Abstract
Background
Recently, patient-specific guides (PSGs) have been introduced, claiming a significant improvement in accuracy and reproducibility of component positioning in TKA. Despite intensive marketing by the manufacturers, this claim has not yet been confirmed in a controlled prospective trial.
Questions/purposes
We (1) compared three-planar component alignment and overall coronal mechanical alignment between PSG and conventional instrumentation and (2) logged the need for applying changes in the suggested position of the PSG.
Methods
In this randomized controlled trial, we enrolled 128 patients. In the PSG cohort, surgical navigation was used as an intraoperative control. When the suggested cut deviated more than 3° from target, the use of PSG was abandoned and marked as an outlier. When cranial-caudal position or size was adapted, the PSG was marked as modified. All patients underwent long-leg standing radiography and CT scan. Deviation of more than 3° from the target in any plane was defined as an outlier.
Results
The PSG and conventional cohorts showed similar numbers of outliers in overall coronal alignment (25% versus 28%; p = 0.69), femoral coronal alignment (7% versus 14%) (p = 0.24), and femoral axial alignment (23% versus 17%; p = 0.50). There were more outliers in tibial coronal (15% versus 3%; p = 0.03) and sagittal 21% versus 3%; p = 0.002) alignment in the PSG group than in the conventional group. PSGs were abandoned in 14 patients (22%) and modified in 18 (28%).
Conclusions
PSGs do not improve accuracy in TKA and, in our experience, were somewhat impractical in that the procedure needed to be either modified or abandoned with some frequency.
Level of Evidence
Level I, therapeutic study. See instructions for authors for a complete description of levels of evidence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Successful outcome of TKA is related to adequate patient selection, three-dimensional (3-D) alignment of the components, ligament tension, rehabilitation, and patient expectations [7, 9, 13, 21–23, 26, 38, 39]. Inferior clinical results have been related to postoperative malalignment. Coronal plane outliers have been shown to have inferior functional outcomes, earlier loosening, and polyethylene wear [3, 22, 32, 34, 42]. Internal rotation of the femoral component has been associated with pain, stiffness, and instability [1, 2, 8, 24, 31, 44]. In contrast, excessive external rotation of the femoral component leads to symptomatic flexion instability [29], increased shear forces on the patella [25], and medial compartment overload in flexion [15]. Excessive tibial slope in the sagittal plane leads to post impingement and flexion instability [6].
Intra- or extramedullary alignment rods guide conventional instrumentation in TKA. Some surgeons use tensioned gaps in addition to the measured resection to position the implants [11, 12]. Postoperative malalignment is reported in a significant number of patients when these conventional instruments are used [4, 14, 26, 35, 36].
Computer-assisted surgery (CAS) was developed in an attempt to improve surgical accuracy and avoid outliers. Despite the reported improvement in coronal alignment, CAS failed to improve alignment in the horizontal plane, mainly because of the difficulty of identifying the correct landmarks intraoperatively [4, 14, 40]. More importantly, the system failed to become generally adopted as a standard orthopaedic instrument in surgical theaters because of issues with cost, time investment, and pin-related complications [30].
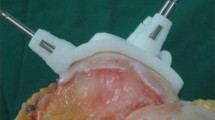
In the past decade, patient-specific guides (PSGs) were developed, based on 3-D models of the patient’s anatomy, by virtue of powerful 3-D software systems and rapid prototyping. This technology gained widespread acceptance in dental surgery [18, 19] and was gradually introduced in TKA [16, 17, 20, 27, 37, 43]. Using a preoperative CT or MRI scan, disposable cutting blocks are produced. These blocks have a single and unique fit on the distal femur and proximal tibia. They direct the femoral and tibial bone cuts, following a virtual preoperative plan. This plan is based on a Cartesian coordinate system, using relevant landmarks [41].
In this prospective randomized controlled trial, we (1) compared three-planar component alignment and overall coronal mechanical alignment between PSG and conventional instrumentation and (2) logged the need for applying changes in the suggested position of the PSG.
Patients and Methods
Between February 2011 and May 2012, we included 128 patients with end-stage knee osteoarthritis scheduled for TKA in the study. Patients with rheumatoid arthritis, previous osteotomy, fractures, retained hardware in the limb, or claustrophobia were excluded. Ethics committee approval was obtained and all patients gave written informed consent.
