Abstract
Purpose
Magnetic resonance imaging with T1ρ mapping is used to quantify the amount of glycosaminoglycan in articular cartilage, which reflects early degenerative changes. The purposes of this study were to evaluate early degenerative changes in knees after anterior cruciate ligament (ACL) reconstruction by comparing T1ρ values before and 2 years after surgery and investigate whether surgical factors and clinical outcomes are related to differences in T1ρ values.
Methods
Fifty patients who underwent unilateral primary ACL reconstruction were evaluated using T1ρ mapping before and 2 years after surgery. Three regions of interest (ROIs) were defined in the cartilage associated with the medial (M) and lateral (L) weight-bearing areas of the femoral condyle (FC) (anterior: MFC1 and LFC1, middle: MFC2 and LFC2, and posterior: MFC3 and LFC3). Two ROIs associated with the tibial plateau (T) were defined (anterior: MT1 and LT1, and posterior: MT2 and LT2). T1ρ values within the ROIs were measured before and 2 years after surgery and compared using the paired t test. Correlations between the difference in T1ρ values at these two time points and patient characteristics, presence of a cartilaginous lesion, graft type, and postoperative anteroposterior laxity were also evaluated using Pearson’s and Spearman’s correlation coefficients.
Results
There was a significant increase in T1ρ before versus 2 years after surgery in the MT1, MT2, LFC1, and LT1 areas, and a significant decrease in the LFC3 and LT2 areas. There was a significant correlation between postoperative anterior-posterior laxity and a postoperative increase in T1ρ values in the MFC3 (r = 0.37, P = 0.013) and MT2 (r = 0.35, P = 0.021) areas. Increases in T1ρ values in the MFC2 area were negatively correlated with KOOS symptoms (ρ = − 0.349, P = 0.027) and quality of life (ρ = − 0.374, P = 0.017) subscale scores.
Conclusion
Early degenerative changes in medial articular cartilage were observed with T1ρ mapping at 2 years after ACL reconstruction. Postoperative anterior-posterior laxity is correlated with an increase in T1ρ values in the posteromedial femur and tibia. An increase in T1ρ values in the central medial femoral condyle was associated with knee symptoms.
Level of evidence
III.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) of the knee has been detected by radiography in 15–85% of patients followed for 10–15 years after anterior cruciate ligament (ACL) reconstruction [1,2,3,4]. Although several studies have attempted to identify risk factors for OA after ACL reconstruction [5,6,7,8], whether ACL reconstruction can prevent OA in knees with ACL injury remains controversial. Furthermore, previous reports regarding the incidence of radiographic OA after ACL reconstruction were based on the long-term results of surgeries performed more than 15 years before the OA finding. Recent progress in surgical techniques, which focus on anatomic reconstruction, could improve the stability and kinematics of knees after ACL reconstruction. Therefore, the incidence of OA after modern ACL reconstruction might be lower than what has been reported in the literature. However, it would take another 10 or 15 years to demonstrate the incidence and characterise the risk factors for radiographic OA after modern ACL reconstruction.
To detect subclinical early degenerative changes before radiographic OA changes emerge, quantitative magnetic resonance imaging (MRI) techniques such as T1ρ relaxometry, T2 relaxometry, and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) have been used recently. These methods provide information on early tissue matrix degeneration substantially earlier than standard morphological assessments based on clinical MRI studies [9,10,11]. Previous studies using these methods reported changes in cartilage composition even in reconstructed knees from 6 months to 3 years after surgery [12,13,14,15]. However, some studies followed patients for only 6 months, while other studies investigated only one time point after surgery and did not compare preoperative and postoperative findings. In particular, the relationship between knee laxity after surgery and differences in T1ρ values has not previously investigated.
The purposes of this study were to evaluate subclinical early degenerative changes in patients who underwent ACL reconstruction by comparing T1ρ values before and 2 years after surgery prior to the emergence of OA changes and to investigate the relationship among surgical factors, clinical outcomes, and differences in T1ρ values.
It was hypothesised that ACL reconstruction can prevent early degenerative changes in articular cartilage and that a greater increase in T1ρ is correlated with a worse postoperative outcome.
