Abstract
Summary
The study aimed to quantify the long-term effects of stroke on tibial bone morphology and hip bone density. Only the trabecular bone mineral density and bone strength index in the hemiparetic tibial distal epiphysis showed a significant decline among individuals who had sustained a stroke 12–24 months ago.
Introduction
This study aims to determine the changes in bone density and morphology in lower limb long bones during a 1-year follow-up period and their relationship to muscle function in chronic stroke patients.
Methods
Twenty-eight chronic stroke patients (12–166 months after the acute stroke event at initial assessment) and 27 controls underwent bilateral scanning of the hip and tibia using dual-energy X-ray absorptiometry and peripheral quantitative computed tomography, respectively. Each subject was re-assessed 1 year after the initial assessment.
Results
Twenty stroke cases and 23 controls completed all assessments. At the end of the follow-up, the paretic tibial distal epiphysis suffered significant decline in trabecular bone density (−1.8 ± 0.6 %, p = 0.006) and bone strength index (−2.7 ± 0.6 %, p < 0.001). More severe decline in the former was associated with poorer leg muscle strength (ρ = 0.447, p = 0.048) and motor recovery (ρ = 0.489, p = 0.029) measured at initial assessment. The loss in trabecular bone density remained significant among those whose stroke onset was 12–24 months ago (p < 0.001), but not among those whose stroke onset was beyond 24 months ago (p > 0.05) at the time of initial assessment. The changes of outcomes in the tibial diaphysis, except for cortical bone mineral content on the non-paretic side (−1.3 ± 0.3 %, p = 0.003), and hip bone density were well within the margin of error for precision.
Conclusions
There is evidence of continuous trabecular bone loss in the paretic tibial distal epiphysis among chronic stroke patients, but it tends to plateau after 2 years of stroke onset. The steady state may have been reached earlier in the hip and tibial diaphysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke survivors have approximately sevenfold higher fracture risk than the age- and sex-matched population [1, 2]. Hip fracture is the most common type of fracture, accounting for 30–58 % of all fractures following stroke [1–3]. Over 80 % of hip fractures occur on the hemiplegic side, perhaps because falls onto this side are more common, and bone mass and quality are more compromised [4–7]. Bone loss and fractures have negative impact on longevity and quality of life following stroke [1, 2, 8, 9] and should warrant attention.
A number of studies have attempted to examine the bone properties in people after stroke but have major limitations. Firstly, previous research that examined longitudinal bone changes after stroke were confined to measurement of bone mineral density (BMD) using dual-energy X-ray absorptiometry (DXA) [8, 10–13]. While these studies consistently found that reduction in bone mineral content (BMC) and BMD on the paretic side was more severe than the non-paretic side [7, 8, 11, 13], they could not provide any information on alterations of bone structural properties due to the planar nature of DXA. Secondly, previous studies that used peripheral quantitative computed tomography (pQCT) to examine lower limb bone morphology following stroke were cross-sectional, which render them unable to capture the time course of bone structural changes post-stroke [14–17]. The immobilization after stroke could induce generalized bone changes in stroke patients [9, 11, 18]. The non-paretic side may hence also suffer bone loss, resulting in potential underestimation of the structural deterioration on the paretic side when side-to-side comparisons were made [16, 17]. To date, no study has examined the longitudinal changes in structural properties of lower limb long bones after stroke.
Another unstudied area pertains to the bone changes in the chronic stage of stroke recovery. While it is known that fracture risk remains elevated long after stroke [2], whether bone loss continues in the longer term is not well established. Sato et al. [19] compared the level of bone resorption and formation markers between people within 1 year of stroke onset and those within 1–2 years of stroke onset. It was found that serum concentration of pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen (ICTP) (a bone resorption marker) was higher in the former group than latter group, whereas the level of bone Gla protein (a bone formation marker) was similar in both groups. Their results indicated that bone resorption, which was exaggerated in the early phase of stroke, had slowed down in the chronic phase. Existing longitudinal studies suggested that during the first year post-stroke, femoral neck areal bone mineral density (aBMD) decreased by ~12 % on the paretic side and ~6 % on the non-paretic side [7, 11]. To date, the changes in lower limb long bones after the first year post-stroke has only been investigated by de Brito et al. [10]. After the follow-up period (mean = 16 months), only a small proportion (15.1 %) of their chronic stroke cases (time since stroke = 33.4 ± 17.9 months) sustained a significant change in femoral neck aBMD. In addition, time since stroke onset was not significantly associated with change in femoral neck aBMD, indicating that the steady-state level may have been reached at first year post-stroke. However, bone geometric changes were not assessed in their study. Overall, existing findings from longitudinal research provide limited information on the time course of the influence of stroke on bone structural and geometric changes in the chronic stroke population.