Sample Size and Randomization
The sample size was calculated based on a power of 0.80 (1 − β) with α = 0.05 to detect a difference in deviation from a target of 1° (required sample size n = 92) and in number of outliers of 20% (required sample size n = 118). Compensating for possible dropouts, a sample size of 128 was chosen for the study.
Patients were randomized into two cohorts. The selection and randomization protocol was as follows. Patients with end-stage osteoarthritis were selected for TKA in the outpatient clinic. An appointment for a preoperative information session on the surgery and hospital stay was scheduled at the time of this outpatient visit. During the preoperative information session, the patient was informed by the coordinating nurse about the proposed study. If the patient agreed to participate and no exclusion criteria were detected, informed consent was obtained and the patient was included in the study. At that time, the nurse opened the sealed randomization envelope. The content of the envelopes was either PSG or conventional. The date of surgery was planned after randomization, allowing sufficient time to plan the preoperative scan and produce the PSGs in the PSG cohort. The PSG cohort (n = 64) was operated on using PSGs and the conventional cohort (n = 64) using conventional instrumentation. In the PSG cohort, PSG designs from four different TKA suppliers (Subgroups PSG 1–4, described below) were used. This subgrouping was not randomized, for logistic reasons; the use of the different systems was consequently consecutive. There were three patients who did not receive the allocated PSG intervention. In the first case, the PSG did not arrive on the day of surgery and the patient refused postponement; in the second case, the PSG was made for the wrong patient; and in the third case, the PSG was made for the contralateral side. These three patients were excluded from the study. The operation was carried out by the senior authors (JV, JB) or fellowship-trained staff members (HV, NA). A flowchart of the study design is shown (Fig. 1).
No statistical differences were found in demographic parameters between the two cohorts. The male:female ratio was identical (21:43). The mean preoperative coronal alignment was 2.7° varus (SD, 7.0°; range, 17° varus to 18° valgus) in the PSG cohort and 3.5° varus (SD, 6.5°; range 13° varus to 15° valgus) in the conventional cohort. The mean preoperative condylar twist angle was respectively −1.7° (SD, 2.1°; range, −4.6° to 3.2°) and −1.9° (SD, 1.7°; range, −5.7° to 2.0°) (Table 1).
Operative Technique
All patients in both cohorts received a posterior-stabilized, fixed-bearing implant. The operation was performed through an anteromedial parapatellar approach, without everting the patella. Cement fixation was used in all patients. The patella was resurfaced in all patients.
In the PSG cohort, PSGs from four different implant suppliers (PSG Subgroups 1–4) were used to align the femoral and tibial implants. The surgical plan was built by the manufacturer and controlled by the senior surgeon. The minimum time between imaging and delivery of the PSG was 4 weeks, except for PSG Subgroup 2, where it was 8 weeks.
In PSG Subgroup 1, Signature® (Biomet Inc, Warsaw, IN, USA) was used. Each patient underwent preoperative MRI of the lower limb, following the prescribed protocol. The images were processed by Materialise (Leuven, Belgium). The following alignment targets were set: a neutral mechanical axis in the coronal plane for the femur and tibia, 3° of flexion in the sagittal plane for the femoral and tibial components, and a femoral component rotation parallel to the surgical epicondylar axis. The implant used was the Vanguard® Complete Knee System (Biomet Inc).
In PSG Subgroup 2, TruMatch® (DePuy Inc, Warsaw, IN, USA) was used. Each patient underwent a CT scan of the leg from hip to ankle, as defined by the manufacturer’s protocol. The following alignment targets were set: a neutral mechanical axis in the coronal plane for the femur and tibia, 3° of flexion in the sagittal plane for the femoral and tibial components, and a femoral component rotation parallel to the surgical epicondylar axis. The implant used was the Sigma® Fixed Bearing Knee System (DePuy Inc).