Materials and methods
A total of 50 patients who underwent unilateral primary ACL reconstruction were recruited for this study. All study patients were evaluated using the MRI protocol mentioned below before and 2 years after surgery. The study group consisted of 25 men and 25 women. Patients who underwent ACL reconstruction more than 2 years after ACL injury were excluded from this study because Osaki et al. reported that the T1ρ values of articular cartilage were significantly higher before ACL reconstruction in patients who underwent surgery more than 2 years after their ACL injury [16]. None of the patients had radiographic OA changes before surgery. Individuals with other ligamentous injuries or surgeries were also excluded from the study. The mean age at surgery was 26.4 ± 10.5 years (range 15–53 years) and body mass index (BMI) was 23.2 ± 3.1 kg/m2 (range 18.7–34.3 kg/m2). The mean time from injury to reconstruction was 117 ± 126 days. Ethical approval was obtained from the institutional review board at our institution, and informed consent was obtained from all patients before their participation. The preoperative femorotibial angle (FTA) was measured using weight-bearing anteroposterior (AP) radiographs. The posterior tibial slope (PTS) was measured using lateral radiographs.
Surgical technique
All ACL reconstructions were performed arthroscopically. The surface of the cartilage was classified using the International Cartilage Repair Society (ICRS) grading system as follows: grade 0: normal cartilage, grade 1: superficial lesions, grade 2: defect less than 50% of the cartilage depth, grade 3: defect more than 50% of the cartilage depth, grade 4: defect down to the subchondral bone [17]. For the cartilage of the medial femur, there were 42 grade 0 knees, 5 grade 1 knees, 2 grade 2 knees, and 1 grade 3 knee. For the lateral femur, there were 48 grade 0 knees and 2 grade 1 knees. For the medial tibia, there were 49 grade 0 knees and 1 grade 1 knee. For the lateral tibia, there were 47 grade 0 knees and 3 grade 1 knees. Meniscal injury was treated appropriately with resection or meniscal sutures according to the type of tear. Patients were divided into two groups based on whether meniscectomy or meniscal suture was needed. Twenty-one patients needed a meniscectomy or meniscal sutures. Thirty-three patients underwent double-bundle ACL reconstruction with an autogenous semitendinosus tendon graft. With this method, two femoral tunnels were drilled within the native ACL femoral footprint through an anteromedial portal and two tibial tunnels were drilled within the tibial footprint in an outside-in fashion. Two double-folded semitendinosus tendon grafts were fixed on the femoral end with TightRope RT implant (Arthrex, Inc., Naples, FL, USA). On the tibial end, the grafts were fixed with a double-spike plate (DSP; MEIRA Corp., Nagoya, Aichi, Japan). Seventeen patients underwent single-bundle reconstruction with an autogenous bone-tendon-bone (BTB) graft. Femoral tunnels were drilled within the femoral footprint through the anteromedial portal and tibial tunnels were drilled in an outside-in fashion. A TightRope BTB was used to fix the femoral end, and an interference screw (Smith and Nephew Inc., Andover, MA, USA) was used for the tibial end. For both methods, range of motion exercises and partial weight-bearing with a functional knee brace (Breg, Inc., Carlsbad, CA, USA) were initiated 1 week after surgery. Full weight-bearing was allowed at 4 weeks after surgery.
MRI protocol and T1ρ mapping imaging assessment
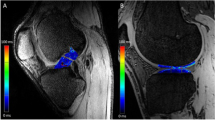
MRI was performed on a 3-T system (Achieva 3.0T, Quasar Dual; Philips Healthcare, Best, The Netherlands) using an eight-channel phased-array knee coil. The MRI protocol was previously described [16]. Two-dimensional (2D) sagittal T1ρ mapping was generated from T1ρ-prepared images using the fast-field echo technique. T1ρ mapping was produced with Philips research integrated development environment (PRIDE) software written in the Interactive Data Language (IDL 6.3; ITT Inc., White Plains, NY, USA). T1ρ mapping was used for quantitative assessment. T1ρ values were calculated using MIPAV (medical image processing, analysis, and visualization) software (Biomedical Imaging Research Services Section, Centre for Information Technology, National Institutes of Health, Bethesda, MD, USA).
