Abstract
Introduction
The rationale of focal articular prosthetic resurfacing used as a primary arthroplasty procedure in the treatment of articular cartilage defects is still under debate. Conflicting reports raise concern about high rates of re-operations and continued development of osteoarthritis, while others have reported good outcomes. The goal of this paper is to present the long-term results of two patients with a 12-year follow-up and to report the results of a literature review.
Materials and methods
Two patients (male, 70 years; female 63 years) with a follow-up of 12 years were reviewed. Patients were evaluated with standard radiographs to assess the progression of osteoarthritis (OA), a clinical examination including the Knee Injury and Osteoarthritis Outcome Score (KOOS) and Tegner activity scale. The literature review was performed using the search terms HemiCAP, focal, femoral, condyle, inlay, and resurfacing to identify articles published in the English language up until September 25, 2016.
Results
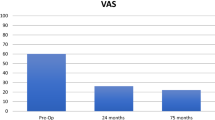
The clinical and radiographic follow-ups of the patients were 11.9 and 11.8 years, respectively. Both patients were satisfied with their outcome and would have the operation again. Comparing the first postoperative to 12-year follow-up X-rays, the radiographic results demonstrated no signs of periprosthetic loosening, preservation of joint space, and no change in the osteoarthritic stage. KOOS Scores were 86 and 83 for pain, 89 and 93 for symptoms, 88 and 100 for activities of daily living (ADL), 75 and 65 for sports and recreation, and 75 and 81 for quality of life (QOL). The Tegner activity level was 5 and 4. The literature review comprised 6 studies with 169 focal articular prosthetic resurfacing procedures in 169 patients (84 male, 85 female) with a mean age at implantation ranging from 44.7 to 53.7 years and a follow-up range of 20 months to 7 years. Five studies were classified as level 4 and one as level 3. Clinical and radiographic results showed mainly good to excellent outcomes but were different among the studies depending on the indication. Re-operation rates ranged from 0 to 23% depending on the length of follow-up.
Conclusions
The results suggest that focal articular prosthetic resurfacing is an effective and safe treatment option in selected cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of full-thickness articular cartilage defects offers a variety of treatment options [26, 34]. Biological repair with marrow stimulation techniques [35], osteochondral autograft [10, 12], osteochondral allograft [6], or cell-based technologies [25, 29] is generally recommended for patients <40 years and results are less predictable with increasing age [17, 19]. Conventional joint arthroplasty is usually recommended for patients >60 years leaving a meaningful treatment gap for the middle-aged patients of 40–60 years.
The HemiCAP® contoured articular prosthetic resurfacing prosthesis (Arthrosurface Inc., Franklin, MA, USA) was introduced in 2003 to offer a focal treatment option among the currently used modalities and close this treatment gap for the middle-aged patients. It is recommended to be used when only one compartment is affected by posttraumatic, degenerative disease or necrosis associated with large unstable articular defects with significant subchondral bone exposure [3, 5, 13, 20]. The device has also been used for revision in failed biological repair attempts [3, 8]. Postulated advantages over biological repair options or conventional unicondylar or bicondylar arthroplasty are a generally shorter rehabilitation and preservation of physiological joint kinematics.
The rationale for focal articular prosthetic resurfacing in the treatment of full-thickness articular cartilage defects is still under debate. The purpose of this report is to demonstrate the clinical and radiographic course of the first two patients treated with the HemiCAP resurfacing prosthesis at the authors’ institution and perform a review of the literature of clinical studies after implantation of the device.
Material and methods
The HemiCAP® resurfacing system comprises a contoured articular prosthetic consisting of two components, a fixation and an articular component. The articular component comes in 15 and 20 mm diameters, which are available in various offset sizes to match the shape and contour of the patient’s individual cartilage surface. The device is implanted using a small para-patellar incision over the center of the defect after asserting the indication with a standard arthroscopy. With mapping instruments, the surface curvature is measured and the matching surface reamer prepares the inlay implant bed. The accurate fit to the adjacent cartilage is confirmed with sizing trials. According to biomechanical and animal studies [2, 23], the implant is aimed to be implanted slightly recessed to the surrounding articular cartilage surface.
