Abstract
Introduction and hypothesis
Overactive bladder (OAB) has a multifactorial aetiology, and for some women symptoms may be associated with chronic urothelial inflammation secondary to bacterial colonisation. One marker of such inflammation may be urinary nerve growth factor (NGF). We hypothesised that for women with OAB and urothelial inflammation, urinary NGF would be reduced following antibiotic therapy.
Methods
Women with overactive bladder and urodynamic diagnosis of detrusor overactivity who were refractory to anticholinergics, and had histological evidence of urothelial inflammation were treated with a 6-week course of rotating antibiotics. Urinary NGF was measured by ELISA before and after treatment. Three-day bladder diaries, the Patients’ Perception of Intensity of Urgency Scale, the King’s Health Questionnaire and the Patients’ Perception of Bladder Condition questionnaire were used to assess subjective and objective outcomes of therapy.
Results
Thirty-nine women with refractory DO were recruited. The NGF levels decreased significantly after antibiotic therapy (Wilcoxon signed rank test; p = 0.015). There were significant improvements in daytime frequency, nocturia and urgency (p < 0.05), and 74 % of women reported improvement in perception of their bladder condition.
Conclusions
Urinary NGF is responsive to antibiotic therapy. Women with refractory overactive bladder and elevated NGF may benefit from antibiotic treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nerve growth factor (NGF) is a neurotrophic factor released by the urothelium and smooth muscle of the bladder [1]. Animal studies have demonstrated a direct link between increased levels of NGF in bladder tissue and bladder function [1]. Intravesical and intramural infusion of NGF have been shown to produce bladder overactivity [2, 3]. Experimentally induced inflammation in animal models has been shown to result in increased NGF levels [4].
These experimental data have been explored in human studies, with increased levels of urinary NGF reported in patients with an overactive bladder (OAB) [5], detrusor overactivity (DO) and interstitial cystitis [6]. NGF levels have also been found to decrease after successful antimuscarinic treatment for OAB [6, 7]. In the light of these data some authors have suggested that NGF level could be considered a biomarker for OAB, as well as an assessment of therapeutic outcome in patients with OAB or DO [6]. Interestingly, NGF levels are higher in OAB patients who do not respond to antimuscarinics, but the reasons for this are unknown [8].
Patients with DO may present with symptoms of OAB such as urgency, urgency urinary incontinence, frequency, and nocturia. Detrusor overactivity is a chronic condition that has a negative impact on life [9] and recent studies have shown that up to 88 % of women with idiopathic DO have persistent symptoms at long-term follow-up [10]. However, there is no consensus on the definition of refractory overactive bladder or refractory DO. In general, patients who have not responded to first-line management therapies such as behavioural modification, bladder retraining and antimuscarinic therapy are considered to have refractory overactive bladder.
Overactive bladder and detrusor overactivity have a multifactorial aetiology [11]. An interesting hypothesis has recently emerged linking OAB symptoms and sub-clinical infection. A subset of women with OAB have persistent pyuria and low count bacteriuria [12, 13]. Underlying untreated infection could therefore be one of the causes of increased NGF levels in women with refractory OAB symptoms.
Therefore, the objective of the present study was to assess change in urinary NGF levels following antibiotic treatment and measure concomitant changes in objective and subjective symptom severity in women with refractory OAB symptoms.
Materials and methods
Women were recruited from a tertiary urogynaecology referral centre following ethical approval (REC reference number: 11/LO/1029) into this prospective six-week cohort study.
Inclusion and exclusion criteria
Adult women with overactive symptoms, and with an urodynamic diagnosis of detrusor overactivity, who had failed to respond to a combination of lifestyle adaptations, bladder retraining and antimuscarinics were included in the study. None of the women were on antimuscarinics during the study period, but they had each previously had a minimum 12-week trial, including at least two antimuscarinics.
Women were excluded if they had a history of neurological dysfunction, bladder pain, bladder cancer, bladder or renal calculi, previous continence surgery, presence of voiding dysfunction, vaginal prolapse beyond the vaginal introitus, urinary tract infection by conventional microbiological criteria (colony count ≥10 5) or history of allergic reaction or side effects to more than one antibiotic of choice.
