Abstract
Summary
The purpose of this study was to identify the determinants of the bone strength index of the distal tibia epiphysis in chronic stroke patients. The results showed that lower cardiovascular fitness, more muscle atrophy, poorer mobility, and more severe spasticity were independently associated with lower tibial bone strength index.
Introduction
To identify the determinants of the bone strength index (BSI) at the distal tibia in chronic stroke patients
Methods
Sixty-three chronic stroke survivors underwent scanning of the distal tibia at the 4% site on both sides using peripheral quantitative computed tomography. The primary outcomes were trabecular bone mineral density (BMD; milligram per cubic centimeter), total BMD (milligram per cubic centimeter), total bone area (square millimeter), and BSI (square gram per centimeter to the power of four). Cardiovascular fitness, leg lean mass, gait velocity, and spasticity were also measured.
Results
Scans from 45 subjects were deemed to have acceptable quality and were included for subsequent analysis. The paretic side had significantly lower trabecular BMD, total BMD, and BSI than the nonparetic side (p < 0.05). However, the total bone area demonstrated no significant side-to-side difference (p > 0.05). After adjusting for relevant biological factors, peak oxygen consumption, leg muscle mass, and gait velocity remained positively associated with tibial BSI on both sides (R 2 change = 6.9–14.2%), whereas spasticity of the paretic leg was negatively associated with tibial BSI on the same side (R 2 change = 4.8%).
Conclusions
Cardiovascular function, muscle atrophy, mobility, and spasticity are independently associated with BSI of the distal tibia epiphysis among chronic stroke patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that individuals with stroke sustain an elevated risk of fragility fractures [1, 2] leading to undesirable complications such as increased mortality [3], increased length of hospital stay [4], and reduced ability to regain independent mobility function [5]. The contributing factors to the increased fracture rate after stroke are many, one of which is compromised bone health status [4]. Pronounced bone loss, particularly on the hemiparetic side, is prevalent among stroke survivors [6, 7].
Besides reduction in bone mineral content, bone geometry may also exert important influence on bone strength, and hence, fracture risk [8]. While numerous studies have examined the stroke-induced areal bone mineral density (aBMD) changes as measured by dual-energy X-ray absorptiometry (DXA) [6, 7], research on how stroke influences bone geometry is scarce. To date, few studies have used peripheral quantitative computed tomography (pQCT) to examine bone volumetric BMD (vBMD) and geometry in cortical bone sites among stroke patients [9–11]. It was found that, while the cortical thickness value and bone strength index (BSI) on the paretic side were significantly lower than on the nonparetic side in both the midshaft radius and midshaft tibia, the cortical vBMD only showed a side-to-side difference in the midshaft radius but not in the midshaft tibia [10, 11]. Apparently, the stroke-induced changes in bone vBMD and geometry are site-specific (i.e., weight-bearing bone vs nonweight-bearing bone).
While previous studies have investigated the bone vBMD and geometry in cortical bone sites (i.e., midshaft of long bones), studying the bone densitometric and geometric properties at trabecular sites (i.e., distal end of long bones) is also important as it would shed light on whether regional areas of bone may respond to the same stroke impairments differently. It is also clinically relevant to study the relationship between various stroke-related impairments (e.g., poor cardiovascular fitness, muscle atrophy, mobility, and spasticity) and bone properties at these sites. Identification of modifiable factors that are also significant determinants of BSI may assist the clinicians in developing effective treatment strategies for enhancing bone health in the stroke population.
The current study was undertaken to examine the bone densitometric and geometric properties of the distal tibia epiphysis in ambulatory, community-dwelling chronic stroke survivors. This subgroup of stroke survivors was selected as their ability to walk tends to expose them to more fall-inducing situations, and hence, increases their fracture risk [12]. The specific objectives were: (1) to examine the differences in vBMD, geometry, and BSI of the distal tibia between the paretic and nonparetic sides and (2) to identify the determinants of BSI of the distal tibia in chronic stroke survivors. It was hypothesized that stroke impairments, namely, cardiovascular fitness, leg lean mass, mobility, and spasticity, would be significantly associated with BSI of the distal tibia in individuals with chronic stroke.
