Abstract
Purpose
The aim of this study was to investigate the long-term outcome of the unicompartmental knee resurfacing prosthesis (UniCAP) using clinical and radiographic assessments, and to evaluate the revision and survival rates.
Methods
This was a prospective cohort study of patients with UniCAP prostheses with 6–9 years of follow-up. The clinical examination included the Knee Society Score (KSS) and Visual Analogue Scale (VAS) score. The radiographic examination included the Kellgren–Lawrence (KL) grading scale. A comparison analysis of the clinical preoperative and follow-up data and a Kaplan–Meier survival analysis were performed.
Results
Of the 64 UniCAP patients, 36 (56%) were revised and one died. Examinations were performed on 23 (85%) of them. When compared with the preoperative data, the examinations showed a significant increase in the KSS objective [mean = 47.4, standard deviation (SD) = 5.8 vs. mean = 90.0, SD = 6.9] and function (mean = 46.7, SD = 6.8 vs. mean = 91.1, SD = 6.9) scores, a decrease in the VAS-score (mean = 7.3, SD = 0.5 vs. mean = 3.4, SD = 1.4) and a significant increase in the KL medial score (mean = 1.7, SD = 0.6 vs. mean = 2.1, SD = 0.5). The Kaplan–Meier survival rate after 5 years indicated good long-term outcomes.
Conclusions
There was a survival rate of approximately 40% after 9 years of follow-up, but in the group of patients (35–65 years old) not eligible for a final total arthroplasty. These patients were often left with pain and disability. This implant can be a temporary or even long-term treatment because it improved the disability and function over the long-term without a major progression in the osteoarthritis, function or pain. Long term results of this mini-prosthesis have not been previously reported.
Level of evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Middle-aged patients with knee pain and disability caused by minor or larger cartilage lesions or even early osteoarthritis (OA) have always been a challenge to treat, mostly because of the decreasing healing capacity of cartilage with advancing age [7, 9]. Several different treatment modalities such as micro-fracturing, True Fit, autologous chondrocyte implantation (ACI), or high tibia osteotomies (HTO) are available [4, 5, 11, 12, 23, 24], some of them in combinations. Many of them have great implications on a patient’s daily life and working ability caused by a long rehabilitation period and the risk of losing work, with enormous costs to society [19, 20] and risks to family life and social functions.
Previous studies have demonstrated that biological treatment options, such as bone marrow stimulation and chondrocyte transplantation, are preferred at the youngest ages (30–35 years old), but these have less favourable outcomes with an increasing patient age [4, 7, 24]. The next operative treatment option has, until recently, been unicompartmental knee arthroplasty (UKA) or total knee arthroplasty (TKA). Thus, these middle-aged patients could only pursue non-operative treatment modalities, such as physiotherapy, weight loss, analgesics and activity modifications [22]. For some of these patients, these interventions are effective, and thus, the optimal treatment because they are able to keep working. However, for other patients, these interventions are ineffective.
The unicompartmental knee resurfacing prosthesis (UniCAP) was introduced in 2006 with a goal of treating larger, localized, full-thickness femoral cartilage defects (and possibly adjacent, smaller cartilage lesions on the tibial side) or early OA [21]. It was first approved for use in Denmark in 2009, with a publication of its specific uses in 2016 [18], which presented a cohort of 64 patients with large symptomatic cartilage lesions or early OA in the medial or lateral femoral chamber. It presented improvements in the clinical function and pain at 2 years, but a prosthesis survival rate of only 50% after 7 years of follow-up.
The aim of this study was to further clarify the long-term outcome of the UniCAP prosthesis. The objectives were to first evaluate the prosthesis outcome at 6–9 years of follow-up using clinical and radiographic assessments, and second, to investigate the prosthesis revision rates and survival. It was hypothesized that the implant would reduce pain and improve knee function.
Long term results of this mini-prosthesis have not been previously reported.
Materials and methods
This was a follow-up study of patients treated with femoral resurfacing from 2009 to 2013 [18]. The follow-up period took place from October through December of 2016. It was run through the research unit for Emergency Medicine at the University of Southern Denmark, Institute for Regional Research, Southern Centre.