To address the above knowledge gaps, a longitudinal study was undertaken to quantify the chronic effects of stroke on density and morphology of the lower limb long bones using both DXA and pQCT during a 1-year follow-up period. This may provide important insight into the reasons underlying the exaggerated hip fracture risk post-stroke [1, 2]. As previous cross-sectional studies have identified a strong relationship between muscle function and tibial and hip bone outcomes in stroke patients [14, 15, 17], it would be interesting to determine whether a similar relationship exists in a longitudinal study. Thus, the secondary objective was to assess the association between muscle function and bone changes in chronic stroke patients.
Methods
Collection of demographic information and bone scan acquisitions were done in both the chronic stroke cases (≥1 year after onset of stroke at initial assessment) and control subjects at initial assessment. Bone scanning on bilateral lower limbs was repeated 1 year after the initial assessment.
Subjects
The stroke cases were recruited from a local stroke self-help group in Hong Kong, China. Inclusion criteria were (i) a diagnosis of stroke, (ii) ≥1 year after onset of stroke, (iii) aged 18 or more, (iv) medically stable, (v) of Chinese origin, and (vi) able to respond to simple verbal commands with an Abbreviated Mental Test score of 7 or higher. Exclusion criteria were other neuromuscular diseases, recent fractures in the lower extremity, metal implants in the lower extremity, and taking prescribed medications for treatment of osteoporosis prior to or after stroke. Controls were identified from an existing database consisting of individuals who had joined previous research in the University, and should fulfill all criteria described above, except that they did not have any history of stroke.
Demographic data
The relevant demographic data (e.g., medical history, etc.) were obtained by interviewing the subjects. The walking capacity was evaluated with the Six-Minute Walk Test [20]. The physical activity level was assessed using the Physical Activity Scale for the Elderly [21]. To determine the leg dominance, each subject was asked to kick a ball in standing position. The leg used to kick the ball was considered the dominant leg.
Bone imaging
The same experienced technician conducted bone imaging initially and at 1-year follow-up using both pQCT and DXA.
pQCT
pQCT (XCT 3000, Stratec Medizintechnik GmbH; Pforzheim, Germany) was used to acquire three-dimensional cross-sectional scans of the tibia. Information on bone mass, density, geometry, and strength indices were generated by pQCT. After obtaining a scout view, an anatomical reference line was placed at the cortical end plate of the distal medial edge of the tibia. A voxel size of 500 μm and scan speed of 25 mm/s were used to obtain 2.3 mm thick scans at (i) tibial distal epiphysis (4 % of the total tibia length proximal to the reference line), a region containing both cortical and trabecular bone, and (ii) tibial diaphysis (66 % of the total tibia length proximal to the reference line), a cortical region. The variables assessed by the pQCT device are listed in Table 1. For the 4 % site, a compressive bone strength index (CBSI, g2/cm4) was computed as an estimation of bone strength against compressive force using the formula: total area × (total volumetric bone mineral density)2 [22, 23]. For the 66 % site, a polar stress–strain index (p-SSI, mm3) was computed as an estimation of bone strength against torsional forces [17, 22, 23] using the following formula [24]:
where A = area of voxel, d = distance between the voxel and corresponding torsion axis, ND = normal physiological bone density at 1200 mg/cm3, and d max = maximal distance to the center of gravity.
For image analysis of the distal tibial epiphysis, CALCB Contour Mode 2 (an algorithm performing an iterative contour procedure to eliminate the soft tissue outside the bone) and Peel Mode 2 (an algorithm used to separate the total bone area into trabecular and (sub)cortical regions) were used. A density threshold of 169 mg/cm3 was used to detect the outer contour of the bone, and trabecular bone was separated from cortical and subcortical bone using a density threshold of 400 mg/cm3 [25]. For tibial diaphysis, cortical bone analysis was performed using CORTBD Mode 1, with a threshold of 710 mg/cm3 [25]. It means that a voxel with a density value higher than 710 mg/cm3 would be detected as cortical. These thresholds were chosen after taking reference from previous pQCT studies in patients with stroke [26, 27]. All image analyses were performed using customized software (Stratec software, Version 6.0). The precision of the pQCT scanner was determined by testing 30 healthy subjects twice (2 women, mean age 72.3 ± 5.1 years), according to the recommendations of the International Society for Clinical Densitometry [28]. The least significant change (LSC) values for the outcome variables were calculated as 2.77 times the absolute precision error and are displayed in Table 1. Any change that exceeds the LSC represents a real change that is beyond the variations stemming from repeated measurements.
DXA
DXA (Hologic Inc, Bedford, MA, USA) was used to assess the total hip aBMD (g/cm2) on both sides. The LSC value for hip aBMD was determined the same way as described for the pQCT device (Table 1).
Muscle function
Knee muscle strength
Peak isometric knee extensor muscle strength of both legs was measured using a hand-held dynamometer (Nicholas MMT, Lafayette Instruments, Lafayette, IN, USA). Subjects were asked to sit upright in a chair with hip and knee in 90° flexion. They were then instructed to perform an isometric knee extension with maximal effort and sustain for 5 s, and the maximal force (N) was registered by the dynamometer. Three trials were conducted to obtain the mean strength score.