Methods
Subjects
The subjects were participants in another clinical study to investigate the effects of a group exercise program on cardiovascular fitness [13]. The original sample size calculation was thus based on the expected change of cardiovascular fitness and was detailed elsewhere [13]. Briefly, with a power of .80 and alpha of 0.05, a desired 15% change in cardiovascular fitness, and a projected attrition rate of 20%, a minimum of 52 participants was required. For the current study, if a sample of 52 subjects was recruited, it would enable us to detect an effect size of r2 = 0.2 (medium to large effect size), given an alpha of 0.05, power of 0.80, and four predictors in the regression model.
All subjects were recruited on a volunteer basis from a local hospital database, local newspaper advertisements, and community stroke self-help groups. The inclusion criteria were: (1) had a single episode of stroke ≥ 1 year onset, (2) age ≥ 50 years, (3) able to walk > 10 meters without physical assistance from other people (with or without walking aids), (4) living at home (i.e., not institutionalized), and (5) no significant cognitive deficits (mini-mental state examination score > 22) [14]. In addition, each individual had to be able to exercise on the ergometer and attain 60% of age-predicted heart rate maximum or above without any cardiac signs and symptoms. Exclusion criteria were: (1) neurological disorders apart from stroke, (2) pain while ambulating, (3) history of serious cardiovascular diseases (i.e., myocardial infarction, uncontrolled hypertension), (4) other serious conditions that precluded the individual from participating in the study, and (5) metal implants within the imaging field. Ethical approval by the local university and hospital ethics committees was obtained before subject recruitment and data collection. Each subject gave informed and written consent prior to participation in the study. All experiments were conducted in accordance with the Declaration of Helsinki.
Outcome measurements
Each subject underwent the following outcome measurements. All the assessments had standardized procedures and were performed by the same research personnel who had relevant experience.
Demographics
Demographic information (e.g., height, weight, medical history) was obtained by a simple physical examination and patient interview. In addition, the Physical Activity Scale for Individuals with Physical Disabilities (PASIPD) was used to measure the level of physical activity [15]. This 13-item questionnaire assessed the amount of participation in physical activities of different intensities for the past 7 days. Based on the assigned metabolic equivalent (MET) value for each activity, a total score (MET hour per day) was computed. The maximal score was 199.5 MET h/day.
Tibial bone density, geometry, and bone strength index
The vBMD and geometric properties of the distal tibia epiphysis were measured by pQCT (Stratec Medizintechnik XCT 2000; software version 5.50; Pforzheim, Germany). The length (millimeters) of the tibia on each side was measured as recommended by the manufacturer. After proper positioning, we obtained a scout view and placed the anatomical reference line at the distal medial edge of the tibia. A 2.5-mm scan at the 4% site of the tibia (proximal to the reference line) was obtained on each side with an in-plane pixel size of 300 microns. For image analysis, we used XCT v.5.50 software and CALCB Contour (outer edge-detection) Mode 3 and Peel Mode 2 and CORTBD Mode 4 with an outer threshold\inner threshold of 169\400 mg/cm3. The variables of interest were total bone area (ToA, square millimeters), total bone mineral content (BMCtotal, milligram per millimeters), total vBMD (vBMDtotal, milligram per cubic centimeter), trabecular vBMD (vBMDtrab, milligram per cubic centimeter), and BSI (g2/cm4). The BSI was computed from the formula \( {\text{vBMD}}_{\text{total}}^2 \times {\text{To}}A \) [16]. The calculation of BSI took into consideration both the density of the structural material and the load-bearing area [16]. This BSI is thought to be an appropriate estimate of the strength of the bony structure against compressive forces at distal end of long bones since the epiphysis is primarily subjected to axial compression rather than bending and torsional loads [17, 18]. A recent cadaver study has shown that the BSI at the 4% tibial site explained 85% and 57% of the variance in the failure load and stiffness, respectively, when tested in axial compression [18]. It thus indicates that the BSI provides an acceptable noninvasive estimate of bone strength. This BSI has also been used in previous studies to estimate the strength of long bones at distal sites [16, 19, 20]. As the cortical thickness at the 4% distal site was <2 mm on both the paretic (mean = 1.4 mm) and nonparetic sides (mean = 1.4 mm) for all subjects, and it is known that cortical thickness < 2 mm may cause the problem of partial volume effect, cortical thickness was not used as an outcome measure in this study [21]. The coefficients of variation (CV) for the pQCT scanner for acquiring images in vivo at the distal tibia were 0.58% for BMCtotal [22]. Each scan was reviewed by two independent researchers to ensure that the pQCT image was of good quality to be accepted for further analysis.