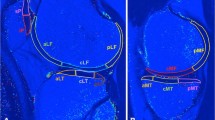
Four compartments of the knee were evaluated: medial femoral condyle (MFC), lateral femoral condyle (LFC), medial tibia (MT), and lateral tibia (LT). Femoral regions of interest (ROIs) in the cartilage were partitioned into three areas. From the centre of the circle marking the approximate circumference of each posterior femoral condyle, a line parallel to the distal femoral axis was drawn. Areas on either side of the line parallel to the femoral axis were defined as follows: the anterior area was 45° from the line (MFC1, LFC1), the middle area was 45° posterior of the line (MFC2, LFC2), and the posterior area was positioned 45° to 90° posterior of the line (MFC3, LFC3). ROIs on the tibial cartilage were divided into anterior (MT1, LT1) and posterior areas (MT2, LT2) (Fig. 1). This method demonstrated excellent agreement according to intraclass and interclass coefficients (intraobserver reliability: 0.95, interobserver reliability: 0.84). Three MR sagittal images, consisting of a centre slice of the medial or lateral compartment and both adjacent slices, were analysed within each ROI. The mean T1ρ value of the three slices within each ROI was calculated using the mean T1ρ value of each slice and the number of pixels in each slice. Segmentation was manually corrected to avoid artefacts caused by synovial fluid or other surrounding tissue. For each ROI, the mean T1ρ value before surgery was compared to the value 2 years after surgery. The difference in T1ρ values between these two time points (2 years after surgery minus before surgery) was defined as ΔT1ρ. The preoperative condition of the cartilage was also assessed with Whole-Organ MRI Scoring (WORMS) using the same compartmentalization as the ROIs for T1ρ mapping [18]. The correlation between ΔT1ρ and variables such as age, sex, BMI, meniscal injury, presence of a cartilaginous lesion, WORMS score for cartilage, and graft type was evaluated. All measurements were performed by one observer and were repeated in a blinded manner during the course of two sessions at least 1 month apart. Another observer independently made measurements of five randomly selected knees.
Each region of interest (ROI) was defined as follows: ROIs on the femoral articular cartilage were divided into an anterior area 45° from the line (MFC1, LFC1), a middle area 45° posterior from the line (MFC2, LFC2), and a posterior area 45° to 90° posterior from the line (MFC3, LFC3). ROIs on the tibial articular cartilage were divided into an anterior (MT1, LT1) and posterior area (MT2, LT2). Left: medial compartment. Right: lateral compartment. MFC medial femoral condyle, MT medial tibia, LFC lateral femoral condyle, LT lateral tibia
Clinical assessment
At 1 year after surgery, clinical assessment of AP knee stability was based on the side-to-side difference relative to the normal contralateral knee on a stress radiograph taken with the knee in 30° of flexion with a 15-kg anterior stress applied by a Telos arthrometer. Anterolateral rotational instability (ALRI) [19,20,21] was measured with open MRI as the translation of the tibia relative to the femur at the centre of the lateral compartment during Slocum’s ALRI test performed during the open MRI examination. This value was compared with that of the normal contralateral knee. At 2 years after surgery, the Lysholm score, Tegner activity score [22], and the Knee injury and Osteoarthritis Outcome Score (KOOS) [23] were obtained using self-administered questionnaires. Correlations between ΔT1ρ values and assessments of knee stability and clinical outcomes were also evaluated. This study was approved by Institutional Review Board of Kyushu University (ID number of the approval: 23-75) and informed consent was obtained from all patients before their participation.
Statistical analysis
A sample size calculation was performed before conducting this study using JMP Pro software version 12.0 (SAS Institute, Cary, NC, USA). The results of a previous study that used the same measurement were used as pilot data [16]. In the present study, the minimal detectable value was set to 4.3 ms, assuming a standard deviation of 6.5 ms and a statistically significant value of P < 0.05. The sample size analysis revealed that a total of 20 knees were needed to obtain a power of 80%. All data are expressed as means ± SD, and analysis was performed using JMP Pro. The paired t test was used to compare differences in T1ρ before and 2 years after surgery in each ROI. To evaluate the effect of cartilaginous lesions, participants were divided into two groups (ICRS grade 1–3 in at least one compartment versus grade 0 in all compartments). To evaluate the effect of WORMS score for cartilage, participants were divided into two groups (WORMS grade > 1 versus grade 0 in each ROI). Student’s t test was used to detect the difference in mean ΔT1ρ between the two groups stratified by sex, presence of meniscal injury, presence of a cartilaginous lesion evaluated by arthroscopy, WORMS score for cartilage evaluated by MRI, and graft type (double-bundle or single-bundle). Pearson’s correlation coefficients were used to investigate the correlation between ΔT1ρ and age, BMI, time to surgery, and side-to-side differences on stress radiographs and ALRI for each ROI. Spearman’s correlation coefficients were used to evaluate the correlation between ΔT1ρ and the Lysholm score, KOOS subscale scores, and Tegner activity score for each ROI. Multiple regression analysis was also used to assess the contribution of these factors.