In PSG Subgroup 3, Visionaire® (Smith & Nephew Inc, Memphis, TN, USA) was used. All patients underwent AP long-leg standing radiography and MRI of the knee, following the protocol prescribed by the manufacturer. The following alignment targets were set: a neutral mechanical axis in the coronal plane for the femur and tibia, 4° of flexion in the sagittal plane for the femoral component and 3° of flexion for the tibial component, and a femoral component rotation parallel to the surgical epicondylar axis, taking the asymmetric nature of the posterior condyles of the implant into account. The implant used was the Genesis® II Total Knee System (Smith & Nephew Inc).
In PSG Subgroup 4, Patient-Specific Instruments® (PSI) (Zimmer Inc, Warsaw, IN, USA) was used. Each patient underwent MRI of the lower limb, following the prescribed protocol. The images were processed by Materialise (Leuven, Belgium). The following alignment targets were set: a neutral mechanical axis in the coronal plane for the femur and tibia, 4° of flexion in the sagittal plane for the femoral component and 7° of flexion for the tibial component, and a femoral component rotation parallel to the surgical epicondylar axis. The implant used was the NexGen® Complete Knee Solution (Zimmer Inc).
In the conventional cohort, standard instrumentation was used. The femoral component was aligned using an intramedullary rod and standard block instrumentation, and the tibial component was aligned using an extramedullary guide and standard block instrumentation. The target was a neutral mechanical axis in the coronal plane for the femur and tibia, 4° of flexion in the sagittal plane for the femoral component and 3° of flexion for the tibial component, and a femoral component rotation parallel to the surgical epicondylar axis, taking the asymmetric nature of the posterior condyles of the implant into account. The implant used was the Genesis® II Total Knee System (Smith & Nephew Inc). In both cohorts, the tibial component was axially aligned to the femoral component in the extended knee.
Surgeon Experience and Pretraining
All participating surgeons were fellowship trained and performed more than 200 TKAs per year. They had a long-standing experience with the use of surgical navigation and the standard instruments used in the control group. Before starting the study, they had performed more than 10 operations with PSGs and at least one procedure within each PSG subgroup.
Abandoning or Modifying the PSG Procedure
In the PSG cohort, the suggested cuts were measured with the surgical navigation system (BrainLAB Knee 2.1 Universal; BrainLAB AG, Munich, Germany), after the block was carefully positioned and pinned in place. If the measured value exceeded the target alignment in any plane and any direction by more than 3°, the use of the PSG was abandoned for the affected bone and the cuts were made following the surgical navigation system. The value before correction, as measured by the navigation system, was recorded and included in the overall analysis, and the patient was marked as an outlier for that specific alignment parameter on the femur or the tibia. This abandoning of the PSG was done independently for femur and tibia.
In case the suggested resection planes remained within 3° of target alignment as measured with the surgical navigation system but the cranial-caudal level of resection was judged incorrect on the basis of insufficient gap space as measured with a spacer block, the position of the distal cut was adapted and marked as modified for the affected bone.
Outcome Measurement
In both cohorts, bipedal standing AP full-leg radiographs were obtained with the patellae facing forward and the knee in full extension [5]. In addition, a lateral radiograph on long film was taken. All patients also underwent a postoperative CT scan, using a scatter reduction protocol. The slice thickness was 2 mm.
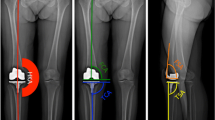
Angular measurements were performed on the hospital picture archiving and communication system (Agfa Gevaert, Mortsel, Belgium). The full-leg standing radiograph was used to measure the mechanical limb alignment and the coronal alignment of the femoral and tibial components. Mechanical limb alignment was defined as the angle formed between the lines connecting the center of the hip to the center of the knee and the center of the knee to the center of the ankle (hip-knee-ankle [HKA] angle). The angle between the femoral mechanical axis and the tangent to the most distal part of the medial and lateral condyles of the femoral component determined its coronal position. The angle between the tibial mechanical axis and the inferior surface of the tibial component determined its coronal position. Sagittal alignment of the femoral and tibial components was measured on the lateral radiograph. The angle between the anterior femoral cortex and the distal femoral cut line determined the femoral component sagittal alignment. The angle between the tangent to the anterior tibial cortex and the inferior surface of the tibial component determined the tibial component sagittal alignment. The rotation of the femoral component was measured on the axial views of the CT scan. The angle between the surgical transepicondylar axis and the tangent to the posterior condyles defined the femoral component position in the axial plane [39]. On the preoperative MRI or CT scan, the condylar twist angle was defined as the angle formed between the surgical transepicondylar axis and the tangent to the posterior condyles. Radiographs and CT scans were measured by an investigator who was blinded to the allocation of the patient.