Cardiovascular fitness
To evaluate the cardiovascular fitness, a maximal exercise test on the Excalibur cycle ergometer (Lode B.V. Medical Technology, Groningen, Netherlands) was conducted to evaluate the peak oxygen consumption (peak VO2). A portable metabolic unit (Cosmed K4 b2 system; COSMED Srl; Rome, Italy) measured the oxygen consumption continuously and performed breath-by-breath gas analysis as the subjects exercised on the cycle ergometer. Subjects were instructed to pedal at 60 rpm. The workload was set at 10–20 W initially and was then gradually increased by 10–20 W/min. If pedaling rate declined to < 30 rpm, the test was terminated. The raw VO2 data were averaged at a rate of every 15 s. The peak value obtained was considered to be the peak VO2 (milliliters per minute), a gold standard for measuring cardiovascular fitness.
Leg lean mass
Lean mass (grams) value of each leg was obtained by a total body scan using DXA (Hologic QDR 4500, Hologic Inc., Waltham, MA, USA). With respect to the precision of our DXA scanner, the CV for left leg and right leg lean mass were 1.0% and 0.7%, respectively.
Gait velocity
The comfortable gait speed test was used to assess gait velocity [23]. Subjects were instructed to walk for 8 m along a corridor at a self-selected speed with walking aid as necessary. The time taken to walk the middle 4 m (in seconds) was recorded by a stopwatch, and the gait velocity value (meter per second) was computed. The test has demonstrated excellent reliability (ICC = 0.94) [23].
Spasticity
To evaluate spasticity level, the Modified Ashworth Scale (MAS) was used [24]. The ankle joint on the paretic side was moved passively into dorsiflexion and plantar flexion. The amount of resistance during the passive movements was noted. The MAS is a six-point ordinal scale with a higher score representing more severe spasticity (0: no increase in muscle tone, 4: affected part rigid in flexion and extension). Subjects were classified into three categories based on the MAS score (0: no spasticity, 1 and 1+: mild spasticity, 2–4: moderate/severe spasticity). The MAS is a common clinical measure used to evaluate spasticity and its reliability has been established [24].
Statistical analysis
All statistical analyses were performed using SPSS 16.0 software. A level of significance at 0.05 (two-tailed) was set for all statistical tests. Normality of data was first checked using Kolmogorov–Smirnov test. It was found that only the data for poststroke duration and physical activity level did not meet the criterion of normality. Paired t tests were then used to compare the tibial pQCT parameters between the paretic and nonparetic sides. The percent side-to-side difference in pQCT parameters was obtained by computing the difference of values between the two sides (paretic minus nonparetic) divided by the value obtained from the nonparetic side and then multiplying it by a factor of 100. Thus, a negative percent difference value indicates that the paretic side has a lower value than the nonparetic side.
To assess the association of pQCT parameters with other continuous variables that met the criterion of normality (age, body mass index (BMI), peak VO2, gait velocity, leg lean mass), Pearson's correlation coefficient was used. Spearman's rho was used to estimate the relationship between pQCT parameters and ordinal variables (spasticity) or continuous variables that failed to meet the criterion of normality (poststroke duration, physical activity level). Finally, separate multiple linear regression analyses were performed to identify the determinants of tibial BSI on both sides. Hierarchical regression models were constructed. Biologically relevant factors including age, gender, and BMI were first forced into the regression models. Stroke impairments that had significant correlation with tibial BSI in the bivariate correlational analysis (e.g., peak VO2, gait velocity, leg lean mass, spasticity) were then entered into the regression models. As the MAS used to measure spasticity is an ordinal scale, dummy variables (DV) were created (DV_1: the group with mild spasticity as referenced to the group with no spasticity; DV_2: the group with moderate/severe spasticity as referenced to the group with no spasticity).