Case reports
Two patients that received the resurfacing device in 2004 were followed with clinical and radiographic assessments in 2010 and 2016. The standard rehabilitation protocol included weight bearing as tolerated using crutches for 1–2 weeks with early functional rehabilitation with no restriction to range of motion.
Demographic data (Table 1) and validated questionnaires (Table 2) were obtained at the follow-up visits 2010 and 2016. The questionnaires included the Knee Injury and Osteoarthritis Outcome Score (KOOS) score [31] that includes five domains: pain, symptoms, activities of daily living (ADL), sports and recreation ability, and knee-related quality of life (QoL) and has been used and validated for the treatment of focal cartilage defects [4, 11] (100 = best possible score, 0 = worst). The Tegner activity scale [36] is a numerical scale measuring the level of competitiveness of an individual sport’s participation. Each activity is measured from 0 to 10 where an elite professional athlete would compete in a sport at a level 10, high level recreational athletes would be at a 6 and persons with knee problems or a disability would score at a level 0. Satisfaction was evaluated by a questionnaire that rated the general satisfaction with the treatment outcomes (1 = excellent, 2 = very good, 3 = good, 4 = fair, 5 = poor) and if they would undergo the procedure again.
Radiographic assessment was performed comparing the pre- and immediate postoperative radiographs to those obtained at the follow-up visits (Rosenberg view, AP and lateral standing X-rays). All radiographs were graded according to the Kellgren–Lawrence classification [15] to compare the stage of osteoarthritis with the preoperative status. Furthermore, signs of radiolucency, implant loosening, and subchondral cyst formation were evaluated.
Literature search
A literature review was performed using the search terms HemiCAP, focal, femoral, condyle, inlay, and resurfacing, at PubMed and EMBASE databases to identify articles published in the English literature up until September 25, 2016.
Abstracts of retrieved studies were reviewed according to the following inclusion criterion: Peer-reviewed level 1–4 studies with description of clinical and radiographic outcomes after focal inlay resurfacing implantation in the femoral condyles or trochlea of the knee and a minimum mean follow-up of 2 years. Indications for the procedure included a chondral or osteochondral defect, spontaneous osteonecrosis, osteochondritis dissecans, or localized osteoarthritis of the condyles.
After screening, all articles that met the search criteria were read entirely and the reference lists were checked for missing relevant publications, with no additional studies being found. The methodological quality of all included studies was calculated with the Coleman Methodology Score [7] and the level of evidence determined with the method described by Wright et al. [38]. A perfect Coleman Score of 100 would represent a study design that largely avoids the influence of chance, various biases, and confounding factors.
Results
Case reports
Both patients were satisfied with their outcome and would have the operation again. Demographics as well as pre- and intraoperative data of both patients are displayed in Table 1. Neither patients underwent a secondary procedure or repeat hospitalization related to their operated knee during the 12-year follow-up. Clinical examination revealed no joint effusion, full range of motion, and stable ligaments. The patients rated the result as either excellent or good (Table 2). Both patients reported good results among all KOOS domains at both follow-ups. Although the scores for pain, symptoms, and ADL were decreased or somewhat similar at the long-term follow-up compared to the midterm follow-up, the sores for knee-related QoL improved from mid- to long-term follow-up (Table 2).
The radiographic evaluation demonstrated no signs of periprosthetic radiolucency, implant loosening, and subchondral cyst formation. There was no change in the osteoarthritic stage (Figs. 1, 2).
Literature search
According to the inclusion criteria of the review, 6 articles [3, 5, 8, 13, 20, 28] were included and analyzed (Tables 3, 4, 5, 6). Five studies were classified as level 4 and one as level 3. The average Coleman Methodology Score was 62 ± 8 (range 49–79) out of 100.
