Abstract
While analytical measurements provide the quantitative estimation of the total amount of metals present in a sample, they do not reflect the truly bioavailable fraction of metal which reflects the adverse biological effect. Hence the development of monitoring tools for detecting bioavailable toxic metals has become a priority in environmental monitoring activities. An optical whole-cell biosensor was constructed using the microalga Scenedesmus subspicatus MM1 immobilizing in inorganic silica hydrogels using the sol-gel technique to detect bioavailable Cadmium (Cd2+), Copper (Cu2+) and Zinc (Zn+) in freshwater. Conditions for optimum biosensor performance have been established regarding effective pH range, cell density, exposure time, and storage stability. The optimum response for the biosensor was dependent on the pH of the matrix, cell concentration and exposure time were derived. The biosensor was operational for four weeks. The limit of detection for the algal biosensor was determined as 9.0 × 10−1, 9.1 × 10−1, and 8.8 × 10−1 mg/L for Cd, Cu and Zn, respectively. Whole-cell cell biosensor will be highly useful since it comprises a single microalgal species able to detect the bioavailable content of Cd2+, Cu2+, and Zn2+ in freshwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
It is well known that toxic metals affect the physiological processes of microalgae. Inhibition of algal photosynthesis is an appealing indicator that reflects the toxic effect of these metals (Chen et al. 2016). Since microalgae are primary producers located at the base of the food chain in the ecosystem, any interference with their activities will potentially affect the food chain (Prata et al. 2019) Therefore, microalgae are often considered as the early indicators of environmental contamination. One of the main advantages of using microalgae in toxicity assays is their adaptability to diverse environments because they are less vulnerable to physicochemical changes than other bioreceptors (Antonacci and Scognamiglio 2020). Owing to their high sensitivity microalgae are often considered in toxicity assessment of the various contaminants in the environment.
Several bioassay systems have been developed for the detection of various toxicants, based on inhibition of microalgal growth which takes several days to respond as well as needs large volumes of cultures. These reasons limit the use of bioassays for rapid detection of toxicity. In order to reduce the assay time and also to keep the apparatus as simple as possible the photosynthetic activity of the algae as estimated by optical or amperometric means has been widely used in constructing the algal biosensors (Turemis et al. 2017). Inhibition of the photosynthetic activity of microalgae is measured either based on chlorophyll a fluorescence contained in the chloroplast (optical sensors) or photosynthetic oxygen evolution (electrochemical sensors) (Tsopela et al. 2016).
One major difficulty in the application of biosensors in environmental monitoring is the broad number and the class range of the environmental pollutants. Development of these non-selective biosensor systems with the ability to detect broad range of pollutants is an emerging area of research. In this regard, algal biosensors have been developed to detect herbicides (Scognamiglio et al. 2019), chemical warfare agents (Antonacci et al. 2018), volatile organic compounds (Podola et al. 2005) etc. There are several algae based biosensors reported in assessing the heavy metal toxicity in the environment (Khishamuddin et al. 2018; Roxby et al. 2020), and whole-cell algal biosensors developed for rapid detection of metal toxicity at low concentrations in the environment are gaining popular (Belaïdi et al. 2019).
Immobilization of microalgae has been proven to be useful in field toxicity assessment. Light transmission is the main limitation if the cells are immobilized in a matrix. Gel entrapment is the most widely used immobilization technique among the techniques used for the algal immobilization (Eroglu et al. 2015; Moreno-Garrido 2013). Being an inert material, immobilization of algae in inorganic silica films has become an emerging area, which helps to overcome the problems such as disruption and dissolution of matrices (alginate), toxicity of the by products (organic sol–gels) (Perullini et al. 2014) associated with other techniques. Hence, the aim of this study was to develop a novel whole cell biosensor using chlorophyll a fluorescence of a single species of microalga, Scenedesmus subspicatus MM1 immobilized in an inorganic silica matrix, in detecting bioavailability of multi metals in freshwater.
Materials and Methods
Stock solutions of Cd, Cu and Zn were prepared by dissolving nitrate salts of Cd, Cu and Zn (Sigma, Analar grade) in Milli Q water. Concentrated metal stocks were diluted to prepare working solutions as required and were sterilized by filtration (0.45 μm). Analytical verification of working metal solution was carried out using Inductively Coupled Plasma Mass Spectroscopy (Agilent 7500 Series ICP-MS). In order to avoid contamination of any metal, all glassware were acid-washed and oven-dried at 105°C before use.