Results
Subject characteristics
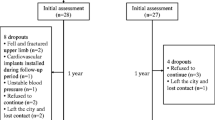
A total of 63 subjects (28 women) volunteered to participate in the study. However, the pQCT scans from 18 subjects (nine women) were excluded due to artifacts caused by movement and tremor. The results reported in this study were thus based on 45 subjects (19 women). These 18 subjects who were excluded from the analysis did not have significant difference in age (p = 0.311), poststroke duration (p = 0.651), BMI (p = 0.815), peak VO2 (p = 0.539), paretic leg lean mass (p = 0.384), spasticity (p = 0.793), and gait velocity (p = 0.405) when compared with other 45 subjects.
Subject characteristics are described in Table 1. The mean peak VO2 value was 21.5 ml/kg/min (men: 22.8 ml/kg/min, women: 19.8 ml/kg/min), which was well below the normative values for this age group (men: 28–32 ml/kg/min; women: 23–26 ml/kg/min) [25]. Leg muscle mass on the paretic side was significantly lower than the nonparetic side by 5.9% (p < 0.001). The mean gait velocity was 0.87 m/s, which was only 50–60% of the reference values [26]. Sixteen subjects had mild spasticity (MAS = 1 or 1+), and another ten subjects had moderate/severe spasticity (MAS = 2–4).
Comparison of pQCT parameters
The paretic side had significantly lower vBMDtotal (p = 0.023), vBMDtrab (p = 0.010), and BSI (p = 0.019) than the nonparetic side (Table 2). The side-to-side difference in BMCtotal almost reached statistical significance (p = 0.053). There was no significant difference in ToA between the two sides (p = 0.531). The percent side-to-side difference in all variables showed no significant difference between men and women (p > 0.200), and thus, the data were pooled for all analyses.
Correlations with pQCT parameters
Bivariate correlational analysis (Table 3) revealed that BMCtotal, BMDtotal, BMDtrab, and BSI on both sides were positively associated with peak VO2, leg lean mass, and gait velocity (p < 0.05). ToA on both sides, on the other hand, was positively correlated with peak VO2 and leg lean mass only. Spasticity of the affected leg was negatively associated with BMCtotal, BMDtotal, BMDtrab, and BSI on the same side, indicating that those with more severe spasticity tended to have more compromised bone density and geometry (p < 0.05).
Determinants of tibial bone strength index
Correlations among the potential determinant variables (i.e., peak VO2, leg lean mass, gait velocity, spasticity) were checked before subsequent multiple regression analysis for predicting tibial BSI. Moderate to high correlations existed among these variables (correlation coefficients ranging from 0.301 to 0.814, p < 0.05) except between peak VO2 and spasticity (ρ = −0.239, p = 0.113). Therefore, separate regression models were thus used to avoid multicolinearity. In the first model (model 1 in Table 4), peak VO2 and spasticity were used to predict BSI on the paretic side. After accounting for age, gender, and BMI, addition of peak VO2 significantly improved the prediction model (Fchange 1,40 = 11.591, p = 0.002) and accounted for 10.7% of the variance in BSI. Spasticity (DV_1 and DV_2) was next entered into the model and explained another 4.8% of the variance in BSI. The final model accounted for a total of 67.7% of the variance in tibial BSI on the paretic side (F6,38 = 13.300, p < 0.001) with gender (p = 0.002), peak VO2 (p = 0.003), and moderate/severe spasticity (DV_2; p = 0.028) as the significant determinants. In the second model, we used leg lean mass to predict paretic tibial BSI (model 2 in Table 4). After accounting for relevant biological factors, leg lean mass remained independently associated with tibial BSI, explaining 8.2% of the variance (F4,40 = 15.297, p < 0.001). The second model accounted for a total of 60.5% of the variance in paretic tibial BSI. In the next model, (model 3 in Table 4), gait velocity was used to predict paretic tibial BSI. Gait velocity accounted for 14.2% of the variance in tibial BSI after accounting for age, gender, and BMI (F4,40 = 19.807, p < 0.001). This model accounted for a total of 66.5% of the variance in paretic tibial BSI.
Separate regression analyses were performed to identify the determinants of BSI on the nonparetic side (Table 5), using peak VO2 (model 1), nonparetic leg lean mass (Model 2), and gait velocity (Model 3) as the independent variables. After accounting for relevant biological factors, peak VO2, leg lean mass, and gait velocity remained significantly associated with tibial BSI on the nonparetic side, accounting for 6.9%, 8.9%, and 11.2% of the variance, respectively. These models accounted for a total of 50.9–55.2% of the variance in nonparetic tibial BSI.