Study design
Study participants were seen prior to and 6 weeks following antibiotic treatment. A clean catch midstream specimen of urine (MSU) was collected at full bladder at baseline and after 6 weeks of antibiotic treatment to measure the urinary NGF levels. Assessment of symptom improvement was done using validated questionnaires at baseline and 6 weeks after treatment. To establish normative ranges MSU samples were also obtained from asymptomatic subjects who did not suffer with overactive bladder symptoms.
Treatment
Participants were treated with a 6-week course of rotational antibiotics. Patients’ previous sensitivities to proven urinary tract infection and drug allergies were taken into consideration prior to treatment. Three consecutive antibiotics were given for 2 weeks each: ciprofloxacin (1 g orally in two divided doses) to cover Gram-positive and Gram-negative organisms, doxycycline (200 mg orally in two divided doses) to cover chlamydia and atypical organisms like Mycoplasma hominis and Ureaplasma urealyticum and cephalexin (1,500 mg orally in three divided doses) or co-amoxiclav (1,875 mg orally in three divided doses) to cover Gram-positive and Gram-negative urinary pathogens.
Methods of assessment
Objective and subjective assessments were performed at baseline and after 6 weeks of antibiotic therapy using a 3-day bladder diary, Patients’ Perception of Intensity of Urgency Scale (PPIUS) [14], King’s Health Questionnaire and Patients’ Perception of Bladder Condition questionnaire (PPBC) [15].
The PPIUS is a tool designed to assess the intensity of urinary urge sensation. It has a five-point scale ranging from 0 to 4, with grades 3 and 4 representing severe urgency and urgency incontinence respectively.
The PPBC is a reproducible validated single item questionnaire that assesses the patients’ subjective impression of their urinary problems. The questionnaire has a six-point scale with responses ranging from “no problems at all” to “many severe problems”. The changes in PPBC score from baseline to week 6 of antibiotic treatment were stratified into four groups: deterioration (difference in scores is positive), no change (difference in scores is 0), minor improvement (difference in scores is negative to a magnitude of 1), and major improvement (difference in scores is negative to a magnitude of 2 or more) as previously described [15].
NGF assay
Collected urine samples were immediately refrigerated and centrifuged at 3,000 rpm at 4 °C for 10 min. Three millilitres of urine were also sent for measurement of urinary creatinine. The centrifuged supernatant urine was stored at −80 °C, until further processing. Urinary NGF levels were measured by enzyme linked immunosorbent assay (ELISA) using the NGF Emax Immunoassay System (Promega, Madison, WI, USA). A standardised protocol was followed as suggested by the manufacturers. Anti-NGF polyclonal antibody, which binds soluble NGF, was coated onto a 96-well plate and incubated overnight. Urine samples were added to the wells, together with a serial dilution of NGF on each plate to provide a reference standard. After a further incubation step, anti-NGF monoclonal antibody was added, which binds to the captured NGF, and incubated overnight again. The following day the amount of specifically bound monoclonal antibody was detected using a species-specific antibody conjugated to horseradish peroxidase. The unbound conjugate is removed by washing, and the final step is then the addition of a chromogenic substrate, which changes the colour of the solution according to the amount of NGF. The colour absorbance is measured at 450 μm using a spectrometer. An NGF standard curve is created from the known concentrations of the NGF solutions and their corresponding optical density. The concentration of NGF in the urine samples was extrapolated from the NGF standard curve. The measured urinary NGF levels were normalised against the concentration of the urinary creatinine to take into account the variation in urine concentration [16].
