Abstract
Summary
This study was conducted to examine the association between the tibial bone strength index and leg muscle strength of different contraction types and speeds among people with chronic stroke. We found that concentric muscle power at moderate speed was more associated with tibial bone strength index than other types.
Introduction
To compare the influence of muscle strength of different contraction types and speeds on the bone strength index of tibial diaphysis in people with chronic stroke.
Methods
Eighty individuals with chronic stroke (age: 62.6 ± 8.0 years; men/women: 46/34; post-stroke duration: 9.0 ± 5.4 years) underwent scanning of the tibia at the 66% site on both sides using peripheral quantitative computed tomography. Each participant was also evaluated for isometric and dynamic (at 60°/s and 120°/s) strength of knee flexors/extensors and ankle dorsiflexors/plantarflexors using an isokinetic dynamometer. For a given contraction type and speed, the strength values of the four muscle groups were summed to yield a composite score. Multiple regression analysis was used to identify the association of the percent side-to-side difference (%SSD) in tibial polar-stress-strain index (p-SSI) with %SSD in muscle strength of different contraction types and speeds.
Results
The p-SSI and all muscle strength parameters on the paretic side had lower values than the non-paretic side (p ≤ 0.001). The %SSD in concentric muscle power at angular speed of 60°/s (R2 = 0.317, p = 0.006) and 120°/s (R2 = 0.298, p = 0.020) remained independently associated with that in p-SSI, after controlling for age, sex, body mass index, post-stroke duration, motor impairment, spasticity, and physical activity level. The effect of isometric strength and eccentric muscle power was not significant in multivariate analysis.
Conclusions
Concentric muscle power was more predictive of %SSD in p-SSI at the tibial diaphysis than other contraction types and may be an important target for intervention to promote bone health in people with chronic stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is one of the most disabling conditions worldwide [1]. It is well known that people with stroke have an elevated risk of fragility fractures [2, 3], leading to complications such as increased morbidity [4], mortality [5], prolonged hospitalization [6], and decreased ability to regain independent mobility [4]. Exaggerated fracture rate after stroke can be partially attributable to compromised bone health status. Reduced bone strength is an important risk factor of fractures [6], which are much more common in individuals with stroke than their age- and sex-matched counterparts [3].
Several studies have shown a close link between muscle mass/strength and the integrity of bone tissue among people with stroke [7,8,9]. For example, Pang et al. have shown muscle mass to be a significant determinant of the bone strength index measured at the distal tibial diaphysis among individuals with chronic stroke [7]. In another study, MacIntyre et al. further demonstrated that calf muscle density was significantly related to the bone strength index of the tibial diaphyseal site in individuals with sub-acute stroke [9]. However, the association between muscle contraction type (i.e., dynamic concentric/ eccentric versus isometric muscle contractions) and bone properties post-stroke is currently unknown. Moreover, the speed of dynamic muscle contractions (e.g., fast versus slow) may also influence bone properties post-stroke. Animal studies have demonstrated that high-frequency dynamic loads are more effective than static loads in enhancing bone formation [10, 11]. Thus, it seems likely that bone tissue integrity may be more associated with the ability to generate force during dynamic muscle contractions at higher speeds rather than isometric contractions but this hypothesis is yet to be tested. This may have important implications for designing muscle strength training programs for enhancing bone health among people with stroke.
The objective of this study was to examine the association between the bone strength index measured at the tibial diaphysis and muscle strength measures consisting of different contraction types and speeds in people with chronic stroke. It was hypothesized that greater dynamic (concentric and eccentric) muscle strength measured at higher speeds would be more strongly associated with a higher bone strength index at the tibial diaphysis.
Methods
Sample size calculation
The sample size calculation was conducted using the Free Statistics Calculators version 4.0 (https://www.danielsoper.com/statcalc/calculator.aspx?id=16). A previous stroke study showed that leg mean mass was independently associated with tibial bone strength index, with R2 value of 0.12 (equivalent to effect size f2 = 0.13) [7]. Another study demonstrated that isometric knee muscle strength was significantly associated with 1-year change in trabecular volumetric bone mineral density (vBMD) at the distal tibia among people with chronic stroke (R2 = 0.20, equivalent to f2 = 0.25) [12]. A more conservative approach was taken by assuming the smaller effect size of f2 = 0.13 attributable to the effect of muscle strength after adjusting for age, sex, post-stroke duration, body mass index, physical activity level, severity of motor impairment, and spasticity. With an alpha of 0.05, power of 0.8, and attrition rate of 10%, a minimum of 76 individuals with stroke would be required.