Discussion
This is the first study to demonstrate that common stroke impairments, namely, low cardiopulmonary fitness, muscle atrophy, poor mobility, and spasticity are independently associated with BSI measured at the distal tibia epiphysis.
Side-to-side difference in bone strength index
Our finding suggests that BSI of the distal tibia on the paretic side was significantly lower than that on the nonparetic side. The lower BSI on the paretic side was primarily due to a lower BMD value, not a smaller total area (Table 2). This is in stark contrast with what was previously reported in the midshaft tibia (a cortical bone site) from the same pool of chronic stroke patients [11]. Although significant side-to-side difference in BSI was also found in the midshaft tibia, the main contributing factor is the smaller total or cortical bone area with cortical vBMD relatively preserved when compared with the nonparetic side [11]. Interestingly, Rittweger et al. [27] showed that the distal tibial epiphysis of the suspended leg (4% site, the same site as measured in this study), but not the diaphysis (38% site), sustained significant bone loss after 24 days of unilateral lower limb suspension in a group of eight young healthy men. These results, when taken together, highlight the phenomenon that different regions of a bone may react to pathology differently.
Our results are also different from the bone changes associated with the aging process [28, 29]. For example, Russo et al. [29] found that while BMDtrab at the 4% site of tibia decreased by approximately 16–23% throughout adult life, the ToA increased by 3–14% during the same period. It has been suggested that the expansion of ToA may reflect a compensatory mechanism against the loss of bone mineral since increase in load-bearing area would increase the BSI [28, 29]. We found no evidence of such compensatory geometric changes on the paretic side against the bone loss induced by the stroke event, indicating that the mechanisms underlying bone alterations poststroke are different from those observed in aging. Perhaps stroke-related impairments may not only lead to lower BMD but also exert direct effects on bone geometry by decreasing the ToA, thereby masking any compensatory expansion of ToA.
Cardiovascular fitness and muscle mass are associated with BSI
It is also well known that stroke patients have impaired cardiovascular health [30, 31]. We found that poorer cardiovascular fitness, as reflected by lower peak VO2, was independently associated with lower BMD, less favorable bone geometry, and hence, lower tibial BSI on both sides. The link between bone parameters and cardiovascular fitness found in this study is not entirely surprising as trabecular bone is highly vascularized and in close contact with endothelial tissue in bone marrow [32]. Increasing evidence has shown a positive link between osteoporosis and cardiovascular disease [29]. As VO2 reflects the overall cardiovascular health, it makes sense that low VO2 would affect bone status on both sides (Tables 4 and 5). It should be noted that VO2 does not provide separate information on the central component (e.g., cardiac output) and peripheral component (e.g., vascular health). Nevertheless, our data have provided a strong basis for further study on the cardiac and vascular mechanisms underlying bone health poststroke.
Similarly, the muscle mass of each leg was significantly associated with all the measured pQCT densitometric and geometric parameters of the distal tibia on the same side, reflecting an intimate relationship between muscle function and bone health. Such muscle-bone link has been established in various populations [33, 34]. One of the factors underlying this muscle–bone link may be vitamin D deficiency. Abnormally low levels of 1,25-dihydroxyvitamin D (the most common circulating form of vitamin D) are prevalent among chronic stroke patients, mainly due to decreased dietary intake and sunlight exposure [35]. Vitamin D level has been identified as an independent determinant of osteopenia in chronic stroke patients [36]. There is also evidence that vitamin D has positive effects on muscle function [37]. Thus, vitamin D deficiency could well contribute to the lower bone and muscle mass observed in our subjects.
A previous study using the same pool of subjects showed that both peak VO2 and leg lean mass were also independently associated with BSI in the paretic midshaft tibia [11], but some interesting differences exist. In this study, peak VO2 contributed slightly more to the prediction of distal tibial BSI on the paretic side than leg lean mass (Table 4, compare model 1 and model 2). In contrast, leg lean mass was found to be a stronger predictor of BSI than peak VO2 in the paretic midshaft tibia [11]. We postulate that such discrepancy in results may be partly related to anatomical differences between the two skeletal sites. The midshaft tibia (50% site) used in the previous study is in close proximity to attachment points of major muscle groups (e.g., soleus, flexor digitorium longus, tibialis posterior), whereas the distal tibia (4% site) used in this study has no such feature. Presumably, the influence of muscle loading would be greater in the midshaft tibia than distal tibia. On the other hand, trabecular bone is a highly vascular structure, more so than cortical bone [32]. It is possible that the integrity of trabecular bone sites may be more sensitive to changes in cardiovascular function.