The six studies comprised 169 HemiCAP implantations in 169 patients (84 male, 85 female) (Table 3). The femoral condyles were involved in 130 cases with the majority of implantations performed on the medial femoral condyle. Trochlear implantations were performed in 39 cases. The follow-up ranged from 20 months to 7 years with a minimum average follow-up of 2.0 years. The mean age of the patients ranged between 44.7 and 53.7 years with an overall range from 38 to 78 years. In all studies, inclusion and exclusion criteria were defined which usually affected the parameters age, BMI, alignment, and ligament stability [3, 5, 8, 13, 20, 28]. In three of six studies, the majority of the patients underwent prior operative procedures for their cartilage defect. One study excluded patients with prior operative procedures for failed previous cartilage procedures [20], one study only included patients with a failed cartilage repair [8], whereas one study provided no information on prior procedures [5] (Table 4). Concomitant procedures regarding the femoral condyle were reported in one study with correction of the mechanical axis in 3 out of 19 patients [5]. Defects were classified as full-thickness chondral or osteochondral defects in all studies. A more detailed etiology was given in only one study with 63% of defects being related to early osteoarthritis, 5% to osteonecrosis, and 32% to a localized traumatic full-thickness defect [5]. The defect size was <4 cm2 in all studies [3, 5, 8, 13, 20, 28].
In a total of 68 patients, KOOS domains scores were gathered with values ranging from 62.7 to 87.6 (pain), 66.8 to 85.6 (symptoms), 71.2 to 91.3 (ADL), 31.7 to 71.9 (sports/recreation), and 37.0 to 73.3 (QoL) (Table 5). WOMAC Scores (49 patients) ranged from 86.2 to 90.8 (pain), 81.3 to 87.0 (stiffness), and 87.0 to 90.9 (function). Generally, the best results were obtained in the study of Bollars et al. [5] that presented no data on any other prior operative procedures, the worst in the study of Dhollander et al. [8] that included only revision cases and used the device as a salvage procedure for a failed cartilage repair. Other results of clinical scores and a satisfaction index are displayed in Tables 5 and 6.
In three out of five studies, no statistically significant change of the osteoarthritic grade according to the Kellgren and Lawrence classification was observed regarding the pre- and postoperative grade (Table 6). However, one study reported a statistically significant increase in a 24-month follow-up in 9 out of 18 included patients [8]. Furthermore, Laursen et al. found a progression of the OA grade after 2-year follow-up in 29 and 25% of medial and lateral compartments, respectively [20]. One study did not report any radiographic findings.
Re-operation rates varied from no revisions at all in the report of Dhollander et al. [8] to 23% in the study of Laursen et al. with a conversion to Total Knee Arthroplasty (TKA) at 7 years postoperatively [20]. In the other four studies, 9/92 (10%) patients were revised of whom three patients were converted to unicompartmental Arthroplasty (UKA), one patient underwent osteochondral plug transplantation, one underwent HTO, and the others with minor procedures such as arthroscopic debridement (Table 6).
Discussion
The rationale for this case report and literature review is the ongoing debate concerning localized prosthetic resurfacing in the treatment of articular cartilage defects. Mainly good results were found in the peer-reviewed literature [3, 5, 8, 13, 20, 28]; however, critical statements were made in a recent case report that questioned the procedure based on a failed outcome in a workers compensation patient who was revised from focal metallic resurfacing to an autologous cancellous bone graft covered with a type I/III collagen membrane [9] using a sandwich technique that to date has been described in the literature with 2-year follow-up in four knees [30].
A major concern when using focal treatment is osteoarthritic progression with concomitant symptomatic failure resulting in revision to unicompartmental or total knee arthroplasty. Data from the Australian Arthroplasty Registry report of 2015 presented a cumulative percent revision of partial resurfacing procedures undertaken for osteoarthritis of 5.6% at 1 year and 35.6% at 7 years in 214 recorded partial resurfacing procedures (all using ‘HemiCAP’ range of prostheses) [1]. This is in contrast to the findings of this review with a maximum revision rate of 23% in one study at 7 years postoperative with complete data without missing drop-outs due to national registry data [20] and considerably lower rates in the other included studies [3, 5, 8, 13, 20, 28].