Participants
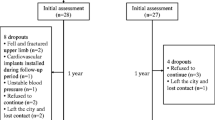
Individuals with stroke were recruited between August 1, 2017, and April 30, 2018, from a stroke group organization in the community through convenience sampling. The screening of individuals and enrolment of participants were conducted by the research personnel through a telephone interview. The inclusion criteria were as follows: (1) a diagnosis of stroke with onset of 6 months or more, (2) medically stable, (3) aged ≥ 18 years, (4) able to walk > 10 m without physical assistance from other people (with or without walking aids), and (5) able to understand simple verbal commands. Exclusion criteria were as follows: (1) other neurological conditions, (2) serious musculoskeletal conditions (e.g., amputations), (3) metal implants in the lower extremity, (4) fracture in the lower extremity within the past 1 year at the time of initial participant screening, (5) taking medications for the treatment of osteoporosis, and (6) other serious illnesses or contraindications that prevented the individual from participating in the study (e.g., neoplasms). Ethical approval was obtained from the Human Subjects Ethics Sub-committee of the University. The details of the study were explained to the participants before informed written consent was obtained. All of the experimental procedures were conducted in accordance with the Helsinki Declaration for human experiments.
Measurements
Demographics
Relevant demographic information (e.g., medications, stroke history) was collected through face-to-face interviews and a hospital discharge summary provided by the participants. The 10-item Abbreviated Mental Test (AMT) was used to assess cognitive function for each participant (possible score range: 0–10). Lower scores indicate greater cognitive impairment. The AMT has been previously validated among geriatric patients [13] and elderly in residential care homes [14] and has demonstrated excellent test-retest and inter-rater reliability (ICC = 0.99) [14]. The 12-item Physical Activity Scale for the Elderly (PASE) questionnaire [15], which has been used in previous stroke research [16], was administered by the researcher to assess the participant’s physical activity levels. Scores were calculated using weights and frequency values corresponding to the type of physical activity being assessed (e.g., leisure, occupational activity). Higher scores suggest greater daily physical activity. The Fugl-Meyer Motor Assessment (FMA) was administered to assess the degree of paresis of the leg and foot on the affected side [17]. It is a 17-item scale with each item rated on a 3-point ordinal scale (0–2). Hemiparesis was considered to be present if one was unable to attain the maximum FMA score of 34 [18].
Bone imaging
Each participant underwent scanning of the tibia on each side using peripheral quantitative computed tomography (pQCT) (XCT 3000, Stratec Medizintechnik GmbH; Pforzheim, Germany). The anatomical reference line was positioned at the cortical end plate of the distal medial edge of the tibia. Scans (2.3 mm in thickness, scan speed at 25 mm/s, voxel size of 500 μm) were acquired at the tibial diaphysis (at 66% of the total bone length proximal to the reference line, mainly a cortical bone site). Cortical bone analysis at the 66% site was performed using CORTBD (model 1), with a threshold of 710 mg/cm3. The variables of interest were total area, cortical bone area, cortical bone mineral content (BMC), cortical volumetric bone mineral density (vBMD), cortical thickness, marrow cavity area, and polar stress-strain index (p-SSI). The coefficients of variation for the aforementioned variables ranged from 0.47 to 1.73%. The marrow cavity area was calculated by subtracting the cortical area from the total area [7]. p-SSI reflects strength of long bone shaft against bending or torsional forces by considering the densitometric and geometric properties of bone [7]. The equation for calculating the p-SSI of the tibial diaphysis has been described in a previous study [19] and is shown below:
where A = area of each pixel, dmax = maximum distance to the center of gravity, dz = distance between the pixel and the corresponding torsional (z) axis, and ND = normal physiological bone density (1200 mg/cm3).