Ethical consideration and data protection
All collected data were stored in accordance with the Danish Data Protection Agency requirements. It was reported according to the principles outlined in the Strengthening the Reporting of Observational Studies in Epidemiology statement [25].
Written consent to participate in the study was obtained. According to Danish law approval by ethical committee was not necessary for follow-up studies. This study was approved by the regional data committee of the Region South Jutland (# 2008-58-0035).
Participants
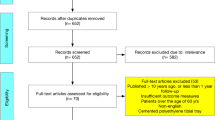
From 2009 to 2013, 64 patients aged 35–65 years were treated with femoral resurfacing using UniCAP-implants at the Orthopaedic Department of the Hospital of Southern Jutland. The indications for using this prosthesis included large symptomatic cartilage lesions or early OA at the femoral condyles, as demonstrated by standing radiographs [Kellgren–Lawrence (KL) grade], magnetic resonance imaging or arthroscopy, with an International Cartilage Repair Society (ICRS) grade of 3–4 and a lesion size exceeding 400 mm2. A patient was not offered treatment if they had either: (1) a valgus or varus malalignment exceeding 5 degrees, (2) ligament instability, (3) previous removal of more than 50% of a meniscus, or (4) a body mass index > 40. The 64 UniCAP patients, that were included in this study, were followed for up to 9 years.
Procedure
The 23 patients who did not have revisions and who did not die during the study (Fig. 1) were invited to participate in the study. If written consent was obtained, a clinically examined by a senior surgeon was performed. Knee Society Score (KSS) [15] objective and function subscale values and a Visual Analogue Scale (VAS) pain score using a numerical rank scale (0–10), with 10 being the worst possible pain, were assigned. In addition, they were radiographically evaluated and assigned KL grades for their medial and lateral tibiofemoral compartments [16].
Device description
TheUniCAP® (UniCAP® Focal Femoral Condyle Resurfacing Prosthesis, Arthrosurface Inc, Franklin, MA, USA) resurfacing implant consists of two components, fixation and modular articular components, and the two are connected by a Morse taper. It is available with a 20 × 35 mm diameter and comes in 16 different offset configurations corresponding to the superior/inferior and medial/lateral radii of the curvatures at the implant site (Fig. 2). A 20 mm polyethylene inlay is available for the tibial lesion, but we have never used this, as we thought the operation would be too comprehensive.
Surgical procedures
A standardized rehabilitation protocol with a free range of motion was allowed immediately after the operation. For the first 2 weeks, the patient performed touch weight-bearing walking followed by full weight-bearing.
Statistical analysis
The demographics and baseline characteristics were presented as the mean and standard deviation (SD). A Wilcoxon signed-rank test was used to compare the paired data. A Kaplan–Meier survival analysis was used with revision or death as the endpoint and a 95% confidence interval (CI). For the statistical analysis, Stata: Data Analysis and Statistical Software for Professionals version 14.1 (StataCorp LLC, College Station, TX, USA) was used. P values of less than 0.05 were considered to be statistically significant.
Results
Of the 64 UniCAP patients, 37 (58%) were excluded from the follow-up. 36 were revised due to progressing OA, functional impairment and pain—no deep infections, but two with aseptic loosening and one died (Fig. 1). Four of the patients were unable to or declined to participate in the follow-up examinations, resulting in the examination of 23 UniCAP patients. Of these, eight (35%) were males, and their median preoperative age was 51 years [interquartile range (IQR) = 47–57 years]. Mean follow-up 7.2 years (SD 1.3) with min. 4.9 and max. 9.1 years.
The objective and subjective outcomes (KSS) and radiographic-OA evaluation (KL-OA) are shown in Table 1. Both the KSS objective and function scores (Table 1; Fig. 3) improved significantly from the preoperative > 2-year control to the follow-up. The VAS scores were reduced significantly (Fig. 4).
KSS objective- and function scores in 23 patients preoperative, at 2 years (mid-term results presented earlier—reference 18) and follow-up till 9 years
VAS-score in 23 patients at 1, 2 years (mid-term results presented earlier—reference 18) and follow-up till 9 years
The KL-OA grade did change significantly from the preoperative medial to the last follow-up, but it was not significant for the lateral chamber.