Motor recovery
The Impairment Inventory of the Chedoke-McMaster Stroke Assessment (CMSA) was used to evaluate the severity of impairment in the paretic leg among the stroke patients [29]. The scoring was based on a seven-point ordinal scale (1–7), with a higher score indicating better motor recovery (e.g., 1 = no motor return, 3 = synergistic movement patterns and marked spasticity, 7 = normal movement patterns). The CMSA has been shown to have high intra-rater reliability (ICC = 0.98) and inter-rater reliability (ICC = 0.97–0.99) [29].
Spasticity
In the stroke group, the Modified Ashworth Scale (MAS) was administered to assess spasticity in the paretic leg [30]. With the participant in a supine position, the ankle joint was moved into dorsiflexion and plantar flexion alternately by the researcher, and the amount of resistance to passive movements was noted. The score range was between 0 and 4, with a higher MAS score denoting more severe spasticity.
Statistical analysis
Comparisons of subjects’ characteristics at initial assessment between the stroke cases and controls for binary variables were conducted using Fisher’s exact test. For continuous variables, Shapiro-Wilk test was used to check normal distribution, and then two-sample t test for the mean was used, if the data were normally distributed; otherwise, non-parametric Mann–Whitney U test was used. The latter test was also used for ordinal data.
For the follow-up data, we computed the percentage change for a variable X as 100{(X follow-up − X initial) / (X initial)}. One-sample t test was used to test whether the percentage change was significantly different from zero. To compare within and between groups for initial differences and percentage change data, we fitted the mixed effects model (within-subject factor, side; between-subject factor, stroke vs control) to each outcome variable, taking into account the correlation between measurements on the two sides within the individuals. We then performed post-hoc testing to compare sides within the stroke cases or controls (i.e., paretic versus non-paretic for stroke cases and dominant versus non-dominant for controls) or between-group comparisons (i.e., paretic side of stroke cases versus non-dominant side of controls, and similarly for non-paretic side versus dominant side).
To further explore the influence of post-stroke duration and bone changes, the stroke subjects were divided into two groups: those between 12 and 24 months after stroke onset at the time of initial assessment (early group), and those beyond 24 months after stroke onset at the time of initial assessment (late group). Within-group (i.e., paretic versus non-paretic) and between-group comparisons (early vs late) of bone parameters were made. To assess the relationship between 1-year percent change in bone variables and muscle function variables, Spearman’s rho was used. All analyses were conducted using commercial software STATA (version 11, StataCorp LP, College Station, Texas, USA). A p value (p) less than 0.05 was considered significant. However, for comparisons of bone outcomes, we further used the false discovery rate (FDR) for adjustment of the potential inflation of type I errors associated with multiple comparisons.
Results
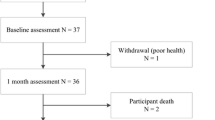
Twenty-eight stroke cases and 27 controls fulfilled the inclusion criteria and completed the initial assessment. Of these, eight cases and four controls were lost to follow-up leaving 20 cases (12 men) and 23 controls (14 men) with evaluable data at 12 months (Fig. 1). The dominant leg was the right leg for all subjects in the stroke and controls group. In the stroke group, the proportion of people with left hemiparesis and right hemiparesis was 60 and 40 %, respectively (ratio = 1.5:1). This left/right hemiparesis ratio was not significant from a ratio of 1:1 (i.e., 50 % left hemiparesis and 50 % right hemiparesis) (Chi-square goodness-of-fit test, p = 0.371).
Differences between cases and controls at initial assessment
In the stroke group, the average onset after stroke was 49 months (range, 12–166) when the initial assessment was conducted. The stroke group had higher proportion of subjects with high cholesterol level (p = 0.003). They also had significantly less physical activity (p = 0.002), lower paretic knee extensor strength (p = 0.006), more comorbidities (p < 0.001), and were prescribed more therapies than controls (p < 0.001). There were no significant between-group differences in age, sex distribution, or body mass index (p > 0.5) (Table 2).
At the 4 % site, there was significant group × side interaction in total volumetric bone mineral density (vBMD) (p = 0.009), trabecular vBMD (p = 0.005), and total cross-sectional area (CSA) (p = 0.035) (Table 2). Specifically, the paretic side had significantly lower total vBMD (p < 0.001), trabecular vBMD (p < 0.001), and CBSI (p < 0.001) than the non-paretic side by 5.9, 4.0, and 7.8 %, respectively, in the stroke group. The total CSA was significantly greater (p = 0.007) on the paretic side than the non-paretic side. None of the variables showed a significant side-to-side difference among controls. Between-group analysis revealed no significant results (Table 3).