Muscle strength
Participants underwent muscle strength testing of the bilateral knee extensors (i.e., quadriceps femoris) and flexors (i.e., biceps femoris) as well as ankle plantar flexors (i.e., gastrocnemius, soleus) and dorsiflexors (i.e., tibialis anterior) using an isokinetic dynamometer (HUMAC ®NORMTM Testing & Rehabilitation System, Computer Sports Medicine; USA), which allows good reliability of strength measurements (ICC = 0.89–0.96) [20]. To test muscle strength during knee flexion-extension, participants were instructed to sit upright in a chair, the knee joint axis was aligned with the mechanical axis of the dynamometer, and straps were used to stabilize the untested body part. Each participant was then instructed to perform maximal concentric/eccentric knee flexion and extension throughout the range of 10°–70° knee flexion on each side at a constant angular speed of 60°/s and 120°/s. Each participant was also required to perform a maximal isometric contraction of the same muscle groups at mid-range (45° flexion). To test muscle strength during ankle plantarflexion-dorsiflexion, participants were placed in a semi-reclined position with 30° of knee flexion. Each participant then performed maximal concentric/eccentric ankle dorsiflexion and plantarflexion throughout the range of 0°–30° ankle plantarflexion on each side at a constant angular speed of 60°/s and 120°/s. Each participant also performed a maximal isometric contraction of the same muscle groups at 10° ankle plantarflexion. The sequence of testing (i.e., paretic vs non-paretic side, type and speed of contractions) was randomized to minimize the order effect. Three trials were recorded for each testing condition, and the data were averaged to obtain the mean peak torque for isometric testing (in newton-meters or Nm), and power for dynamic testing (in watts or W) of each leg using customized software. As contraction type and speed were of primary interest, the strength values of knee flexion, knee extension, ankle dorsiflexion, and ankle plantarflexion were summed to yield a composite muscle strength score to reflect overall leg muscle strength for each of the five testing conditions (i.e., isometric, concentric at 60°/s and 120°/s, eccentric at 60°/s and 120°/s) [21].
H-reflex
The H-reflex was measured to assess the degree of excitability of the monosynaptic spinal reflex arc and was commonly used as an indicator of spasticity. Participants were instructed to lie in a comfortable supine position. The soleus H-reflex was induced and recorded using a Viking Quest device (Nicolet Biomedical, Madison, WI, USA). We used self-adhesive Ag–AgCl electrodes (1.0 cm diameter) to record surface electromyography signals. The recording electrodes were fixed to the corresponding skin over the muscle bellies, and the reference electrode was secured to the skin over the muscle tendon. The ground electrode was secured between the recording and reference electrodes.
Before the application of the electrodes, the skin was properly prepared until a skin impedance of less than 20 kohm was obtained. The tibial nerve was stimulated with a rectangular electrical pulse of 1 ms duration and a stimulus frequency of 1 per 5 s. The stimulation procedure described by Braddom and Johnson [22] was followed. Initially the optimal position for stimulating the tibial nerve in the popliteal fossa was determined by moving the stimulating electrode around until a visible contraction of the gastrocnemius muscle was seen. Then, the current was gradually increased until an H-reflex without an M response was recorded. The H reflex was identified as a triphasic wave with a small initial positive deflection followed by a larger negative one. The eleventh response with the largest amplitude was selected as the Hmax and other values were rejected. The stimulus intensity was then increased in small increments until the maximum M response was obtained. The maximum amplitudes of the H-reflex and the M wave were measured from the peak of the positive to the peak of the negative deflections. The Hmax to Mmax ratio was calculated by dividing the maximum amplitudes of the H reflex by that of the M wave.
Statistical analyses
All statistical analyses were conducted using SPSS 23.0 software (IBM, Armonk, NY, USA). A significance level of 0.05 (2-tailed) was set for all statistical tests. Descriptive statistics were used to report all variables of interest. Paired t tests were used to compare the pQCT and muscle strength parameters between the paretic and non-paretic sides. The percent side-to-side difference (%SSD) in pQCT and composite muscle strength parameters was obtained by calculating the difference of values between the two sides (non-paretic minus paretic) divided by the value obtained from the non-paretic side and then multiplying it by a factor of 100. A positive value thus indicates a lower value on the paretic side when compared with the non-paretic side. Pearson’s correlation coefficients were used to assess the association of %SSD in p-SSI with other variables. Multiple linear regression analyses were then performed to identify the association of %SSD in p-SSI with that in muscle strength outcomes. The %SSD in p-SSI was of interest here. It is derived from comparing the paretic and non-paretic side of the same individual. It can thus provide a more specific evaluation of the influence of stroke on bone strength index on the paretic side, while providing appropriate control for the different cofactors (i.e., genetic, age, nutrition, other environmental factors) which may affect bone metabolism across different people [23, 24]. Being a standardized score, it also facilitates the comparison of the degree of impact of stroke on bone properties between individuals. The %SSD has also been used in previous research assessing post-stroke bone status [25]. In each hierarchical regression model, age, sex, body mass index (BMI), years since stroke onset, physical activity level, and H-reflex were first forced into the model. Next, the muscle strength variable was entered.