Statistical methods
Based on previous studies [6] a sample size of 34 was estimated to provide 80 % power for a half standard deviation change in NGF, and 90 % power for a 60 % standard deviation change, with alpha set at 0.05. Spearman correlation test was used to assess the correlation between the baseline NGF values and the PPBC scores. Urinary NGF levels were compared before and after antibiotic therapy using Wilcoxon signed rank test, and with levels from a parallel control group of asymptomatic women using the Mann–Whitney U test. The daytime frequency, nocturia, urgency scores and PPBC scores were compared before and after treatment using Student’s t test or Wilson signed rank test as appropriate. The Mann–Whitney U test was used to compare the NGF levels after antibiotic treatment between the group of women who had no improvement in their PPBC scores and the group who did. (SPSS version 14.0; SPSS, Chicago, IL, USA)
Results
Thirty-nine symptomatic women with refractory detrusor overactivity were recruited. In addition, MSU samples were collected from 27 asymptomatic women who served as controls. Four patients (10.2 %) were excluded from the final analysis owing to discontinuation of antibiotics, commonly because of gastrointestinal side effects (n = 3)and vulvovaginal candidiasis (n = 1).
The median NGF level in the control group was 0.19 (25th–75th interquartile range, 0.06–1.14). The pre-treatment median NGF level in the symptomatic group was 2.12 (25th to 75th interquartile range, 0.65–12.43). There was a significant difference in the median NGF levels between the control and the symptomatic group at baseline (Mann–Whitney test, Z value −3.060; p value < 0.05).
The baseline demographics and the clinical characteristics of the symptomatic group (n = 35) are listed in Table 1. There was a statistically significant difference in the median age between the symptomatic and asymptomatic group (Mann–Whitney U test, Z value −4.43; p value < 0.05). However, there was no correlation between the NGF values and age (Spearman correlation test, rho 0.06; p value > 0.05). There was a significant correlation between the baseline NGF values and the PPBC scores (Spearman’s rho 0.34; p value < 0.05).
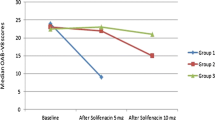
The NGF levels decreased significantly after 6 weeks of antibiotic therapy, as shown in Table 2 (Wilcoxon signed rank test, Z value −2.424; p = 0.015). OAB symptoms including daytime frequency, nocturia and urgency were also significantly improved by antibiotic therapy, as shown in Table 3. The magnitude of improvement in PPBC after antibiotics therapy is shown in Table 4. Overall 74 % of women found a minor to major improvement in their symptoms after antibiotic treatment. The median NGF values after antibiotic treatment were lower in this group compared with the group of women who had no improvement in their PPBC scores. However, this difference was not statistically significant (1.94 vs 1.06; Mann–Whitney U test; p = 0.52).
Discussion
Urinary NGF levels were significantly higher in our symptomatic study group with refractory OAB symptoms compared with the control subjects at baseline, which is in keeping with the results of other studies [5, 6]. Significant correlation existed between the baseline NGF values and the severity of urinary symptoms. Antibiotic treatment in women with refractory OAB symptoms was associated with a significant decrease in the NGF levels as well as improvement in patients’ symptoms and PPBC (Tables 2, 3). Supporting our data, animal studies have shown a link between increased NGF levels and infection. Experimentally induced bacterial cystitis in animal bladders was found to result in increased NGF levels [4]. NGF is produced by the urothelium and detrusor in the bladder, but other possible sources are eosinophils and macrophages [17]. Therefore, women with refractory OAB symptoms may have inflammation secondary to infection, associated with increased NGF levels.
The diagnosis of overactive bladder or detrusor overactivity is made only in the absence of urinary tract infection with a culture of ≥10 5 CFU/ml or other obvious pathology [18]. Interestingly, it has been shown that the likelihood of asymptomatic bacterial cystitis in women undergoing urodynamics is 6 % in those with detrusor overactivity compared with 1 % in women with urodynamic stress incontinence [19] and women who develop OAB in later life are more likely to have suffered from urinary tract infections in their childhood [20]
In a study in women with lower urinary tract symptoms (LUTS) the incidence of bacteriuria was 29 % when a threshold of 102 CFU/ml was chosen, compared with 15 % at the routinely used threshold of 10 5 CFU/ml [12]. Similarly, in a study using a threshold of 103 CFU/ml, 28 % of women with OAB were found to have bacteriuria [21]. In women with acute exacerbation of OAB symptoms, the prevalence of bacteriuria on MSU samples at a low count of ≥103 was 39 % compared with 6 % in controls [13]. Concerns have therefore been raised that urinary tract infections may be missed or under-diagnosed in women with symptoms of overactive bladder if a threshold of 105 CFU/ml is used for diagnosis. Alternatively, this could reflect an increased contamination rate in patients who have OAB as they may void smaller volumes and have higher post-void residuals [22, 23].