At the 66 % site, there was significant group × side interaction in cortical BMC (p = 0.004), cortical vBMD (p = 0.016), cortical CSA (p = 0.017), and p-SSI (p = 0.001). Post-hoc analysis showed that the cortical area (p = 0.001) was smaller and the cortical BMC (p < 0.001), cortical vBMD (p = 0.001), and p-SSI (p = 0.001) were lower on the paretic side than the non-paretic side in the stroke group, by 5.3, 6.8, 2.2, and 6.1 %, respectively. Similar to the 4 % site, none of the pQCT variables showed a significant side-to-side difference in controls. Between-group analysis also revealed no significant results.
In the stroke group, the total hip aBMD on the paretic side was also significantly lower than the non-paretic side (p < 0.001) (Table 3). The side-to-side comparisons for the control group and the between-group analysis did not yield significant results.
Bone changes during 1-year follow-up
After the 12-month follow-up period at the 4 % site, trabecular vBMD (p = 0.006) and CBSI (p < 0.001) on the paretic side showed a significant decline by 1.8 and 2.7 %, respectively, which exceeded their respective LSC values (Table 4). On the non-paretic side, only trabecular vBMD (p < 0.001) suffered a significant decline (by 1.3 %) that exceeded the LSC values (Table 4). No other bone changes at this site exceeded the LSC values among both stroke cases and controls.
Few statistically significant changes were detected during the follow-up period at the 66 % site among the stroke cases (Table 4), and only the decline in cortical BMC (by 1.3 %; p = 0.003) on the non-paretic side exceeded the LSC value. During the follow-up period, the total hip aBMD (p = 0.002) decreased significantly on the paretic side (Table 4), but it did not exceed the LSC value.
Influence of time since stroke onset: subgroup analysis
The stroke group was divided into two subgroups (early group, onset at 12–24 months; late group, onset >24 months) (Supplemental Table 1). Besides the difference in duration after stroke, the late group was found to have a significantly shorter 6-min walk test distance than the early group (early group, 318.1 ± 31.4 m; late group, 201.7 ± 4.5 m; p = 0.035). The women in the late group also had a significantly longer post-menopausal years than the early group (early group, 9.3 ± 4.3 years; late group, 25.9 ± 2.1 years; p = 0.029). Otherwise, no significant difference in other demographic variables was found between the two groups.
At the 4 % site, the early group suffered a significant decline in trabecular vBMD (p < 0.001) on the paretic side, which exceeded the LSC (Table 5). Similar change was not found in the late group. Besides, decline in CBSI on the paretic side also exceeded the LSC value in the early group (p = 0.006), but the change was only marginally significant after adjusting for multiple comparisons (p = 0.055). On the non-paretic side, the decline in trabecular vBMD exceeded the LSC value in the late group (p = 0.012). However, the change was only marginally significant after adjusting for multiple comparisons (p = 0.058).
At the 66 % site, only the decline in cortical BMC on the non-paretic side in the late group exceeded the LSC value (p = 0.009), but the change was only marginally significant after correction for multiple comparisons (p = 0.056).
At the hip, none of the 1-year percentage change values reached statistical significance in either the early or the late group after correction for multiple comparisons.
Correlation with leg muscle function
Correlation analysis was only performed for variables that showed a significant decline that exceeded the LSC values during the follow-up period. At the 4 % site, it was found that more reduction in trabecular vBMD at the 4 % site on the paretic side during the 1-year follow-up period was significantly associated with weaker knee muscle strength (ρ = 0.447, p = 0.048) and poorer CMSA leg motor score (ρ = 0.489, p = 0.029), but not spasticity (ρ = −0.162, p = 0.495) measured at initial assessment.
Discussion
This is the first study to examine the changes in bone density and macrostructure in lower limb long bones in people after 1 year of stroke onset. The principal finding is that the change in bone density and strength index at the tibial distal epiphysis on the paretic side was more pronounced than that at the tibial diaphysis. The bone changes reported here were modest compared with acute and subacute stroke patients (less than 1 year post-stroke onset) reported in previous studies [7, 11].
Differences in bone properties between stroke cases and controls
The bone outcomes measured at the three skeletal sites (tibia 4 and 66 % sites, hip) were generally more compromised on the paretic than non-paretic side at the initial assessment. The findings are largely in line with previous cross-sectional DXA and pQCT studies comparing the bone properties between the two legs in people with chronic stroke [13, 16, 17]. To some degree, the side-to-side differences in bone outcomes among chronic stroke patients as reported in the current study and previous research may reflect the impact of stroke on bone health during the period between stroke onset and the time when the initial assessment was conducted.
We found that the total CSA at the 4 % site on the paretic side was significantly larger than that on the non-paretic side, a finding that is in contrast with a few previous cross-sectional studies in chronic stroke where no side-to-side difference in the same variable was identified [15, 31]. The underlying reasons are unknown. Upon comparing the subjects’ characteristics and side-to-side differences in total vBMD and trabecular vBMD at the same skeletal site among our stroke patients with previous studies, no determining factors could be identified to explain the discordance in results found. The larger total CSA found at the 4 % site on the paretic side may be due to an increase in periosteal apposition. It has been found in post-menopausal women that more endosteal resorption is associated with more periosteal apposition [32]. We postulate that a similar phenomenon may occur in the post-stroke population. It has been suggested that the observed increase in bone size could be an adaptive response to the reduction in bone content, as a mean to increase bone strength [33].
