puhaindran ME, Pho RWH (2013) The reconstruction of large bone defects in the upper limb. Injury 44(3):313–317. doi:10.1016/j.injury.2013.01.014
Atkins RM, Madhavan P, Sudhakar J, Whitwell D (1999) Ipsilateral vascularised fibular transport for massive defects of the tibia. J Bone Joint Surg Br 81(6):1035–1040
Ducher G, Daly RM, Hill B et al (2009) Relationship between indices of adiposity obtained by peripheral quantitative computed tomography and dual-energy X-ray absorptiometry in pre-pubertal children. Ann Hum Biol 36(6):705–716. doi:10.3109/03014460903055139
Burt LA, Naughton GA, Greene DA, Ducher G (2011) Skeletal differences at the ulna and radius between pre-pubertal non-elite female gymnasts and non-gymnasts. J Musculoskelet Neuronal Interact 11(3):227–233
Acknowledgments
The Academy of Finland (250683, 253198), Ministry of Education and Culture, KELA (Dnro 34/26/2011). Dr Rantalainen was supported by a Grant from Emil Aaltonen Foundation during the preparation of the mansucript.
Conflict of Interest
All authors state that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All procedures followed were in accordance with the ethical standards of the ethical comittee of the University of Jyväskylä, Finland responsible on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rantalainen, T., Duckham, R.L., Suominen, H. et al. Tibial and Fibular Mid-Shaft Bone Traits in Young and Older Sprinters and Non-Athletic Men. Calcif Tissue Int 95, 132–140 (2014). https://doi.org/10.1007/s00223-014-9881-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9881-4