Bold’s basal medium was used for maintenance of algae. The Test Medium 1 (TM1) (Peterson et al. 2005), was used in the current study for the biosensor development experiments. The TM1 is a modified version of a freshwater inhibition test developed by the International Standards Organization (ISO 1989).
Axenic culture of S. subspicatus MM1 previously isolated (Megharaj et al. 2000), identified (Rathnayake 2010) and maintained at the algal laboratory of the University of South Australia, was grown in the algal growth medium for inoculum preparation. The cultures were incubated at 25 ± 2°C in a growth chamber under continuous illumination (200 µE m−2 s−1 PPFD). After 96 h of incubation, the cells were centrifuged at 4000×g for 20 min, and the supernatant was discarded. Resuspended pellet in the same medium was used as the inocula of different cell densities as and when needed.
Optimum pH range of the microalgal culture was determined by inoculating the S. subspicatus MM1 culture into algal growth medium (TM1) with 1.0 pH increments ranging between 3.0 and 10.0 and incubated at 25 ± 2°C in an algal inubation room under continuous illumination (200 µE m−2 s−1 PPFD) for 96 h. Growth of the algae was measured in terms of chlorophyll a fluorescence at 680/440 ± 20 (excitation/ emission) nm using the Biotek® SynergyHT microplate reader equipped with KC4 software.
Optimization of the S. subspicatus MM1 cell exposure time for the selected heavy metals was also investigated. Microalgal culture was grown in the TM1 (Peterson et al. 2005) culture medium with different metal concentrations (0–10 mg/L) in Iwaki 96 well sterile polystyrene microplates with growth (alga without metal) and abiotic (metal only) controls in the same plate and incubated at 25°C. Chlorophyll a fluorescence was measured at 680/440 ± 20 (excitation/ emission) nm at different time intervals until 96 h of incubation.
Different microalgal inocula sizes corresponding to relative fluorescence 800; 1000; 1500; 3000 (Approx) at 680/440 ± 20 nm (excitation/ emission) were used in solution, amended with different metal concentrations (0–10 mg/L) in Iwaki 96 well sterile polystyrene microplates in quadruplicates and incubated at 25°C in determining the optimum microalgal cell concentration. Growth control and abiotic controls were also prepared. Chlorophyll a fluorescence was measured at 680/440 ± 20 (excitation/ emission) nm at different time intervals until 96 h of incubation.
The method of Perullini et al. (2007) was modified in order to prepare the immobilization matrix (Rathnayake et al. 2021). Different volumes of the precursor solutions [Sodium silicate (Riedel-de Haën) and commercial colloidal silica (LODOX HS40, Aldrich)] were used to obtain different ratios of the sodium silicate, and colloidal silica and the sol–gels were prepared by adding 10 µL of the algal cell suspension, with 10% (wt/v) glycerol (fluorescence 800 approx. at 440/680 nm) into microplate wells (Iwaki® 96 well polystyrene) at pH 7.0 and kept undisturbed in order to form the sol–gel, and time taken was also noted. Experiments were conducted in quadruplicates in each occasion.
Sol–gel matrices were prepared by entrapping S. subspicatus MM1 cells using different precursor combinations (4/1; 3/1; 2/1; & 1/1 of SiNa/LUDOX) according to the procedure described above (Fig. 1). Prepared microplates were stored at 4°C and − 20°C, and checked for the chlorophyll a fluorescence over 8 weeks at weekly intervals. The change in the fluorescence was monitored over the duration of the experiment. Change in the fluorescence was monitored. S. subspicatus MM1 in suspension at 4°C was also tested for the storage stability upon long term storage. Change of the algal fluorescence was checked weekly up to 8 weeks. Quadruplicates samples were used in all the experiments.
Percentage survival of microalgae based on fluorescence was calculated as a percentage in relation to the untreated control for each tested concentration of each metal ion. All the values are means of n = 4 with ± SD. A linear model was used in fitting the data obtained based on algal biosensor response to the three metals tested using linear regression. EC25 values correspond to Cd, Cu and Zn were derived using the fitted model. The graphical method described by Meier and Zund (2000) was used to calculate the limit of detection (LOD) at 95% confidence level.
Results and Discussion
This whole-cell optical biosensor comprised of the microalga S. subspicatus MM1 as the biological component which responded to different concentrations of selected heavy metals and produced a detectable signal in terms of chlorophyll a fluorescence using a fluorometer. Growth of the S. subspicatus MM1 was measured at 24, 48 and 96 h incubation as fluorescence is shown in Fig. 2. Optimum stable pH range for the microalgae (based on the stable high fluorescence) appeared to be from 5 to 8.5 at 96 h. Furthermore it was evdent that aforesaid chlorophyll a fluorescence intensity optimum range was increased with the time of incubation.