It is unlikely that the significant side-to-side difference reported in the stroke group was due to the effect of leg dominance. First, the dominant leg is the right leg for all stroke and control subjects, but only the stroke group demonstrated significant side-to-side differences in many of the bone variables. Second, the proportion of subjects with the dominant leg affected did not significantly differ from that with the non-dominant leg affected. Thus, the reported differences between the paretic and non-paretic sides among stroke patients can be mainly attributable to the effect of the stroke rather than that of leg dominance.
The magnitude of the side-to-side differences in bone parameters was also much lower than that in the upper limb bone sites previously reported [34–36]. The observation could be due to the difference in course of recovery between the affected upper limb and lower limb post-stroke. Previous studies revealed that only 44 % of stroke survivors with severe paresis in the upper limb had a partial or complete recovery in their life time [37] versus 75 % in the lower limb [38]. The unaffected upper limb could be used to compensate for the loss of function of the paretic upper limb, and may thus further magnify the side-to-side difference in bone parameters in the upper limbs. In contrast, during daily activities such as standing and walking, both lower limbs have to be involved, although some degree of asymmetry in weight-bearing may exist. These factors may explain why the side-to-side differences in bone parameters measured in the lower limbs are smaller in magnitude than those in the upper limb bone sites.
Tibial bone changes during 1-year follow-up
The follow-up data revealed that the bone changes in the tibial distal epiphysis on the paretic side were more pronounced than the diaphyseal site. At the 4 % site, the reduction in trabecular vBMD (−1.8 %) and CBSI (−2.7 %) on the paretic side during the follow-up period exceeded their respective LSC values. No longitudinal study has measured the changes in pQCT outcomes in lower limb long bones in the acute and subacute phases of stroke (<1 year post-stroke). Therefore, we could not determine how these values would compare with the possible changes that occur in the acute and subacute phases of stroke recovery (<1 year post-stroke). However, we postulate that the changes detected at the 4 % site here are more modest than acute and subacute phases of stroke. First, our subgroup analysis revealed that the changes in trabecular vBMD were significant only in those stroke patients who had sustained the stroke for 12–24 months at the time of initial assessment (early group), but not those whose stroke onset was more than 24 months ago (late group). A similar trend was identified for the CBSI on the paretic side. These findings suggest a decelerating rate of bone changes at tibial epiphysis as post-stroke duration increased. Second, previous studies suggested that the changes in upper limb bone properties also showed a decelerating trend after 1 year of stroke onset. In the same group of chronic stroke cases, we had previously reported that none of the pQCT outcomes at the paretic radius distal epiphysis (4 % site) sustained any changes that were above the LSC values during the 1-year follow-up period [35]. In contrast, Lazoura et al. [7] showed that during the follow-up period from 3 to 12 months after stroke, the reduction in trabecular vBMD in both the paretic and non-paretic radius distal epiphyses was much more impressive, at 14.0 and 6.8 %, respectively. We speculate that similar trends may also take place in the lower limb long bones. It is likely that the bone changes at the tibial distal epiphysis continue to stabilize as time progresses and reach a plateau at approximately 2 years post-stroke.
Although there were significant differences in post-menopausal years and mobility (6-min-walk distance) between the early group and late group, it is unlikely that these would affect our interpretation of results. We found that the 1-year decline in trabecular vBMD at the 4 % site on the paretic side was significant in the early group, but not the late group, despite that the latter group had longer post-menopausal period and shorter 6-min-walk distance (both factors presumed to be detrimental to bone health). Hence, it should not affect our conclusion that the bone changes at the tibial 4 % site may start to plateau at approximately 2 years post-stroke.
In contrast, the bone changes at the tibial diaphysis site in the paretic leg were less remarkable compared with the distal epiphyseal region, even for the early group. The results thus indicated that the bone changes at the tibial diaphysis had leveled off as the time of stroke onset passed the 1-year mark, earlier than what was observed at the tibial distal epiphysis. The overall results thus suggested that the distal epiphysis, a region with abundant trabecular bone, may have a more protracted course of bone changes than the diaphysis, a cortical bone site. However, we could not rule out the possibility that the less pronounced bone changes at the 66 % site may be partially related to the pQCT scanning resolution used. With an in-plane voxel size of 0.5 and 2.3 mm thick sections, there may be inaccuracies in the segmentation of the cortical compartment that may result in limiting the ability to detect changes in the cortical bone parameters. This factor may affect the 66 % site more, where the bone tissue is exclusively cortical.