Results
Mean preoperative FTA and PTS were 174.6 ± 1.5° and 9.3 ± 2.5°, respectively. Mean T1ρ values in each area of cartilage on the femoral condyles and tibial plateau are summarised in Table 1. The paired t test revealed a significant increase in T1ρ before versus 2 years after surgery in the MT1, MT2, LFC1, and LT1 areas, and a decrease in the LFC3 and LT2 areas. Table 2 summarises WORMS score for cartilage. Correlations between ΔT1ρ in each ROI and surgical factors were also demonstrated in Table 3.
The mean anterior side-to-side difference was 1.6 ± 2.0 mm on stress radiographs. The mean difference in ALRI was 1.5 ± 4.1 mm. Pearson’s correlation coefficient analysis showed a significant correlation between side-to-side difference on stress radiographs and ΔT1ρ in the MFC3 (r = 0.37, P = 0.013) and MT2 (r = 0.35, P = 0.021) areas (Table 3). Multiple regression analysis of these surgical factors showed a significant correlation only between side-to-side difference on stress radiographs and ΔT1ρ in the MFC3 (β = 0.36, P = 0.020) and MT2 (β = 0.38, P = 0.013) areas.
The results of the Lysholm score, Tegner score, and KOOS are shown in Table 4 and Fig. 2. ΔT1ρ for the MFC2 area was negatively correlated with KOOS symptoms (ρ = -0.349, P = 0.027) and quality of life (QOL) (ρ = − 0.374, P = 0.017) subscale scores.
Discussion
The most important finding of this study was that an increase in T1ρ in the medial compartment is likely to occur even with ACL reconstruction. Significant increases in T1ρ were observed in the anterior femur and tibia (MT1, MT2, LFC1, and LT1). In contrast, T1ρ in the posterolateral femur and tibia (LFC3 and LT2) decreased. These trends are consistent with previous studies of T1ρ and T2 mapping for knees after ACL reconstruction [14, 15, 24]. On the medial side, altered kinematics of the knee after ACL reconstruction may cause articular cartilage damage [25], leading to higher T1ρ values [26]. The reason for the decrease on the lateral side is that the posterolateral area had experienced cartilage damage at the time of injury [27], which led to decreased T1ρ values during the postoperative period. Whereas previous studies had short follow-ups (e.g., 6 months or 1 year) or MRI examination at only one time point, this study followed patients for 2 years postoperatively and MRI was performed at preoperative and postoperative visits. Longitudinal analysis with the paired t test enabled a more robust evaluation. Moreover, our method for determining ROI was possibly more reliable for longitudinal studies than past studies [9, 12, 14]. It enabled the determination of ROIs independent of the position of the meniscus, which depends on the angle of knee flexion. Instead, our method was based on bone morphology and enabled us to have minimal impact on the area of interest while identifying the same location during both time points.