Three-planar alignment was the primary end point. Deviation of more than 3° from the target in any plane, as measured with surgical navigation or postoperative radiographic imaging, was defined as an outlier.
Statistical Analysis
We used a nonparametric approach [33] to compare the coronal, sagittal, and axial alignment measurements between the two cohorts and among the four PSG subgroups. This was done for the actual measurement and for the deviation of the actual measurement from the target. Fisher exact tests (and an extension thereof for multiple groups) were used to make comparisons for proportions. We considered p values smaller than 0.05 significant. All analyses were performed using SAS® Version 9.2 software (SAS Institute Inc, Cary, NC, USA).
Results
Deviation from target alignment in the three planes was not significantly different between the two cohorts (Table 2). No significant differences were noted in deviation from target alignment among PSG Subgroups 1 to 4, except for sagittal alignment of the femoral component, which was significantly better for PSG Subgroup 3 (p = 0.02) (Table 3). Both cohorts showed a similar number of outliers in coronal long-leg alignment, in femoral coronal alignment, and in the axial plane (Table 4). The PSG cohort had more outliers in coronal tibial alignment (p = 0.03) and sagittal tibial alignment (p = 0.002) than the conventional cohort. When PSG Subgroups 1 to 4 were compared with each other, no significant differences were noted in the number of outliers, except for PSG Subgroup 3, which had more overall coronal alignment outliers (p = 0.04) but fewer femoral component sagittal alignment outliers (p = 0.001) (Table 5). The percentage of knees situated within 3° for all alignment parameters was 51% in the PSG cohort versus 47% in the conventional (p = 0.72). For PSG Subgroups 1, 2, 3, and 4, this was 59%, 38%, 45%, and 57%, respectively (p = 0.70).
The PSG procedure was abandoned in 14 patients (22%) and modified in 18 patients (28%). The values recorded when the PSG was abandoned ranged from −4.5° to 5° on the femur and −7° to 5° on the tibia in the coronal plane, −7° to 12° on the femur and −6° to 10° on the tibia in the sagittal plane, and 3.5° to 7° on the femur in the axial plane (negative values mean varus in the coronal plane, extension in the sagittal plane, and internal rotation in the axial plane) (Table 6). A change in sizing was the most common reason for modifying the use of the PSG. In 13 patients, the implant size as determined by the PSG preoperative planning was incorrect. This occurred three times for the femur (5%) and 10 times for the tibia (16%). In nine patients in this cohort, the level of the cut was inappropriate and required intraoperative correction: five times at the level of the femur (8%) and four times at the level of the tibia (6%). Administrative errors not leading to abandoning or modifying the PSG were noted in the PSG cohort. In one patient, the name of the patient was incorrectly labeled on the guide. In another patient, the cutting guide was delivered for a femoral component size 7, whereas size 6 had been ordered.
Discussion
PSGs are a new tool in the orthopedic armamentarium. They have the potential of relaying preoperative 3-D imaging into the surgical field in a reproducible way. The combination of detailed preoperative virtual planning with controlled execution of the bone cuts holds a promise of more accurate positioning of the implants. We investigated this alleged improvement in 3-D, using intraoperative surgical navigation and postoperative imaging as measurement tools and conventional instrumentation as a control cohort. We (1) compared three-planar component alignment and overall coronal mechanical alignment between PSG and conventional instrumentation and (2) logged the need for applying changes in the suggested position of the PSG.
This study had a number of limitations. First, we acknowledge the potential influence of the learning curve. Despite the fact that all participating surgeons had prior experience with PSGs, the introduction of a new implant system is a potential bias. We limited the influence of this bias in doing a pilot case for every subgroup. Also, the manufacturer provided technical support for every intervention and helped with the logistic workflow in the hospital. We did not encounter technical problems related to the change in implant system between the PSG subgroups. Second, the conventional cohort lacked intraoperative surgical navigation control. However, conventional instrumentation is the standard of care and we decided to measure component position in this cohort with postoperative radiographs and CT only. We believed the lack of clinical proof of reliability and accuracy of PSGs justified the use of an intraoperative control to avoid major errors in the PSG cohort. The range of values corrected in the outliers by virtue of the surgical navigation, especially in the sagittal plane, proves the value of this approach. As the outlier values overruled by the surgical navigation were included as the original uncorrected value in the statistical analysis, bias between the two cohorts was avoided. Radiographic analysis was identical in the two cohorts.