Freshwater samples have a range of pH. Also, it is a known factor that the working pH of the solution has an effect on the activity of the alga (Jayaraman and Rhinehart 2015). Therefore, ability of the microalgae S. subspicatus MM1 used in the present study to function in a broader pH range will be highly useful in detecting bioavailable metals in samples with different pH.
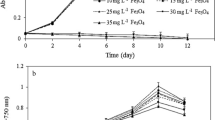
Microalgae show different sensitivities to different heavy metals as observed in the study described in Rathnayake (2010), based on 96 h exposure time. Since the focus of this whole-cell biosensor was to optimize the conditions such as to use this in rapid detection of the heavy metal toxicity. Therefore the exposure time was reduced to few hours after taking the time taken by the algae to respond to the lowest concentration tested (0.05 mg/L) to inhibit its growth at least by 90% compared to that of the control (Fig. 3). This biosensor was tested in the presence as well as in the absence of heavy metals to compare the effect of the individual heavy metal on the kinetics of fluorescence induction in algal chlorophyll a (Fig. 3). Time-dependent decline in the chlorophyll a fluorescence was evaluated at 1 h intervals in the presence of Cd, Cu and Zn.
The optimum exposure time for Cd, Cu, and Zn were determined as 7 h, 4 h, and 7 h respectively, for the S. subspicatus MM1 to respond to the lowest tested concentration of heavy metals at 20% inhibition (Cd and Cu) and 10% inhibition (Zn) compared to that of the control.
The effect of algal density on the fluorescence intensity was given in Fig. S1 (Supplementary materials). The optimum cell concentration of algae to detect lower range of heavy metal concentration was observed to be the fluorescence 800 approx. at 440/680 nm. Increasing algal density resulted in increasing fluorescence intensity and a decrease in heavy metal sensitivity during the exposure time. A similar trend was reported elsewhere (Pham et al. 2010). The initial inoculum with a high cell density will prevent the heavy metals from coming into close contact with algal cells hence not able to trigger much toxicity (Jaafari and Yaghmaeian 2019). The initial cell concentration shown to have an effect on the fluorescence response effectiveness in the presence of the heavy metals investigated was in agreement with the previous reports (Teo and Wong 2014).
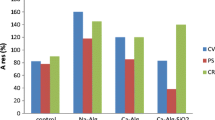
Effect of different silica precursor formulations (SiNa/LUDOX) on the toxicity response of S. subspicatus MM1 is presented in the Fig. S2 (Supplementary materials). The response to heavy metals given by the immobilized algae in the algal biosensor is in agreement with the results obtained using the algae in suspension, even though a minor difference was observed. This might be due to the time taken for the heavy metals to diffuse through the sol-gel matrix to reach the biological component of the sensor. Furthermore, since the different heavy metals have different diffusion coefficients, the time taken for their movement can be different from each other (Żur et al. 2016). Immobilization of algal cells in sol-gels should not block the fluid flow into sol-gel matrix but must prevent the immobilized cells from escaping the matrix. Porosity of the sol-gel is a critical factor in this regard because the pores allow the exchange of liquids and gases (Perullini et al. 2014). The differences in sodium silicate concentration used in the tested formulations accounts to the structural difference in the sol-gel. Increasing concentrations of sodium silicate caused a surface area and pore volume increase but a decrease in pore size. As a result, a high pore volume and high surface area facilitate a higher diffusion rate (Zhao et al. 2013).
Figure 4 shows the algal fluorescence changes upon immobilization in four different matrices and effect of storage. There was about 85% fluorescence intensity compared to the freshly prepared sensors after 1 week of storage. It was observed that the cell activity (measured as the fluorescence) was reduced during storage. There was a drastic reduction compared to that of the initial fluorescence signal, response to heavy metal exposure after 4th week.
The surface area, and pore volume are the major factors controlling the diffusion of the liquids through the sol–gel. Although immobilization was found to be effective in using the microalgae for immediate use (within a week) as a biosensor, its long term storage resulted in less effectiveness.