This discrepancy might be explained by various factors. All articled included in this literature review had clear inclusion and exclusion criteria, which are comparable to large prospective randomized multicenter analyses when comparing different options for localized full-thickness articular cartilage defects such as autologous chondrocyte transplantation (ACT) and microfracture [32, 33]. The Australian registry reports that with 86.9% the most common reason for undertaking the partial resurfacing procedure was osteoarthritis and that of the 214 procedures, 161 (75.2%) have one cap implanted, 48 (22.4%) have two and five procedures (2.3%) have three caps implanted [1] and thus can be clearly regarded as out of the normal indication range for the device.
Data from the Food and Drug Administration (FDA) regarding Carticel implantation, the only FDA approved ACT product, reveal a surgical revision rate in 93% of the patients in a 7-year period, of which 48% involved subsequent cartilage procedures for the treatment of problems directly related to the graft [37]. In a retrospective cohort study, with comparable exclusion criteria as in the included studies of this literature review, following 413 patients that underwent ACT (first, second, and third generation), operated in a center that is specialized on cartilage repair, a total of 88 patients (21.3%) had undergone surgical revision at a follow-up of 4.4 ± 0.9 years [14]. Thus, in the light of these data for ACT treatment, the re-operation rates for the HemiCAP® implant of this review and of the Australian joint registry appear not concerning, but pointing out to acknowledge that with leaving the range of common indication criteria for the use of the implant, a higher failure rate can be expected.
A recently comparative study showed that a group of HemiCAP® patients compared with a matched group with various biological procedures (microfracture, osteochondral autograft transplantation, debridement, autologous chondrocyte transplantation, or osteochondral allograft), showed significantly better clinical success and required fewer subsequent procedures [28].
Conflicting statements were found in the literature for the use of the HemiCAP® might lead to osteoarthritic progression [3, 5, 8, 13, 20]. However, a statistically significant increase of degenerated changes according to the Kellgren and Lawrence classification in the treated knees was only found in the study of Dhollander et al. based on 9 out of 18 patients that were radiographically evaluated at 24 months [8]. The 29 and 25% OA grade deterioration after 2-year follow-up in medial and lateral compartments in the study of Laursen et al. remains unclear but explains to some degree the increased re-operation rate in comparison to the other included studies of this review.
Due to the concern that a metal implant may result in damage to the opposing articulating structures, several basic science studies using large animal models, finite element, or biomechanical analysis have been performed [2, 16, 22,23,24]. Two animal studies using a caprine or ovine model showed limited cartilage wear of opposing and surrounding joint cartilage at 1 year [16, 24]. The importance of slightly recessed implant positioning was highlighted in a further study in a sheep model [23] and biomechanical study using fresh frozen human cadaver specimens [2]. The long-term follow-up of our two cases showed encouraging results with no radiographic increase of the OA stage and suggests that the correct use of metal implants is not responsible for OA progression but rather a possible wrong indication or technical errors occurring during implantation.
The clinical results of the two cases as well as the results from the literature review demonstrate a meaningful improvement among all domains of the KOOS and WOMAC Score and other used modalities used for evaluation. The clinical scores are, apart from lower scores in the cohort of Dhollander et al., comparable to the vast majority of studies after microfracture or ACT [27]. Since many patients have had single or repeat biological procedures prior to focal prosthetic resurfacing, the device has the ability to serve as a treatment option thereby extending the range of focal procedures for middle-aged patients who frequently find themselves between biological solutions and conventional arthroplasty; particularly since unicompartmental knee arthroplasty has shown higher revision rates for patients with partial cartilage thickness loss compared to patients with full cartilage thickness loss in the medial compartment [21].
This report has limitations that need to be acknowledged. It is premature to draw general conclusions from two cases and a total number of 169 HemiCAP® implantations in 6 peer-reviewed studies. However, based on existing evidence, it can be postulated that the use of the HemiCAP® resurfacing prosthesis in an otherwise healthy knee joint environment does not increase the rate or speed of joint degeneration, even at the long-term follow-up. According to Coleman Score, the quality of the reviewed studies was acceptable and not inferior compared to a review in the evaluation of cartilage repair that also included higher level studies [18].