There is also evidence to suggest that infection caused by atypical organisms, such as Mycoplasma hominis, Ureaplasma urealyticum and chlamydia not detected by routine urine culture methodology may be associated with overactive bladder symptoms. A retrospective observational study in patients with urinary symptoms showed a prevalence of 13.7 % of atypical organisms [1, 3, 24]
Based on the data available, women with OAB seem to have an increased likelihood of having asymptomatic bacterial infection that may be missed because of usage of a higher threshold of 105 for diagnosis or lack of specific culture techniques.
Bacterial infection has been shown to trigger neutrophils infiltration into bladder mucosa and also production of cytokine IL-6. Therefore, untreated infection could result in chronic cystitis and non-responsiveness to antimuscarinics.
Inflammation has been postulated to be one of the causative factors for the development of OAB [25, 26]. Bladder biopsies from patients with detrusor overactivity before intravesical botulinum toxin injection revealed the presence of chronic inflammation in 59 % of cases [27]. Animal and clinical studies on inflammatory biomarkers have provided evidence of the role of cytokines, prostaglandins and nerve growth factor in the development of lower urinary tract symptoms [1, 3, 5, 6, 16].
We postulated that bladder inflammation might be the underlying pathogenesis for their refractory overactive bladder symptoms, which may have occurred as a result of chronic subclinical infection. Therefore, we treated our patients with a combination of antibiotics to treat common urinary tract pathogens such as Gram-negative and Gram-positive organisms as well as atypical organisms such as Mycoplasma hominis, Ureaplasma urealyticum and chlamydia. The main limitation of our study is the lack of evidence of infective aetiology in the treatment group. However, treatment with antibiotics in women with refractory overactive bladder significantly decreased the urinary NGF levels (Table 2). Our study also shows a statistically significant improvement in the daytime frequency, nocturia and urgency and PPBC scores after antibiotic treatment (Table 3) Twenty-six percent of women who received antibiotic treatment did not find any improvement in their symptoms, whereas 74 % of them found a minor to major improvement in their symptoms (Table 4). The mean NGF values after antibiotic treatment in women who had improvement in their PPBC scores were lower compared with those of women who had no improvement. However, this difference did not reach statistical significance, which could be due to small numbers in the subgroups.
Antibiotics could have indirect immune modulatory effects in addition to antimicrobial effects [28]. There is evidence that drugs such as doxycycline, ciprofloxacin, cephalexin and co-amoxiclav exert anti-inflammatory properties. Therefore, it is possible that the antibiotics that were used had an anti-inflammatory effect, which resulted in improvement of the overactive bladder symptoms rather than an anti-infective effect. It is also possible that the treatment with antibiotics had a placebo effect. However, the improvement in the PPBC scores is far larger at 74 % than most placebo studies where improvement levels of only 40–45 % have been reported with placebo [29]. There is one study using sequential antibiotics compared with placebo, but the study group consisted of patients with interstitial cystitis, in whom no significant difference was shown between the two treatment arms [30]. This report is not applicable to the present study because of the differences in pathology as the patients had pain and no incontinence. Therefore, we can be confident that this is the first report of sequential antibiotic treatment in this group of patients. Although we found a small number of adverse events in this short-term study, it is important to keep in mind that microbial resistance could occur with long-term use of antibiotics, particularly if the treatment is repeated for symptom recurrence.
The limitations of this study are inherent in the uncontrolled design, which could create a risk of biases, and the use of patients in whom first-line therapies had failed limits the generalisability. Although the NGF measurements were performed blind, limiting the risk of experimenter bias, without a group of untreated controls the changes observed may result from unmeasured factors. A placebo-controlled study is therefore required to quantify the therapeutic effect. These data provide a convincing rationale for the design of a controlled trial of this therapy, and it is important for such a study to assess the long-term outcome of treatment.