Model comparisons were performed using the Bayesian Information Criterion (BIC) and Akaike Information Criterion (AIC) functions in R Studio (Version 1.2.5033, R Studio Inc., Boston, MA URL http://www.rstudio.com/). The AIC [26] and BIC [27] are commonly used criterion for determining model selection between two competing or comparable models. Given that the models arising from the analyses in the current study may be either true (asymptotically consistent) or a likely approximation of the data (asymptotically inconsistent), both BIC and AIC were interpreted [28].
The AIC value difference (∆AIC) and the BIC value difference (∆BIC) were compared between two models with equal numbers of predictor variables. The model with the lesser AIC or BIC value was considered optimal while the other was considered to be a secondary candidate model. ∆AIC was interpreted using the following criteria: < 2 = substantial evidence candidate model is likely to be as good as the optimal model; 4–7 = less evidence that the candidate model is as good as the optimal model; > 10 = no support for the candidate model over the optimal model [29]. ∆BIC was interpreted using the following criteria: < 2 = negligible difference between models; 2–6 = evidence against the candidate model is positive; 6–10 = evidence against the candidate model is strong; > 10 = evidence against the candidate model is very strong [30].
Results
Eighty individuals fulfilled all selection criteria. A summary of demographic and stroke-specific characteristics is provided in Table 1. Bilateral comparisons showed significant side-to-side differences in cortical area, thickness, BMC, vBMD, and p-SSI, with higher values for the non-paretic side (p ≤ 0.001) (Table 2). There was no difference observed for the total area between the two sides (p = 0.773).
All composite leg muscle strength variables showed significant side-to-side differences, indicating diminution of leg muscle strength on the paretic side during static and dynamic contractions at all measured speeds (p ≤ 0.001) (Table 2).
A summary of bivariate correlations is provided in Table 3. The %SSD of p-SSI was significantly correlated with the %SSD of all composite muscle strength measures (p ≤ 0.026). There were also significant correlations between the %SSD of p-SSI and body mass index (BMI) (p = 0.027) and motor impairment level (FMA) (p = 0.038).
To avoid multicollinearity among independent predictor variables, a series of separate regression models were used to predict the %SSD of p-SSI. After adjusting for the effects of sex, age, stroke duration, BMI, physical activity level, motor impairment level, and spasticity (paretic leg H-reflex), the regression models also addressed the relative predictive contribution of the %SSD in composite muscle strength variables with regard to differences in contraction type and speed (Table 4). Overall, the models explained 25.6–31.7% of the variance in %SSD of p-SSI. Among the five models, model 2 and 3 accounted for the most variance in %SSD of p-SSI (29.8–31.7%). Also, only in these two models was the muscle power variable (concentric muscle contraction at 60°/s and 120°/s, respectively) independently associated with %SSD of p-SSI (p < 0.05), accounting for an additional 7.5% (model 2) and 5.6% (model 3) of the variance.
The information criteria used to determine the optimal regression model (i.e., appropriate model complexity and explanatory power or fit of the model to the data) are summarized in Table 5. Model 2 had lower values (BIC = 550.84, AIC = 527.02) in comparison to model 3 (BIC = 553.07, AIC = 529.25). The difference in BIC and AIC also demonstrated positive evidence that the candidate model (model 3) was not comparable to the optimal model (model 2) (∆BIC = 2.23, ∆AIC = 2.23). As there were an equal number of predictors between models with no unknown parameters, the use of the likelihood-ratio test was unwarranted. The distinguishing feature between these models was the speed of the muscle contraction variable used. The results of the model comparison suggested that the power output during concentric muscle contractions at a lower relative contraction velocity (60°/s, model 2) was more predictive of %SSD in p-SSI than at a higher contraction velocity (120°/s, model 3).