In summary, meaningful clinical improvements, radiological safety, and low revision rates can be expected when using the HemiCAP® resurfacing prosthesis in localized cartilage defects of the knee. Both surgeons and patients should recognize the importance of adhering to proper indications and place appropriate expectations on focal treatment with an acknowledgement of less predictable outcomes with increasing disease complexity such as revision cases, presence of osteoarthritic changes, and decreased meniscus function as already known for any kind of cartilage repair procedures. Studies with larger patient cohorts in registries and controlled trials are necessary.
References
(2015) Australian Orthopaedic Association National Joint Replacement Registry (ed)
Becher C, Huber R, Thermann H, Tibesku CO, von Skrbensky G (2009) Tibiofemoral contact mechanics with a femoral resurfacing prosthesis and a non-functional meniscus. Clin Biomech (Bristol, Avon) 24(8):648–654
Becher C, Kalbe C, Thermann H et al (2011) Minimum 5-year results of focal articular prosthetic resurfacing for the treatment of full-thickness articular cartilage defects in the knee. Arch Orthop Trauma Surg 131(8):1135–1143
Bekkers JE, de Windt TS, Raijmakers NJ, Dhert WJ, Saris DB (2009) Validation of the Knee Injury and Osteoarthritis Outcome Score (KOOS) for the treatment of focal cartilage lesions. Osteoarthr Cartil 17(11):1434–1439
Bollars P, Bosquet M, Vandekerckhove B, Hardeman F, Bellemans J (2012) Prosthetic inlay resurfacing for the treatment of focal, full thickness cartilage defects of the femoral condyle: a bridge between biologics and conventional arthroplasty. Knee Surg Sports Traumatol Arthrosc 20(9):1753–1759
Briggs DT, Sadr KN, Pulido PA, Bugbee WD (2015) The use of osteochondral allograft transplantation for primary treatment of cartilage lesions in the knee. Cartilage 6(4):203–207
Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD (2000) Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports 10(1):2–11
Dhollander AA, Almqvist KF, Moens K et al (2015) The use of a prosthetic inlay resurfacing as a salvage procedure for a failed cartilage repair. Knee Surg Sports Traumatol Arthrosc 23(8):2208–2212
Goebel L, Kohn D, Madry H (2016) Biological reconstruction of the osteochondral unit after failed focal resurfacing of a chondral defect in the knee. Am J Sports Med 44(11):2911–2916
Goyal D, Keyhani S, Goyal A, Lee EH, Hui JH, Vaziri AS (2014) Evidence-based status of osteochondral cylinder transfer techniques: a systematic review of level I and II studies. Arthroscopy 30(4):497–505
Hambly K, Griva K (2008) IKDC or KOOS? Which measures symptoms and disabilities most important to postoperative articular cartilage repair patients? Am J Sports Med 36(9):1695–1704
Hangody L, Fules P (2003) Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Jt Surg Am 85-A(Suppl 2):25–32
Hobbs H, Ketse-Matiwani N, Van der Merwe W, Posthumus M (2013) Focal full thickness articular cartilage lesions treated with an articular resurfacing prosthesis in the middle-aged. S Afr Orthop J 12(4):6
Jungmann PM, Salzmann GM, Schmal H, Pestka JM, Sudkamp NP, Niemeyer P (2012) Autologous chondrocyte implantation for treatment of cartilage defects of the knee: what predicts the need for reintervention? Am J Sports Med 40(1):58–67
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16(4):494–502
Kirker-Head CA, Van Sickle DC, Ek SW, McCool JC (2006) Safety of, and biological and functional response to, a novel metallic implant for the management of focal full-thickness cartilage defects: preliminary assessment in an animal model out to 1 year. J Orthop Res 24(5):1095–1108
Knutsen G, Drogset JO, Engebretsen L et al (2007) A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Jt Surg Am 89(10):2105–2112
Kon E, Verdonk P, Condello V et al (2009) Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am J Sports Med 37(Suppl 1):156S–166S
Kreuz PC, Erggelet C, Steinwachs MR et al (2006) Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy 22(11):1180–1186
Laursen JO, Lind M (2015) Treatment of full-thickness femoral cartilage lesions using condyle resurfacing prosthesis. Knee Surg Sports Traumatol Arthrosc 25(3):746–751