One of the most fundamental criticisms of PSGs is the pure geometric nature of the approach, not taking soft tissue status into account. This study did not specifically focus on this problem, but we acknowledge this drawback. The frequent changes made in the level of the distal femoral and proximal tibial cut and in component size do reflect the difficulty of adequately assessing resection levels and flexion-extension gaps without clinical information on the patient’s ligament status.
In our prospective randomized controlled trial, the use of PSGs did not reduce the number of outliers in any plane in space. Few studies assessing the accuracy of PSGs are available in the literature [10, 26, 27]. To our knowledge, no prospective randomized studies assessing 3-D alignment have been published so far. Two retrospective studies looked at coronal plane alignment in large cohorts of patients [26, 27]. Nunley et al. [27] compared coronal alignment after TKA performed with PSGs to conventional instrumentation in a retrospective nonrandomized study using a coronal nonweightbearing CT scanogram. They found 16% outliers in HKA angle in the conventional group versus 18% in the PSG subgroup that targeted neutral mechanical alignment and 44% in the PSG subgroup that targeted kinematic alignment. They concluded that PSGs did not improve coronal alignment. These reported figures are in the same order of magnitude as the number of outliers we found in the PSG cohort (24.6%). In a retrospective review, Ng et al. [26] used long-leg films to measure coronal alignment after TKA performed with PSGs. The percentage of outliers in overall leg alignment in the PSG group was 9% versus 22% with manual instrumentation. However, coronal component angles for the tibia and femur separately showed similar numbers of outliers for PSGs and manual instrumentation (tibia: 10% versus 7%, respectively; femur: 22% versus 18%, respectively). Conteduca et al. [10] assessed the intraoperative position of PSGs in 12 patients with surgical navigation and concluded that PSG-guided alignment was not reliable. They recommended that an accurate control of the alignment should be performed before making the cuts, for any step of the procedure.
In addition, the range of error values that needed to be corrected intraoperatively showed some unacceptable outliers. This is particularly disturbing given the high frequency by which these errors occurred: the PSG procedure had to be abandoned in more than one of five patients.
One would hope that an experienced surgeon would recognize and correct major errors of more than 5° deviation from the target, as was the case in some of the PSGs, but in the hands of less experienced surgeons, this could cause malalignment necessitating early revision. Especially the sagittal plane is a cause for concern, with the widest range in outlier values (−8° to 12° on the femur and −6° to 7° on the tibia). This can be explained by the nature of the technology. Capturing a 3-D irregular body is less reliable as the available surface area decreases. It is particularly difficult to obtain a unique fit on the proximal tibia and distal femur, within the boundaries of a standard surgical exposure. A small toggle in the sagittal plane irrevocably leads to sagittal plane outliers. In our experience, the flexion-extension position of the PSG was the most difficult to locate unequivocally, with all four systems used.
Manufacturers of knee implants often claim a reduction of operative time as an advantage of PSGs. We did not include operative times in our analysis as the use of surgical navigation as a control measure slowed down the procedure. However, previous work by Nunley et al. [28] showed only a marginal improvement in tourniquet time (56 versus 61 minutes) for the PSG group. Taking into account the time needed for preoperative planning and confirming the final plan, this claim should be seriously challenged. In addition, a crucial part of the operation, the planning of implant positioning, is outsourced. We believe this outsourcing is a potential danger. To the surgeon, it is unclear who is dealing with data processing and positioning of the implant. Most steps in the planning process require surgical experience, and it can be questioned whether the technicians involved in the planning are sufficiently skilled. Greater transparency and quality control from the manufacturer side are needed. We had concerns about the logistical elements of PSG fabrication; incorrect patient and size labeling occurred with several PSGs in our series.