Discussion
Side-to-side difference in bone strength index
Our findings suggest that the p-SSI of the tibial diaphysis on the paretic side was significantly lower than that on the non-paretic side. This was primarily due to the side-to-side differences in cortical vBMD, cortical bone mass and area, but not total area (Table 2). These findings are consistent with what were previously reported for the tibial diaphysis in people with chronic stroke [7, 17], and indicative of possible endosteal resorption.
Concentric strength at moderate speed is more strongly associated with bone strength index
An important finding of this study is that concentric muscle contraction power (models 2 and 3 in Table 4) was more associated with the %SSD of p-SSI than isometric muscle strength (model 1). Animal studies have revealed that dynamic loads are more effective than static loads in inducing bone formation [10, 11, 31]. The comparatively stronger association with concentric over isometric muscle contraction observed in our study confirms our initial hypothesis in this regard. A previous human study has also demonstrated a significant correlation between dynamic concentric muscle contraction power and the bone strength index measured at the tibial mid-shaft in older adults [32].
Our results also showed that the concentric leg muscle power (models 2 and 3) yielded a stronger association with %SSD in p-SSI than eccentric muscle power (models 4 and 5). Eng et al. found that eccentric torque production in the paretic side was less affected by stroke than concentric torque production [33]. This is also largely in line with our findings, particularly at a higher speed of 120°/s, where a greater strength deficit was observed with concentric contraction (44.2%) compared to eccentric contraction (34.8%) (Table 2). The relative preservation of eccentric muscle strength may partly explain why it yielded a weaker association with %SSD in p-SSI compared to concentric muscle contraction.
Concentric muscle power at an angular speed of 60°/s (model 2) had a slightly stronger association with %SSD in p-SSI than that at 120°/s (model 3). Therefore, it did not support our hypothesis that a greater contraction speed was more strongly associated with %SSD in p-SSI. Theoretically, high-speed muscle contractions produce more rapid fluid flow in bone canaliculi, thereby providing a stimulus that may magnify mechanotransduction [34, 35]. Animal work has also shown that mechanical stimulation at higher frequencies is more osteogenic than that at lower frequencies [11]. Nevertheless, the difference in contribution of the concentric muscle power at 60°/s versus 120°/s is modest (R2 change: 7.5% vs 5.6%). The relationship between muscle power measured at different contraction speeds and bone strength index may not be linear. Future studies may benefit by testing more angular speeds in order to more accurately extrapolate the effect of contraction speed on the bone outcomes among people with stroke.
Clinical and research implications
Our findings may have important implications in designing muscle strength training programs for individuals with stroke. It is known that resistance training is effective in maintaining or enhancing bone health in different populations, including post-menopausal women [36, 37] and older adults [38,39,40]. A previous study [41] found beneficial effects on tibial bone architecture (using PQCT) and femoral neck BMD in people with chronic stroke as a result of a multi-dimensional exercise intervention which included a resistance training component. However, these studies did not specifically address the effectiveness of muscle strength training on bone outcomes. In addition, the issue concerning the type of strength training exercise (isometric, concentric, or eccentric contractions) was not considered. Our findings may help inform further research and clinical practice, particularly with regard to the approach and application of muscle strengthening in bone health management. Specifically, as concentric power at moderate speed shows the greatest deficit and demonstrates the strongest association with %SSD in p-SSI, more emphasis should be placed on concentric muscle training at similar speeds in the overall resistance exercise training protocol in order to enhance bone health post-stroke. This hypothesis will require further study.