In this study, the difference between preoperative and postoperative T1ρ values (ΔT1ρ) was calculated and a relationship between ΔT1ρ and surgical factors was detected. There was a significant correlation between sex and ΔT1ρ in the MFC1 area. According to a systematic review, many studies assessed female sex as a potential risk factor for OA [28]. Higher age was correlated with smaller ΔT1ρ in MFC3 and MT2. It is important that the difference in T1ρ values before and after surgery was evaluated using MRI to overcome the concerns due to the baseline variations among each individual. For example, if the T1ρ value were already elevated preoperatively, the difference would potentially be small. Moreover, there was a significant negative correlation between age and sports activity as reflected by the Tegner score at 2 years after surgery (Spearman’s correlation; ρ = − 0.341, P = 0.034), which might have affected the results. There was no significant correlation between ΔT1ρ and duration to surgery. It is well known that a longer duration to surgery is associated with greater degenerative changes in cartilage, resulting in clinical OA changes of the knee [29,30,31]. Because this study recruited patients who underwent surgery within 2 years of injury, ΔT1ρ might not be correlated with duration to surgery. The presence of meniscal injury did not affect ΔT1ρ of any ROI. Past studies noted that meniscal injury is a risk factor for degenerative changes in knee articular cartilage [5, 15, 16]. In contrast, some studies have demonstrated no significant difference between patients with or without meniscal tears [13] and between patients who underwent meniscectomy versus meniscal repair [32]. In this study, we treated meniscus injury with repair as much as possible in accordance with current trends to save the meniscus [33,34,35], which might result in no significant increase in T1ρ. However, meniscus injuries involve many factors, such as type, grade, and location of the injury, and treatment type. Therefore, it is difficult to assess the influence of meniscus injury precisely, which might have affected the results. Regarding the presence of a cartilaginous lesion observed with arthroscopy during ACL reconstruction and WORMS score for cartilage evaluated by preoperative MRI, there was no significant difference whether a cartilaginous lesion was present, although Hirose et al. showed that cartilaginous lesions are related to progressive degenerative changes in cartilage [13]. Few patients with cartilaginous lesions and WORMS grade > 1 were included in our study, so a significant difference might not have emerged. There was no significant difference in ΔT1ρ between the two graft types in this study. Clinical results for both graft types have been inconsistent [36, 37]. Further study with more patients that have varying degrees of cartilaginous lesions with reconstruction using different graft types could clarify the influence of such factors on T1ρ values.
AP laxity demonstrated on stress radiographs affected ΔT1ρ values in the MFC3, MT2, and LFC2 areas. Multiple regression in a model with other surgical factors showed a significant relationship between AP laxity and ΔT1ρ in the MFC3 and MT2 areas. Past studies with long-term follow-up showed significantly more severe degenerative changes and OA progression in patients with increased AP laxity [38]. When the reconstructed ACL is not functional and has laxity, the loading pattern and amount of load might differ. Otherwise, there was no significant relationship between ΔT1ρ in each ROI and rotational laxity expressed by the ALRI test, which was inferred to be due to rotational laxity being less detectable than AP laxity [19, 20]. No other studies have demonstrated a relationship between knee laxity and quantitative MRI values. Thus, AP laxity is possibly a risk factor for early cartilage degeneration, especially in the posteromedial area. Elevated T1ρ values on the lateral side on the preoperative MRI might offset the increase in T1ρ due to laxity, which might explain why there was no correlation between AP laxity and ΔT1ρ of the lateral area and why the ALRI results did not affect ΔT1ρ in any ROI.
ΔT1ρ for the MFC2 area was negatively associated with KOOS symptoms and QOL subscale scores. There have a few reports about the relationship between MRI relaxation time and clinical outcomes [12, 15]. However, these reports were based on short follow-up periods and did not consider differences before versus after surgery. In this study, the difference in T1ρ before versus 2 years after surgery was calculated and a relationship between this difference and clinical outcomes was detected.
This study has several limitations. First, our study included a relatively small number of patients even though it was large enough from a statistical perspective to detect a difference in T1ρ values. Some multiple regression results might have been affected by the study’s sample size. In addition, various surgical factors potentially acted as confounders, so there was a possibility that the factors that lead to subtle but important changes in T1ρ had been overlooked. Second, we evaluated cartilage degeneration only with T1ρ values. Although T2 and dGEMRIC are also established methods for detecting early cartilage degeneration, a systematic review and meta-analysis showed T1ρ relaxometry is superior to T2 relaxometry and dGEMRIC for discriminative validity [39]. Therefore, we believe we could conduct a reliable evaluation with T1ρ relaxometry. Third, uninjured contralateral knees in patients with ACL tears were not assessed. Previous studies have shown increases in T1ρ values in the uninjured contralateral knee in a defined period of time [12, 40]. However, a longitudinal assessment was conducted and differences between preoperative and 2-year postoperative T1ρ values were evaluated, which enabled the identification of factors affecting increases in T1ρ. Fourth, some intensity alternation in the condyles because of the impact during injury might affect T1ρ values. Patients who underwent surgery less than 2 years after their ACL injury were recruited for this study based on a previous study [16] to minimise the influence of differences in T1ρ values among patients. However, there were some differences even among patients who received surgery within 2 years of injury, especially on the lateral side. Finally, this cohort of patients was only followed for 2 years whereas past studies had longer follow-up periods. Although it is unclear whether the increase in T1ρ results in clinical OA, we believe we were able to detect subclinical degenerative changes in cartilage in this study. Longer follow-up is needed to determine the threshold T1ρ value for clinical OA.