Maier MW, Kuhs F, Streit MR et al (2015) Unicompartmental knee arthroplasty in patients with full versus partial thickness cartilage loss (PTCL): equal in clinical outcome but with higher reoperation rate for patients with PTCL. Arch Orthop Trauma Surg 135(8):1169–1175
Manda K, Ryd L, Eriksson A (2011) Finite element simulations of a focal knee resurfacing implant applied to localized cartilage defects in a sheep model. J Biomech 44(5):794–801
Martinez-Carranza N, Berg HE, Hultenby K, Nurmi-Sandh H, Ryd L, Lagerstedt AS (2013) Focal knee resurfacing and effects of surgical precision on opposing cartilage. A pilot study on 12 sheep. Osteoarthr Cartil 21(5):739–745
Martinez-Carranza N, Ryd L, Hultenby K et al (2016) Treatment of full thickness focal cartilage lesions with a metallic resurfacing implant in a sheep animal model, 1 year evaluation. Osteoarthr Cartil 24(3):484–493
Mobasheri A, Kalamegam G, Musumeci G, Batt ME (2014) Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas 78(3):188–198
Niemeyer P, Feucht MJ, Fritz J, Albrecht D, Spahn G, Angele P (2016) Cartilage repair surgery for full-thickness defects of the knee in Germany: indications and epidemiological data from the German Cartilage Registry (KnorpelRegister DGOU). Arch Orthop Trauma Surg 136(7):891–897
Oussedik S, Tsitskaris K, Parker D (2015) Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: a systematic review. Arthroscopy 31(4):732–744
Pascual-Garrido C, Daley E, Verma NN, Cole BJ (2016) A Comparison of the outcomes for cartilage defects of the knee treated with biologic resurfacing versus focal metallic implants. Arthroscopy 33(2):364–373
Peterson L, Minas T, Brittberg M, Lindahl A (2003) Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am 85-A(Suppl 2):17–24
Petri M, Ettinger M, von Falck C, Hawi N, Jagodzinski M, Haasper C (2013) Reconstruction of osteochondral defects by combined bone grafting and a bilayer collagen membrane as a sandwich technique. Orthop Rev (Pavia) 5(4):e36
Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD (1998) Knee Injury and Osteoarthritis Outcome Score (KOOS)–development of a self-administered outcome measure. J Orthop Sports Phys Ther 28(2):88–96
Saris D, Price A, Widuchowski W et al (2014) Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med 42(6):1384–1394
Saris DB, Vanlauwe J, Victor J et al (2009) Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med 37(Suppl 1):10S–19S
Spahn G, Fritz J, Albrecht D, Hofmann GO, Niemeyer P (2016) Characteristics and associated factors of Klee cartilage lesions: preliminary baseline-data of more than 1000 patients from the German cartilage registry (KnorpelRegister DGOU). Arch Orthop Trauma Surg 136(6):805–810
Steadman JR, Rodkey WG, Rodrigo JJ (2001) Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res 391 Suppl:S362–S369
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 198:43–49
Wood JJ, Malek MA, Frassica FJ et al (2006) Autologous cultured chondrocytes: adverse events reported to the United States Food and Drug Administration. J Bone Jt Surg Am 88(3):503–507
Wright JG, Swiontkowski MF, Heckman JD (2003) Introducing levels of evidence to the journal. J Bone Joint Surg Am 85-A(1):1–3
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A fee for speaking and reimbursement for attending a symposium was received by the corresponding author from 2med GmbH, Hamburg, Germany (Distributor of Arthrosurface in Germany) in 2015. The authors declare no other conflict of interest.
Funding
There is no funding source.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The follow-up examinations were performed on a regular base of follow-up and not for study reasons.
Informed consent
Informed consent was obtained from both participants included in the report of the two cases.
Rights and permissions
About this article
Cite this article
Becher, C., Cantiller, E.B. Focal articular prosthetic resurfacing for the treatment of full-thickness articular cartilage defects in the knee: 12-year follow-up of two cases and review of the literature. Arch Orthop Trauma Surg 137, 1307–1317 (2017). https://doi.org/10.1007/s00402-017-2717-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-017-2717-8