Failures and complications
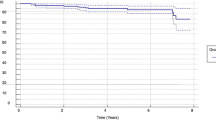
Of the UniCAP procedures, 36 (56%) were revisions. The Kaplan–Meier survival rate was 40% up to 9 years postoperatively, and no failures (revisions) were seen when they survived more than 5 years, with no difference between the males and females (Fig. 5).
Discussion
The most important finding in this cohort study with up to 9 years of follow-up clinically and radiographically was the survival rate at 40%. More important and interesting were the prosthesis survival rates between 5 and 9 years, which was one of the subjects of this paper. No further revisions were seen in this group of “long-term survivors”, and the clinical and function scores remained positive, but the KL grade worsened significantly in the knee compartment operated on. These findings have not been previously reported, and they may indicate that with proper selection, a larger inlay mini-prosthesis can serve as a long term treatment modality for these patients during the “treatment gap” between 35 and 65 years old [3, 6, 10, 12,13,14, 17,18,19,20], but still with the risk of progressing OA.
When evaluating the UniCAP, only one mid-term cohort study has been published [18], which reported an already concerning 7-year prosthesis survival rate of 50%, but it only provided a clinical and radiographic follow-up during the first 2 years after the prosthesis placement. This current study expands the time horizon for the prosthesis outcomes, specifically the clinical outcomes, radiographic progression and prosthesis survival, for up to 9 years. It confirms the concerning overall survival rate of 40. There was a clinical follow-up for 92% of the patients who did not require revisions, and interestingly, these patients had high objective and function scores, with no significant increase in the VAS and only a slight progression in the generalized OA (KL grade). In the first study [18], we saw that at the revision time, the patient’s KL grade was significantly worse when compared to the unrevised patients at the 2-year follow-up. The unrevised patients clinical status did not progress significantly from 2 years until this follow-up, indicating there is good evidence that even the UniCAP may provide long-term improvement and obviate the need for a UKA or TKA in these patients with even larger full-thickness cartilage lesions (ICRS 3–4) of more than 400 mm2 in one compartment and a KL grade maximum of 1–2 with no kissing lesions [2, 3, 6, 14, 18].
For the UniCAP resurfacing mini-prosthesis, we found a concerning long-term survival rate of approximately 40% in the 9 years after the prosthesis placement. This is unacceptable when compared to HTO, UKA or TKA [1, 8, 13], but overall, the results suggest that for a subgroup of patients with larger, but isolated cartilage lesions that do not require early revisions, there is the potential for long-lasting treatment effectiveness.
The strengths of this present study included its large sample-size, the 9-year follow-up duration and the comprehensive data concerning the revisions, which were a consequence of having a national registry. This study was limited because it was a single-centre case-cohort study with only one operating surgeon, which, at the same time, was the clinical investigator, thereby weakening the external validity.
Conclusions
As hypothesized, we found acceptable clinical and radiographic outcomes in patients not revised. However, there was a concerning low survival rate of 40% up to 9 years of follow-up in this group of patients (35–65 years old) who would generally be considered ineligible for a UKA or TKA. However, for those patients who did not require revisions, there were long-term improvements in their function.