Mobility and spasticity are associated with BSI
We showed that higher gait velocity was positively associated with higher BMC, BMD, and tibial BSI on both sides, whereas moderate/severe spasticity in the affected leg was negatively associated with these same pQCT parameters on the same side. Spasticity has been shown to be the most important determinant of reduced single limb support time of the paretic limb during the gait cycle and reduced gait velocity [38]. As ground reaction force produced during walking increases with gait velocity [39], the mechanical loading applied to both legs would presumably be less for those patients with slower gait velocity. Patients with slower gait velocity also tend to have poorer endurance with limited ambulatory activity [40, 41]. The combination of reduced ground reaction force and low ambulatory activity may account for the low tibial BSI on both sides.
Interestingly, the results here are in contrast with what was previously found in the midshaft tibia where no association of BSI with mobility and spasticity exists [13]. We postulate that the distal tibia may be more subject to compressive forces during mobility tasks [17], whereas the midshaft tibia, a site of attachment of major muscle groups, may be more subject to muscle loading. Although spasticity may impair gait function and bone loading as a result, the tonic muscle activity associated with spasticity may exert some protective effect on cortical bone at the midshaft tibia. It is important to stress that this is a pure speculation. The relationship between spasticity and bone parameters may be very complex. It remains uncertain whether a spasticity threshold exists, beyond which bone mass would not be significantly affected. [11].
Clinical and research implications
Our findings have important clinical and research implications. For example, common antispastic agents, such as baclofen and botulinum toxin type A, have been found to be effective in reducing spasticity in affected limbs in stroke patients [42, 43]. It would be valuable to study the effects of different spasticity management techniques on bone health in stroke patients. In addition, physical exercise may be a viable method to enhance bone health in this group of individuals as it could potentially modify muscle mass, cardiovascular function, and mobility, which are all significant determinants of tibial BSI. Various forms of exercise such as resistance training, aerobic impact exercises, and agility training have been shown to improve bone health in older adults [44, 45]. Further research on the effects of different types of exercise on bone density and geometry is much needed in this important area.
Limitations
The study has several limitations. First, the methods employed in this study do not have adequate resolution to examine potential mechanisms underlying low vBMDtrab, such as disruption of trabecular microstructure, loss of trabecular elements, or/and thinning of trabeculae [27]. Nevertheless, the significant finding in this study would warrant future research on stroke-induced changes in bone microstructure.
Second, the study is cross-sectional and cannot establish the temporal relationship between tibial BSI and stroke impairments. For example, many stroke patients have a positive history of cardiovascular disease (Table 1). We could not determine to what extent the cardiovascular impairment contributed to the bone changes prior to stroke onset versus after stroke onset.
Third, there was no healthy age-matched control group. We compared the side-to-side difference in bone parameters within each stroke patient because it enabled us to evaluate the effect of stroke on bone health while controlling for important extraneous variables such as genetics and difference in lifestyle and dietary habits. However, the nonparetic side is not an ideal control. We could not rule out that the nonparetic side might have also undergone some degree of changes in bone density and geometry over time due to favored use of the nonparetic side during functional activities. Alternatively, decreased ambulatory activity may lead to similar bone changes on both sides.
Finally, the distal tibia is not the most common site of fragility fractures in stroke patients, accounting for only 6.8–9.1% of all fractures [1, 2]. Rather, the hip is the most common site of fractures in this population. Whether the lower BSI at the 4% tibial site on the paretic side is related to an increased risk of hip fracture remains unknown. However, peripheral skeletal sites are what we were able to measure with available technology and, in theory, are subject to similar loading patterns as the femur.
Conclusion
This study provides novel results on bone densitometric and geometric properties at the distal tibia epiphysis poststroke. Specific stroke impairments, namely, low cardiovascular fitness, muscle atrophy, poor mobility, and spasticity, may all contribute to the compromised BSI in the distal tibia. Strategies to modify these factors may be instrumental in improving bone health in this group and will need further investigations.