Limitations
This study was a cross-sectional design and does not provide information on the changes in bone outcomes over time. While significant correlations between %SSD in p-SSI and muscle strength variables were found, cause-and-effect cannot be inferred. Our various regression models explained only 29.8–31.7% of the variance in %SSD of p-SSI, indicating that other potentially important factors underlying bone health post-stroke (e.g., nutrition) were under-explored in the current study. Further studies should use a larger sample size and address the relationship between these factors and bone health in people with chronic stroke. There was a risk of recall bias with the use of the self-reported activity questionnaire used in the present study (i.e., PASE), although the reported activities undertaken by participants were limited to the previous 7 days. A more objective measurement of physical activity would strengthen future studies by providing information regarding the actual ambulatory and sedentary activities undertaken. Finally, it is possible that some of the participants may have undiagnosed osteoporosis. The purpose of excluding those who were taking osteoporosis medications was to minimize the influence of these medications on the results (e.g., bone properties). The possibility of having people with undiagnosed osteoporosis should not have major impact on the results, especially when we were interested in %SSD, which was derived from comparing the two sides within the same individual. However, the results can only be generalized to individuals with stroke who have similar demographic and clinical characteristics to our sample (e.g., people not taking osteoporosis medications).
Conclusion
In summary, concentric muscle power was more predictive of %SSD in bone strength index at the tibial diaphyseal site than eccentric muscle power or isometric muscle strength in people with chronic stroke. Promoting concentric leg muscle power may be an important intervention strategy to improve or maintain lower extremity bone health post-stroke, and will need further investigation.
Data availability
All data generated or analyzed during this study will be made available at the time of publication and can be obtained upon request.
References
Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V (2009) Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 8:355–369
Ramnemark A, Nyberg L, Borssén B, Olsson T, Gustafson Y (1998) Fractures after stroke. Osteoporos Int 8:92–95
Dennis M, Lo K, McDowall M, West T (2002) Fractures after stroke: frequency, types, and associations. Stroke 33:728–734
Di Monaco M, Vallero F, Di Monaco R, Mautino F, Cavanna A (2003) Functional recovery and length of stay after hip fracture in patients with neurologic impairment. Am J Phys Med Rehabil 82:143–148
Ramnemark A, Nilsson M, Borssén B, Gustafson Y (2000) Stroke, a major and increasing risk factor for femoral neck fracture. Stroke 31:1572–1577
Poole KE, Reeve J, Warburton EA (2002) Falls, fractures, and osteoporosis after stroke: time to think about protection? Stroke 33:1432–1436
Pang MY, Ashe MC, Eng JJ (2008) Tibial bone geometry in chronic stroke patients: influence of sex, cardiovascular health, and muscle mass. J Bone Miner Res 23:1023–1030
Pang MY, Ashe MC, Eng JJ (2010) Compromised bone strength index in the hemiparetic distal tibia epiphysis among chronic stroke patients: the association with cardiovascular function, muscle atrophy, mobility, and spasticity. Osteoporos Int 21:997–1007
MacIntyre N, Rombough R, Brouwer B (2010) Relationships between calf muscle density and muscle strength, mobility and bone status in the stroke survivors with subacute and chronic lower limb hemiparesis. J Musculoskelet Neuronal Interact 10:249–255
Burr D, Robling AG, Turner CH (2002) Effects of biomechanical stress on bones in animals. Bone 30:781–786
Turner CH, Robling AG (2003) Designing exercise regimens to increase bone strength. Exerc Sport Sci Rev 31:45–50
Lam FM, Bui M, Yang FZ, Pang MY (2016) Chronic effects of stroke on hip bone density and tibial morphology: a longitudinal study. Osteoporos Int 27:591–603
Chu L, Pei C, Ho M, Chan P (1995) Validation of the Abbreviated Mental Test (Hong Kong version) in the elderly medical patient. Hong Kong Med J 1:207–211
Lam SC, Wong Y-y, Woo J (2010) Reliability and validity of the abbreviated mental test (Hong Kong version) in residential care homes. J Am Geriatr Soc 58:2255–2257
Washburn RA, Zhu W, McAuley E, Frogley M, Figoni SF (2002) The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil 83:193–200
Vaughan K, Miller WC (2013) Validity and reliability of the Chinese translation of the Physical Activity Scale for the Elderly (PASE). Disabil Rehabil 35:191–197