The strengths of the study included its randomized design and the inclusion of four different PSG systems. The use of both surgical navigation and 3-D radiographic analysis allowed for reliable measurement of the PSG position both intra- and postoperatively. Using these approaches, we found that, although PSGs offer a very interesting opportunity to transfer 3-D imaging data into the surgical field, the systems did not improve the accuracy of TKA. The magnitude and frequency of erroneous cuts resulting from the use of PSGs do not currently support their use in clinical practice. In addition, conceptual and logistical aspects of outsourcing of the surgical procedure need to be addressed.
References
Akagi M, Matsusue Y, Mata T, Asada Y, Horiguchi M, Iida H, Nakamura T. Effect of rotational alignment on patellar tracking in total knee arthroplasty. Clin Orthop Relat Res. 1999;366:155–163.
Anouchi YS, Whiteside LA, Kaiser AD, Milliano MT. The effects of axial rotational alignment of the femoral component on knee stability and patellar tracking in total knee arthroplasty demonstrated on autopsy specimens. Clin Orthop Relat Res. 1993;287:170–177.
Bargren JH, Blaha JD, Freeman MA. Alignment in total knee arthroplasty: correlated biomechanical and clinical observations. Clin Orthop Relat Res. 1983;173:178–183.
Bauwens K, Matthes G, Wich M, Gebhard F, Hanson B, Ekkernkamp A, Stengel D. Navigated total knee replacement: a meta-analysis. J Bone Joint Surg Am. 2007;89:261–269.
Bellemans J, Colyn W, Vandenneucker H, Victor J. The Chitranjan Ranawat Award. Is neutral mechanical alignment normal for all patients? The concept of constitutional varus. Clin Orthop Relat Res. 2012;470:45–53.
Bellemans J, Robijns F, Duerinckx J, Banks S, Vandenneucker H. The influence of tibial slope on maximal flexion after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2005;13:193–196.
Berend ME, Ritter MA, Meding JB, Faris PM, Keating EM, Redelman R, Faris GW, Davis KE. Tibial component failure mechanisms in total knee arthroplasty. Clin Orthop Relat Res. 2004;428:26–34.
Berger RA, Crossett LS, Jacobs JJ, Rubash HE. Malrotation causing patellofemoral complications after total knee arthroplasty. Clin Orthop Relat Res. 1998;356:144–153.
Choong PF, Dowsey MM, Stoney JD. Does accurate anatomical alignment result in better function and quality of life? Comparing conventional and computer-assisted total knee arthroplasty. J Arthroplasty. 2009;24:560–569.
Conteduca F, Iorio R, Mazza D, Caperna L, Bolle G, Argento G, Ferretti A. Evaluation of the accuracy of a patient-specific instrumentation by navigation. Knee Surg Sports Traumatol Arthrosc. 2012 June 27 [Epub ahead of print].
Dennis DA. Measured resection: an outdated technique in total knee arthroplasty. Orthopedics. 2008;31:940, 943–944.
Dennis DA, Komistek RD, Kim RH, Sharma A. Gap balancing versus measured resection technique for total knee arthroplasty. Clin Orthop Relat Res. 2010;468:102–107.
Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24:39–43.
Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152–159.
Hanada H, Whiteside LA, Steiger J, Dyer P, Naito M. Bone landmarks are more reliable than tensioned gaps in TKA component alignment. Clin Orthop Relat Res. 2007;462:137–142.
Harrysson OL, Hosni YA, Nayfeh JF. Custom-designed orthopedic implants evaluated using finite element analysis of patient-specific computed tomography data: femoral-component case study. BMC Musculoskelet Disord. 2007;8:91.
Klatt BA, Goyal N, Austin MS, Hozack WJ. Custom-fit total knee arthroplasty (OtisKnee) results in malalignment. J Arthroplasty. 2008;23:26–29.
Lal K, White GS, Morea DN, Wright RF. Use of stereolithographic templates for surgical and prosthodontic implant planning and placement. Part I. The concept. J Prosthodontics. 2006;15:51–58.
Lal K, White GS, Morea DN, Wright RF. Use of stereolithographic templates for surgical and prosthodontic implant planning and placement. Part II. A clinical report. J Prosthodontics. 2006;15:117–122.
Lombardi AV Jr, Berend KR, Adams JB. Patient-specific approach in total knee arthroplasty. Orthopedics. 2008;31:927–930.