The findings are in contrast with the upper limb changes in the same cohort of stroke cases in a previous report [35]. In the radius, none of the changes exceeded the LSC values at the distal epiphysis whereas the diaphysis showed real decline in cortical BMC and thickness during a 1-year follow-up period. The results may indicate that the changes in the tibia (a weight-bearing bone) are distinct from those in the radius (a non-weight-bearing bone) after chronic stroke. Another potential explanation is that the precision of the pQCT device was lower for measuring the radius at the 4 % site, leading to higher LSC values (4.9–14.7 %) [39]. More substantial bone changes at the 4 % radius site are thus required to exceed the LSC values.
Changes of hip bone density during 1-year follow-up
The decline in hip aBMD reported in this study, albeit statistically significant, did not exceed the LSC value. This is in agreement with the findings of de Brito et al. [10], who reported an overall <1 % loss in paretic and non-paretic total femur and femoral neck aBMD in a group of 51 chronic stroke patients with post-stroke onset of >1 year (mean onset 33.4 months) during a mean follow-up period of 16 months. This was much less than the ~12 and ~6 % loss in femoral neck BMD on the paretic and non-paretic side, respectively, within the first year post-stroke previously reported [7, 11]. Moreover, de Brito et al. [10] also showed that the change in hip aBMD was not significantly associated with time since stroke. Taken together, the results suggested that bone loss at the hip had largely leveled off after 1 year post-stroke.
Correlation with muscle function
Strong relationship between muscle strength/mass and bone strength index of the paretic tibia among chronic stroke patients has been reported in previous cross-sectional pQCT studies [14, 15, 17]. For the first time, we demonstrated in a longitudinal study that those who had weaker knee muscle strength and poorer leg motor recovery had more substantial decline in trabecular vBMD at the 4 % tibial site on the paretic side during the 1-year follow-up period. The results thus highlighted the potential importance of muscle strengthening in promoting bone health/preventing bone loss in individuals with chronic stroke. A recent systematic review revealed that research on exercise intervention on lower limb bone health is scarce, with only one randomized controlled trial [40]. In that particular chronic stroke trial, it was found that a multidimensional exercise program incorporating muscle strengthening and impact exercises successfully maintained paretic hip aBMD, increased cortical thickness at the tibial diaphysis and trabecular BMC at the distal epiphysis relative to the control group after 5 months of training [27, 39]. In addition, a non-randomized controlled trial in chronic stroke patients reported a significant gain in cortical thickness measured at the tibial diaphysis in the paretic leg after 6 months of treadmill gait training. More research is required to further evaluate the optimal exercise protocols for preventing bone loss and detrimental structural bone changes in the chronic stage of stroke.
Interestingly, spasticity did not have a significant relationship with bone changes. The results from previous cross-sectional studies are mixed, with some studies reporting significant relationship between spasticity and BMD/bone strength indices in lower limb long bones among chronic stroke patients [15], but not others [12, 17]. This is in contrast with the findings of de Brito et al. [10], which is the only longitudinal study that evaluates bone changes after the first year post-stroke. They found that those who had more substantial spasticity (MAS score ≥2) had greater risk of sustaining significant bone loss of the total femur (OR = 9.75, 95 % CI = 1.52, 62.6), compared with those without significant spasticity (MAS sore <2). However, their data should be interpreted with caution, as the overall change in paretic total femur aBMD was marginal (<1 %), with only a small proportion of their participants (22.6 %) having sustained bone loss at that skeletal site [10]. The characteristics of the participants also differed. Over 40 % of their stroke cases were taking alendronate whereas none of our subjects were taking prescribed medications for treating osteoporosis. Another potential explanation of the non-significant finding in our study may be related to the use of MAS to measures spasticity. The MAS is only a six-point ordinal scale and may not adequately differentiate individuals with varying degree of spasticity [41]. The relatively small sample size, and hence reduced statistical power, may also contribute to the non-significant finding. Moreover, the relationship between spasticity and bone changes may not be a straightforward one. While spasticity may impair leg function and functional activities such as walking [20, 42], may thus have detrimental effect on bone, mechanical loading as a result of spasticity may have a protective effect. It is unknown whether a threshold of spasticity exists above which detrimental effects on bone occur. The relationship between spasticity and bone changes warrants further investigations.
Limitations
The results reported in this study reflect the bone changes in stroke patients who had regained their ambulatory function. These individuals were also quite physically active as they attended regular activities in a community self-help group. The results thus cannot be generalized to those patients who suffered from a severe stroke and failed to regain their ambulatory function. Further study on people with severe stroke is needed, as the time course and characteristics of bone changes may be very different. As we were interested in studying bone changes in chronic stroke, the effects of stroke on bone during acute and subacute phases (<1 year post-stroke) were not evaluated. We thus could not determine whether the bone changes observed in this study were indeed attenuated compared with acute and subacute phases of stroke recovery in the same individuals. In the subgroup comparison (early group vs late group), there are potential confounding variables (i.e., post-menopausal years and 6-min walk test distance). However, as explained earlier, it should not have major impact on our conclusion. Furthermore, pQCT scanning was done at the tibia, which is far from the hip, where fracture occurs the most frequently post-stroke. However, QCT scanning of the hip involves a relatively high level of radiation exposure [43]. The pQCT scanning at the tibia was previously found to correlate with hip BMD [44] and is commonly used in previous research [16, 27]. Thus, pQCT scanning at the tibia was adopted in this study despite the stated limitations. Finally, the sample size was not large enough for us to perform multivariate analysis to identify the determinants of bone changes.