References
Australian Orthopaedic Association National Joint Replacement Registry (2016) Annual report, pp 183
Becher C, Huber R, Cantiller EB (2017) Focal articular prosthesis resurfacing for the treatment of full-thickness articular cartilage defects in the knee: 12-year follow-up of two cases and review of the literature. Arch Orthop Trauma Surg 137(9):1307–1317
Becher C, Kalbe C, Thermann H, Paessler HH, Laprell H, Kaiser T, Fechner A, Bartsch S, Windhagen H, Ostermeier S (2011) Minimum 5-year results of focal articular prosthetic resurfacing for the treatment of full-thickness articular cartilage defects in the knee. Arch Orthop Trauma Surg 131:1135–1143
Biant LC, Bentley G, Vijayan S, Skinner JA, Carrington RW (2014) Long-term results of autologous chondrocyte implantation in the knee for chronic chondral and osteochondral defects. Am J Sports Med 42(9):2178–2183
Bollars P, Bosquet M, Vandekerckhove B, Hardeman F, Bellemans J (2012) Prosthetic inlay resurfacing for the treatment of focal, full thickness cartilage defects of the femoral condyle: a bridge between biologics and conventional arthroplasty. Knee Surg Sports Traumatol Arthrosc 20:1753–1759
Brennan SA, Devitt BM, O’Neill CJ, Nicholson P (2013) Focal femoral condyle resurfacing. Bone Jt J 95-B:301–304
Cicuttini F, Ding C, Wluka A, Davis S, Ebeling PR, Jones G (2005) Association with cartilage defects with loss of knee cartilage in healthy, middle-age adults: a prospective study. Arthritis Rheum 52:2033–2039
Danish Orthopaedic Association (2015) National joint replacement registry (DKR) annual report
Davies-Tuck ML, Wluka AE, Wang Y, Teichtahl AJ, Jones G, Ding C, Cicuttini FM (2008) The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthr Cartil 16(3):337–342
Dhollander AAM, Almquist KF, Moens K, Vandekerckhove PJ, Verdonk R, Verdonk P, Victor J (2015) The use of a prosthetic inlay resurfacing as a salvage procedure for a failed cartilage repair. Knee Surg Sports Traumatol Arthrosc 23:2208–2212
Feucht MJ, Cotic M, Beitzel K, Baldini JF, Meidinger G, Schöttle PB, Imhoff AB (2017) A matched-pair comparison of inlay and onlay trochlear designs for patellofemoral arthroplasty: no differences in clinical outcome but less progression of osteoarthritis with inlay designs. Knee Surg Sports Traumatol Arthrosc 25:2784–2791
Fuchs A, Eberbach H, Izadpanah K, Bode G, Südkamp NP, Feucht MJ (2018) Focal metallic inlay resurfacing prosthesis for the treatment of localized cartilage defects of the femoral condyles: a systematic review of clinical studies. Knee Surg Sports Traumatol Arthrosc 26:2722–2732
Harrysson OL, Robertsson O, Nayfeh JF (2004) Higher cumulative revision rate of knee arthroplasties in younger patients with osteoarthritis. Clin Orthop Relat Res 421:162–168
Imhoff AB, Feucht MJ, Meidinger G, Schötte PB, Cotic M (2015) Prospective evaluation of anatomic patellofemoral inlay resurfacing: clinical, radiographic and sports-related results after 24 months. Knee Surg Sports Traumatol Arthrosc 23:1299–1307
Insall JN, Dorr LD, Scott RD, Scott WN (1989) Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res 248:13–14
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteoarthrosis. Ann Rheum Dis 16(4):494–502
Laursen JO, Lind M (2017) Treatment of full-thickness femoral cartilage lesions using condyle resurfacing prosthesis. Knee Surg Sports Traumatol Arthrosc 25:746–751
Laursen JO (2016) Treatment of full-thickness cartilage lesions and early OA using large condyle resurfacing prosthesis: UniCAP. Knee Surg Sports Traumatol Arthrosc 24:1695–1701
Li CS, Karlsson J, Winemaker M, Sancheti P, Bhandari M (2014) Orthopaedic surgeons feel that there is a treatment gap in management of early OA: international survey. Knee Surg Sports Traumatol Arthrosc 22(2):363–378
London NJ, Miller LE, Block JE (2011) Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypotheses 76:887–892
Miniaci A (2014) UniCAP as an alternative for unicompartmental arthritis. Clin Sports Med 33(1):57–65
Skou ST, Derosche CA, Andersen MM, Rathleff MS, Simonsen O (2015) Nonoperative treatment improves pain irrespective of radiographic severity. A cohort study of 1,414 patients with knee osteoarthritis. Acta Orthop 86(5):599–604
Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG (2003) Outcomes of microfracture for traumatic chondral defects of the knee: 11-year follow-up. J Arthroscopy 19:477–480
Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP (2011) Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med 39(12):2566–2574
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The strengthening in the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet 370(9596):1453–1457
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There have been no conflicts of interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laursen, J.O., Mogensen, C.B. & Skjøt-Arkil, H. UniCAP offers a long term treatment for middle-aged patients, who are not revised within the first 9 years. Knee Surg Sports Traumatol Arthrosc 27, 1693–1697 (2019). https://doi.org/10.1007/s00167-019-05356-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05356-y