Longstaff LM, Sloan K, Stamp N, Scaddan M, Beaver R. Good alignment after total knee arthroplasty leads to faster rehabilitation and better function. J Arthroplasty. 2009;24:570–578.
Lotke PA, Ecker ML. Influence of positioning of prosthesis in total knee replacement. J Bone Joint Surg Am. 1977;59:77–79.
Mahoney OM, Kinsey TL. 5- to 9-year survivorship of single-radius, posterior-stabilized TKA. Clin Orthop Relat Res. 2008;466:436–442.
Matsuda S, Miura H, Nagamine R, Urabe K, Hirata G, Iwamoto Y. Effect of femoral and tibial component position on patellar tracking following total knee arthroplasty: 10-year follow-up of Miller-Galante I knees. Am J Knee Surg. 2001;14:152–156.
Miller MC, Berger RA, Petrella AJ, Karmas A, Rubash HE. Optimizing femoral component rotation in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:38–45.
Ng VY, DeClaire JH, Berend KR, Gulick BC, Lombardi AV Jr. Improved accuracy of alignment with patient-specific positioning guides compared with manual instrumentation in TKA. Clin Orthop Relat Res. 2012;470:99–107.
Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470:895–902.
Nunley RM, Ellison BS, Zhu J, Ruh EL, Williams BM, Foreman K, Ford AD, Barrack RL. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470:889–894.
Olcott CW, Scott RD. The Ranawat Award. Femoral component rotation during total knee arthroplasty. Clin Orthop Relat Res. 1999;367:39–42.
Quack VM, Kathrein S, Rath B, Tingart M, Luring C. Computer-assisted navigation in total knee arthroplasty: a review of literature. Biomed Tech (Berl). 2012;57:269–275.
Rhoads DD, Noble PC, Reuben JD, Mahoney OM, Tullos HS. The effect of femoral component position on patellar tracking after total knee arthroplasty. Clin Orthop Relat Res. 1990;260:43–51.
Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement: its effect on survival. Clin Orthop Relat Res. 1994;299:153–156.
Shah DA, Madden LV. Nonparametric analysis of ordinal data in designed factorial experiments. Phytopathology. 2004;94:33–43.
Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13.
Song EK, Seon JK, Park SJ, Jung WB, Park HW, Lee GW. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19:1069–1076.
Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471:118–126.
Spencer BA, Mont MA, McGrath MS, Boyd B, Mitrick MF. Initial experience with custom-fit total knee replacement: intra-operative events and long-leg coronal alignment. Int Orthop. 2009;33:1571–1575.
Tew M, Waugh W. Tibiofemoral alignment and the results of knee replacement. J Bone Joint Surg Br. 1985;67:551–556.
Victor J. Rotational alignment of the distal femur: a literature review. Orthop Traumatol Surg Res. 2009;95:365–372.
Victor J, Hoste D. Image-based computer-assisted total knee arthroplasty leads to lower variability in coronal alignment. Clin Orthop Relat Res. 2004;428:131–139.
Victor J, Van Doninck D, Labey L, Innocenti B, Parizel PM, Bellemans J. How precise can bony landmarks be determined on a CT scan of the knee? Knee. 2009;16:358–365.
Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31–43.
White D, Chelule KL, Seedhom BB. Accuracy of MRI vs CT imaging with particular reference to patient specific templates for total knee replacement surgery. Int J Met Robot. 2008;4:224–231.
Yoshii I, Whiteside LA, White SE, Milliano MT. Influence of prosthetic joint line position on knee kinematics and patellar position. J Arthroplasty. 1991;6:169–177.
Acknowledgments
The authors thank BrainLAB AG (Munchen, Germany) for providing a free license for the generic Knee 2.1 Universal software used for surgical navigation in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at AZ St-Lucas (Brugge, Belgium) and UZ Leuven (Leuven, Belgium).
About this article
Cite this article
Victor, J., Dujardin, J., Vandenneucker, H. et al. Patient-specific Guides Do Not Improve Accuracy in Total Knee Arthroplasty: A Prospective Randomized Controlled Trial. Clin Orthop Relat Res 472, 263–271 (2014). https://doi.org/10.1007/s11999-013-2997-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-013-2997-4