Urinary NGF levels may be responsive to antibiotic treatment in women with refractory OAB. Furthermore, targeted antibiotic treatment is associated with subjective and objective improvement in OAB symptoms. Antibiotics may be acting by producing an antibacterial or anti-inflammatory effect. Antibiotic treatment could be considered in women with refractory OAB symptoms before invasive treatment modalities such as botulinum toxin, neuromodulation and bladder augmentation. The use of NGF as a predictor of antibiotic treatment response merits testing in a blinded randomised trial.
References
Steers WD, Tuttle JB (2006) Mechanisms of disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol 3(2):101–110. doi:10.1038/ncpuro0408
Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N (2001) The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol 165(3):975–979
Zvara P, Vizzard MA (2007) Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7:9. doi:10.1186/1472-6793-7-9
Dupont MC, Spitsbergen JM, Kim KB, Tuttle JB, Steers WD (2001) Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol 166(3):1111–1118
Kim JC, Park EY, Seo SI, Park YH, Hwang TK (2006) Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol 175(5):1773–1776. doi:10.1016/s0022-5347(05)00992-4, discussion 1776
Liu HT, Chen CY, Kuo HC (2010) Urinary nerve growth factor levels in overactive bladder syndrome and lower urinary tract disorders. J Formos Med Assoc 109(12):862–878. doi:10.1016/s0929-6646(10)60133-7
Cho KJ, Kim HS, Koh JS, Kim JC (2012) Changes in urinary nerve growth factor and prostaglandin E(2) in women with overactive bladder after anticholinergics. Int Urogynecol J. doi:10.1007/s00192-012-1854-4
Liu HT, Lin H, Kuo HC (2011) Increased serum nerve growth factor levels in patients with overactive bladder syndrome refractory to antimuscarinic therapy. Neurourol Urodyn 30(8):1525–1529. doi:10.1002/nau.21118
Banakhar MA, Al-Shaiji TF, Hassouna MM (2012) Pathophysiology of overactive bladder. Int Urogynecol J 23(8):975–982. doi:10.1007/s00192-012-1682-6
Garnett S, Swithinbank L, Ellis-Jones J, Abrams P (2009) The long-term natural history of overactive bladder symptoms due to idiopathic detrusor overactivity in women. BJU Int 104(7):948–953. doi:10.1111/j.1464-410X.2009.08535.x
Diamond P, Hassonah S, Alarab M, Lovatsis D, Drutz HP (2012) The prevalence of detrusor overactivity amongst patients with symptoms of overactive bladder: a retrospective cohort study. Int Urogynecol J 23(11):1577–1580. doi:10.1007/s00192-012-1781-4
Khasriya R, Khan S, Lunawat R, Bishara S, Bignal J, Malone-Lee M, Ishii H, O’Connor D, Kelsey M, Malone-Lee J (2010) The inadequacy of urinary dipstick and microscopy as surrogate markers of urinary tract infection in urological outpatients with lower urinary tract symptoms without acute frequency and dysuria. J Urol 183(5):1843–1847. doi:10.1016/j.juro.2010.01.008
Walsh CA, Siddins A, Parkin K, Mukerjee C, Moore KH (2011) Prevalence of “low-count” bacteriuria in female urinary incontinence versus continent female controls: a cross-sectional study. Int Urogynecol J 22(10):1267–1272. doi:10.1007/s00192-011-1506-0
Cartwright R, Srikrishna S, Cardozo L, Robinson D (2011) Validity and reliability of the patient’s perception of intensity of urgency scale in overactive bladder. BJU Int 107(10):1612–1617. doi:10.1111/j.1464-410X.2010.09684.x
Coyne KS, Matza LS, Kopp Z, Abrams P (2006) The validation of the patient perception of bladder condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol 49(6):1079–1086. doi:10.1016/j.eururo.2006.01.007