Conclusion
Despite the apparent attenuation of bone loss in the chronic stage of stroke, the tibial distal epiphysis still suffered significant bone loss among those who had sustained a stroke 12–24 months ago, resulting in reduced bone strength index. Such changes were significantly associated with knee muscle strength and leg motor recovery measured at the initial assessment. Further research should explore the possibility of modifying these factors in the endeavor to prevent bone loss in this patient population.
References
Dennis MS (2002) Fractures after stroke: frequency, types, and associations. Stroke 33:728–734. doi:10.1161/hs0302.103621
Kanis J, Oden A, Johnell O (2001) Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke 32:702–706. doi:10.1161/01.STR.32.3.702
Ramnemark A, Nyberg L, Borssen B et al (1998) Fractures after stroke. Osteoporos Int 630:92–95
Chiu KY, Pun WK, Luk KD, Chow SP (1992) A prospective study on hip fractures in patients with previous cerebrovascular accidents. Injury 23:297–299
Hyndman D, Ashburn A, Stack E (2002) Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil 83:165–170
Ramnemark A, Nyberg L, Lorentzon R et al (1999) Progressive hemiosteoporosis on the paretic side and increased bone mineral density in the nonparetic arm the first year after severe stroke. Osteoporos Int 9:269–275. doi:10.1007/s001980050147
Lazoura O, Groumas N, Antoniadou E et al (2008) Bone mineral density alterations in upper and lower extremities 12 months after stroke measured by peripheral quantitative computed tomography and DXA. J Clin Densitom 11:511–517. doi:10.1016/j.jocd.2008.05.097
Hamdy R, Moore S, Cancellaro B (1995) Long-term effects of strokes on bone mass. Am J Phys Med Rehabil 74:351–356
Ramnemark A, Nyberg L, Lorentzon R et al (1999) Hemiosteoporosis after severe stroke, independent of changes in body composition and weight. Stroke 30:755–760. doi:10.1161/01.STR.30.4.755
De Brito CMM, Garcia ACF, Takayama L et al (2013) Bone loss in chronic hemiplegia: a longitudinal cohort study. J Clin Densitom 16:160–167. doi:10.1016/j.jocd.2012.02.011
Jørgensen L, Jacobsen BK, Wilsgaard T, Magnus JH (2000) Walking after stroke: does it matter? Changes in bone mineral density within the first 12 months after stroke. A longitudinal study. Osteoporos Int 11:381–387. doi:10.1007/s001980070103
Jørgensen L, Jacobsen B (2001) Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: a 1 year prospective study. Bone 28:655–659
Worthen LC, Kim CM, Kautz SA et al (2005) Key characteristics of walking correlate with bone density in individuals with chronic stroke. J Rehabil Res Dev 42:761. doi:10.1682/JRRD.2005.02.0036
MacIntyre NJ, Rombough R, Brouwer B (2010) Relationships between calf muscle density and muscle strength, mobility and bone status in the stroke survivors with subacute and chronic lower limb hemiparesis. J Musculoskelet Neuronal Interact 10:249–255
Pang MYC, Ashe MC, Eng JJ (2010) Compromised bone strength index in the hemiparetic distal tibia epiphysis among chronic stroke patients: the association with cardiovascular function, muscle atrophy, mobility, and spasticity. Osteoporos Int 21:997–1007. doi:10.1007/s00198-009-1038-3
Talla R, Galea M, Lythgo N et al (2011) Contralateral comparison of bone geometry, BMD and muscle function in the lower leg and forearm after stroke. J Musculoskelet Neuronal Interact 11:306–313
Pang MYC, Ashe MC, Eng JJ (2008) Tibial bone geometry in chronic stroke patients: influence of sex, cardiovascular health, and muscle mass. J Bone Miner Res 23:1023–1030. doi:10.1359/jbmr.080224
Poole KES, Vedi S, Debiram I et al (2009) Bone structure and remodelling in stroke patients: early effects of zoledronate. Bone 44:629–633. doi:10.1016/j.bone.2008.11.017
Sato Y, Kuno H, Kaji M et al (1998) Increased bone resorption during the first year after stroke. Stroke 29:1373–1377. doi:10.1161/01.STR.29.7.1373
Eng JJ, Chu KS, Dawson AS et al (2002) Functional walk tests in individuals with stroke: relation to perceived exertion and myocardial exertion. Stroke 33:756–761
Washburn RA, Smith KW, Jette AM, Janney CA (1993) The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162. doi:10.1016/0895-4356(93)90053-4
Kontulainen S, Sievänen H, Kannus P et al (2002) Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res 17:2281–2289. doi:10.1359/jbmr.2002.17.12.2281
Macdonald H, Kontulainen S, Petit M et al (2006) Bone strength and its determinants in pre- and early pubertal boys and girls. Bone 39:598–608. doi:10.1016/j.bone.2006.02.057