Conclusion
An increase in quantitative MRI T1ρ values in the medial compartment likely occurs even with ACL reconstruction. AP laxity made T1ρ values increase significantly in the posteromedial femur and tibia. Increased T1ρ in the central medial femoral condyle is associated with knee symptoms.
Abbreviations
- ACL:
-
: Anterior cruciate ligament
- MRI:
-
: Magnetic resonance imaging
- ROI:
-
: Region of interest
- MFC:
-
: Medial femoral condyle
- LFC:
-
: Lateral femoral condyle
- MT:
-
: Medial tibia
- LT:
-
: Lateral tibia
- OA:
-
: Osteoarthritis
- dGEMRIC:
-
: Delayed gadolinium-enhanced MRI of cartilage
- BMI:
-
: Body Mass Index
- FTA:
-
: Femorotibial angle
- AP:
-
: Anteroposterior
- PTS:
-
: Posterior tibial slope
- ICRS:
-
: International Cartilage Repair Society
- BTB:
-
: Bone-tendon-bone
- WORMS:
-
: Whole-Organ MRI Scoring
- ALRI:
-
: Anterolateral rotational instability
- KOOS:
-
: Knee Injury and Osteoarthritis Outcome Score
- ADL:
-
: Activity of daily living
- QOL:
-
: Quality of life
References
Hanypsiak B, Spindler K, Rothrock C, Calabrese G, Richmond B, Herrenbruck T, Parker R (2008) Twelve-year follow-up on anterior cruciate ligament reconstruction: long-term outcomes of prospectively studied osseous and articular injuries. Am J Sports Med 36:671–677
Lohmander LS, Östenberg A, Englund M, Roos H (2004) High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum 50:3145–3152
Von Porat A, Roos EM, Roos H (2004) High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis 63:269–273
Spindler KP, Huston LJ, Chagin KM, Kattan MW, Reinke EK, Amendola A, Andrish JT, Brophy RH, Cox CL, Dunn WR, Flanigan DC, Jones MH, Kaeding CC, Magnussen RA, Marx RG, Matava MJ, McCarty EC, Parker RD, Pedroza AD, Vidal AF, Wolcott ML, Wolf BR, Wright RW (2018) Ten-year outcomes and risk factors after anterior cruciate ligament reconstruction: a MOON longitudinal prospective cohort study. Am J Sports Med 46:815–825
Barenius B, Ponzer S, Shalabi A, Bujak R, Norlén L, Eriksson K (2014) Increased risk of osteoarthritis after anterior cruciate ligament reconstruction. Am J Sports Med 42:1049–1057
Janssen RPA, du Mée AWF, van Valkenburg J, Sala HAGM, Tseng CM (2013) Anterior cruciate ligament reconstruction with 4-strand hamstring autograft and accelerated rehabilitation: A 10-year prospective study on clinical results, knee osteoarthritis and its predictors. Knee Surg Sports Traumatol Arthrosc 21:1977–1988
Li R, Lorenz S, Xu Y, Harner C, Fu F, Irrgang J (2011) Predictors of radiographic knee osteoarthritis after anterior cruciate ligament reconstruction. Am J Sports Med 39:2595–2603
Risberg M, Oiestad B, Gunderson R (2016) Changes in knee osteoarthritis, symptoms, and function after anterior cruciate ligament reconstruction: a 20-year prospective follow-up study. Am J Sports Med 44:1215–1224
Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, Boutin RD, Gray ML (2001) Protocol issues for delayed Gd(DTPA)2–enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med 45:36–41
Nieminen MT, Rieppo J, Töyräs J, Hakumäki JM, Silvennoinen J, Hyttinen MM, Helminen HJ, Jurvelin JS (2001) T2 relaxation reveals spatial collagen architecture in articular cartilage: A comparative quantitative MRI and polarized light microscopic study. Magn Reson Med 46:487–493
Wheaton AJ, Casey FL, Gougoutas AJ, Dodge GR, Borthakur A, Lonner JH, Schumacher HR, Reddy R (2004) Correlation of T1ρ with fixed charge density in cartilage. J Magn Reson Imaging 20:519–525