Tyagi P, Barclay D, Zamora R, Yoshimura N, Peters K, Vodovotz Y, Chancellor M (2010) Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol 42(3):629–635. doi:10.1007/s11255-009-9647-5
Aloe L, Bracci-Laudiero L, Bonini S, Manni L (1997) The expanding role of nerve growth factor: from neurotrophic activity to immunologic diseases. Allergy 52(9):883–894
Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK, Schaer GN (2010) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J 21(1):5–26. doi:10.1007/s00192-009-0976-9
Moore KH, Simons A, Mukerjee C, Lynch W (2000) The relative incidence of detrusor instability and bacterial cystitis detected on the urodynamic-test day. BJU Int 85(7):786–792
Salvatore S, Serati M, Origoni M, Candiani M (2012) Is overactive bladder in children and adults the same condition? ICI-RS 2011. Neurourol Urodyn 31(3):349–351. doi:10.1002/nau.22223
Hessdoerfer E, Jundt K, Peschers U (2011) Is a dipstick test sufficient to exclude urinary tract infection in women with overactive bladder? Int Urogynecol J 22(2):229–232. doi:10.1007/s00192-010-1263-5
Fitzgerald MP, Ayuste D, Brubaker L (2005) How do urinary diaries of women with an overactive bladder differ from those of asymptomatic controls? BJU Int 96(3):365–367. doi:10.1111/j.1464-410X.2005.05632.x
Digesu GA, Khullar V, Cardozo L, Salvatore S (2003) Overactive bladder symptoms: do we need urodynamics? Neurourol Urodyn 22(2):105–108. doi:10.1002/nau.10099
Latthe PM, Toozs-Hobson P, Gray J (2008) Mycoplasma and ureaplasma colonisation in women with lower urinary tract symptoms. J Obstet Gynaecol 28(5):519–521. doi:10.1080/01443610802097690
Saini R, Gonzalez RR, Te AE (2008) Chronic pelvic pain syndrome and the overactive bladder: the inflammatory link. Curr Urol Rep 9(4):314–319
Hsiao SM, Lin HH, Kuo HC (2012) The role of serum C-reactive protein in women with lower urinary tract symptoms. Int Urogynecol J 23(7):935–940. doi:10.1007/s00192-012-1715-1
Apostolidis A, Jacques TS, Freeman A, Kalsi V, Popat R, Gonzales G, Datta SN, Ghazi-Noori S, Elneil S, Dasgupta P, Fowler CJ (2008) Histological changes in the urothelium and suburothelium of human overactive bladder following intradetrusor injections of botulinum neurotoxin type A for the treatment of neurogenic or idiopathic detrusor overactivity. Eur Urol 53(6):1245–1253. doi:10.1016/j.eururo.2008.02.037
Tauber SC, Nau R (2008) Immunomodulatory properties of antibiotics. Curr Mol Pharmacol 1(1):68–79
Van Kerrebroeck PE, Kelleher CJ, Coyne KS, Kopp Z, Brodsky M, Wang JT (2009) Correlations among improvements in urgency urinary incontinence, health-related quality of life, and perception of bladder-related problems in incontinent subjects with overactive bladder treated with tolterodine or placebo. Health Qual Life Outcomes 7:13. doi:10.1186/1477-7525-7-13
Warren JW, Horne LM, Hebel JR, Marvel RP, Keay SK, Chai TC (2000) Pilot study of sequential oral antibiotics for the treatment of interstitial cystitis. J Urol 163(6):1685–1688
Conflicts of interest
G. Vijaya and R. Fernando have accepted paid travel expenses to attend a conference from Pfizer. Dr. A. Derpapas has accepted paid travel expenses to attend a conference from Astellas. V. Khullar has accepted paid travel expenses to attend a conference from Pfizer and payment for research from Astellas and Pfizer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vijaya, G., Cartwright, R., Derpapas, A. et al. Changes in nerve growth factor level and symptom severity following antibiotic treatment for refractory overactive bladder. Int Urogynecol J 24, 1523–1528 (2013). https://doi.org/10.1007/s00192-012-2038-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-2038-y