Leonard M, Shore R (2003) Radiologic evaluation of bone mineral in children. In: Favus M (ed) Primer on the metabolic bone disease and disorders of mineral metabolism, 5th edn. American Society for Bone and Mineral Research, Washington, pp 173–189
Stratec Medizintechnik Gmbh (2004) XCT 2000 Manual Software Version 5.50
Ashe MC, Fehling P, Eng JJ et al (2006) Bone geometric response to chronic disuse following stroke: a pQCT study. J Musculoskelet Neuronal Interact 6:226–233
Pang M, Ashe M, Eng J et al (2006) A 19-week exercise program for people with chronic stroke enhances bone geometry at the tibia: a peripheral quantitative computed tomography study. Osteoporos Int 17:1615–1625. doi:10.1007/s00198-006-0168-0
Baim S, Wilson CR, Lewiecki EM et al (2005) Precision assessment and radiation safety for dual-energy X-ray absorptiometry: position paper of the International Society for Clinical Densitometry. J Clin Densitom 8:371–378
Gowland C, Stratford P, Ward M et al (1993) Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke 24:58–63. doi:10.1161/01.STR.24.1.58
Pandyan AD, Johnson GR, Price CI et al (1999) A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clin Rehabil 13:373–383
Yang FZH, Pang MYC (2015) Influence of chronic stroke impairments on bone strength index of the tibial distal epiphysis and diaphysis. Osteoporos Int 26:469–480. doi:10.1007/s00198-014-2864-5
Ahlborg HG, Johnell O, Turner CH et al (2003) Bone loss and bone size after menopause. N Engl J Med 349:327–334. doi:10.1056/NEJMoa022464
Seeman E (2003) Periosteal bone formation—a neglected determinant of bone strength. N Engl J Med 349:320–323. doi:10.1056/NEJMp038101
Pang MYC, Ashe MC, Eng JJ (2007) Muscle weakness, spasticity and disuse contribute to demineralization and geometric changes in the radius following chronic stroke. Osteoporos Int 18:1243–1252. doi:10.1007/s00198-007-0372-6
Pang MYC, Zhang M, Li LSW, Jones AYM (2013) Changes in bone density and geometry of the radius in chronic stroke and related factors: a one-year prospective study. J Musculoskelet Neuronal Interact 13:77–88
Pang MYC, Yang FZH, Jones AYM (2013) Vascular elasticity and grip strength are associated with bone health of the hemiparetic radius in people with chronic stroke: implications for rehabilitation. Phys Ther 93:774–785. doi:10.2522/ptj.20120378
Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS (1994) Compensation in recovery of upper extremity function after stroke: the Copenhagen Stroke Study. Arch Phys Med Rehabil 75:852–857
Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS (1995) Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil 76:27–32
Pang MYC, Eng JJ, Dawson AS et al (2005) A community-based fitness and mobility exercise program for older adults with chronic stroke: a randomized, controlled trial. J Am Geriatr Soc 53:1667–1674. doi:10.1111/j.1532-5415.2005.53521.x
Borschmann K, Pang MYC, Bernhardt J, Iuliano-Burns S (2012) Stepping towards prevention of bone loss after stroke: a systematic review of the skeletal effects of physical activity after stroke. Int J Stroke 7:330–335. doi:10.1111/j.1747-4949.2011.00645.x
Fleuren JFM, Voerman GE, Erren-Wolters CV et al (2010) Stop using the Ashworth Scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry 81:46–52. doi:10.1136/jnnp.2009.177071
Hsu A-L, Tang P-F, Jan M-H (2003) Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil 84:1185–1193
Damilakis J, Adams JE, Guglielmi G, Link TM (2010) Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur Radiol 20:2707–2714. doi:10.1007/s00330-010-1845-0
Liu XS, Cohen A, Shane E et al (2010) Bone density, geometry, microstructure, and stiffness: relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Min Res 25:2229–2238. doi:10.1002/jbmr.111
Acknowledgments
We thank the International Osteoporosis Foundation for their constructive criticisms and assistance in the writing of the parts of the manuscript. F.M.H. Lam was granted a full-time research studentship by the Hong Kong Polytechnic University. F.M.H. Lam was granted a Young Investigator Award from the International Osteoporosis Foundation to present the preliminary results of this study at the 3rd Asia-Pacific Osteoporosis Meeting, Kuala Lumpur, Malaysia, 2012. M.Y.C. Pang was granted a General Research Fund by the Research Grants Council.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Subjects’ characteristics of the subgroups of stroke cases(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Lam, F.M.H., Bui, M., Yang, F.Z.H. et al. Chronic effects of stroke on hip bone density and tibial morphology: a longitudinal study. Osteoporos Int 27, 591–603 (2016). https://doi.org/10.1007/s00198-015-3307-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3307-7