Yang FZ, Pang MY (2015) Influence of chronic stroke impairments on bone strength index of the tibial distal epiphysis and diaphysis. Osteoporos Int 26:469–480
Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S (1975) The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 7:13–31
Lam FMH, Pang MYC (2016) Correlation between tibial measurements using peripheral quantitative computed tomography and hip areal bone density measurements in ambulatory chronic stroke patients. Brain Inj 30:199–207
Flansbjer U-B, Holmbäck AM, Downham D, Lexell J (2005) What change in isokinetic knee muscle strength can be detected in men and women with hemiparesis after stroke? Clin Rehabil 19:514–522
Pang MYC, Lau RWK (2010) The effects of treadmill exercise training on hip bone density and tibial bone geometry in stroke survivors: a pilot study. Neurorehabil Neural Repair 24:368–376
Braddom R, Johnson E (1974) Standardization of H reflex and diagnostic use in Sl radiculopathy. Arch Phys Med Rehabil 55:161–166
del Puente A, Pappone N, Mandes MG, Mantova D, Scarpa R, Oriente P (1996) Determinants of bone mineral density in immobilization: a study on hemiplegic patients. Osteoporos Int 6:50–54
Pang MY, Eng JJ, McKay HA, Dawson AS (2005) Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporos Int 16:1769–1779
Pang MY, Ashe MC, Eng JJ (2007) Muscle weakness, spasticity and disuse contribute to demineralization and geometric changes in the radius following chronic stroke. Osteoporos Int 18:1243–1252
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6:461–464
Vrieze SI (2012) Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods 17:228–243
Burnham KP (2002) Model selection and multimodel inference a practical information-theoretic approach. Springer, New York
Kass RE, Raftery AE (1995) Bayes factors. J Am Stat Assoc 90:773–795
Akyuz E, Braun JT, Brown NA, Bachus KN (2006) Static versus dynamic loading in the mechanical modulation of vertebral growth. Spine 31:E952–E958
Ashe M, Liu-Ambrose T, Cooper D, Khan K, McKay H (2008) Muscle power is related to tibial bone strength in older women. Osteoporos Int 19:1725–1732
Eng JJ, Lomaglio MJ, MacIntyre DL (2009) Muscle torque preservation and physical activity in individuals with stroke. Med Sci Sports Exerc 41:1353
Turner CH, Owan I, Takano Y (1995) Mechanotransduction in bone: role of strain rate. Am J Physiol-Endoc M 269:E438–E442
Burger EH, Klein-Nulend J (1999) Mechanotransduction in bone—role of the lacuno-canalicular network. FASEB J 13:S101–S112
Zehnacker CH, Bemis-Dougherty A (2007) Effect of weighted exercises on bone mineral density in post menopausal women a systematic review. J Geriatr Phys Ther 30:79–88
Martyn-St James M, Carroll S (2009) A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sport Med 43:898–908
Marques EA, Mota J, Carvalho J (2012) Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age 34:1493–1515
Hong AR, Kim SW (2018) Effects of resistance exercise on bone health. Endocrinol Metab 33:435–444
Liu-Ambrose TY, Khan KM, Eng JJ, Heinonen A, McKay HA (2004) Both resistance and agility training increase cortical bone density in 75-to 85-year-old women with low bone mass: a 6-month randomized controlled trial. J Clin Densitom 7:390–398
Pang MY, Ashe MC, Eng JJ, McKay HA, Dawson AS (2006) A 19-week exercise program for people with chronic stroke enhances bone geometry at the tibia: a peripheral quantitative computed tomography study. Osteoporos Int 17:1615–1625
Funding
Zhenhui Yang and Tiev Miller were granted full-time research studentships by the Hong Kong Polytechnic University (RUBE and RL27). This study was supported by the Hong Kong Research Grants Council (General Research Fund no. PolyU 151025/14M).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethics approval
Ethical approval was obtained from the Human Subjects Ethics Sub-committee of the University. All of the experimental procedures were conducted in accordance with the Helsinki Declaration for human experiments.
Consent to participate
The details of the study were explained to the participants before informed written consent was obtained.
Consent for publication
Consent to use information and data collected during the course of the study for education and knowledge dissemination purposes was granted by the participants
Code availability
No custom software or code was used. R Studio is an open source program and SPSS is commercially available.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Z., Miller, T. & Pang, M.Y.C. Relationship between bone strength index of the hemiparetic tibial diaphysis and muscle strength in people with chronic stroke: influence of muscle contraction type and speed. Osteoporos Int 32, 951–959 (2021). https://doi.org/10.1007/s00198-020-05716-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05716-2