Amano K, Li AK, Pedoia V, Koff MF, Krych AJ, Link TM, Potter H, Rodeo S, Li X, Ma CB, Majumdar S, Goldring SR, Goldring M, Hannafin JA, Marx RG, Nawabi DH, Otero M, Shah P, Warren RF, Amrami KK, Felmlee JP, Frick MA, Stuart MJ, Williams SL, Kretzchmar M, Lansdown DA, Okazaki N, Russell C, Savic D, Schwaiger B, Su F, Wyatt C, Cheong M, Hardin JA (2017) Effects of surgical factors on cartilage can be detected using quantitative magnetic resonance imaging after anterior cruciate ligament reconstruction. Am J Sports Med 45:1075–1084
Hirose J, Nishioka H, Okamoto N, Oniki Y, Nakamura E, Yamashita Y, Usuku K, Mizuta H (2013) Articular cartilage lesions increase early cartilage degeneration in knees treated by anterior cruciate ligament reconstruction: T1 mapping evaluation and 1-year follow-up. Am J Sports Med 41:2353–2361
Russell C, Pedoia V, Amano K, Potter H, Majumdar S, Koff MF, Goldring SR, Goldring M, Hannafin JA, Marx RG, Nawabi DH, Otero M, Rodeo SA, Shah P, Warren RF, Amrami KK, Felmlee JP, Frick MA, Krych AJ, Stuart MJ, Williams SL, Kretzchmar M, Lansdown DA, Li A, Li X, Link TM, Benjamin Ma C, Okazaki N, Savic D, Schwaiger B, Su F, Wyatt C, Hardin JA (2017) Baseline cartilage quality is associated with voxel-based T1ρ and T2 following ACL reconstruction: a multicenter pilot study. J Orthop Res 35:688–698
Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, Ma CB, Li X (2013) Cartilage morphology and T1ρ and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthr Cartil 21:1058–1067
Osaki K, Okazaki K, Takayama Y, Matsubara H, Kuwashima U, Murakami K, Doi T, Matsuo Y, Honda H, Iwamoto Y (2015) Characterization of biochemical cartilage change after anterior cruciate ligament injury using T1ρ mapping magnetic resonance imaging. Orthop J Sport Med 3:1–7
Brittberg M, Winalski C (2003) Evaluation of cartilage injuries and cartilage repair. J Bone Joint Surg Am 85:58–69
Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK (2004) Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil 12:177–190
Okazaki K, Miura H, Matsuda S, Yasunaga T, Nakashima H, Konishi K, Iwamoto Y, Hashizume M (2007) Assessment of anterolateral rotatory instability in the anterior cruciate ligament-deficient knee using an open magnetic resonance imaging system. Am J Sports Med 35:1091–1097
Okazaki K, Tashiro Y, Izawa T, Matsuda S, Iwamoto Y (2012) Rotatory laxity evaluation of the knee using modified Slocum’s test in open magnetic resonance imaging. Knee Surg Sports Traumatol Arthrosc 20:679–685
Slocum DB, James SL, Larson RL, Singer KM (1976) Clinical test for anterolateral rotary instability of the knee. Clin Orthop Relat Res 118:63–69
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 198:43–49
Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD (1998) Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther 28:88–96
Theologis AA, Haughom B, Liang F, Zhang Y, Majumdar S, Link TM, Ma CB, Li X (2014) Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc 22:298–307
Thompson SM, Salmon LJ, Waller A, Linklater J, Roe JP, Pinczewski LA (2016) Twenty-year outcome of a longitudinal prospective evaluation of isolated endoscopic anterior cruciate ligament reconstruction with patellar tendon or hamstring autograft. Am J Sports Med 44:3083–3094
Lansdown DA, Allen C, Zaid M, Wu S, Subburaj K, Souza R, Feeley BT, Li X, Ma CB (2015) A comprehensive in vivo kinematic, quantitative MRI and functional evaluation following ACL reconstruction—a comparison between mini-two incision and anteromedial portal femoral tunnel drilling. Knee 22:547–553
Potter H, Jain S, Ma Y, Black B, Fung S, Lyman S (2012) Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med 40:276–285
Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP (2015) Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr Cartil 23:507–515
Fok AWM, Yau WP (2013) Delay in ACL reconstruction is associated with more severe and painful meniscal and chondral injuries. Knee Surg Sports Traumatol Arthrosc 21:928–933