References
Ramnemark A, Nyberg L, Borssén B et al (1998) Fractures after stroke. Osteoporos Int 8:92–95
Dennis MS, Lo KM, McDowall M et al (2002) Fractures after stroke: frequency, types, and associations. Stroke 33:728–734
Ramnemark A, Nilsson M, Borssén B et al (2000) Stroke, a major and increasing risk factor for femoral neck fracture. Stroke 31:1572–1577
Poole KES, Reeve J, Warburton EA (2002) Falls, fractures, and osteoporosis after stroke. Time to think about protection? Stroke 33:1432–1436
Di Monaco M, Vallero F, Di Monaco R et al (2003) Functional recovery and length of stay after hip fracture in patients with neurologic impairment. Am J Phys Med Rehabil 82:143–148
Jorgensen L, Jacobsen BK, Wilsgaard T et al (2000) Walking after stroke: does it matter? Changes in bone mineral density within the first 12 months after stroke. A longitudinal study. Osteoporos Int 11:381–387
Jorgensen L, Crabtree NJ, Reeve J et al (2000) Ambulatory level and asymmetrical weight bearing after stroke affects bone loss in the upper and lower part of the femoral neck differently: bone adaptation after decreased mechanical loading. Bone 27:701–707
Peacock M, Turner CH, Liu G et al (1995) Better discrimination of hip fracture using bone density, geometry, and architecture. Osteoporos Int 5:167–173
Ashe MC, Fehling P, Eng JJ et al (2006) Bone geometric response to chronic disuse following stroke: a pQCT study. J Musculoskelet Neuronal Interact 6:226–233
Pang MYC, Ashe MA, Eng JJ (2007) Muscle weakness, spasticity and disuse contribute to demineralization and geometric changes in the radius following chronic stroke. Osteoporosis Int 18:1243–1252
Pang MYC, Ashe MA, Eng JJ (2008) Tibial bone geometry in chronic stroke patients: influence of sex, cardiovascular health, and muscle mass. J Bone Miner Res 23:1023–1030
Melton LJ III, Brown RD Jr, Achenbach SJ et al (2001) Long-term fracture risk following ischemic stroke: a population study. Osteoporos Int 12:980–986
Pang MYC, Eng JJ, Dawson AS et al (2005) A community-based fitness and mobility exercise program for individuals with chronic stroke: a randomized controlled trial. J Am Geriatr Soc 53:1667–1674
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: a practical method for grading the state of patients for the clinician. J Psychiat Res 12:189–198
Washburn RA, Zhu W, McAuley E (2002) The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil 83:193–200
Kontulainen S, Sievanen H, Kannus P et al (2002) Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res 17:2281–2289
Hangartner TN, Gilsanz V (1996) Evaluation of cortical bone by computed tomography. J Bone Miner Res 11:1518–525
Hayes W, Bouxsein ML (1997) Biomechanics of cortical and trabecular bone: implications for assessment of fracture risk. Basic orthopaedic biomechanics. Lippincott-Raven, Philadelphia
Kontulainen SA, Johnston JD, Liu D et al (2008) Strength indices from pQCT imaging predict up to 85% of variance in bone failure properties at tibial epiphysis and diaphysis. J Musculoskelet Neuronal Interact 8:401–409
Macdonald HM, Kontulainen S, Petit M et al (2006) Bone strength and its determinants in pre-and early pubertal boys and girls. Bone 39:598–608
Wetzsteon RJ, Petit MA, Macdonald HM et al (2008) Bone structure and volumetric BMD in overweight children: a longitudinal study. J Bone Miner Res 23:1946–1953
Ashe MC, Liu-Ambrose T, Khan KM et al (2005) Optimizing results from pQCT: reliability of operator-dependent pQCT variables in cadavers and humans with low bone mass. J Clin Densitom 8:335–340
Flansbjer U-B, Holmback AM, Downham D et al (2005) Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med 37:75–82
Bohannon BW, Smith MB (1987) Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67:206–207
Talbot LA, Metter EJ, Fleg JL (2000) Leisure-time physical activities and their relationship to cardiorespiratory fitness in healthy men and women 18–95 years old. Med Sci Sports Exerc 32:417–425