Magnussen R, Pedroza AD, Donaldson CT, Flanigan DC, Kaeding CC (2013) Time from ACL injury to reconstruction and the prevalence of additional intra-articular pathology: Is patient age an important factor? Knee Surg Sports Traumatol Arthrosc 21:2029–2034
Nishioka H, Hirose J, Nakamura E, Okamoto N, Karasugi T, Taniwaki T, Okada T, Yamashita Y, Mizuta H (2013) Detecting ICRS grade 1 cartilage lesions in anterior cruciate ligament injury using T1ρ and T2 mapping. Eur J Radiol 82:1499–1505
Li H, Chen S, Tao H, Chen S (2015) Quantitative MRI T2 relaxation time evaluation of knee cartilage: comparison of meniscus-intact and -injured knees after anterior cruciate ligament reconstruction. Am J Sports Med 43:865–872
Lubowitz JH, Poehling GG (2011) Save the meniscus. Arthroscopy 27:301–302
Noyes FR, Barber-Westin SD (2002) Arthroscopic repair of meniscal tears extending into the avascular zone in patients younger than twenty years of age. Am J Sports Med 30:589–600
Seil R, Becker R (2016) Time for a paradigm change in meniscal repair: save the meniscus! Knee Surg Sports Traumatol Arthrosc 24:1421–1423
Aga C, Risberg MA, Fagerland MW, Johansen S, Trøan I, Heir S, Engebretsen L (2018) No Difference in the KOOS Quality of Life Subscore Between Anatomic Double-Bundle and Anatomic Single-Bundle Anterior Cruciate Ligament Reconstruction of the Knee: A Prospective Randomized Controlled Trial With 2 Years’ Follow-up. Am J Sports Med 46:2341–2354
Li X, Xu CP, Song JQ, Jiang N, Yu B (2013) Single-bundle versus double-bundle anterior cruciate ligament reconstruction: an up-to-date meta-analysis. Int Orthop 37:213–226
Struewer J, Frangen TM, Ishaque B, Bliemel C, Efe T, Ruchholtz S, Ziring E (2012) Knee function and prevalence of osteoarthritis after isolated anterior cruciate ligament reconstruction using bone-patellar tendon-bone graft: long-term follow-up. Int Orthop 36:171–177
MacKay JW, Low SBL, Smith TO, Toms AP, McCaskie AM, Gilbert FJ (2018) Systematic review and meta-analysis of the reliability and discriminative validity of cartilage compositional MRI in knee osteoarthritis. Osteoarthr Cartil 26:1140–1152
Pedoia V, Su F, Amano K, Li Q, McCulloch CE, Souza RB, Link TM, Ma BC, Li X (2017) Analysis of the articular cartilage T1ρand T2relaxation times changes after ACL reconstruction in injured and contralateral knees and relationships with bone shape. J Orthop Res 35:707–717
Acknowledgements
The authors thank Mr. Junji Kishimoto, a statistician from the Digital Medicine Initiative of Kyushu University, for his advice on the statistical analysis.
Funding
No financial support was provided to this study.
Author information
Authors and Affiliations
Contributions
TU collected and analysed data and drafted the manuscript. KO conceived the study, contributed to its design, collected and analysed data, coordinated the study, and helped to draft the manuscript. KO is also the corresponding author. YT, KS, and HH collected and analysed data. HM, SH, and YA assisted in drafting the manuscript. YN gave final approval to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
KO has received speaker honoraria from Zimmer Biomet and Smith & Nephew. HM has received a speaker honorarium from Zimmer Biomet.
Ethical approval
Ethical approval was provided by the IRB of Kyushu University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ushio, T., Okazaki, K., Osaki, K. et al. Degenerative changes in cartilage likely occur in the medial compartment after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 27, 3567–3574 (2019). https://doi.org/10.1007/s00167-019-05468-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05468-5