Steffen TM, Hacker TA, Mollinger L (2002) Age- and gender-related test performance in community-dwelling elderly people: six-minute walk test, Berg balance scale, timed up and go test, and gait speeds. Phys Ther 82:128–137
Rittweger J, Winwood K, Seynnes O et al (2006) Bone loss from the human distal tibia epiphysis during 24 days of unilateral lower limb suspension. J Physiol 577:331–337
Riggs LB, Melton LJ, Robb RA et al (2004) A population-based study of age and sex differences in bone volumetric density, size, geometry and structure at different skeletal sites. J Bone Miner Res 19:1945–1954
Russo CR, Lauretani F, Badinelli S et al (2003) Aging bone in men and women: beyond changes in bone mineral density. Osteoporos Int 14:531–538
Roth EJ (1993) Heart disease in patients with stroke: incidence, impact, and implications for rehabilitation. Part I: classification and prevalence. Arch Phys Med Rehabil 74:752–760
Kopunek SP, Michael KM, Shaughnessy M et al (2007) Cardiovascular risk in survivors of stroke. Am J Prev Med 32:408–412
Whitney C, Warburton ER, Frohlich J et al (2004) Are cardiovascular disease and osteoporosis directly linked? Sports Med 34:779–807
Osei-Hyiaman D, Ueji M, Toyakawa S (1999) Influence of hand grip strength on metacarpal bone mineral density in postmenopausal Japanese women: a cross-sectional study. Calcif Tissue Int 64:263–266
Chen Z, Lohman TG, Stini WA et al (1997) Fat or lean mass: which one is the major determinant of bone mineral mass in healthy postmenopausal women? J Bone Miner Res 12:144–151
Sato Y, Tsuru T, Oizumi K et al (1999) Vitamin K deficiency and osteopenia in disuse-affected limbs of vitamin D-deficient elderly stroke patients. Am J Phys Med Rehabil 78:317–322
Sato Y, Kuno H, Kaji M et al (1998) Increased bone resorption during the first year after stroke. Stroke 29:1373–1377
Sato Y, Iwamoto J, Kanoko T et al (2005) Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis 20:187–192
Hsu AL, Tang PF, Jan MH (2003) Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil 84:1185–1193
Lau RWK, Pang MYC (2009) An assessment of the osteogenic index of therapeutic exercises for stroke patients: relationship to severity of leg motor impairment. Osteoporos Int 6:979–987
Eng JJ, Chu KS, Dawson AS et al (2002) Functional walk tests in individuals with stroke. Relation to perceived exertion and myocardial exertion. Stroke 33:756–561
Michael K, Macko RF (2007) Ambulatory activity intensity profiles, fitness, and fatigue in chronic stroke. Top Stroke Rehabil 14:5–12
Francisco GE, Hu MM, Boake C et al (2005) Efficacy of early use of intrathecal baclofen therapy for treating spastic hypertonia due to acquired brain injury. Brain Injury 19:359–364
Hesse S, Brandi-Hesse B, Bardeleben A et al (2001) Botulinum toxin A treatment of adult upper and lower limb spasticity. Drugs Aging 18:255–262
Kemmler W, Lauber D, Weineck J et al (2004) Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women; results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS). Arch Int Med 164:1084–1091
Liu-Ambrose TY, Khan KM, Eng JJ et al (2004) Both resistance and agility training increase cortical bone density in 75- to 85-year-old women with low bone mass: a 6-month randomized controlled trial. J Clin Densitom 7:390–398
Acknowledgments
MYCP was supported by a postdoctoral fellowship from Natural Sciences and Engineering Research Council of Canada. MCA was supported by a postdoctoral fellowship from the Michael Smith Foundation for Health. This study was supported by a grant-in-aid from the Heart Stroke Foundation of British Columbia and Yukon (JJE), and from career scientist awards from Canadian Institute of Health Research (JJE) and the Michael Smith Foundation for Health Research (JJE).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pang, M.Y.C., Ashe, M.C. & Eng, J.J. Compromised bone strength index in the hemiparetic distal tibia epiphysis among chronic stroke patients: the association with cardiovascular function, muscle atrophy, mobility, and spasticity. Osteoporos Int 21, 997–1007 (2010). https://doi.org/10.1007/s00198-009-1038-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-1038-3