The instability of the biosensor during the long term storage was reported previously (Teo and Wong 2014; Antonacci and Scognamiglio 2020; Shitanda et al. 2005) reported that the microalga C. vulgaris immobilized in polyion complex membrane was able to retain 80% of its photosynthetic ability upon a week of storage at 4°C. These differences in storage stability of algae in immobilized state might be dependent on the nature of microalga used as well as the immobilizing matrix and the storage temperature. Use of algae in suspension showed more stable fluorescence upon long term storage than the immobilized cells. The concentration ranges of the heavy metals that showed a linear range was selected to derive the standard curves (Fig. S2 – Supplementary materials).
Based on the results derived from the graphical method described by Meier and Zund (2000), limit of detection corresponding to the three heavy metals tested are derived and presented in the Table 1. The linear range of the effect-concentration curve for survival of S. subspicatus MM1 (Fig. S3) fits precisely with a coefficient of determination (R2) of more than 0.90 and p = 1.4 × 10−7; 8.41 × 10−8; 5.6 × 10−9 for Cd, Cu and Zn respectively with 95% confidence. Microalgal biosensors developed for detection of heavy metals are reported elsewhere (Table S1 – Supplementary materials). Their sensitivities vary with the microalgae used and the method of signal transduction hence the LODs are different (D’Souza 2001) therefore, comparison with the one that developed during the present study was not feasible.
An optical whole-cell algal biosensor was constructed to detect heavy metal toxicity based on the inhibition of the chlorophyll a fluorescence. It was revealed that this algal biosensor is suitable for the detection of Cd, Cu and Zn in laboratory for aquous samples within 4 weeks of storage.
References
Antonacci A, Scognamiglio V (2020) Biotechnological advances in the design of algae-based biosensors. Trends Biotechnol 38(3):334–347. https://doi.org/10.1016/j.tibtech.2019.10.005
Antonacci A, Lambreva MD, Arduini F, Moscone D, Palleschi G, Scognamiglio V (2018) A whole-cell optical bioassay for the detection of chemical warfare mustard agent simulants. Sens Actuators B 257:658–665. https://doi.org/10.1016/j.snb.2017.11.020
Belaïdi FS, Farouil L, Salvagnac L, Temple-Boyer P, Séguy I, Heully JL, Alary F, Bedel-Pereira E, Launay J (2019) Towards integrated multi-sensor platform using dual electrochemical and optical detection for on-site pollutant detection in water. Biosens Bioelectron 132:90–96. https://doi.org/10.1016/j.bios.2019.01.065
Chen Z, Song S, Wen Y, Zou Y, Liu H (2016) Toxicity of Cu (II) to the green alga Chlorella vulgaris: a perspective of photosynthesis and oxidant stress. Environ Sci Pollut Res 23(18):17910–17918. https://doi.org/10.1007/s11356-016-6997-2
D’souza SF (2001) Microbial biosensors. Biosens Bioelectron 16(6):337–353. https://doi.org/10.1016/S0956-5663(01)00125-7
Eroglu E, Smith SM, Raston CL (2015) Application of various immobilization techniques for algal bioprocesses. In: Moheimani NR, McHenry MP, de Boer K, Bahri PA (eds) Biomass and biofuels from microalgae, vol 2. Springer, Cham, pp 19–44. https://doi.org/10.1007/978-3-319-16640-7_2
ISO (International Standard Organization) (1989) Water quality – fresh water algal growth inhibition test with Scenedesmums subspicatus and Selenastrum capricornutum, International Standards Organization (ISO) 8692-1989(E), International Organization for Standardization, Geneva, Switzerland, p 5
Jaafari J, Yaghmaeian K (2019) Optimization of heavy metal biosorption onto freshwater algae (Chlorella coloniales) using response surface methodology (RSM). Chemosphere 217:447–455. https://doi.org/10.1016/j.chemosphere.2018.10.205
Jayaraman SK, Rhinehart RR (2015) Modeling and optimization of algae growth. Indus Eng Chem Res 54(33):8063–8071. https://doi.org/10.1021/acs.iecr.5b01635
Khishamuddin NA, Shing WL, Kin CM, Niu VB (2018) Fluorometric response of photosynthetic microorganism consortium as potential bioindicator for heavy metals detection in water. EnvironmentAsia 11(1):80–86. https://doi.org/10.14456/ea.2018.6
Meier P, Zund R (2000) Statistical methods in analytical chemistry, 2nd edn. Wiley, Hoboken, pp 115–118
Megharaj M, Singleton I, McClure NC, Naidu R (2000) Influence of petroleum hydrocarbon contamination on microalgae and microbial activities in a long-term contaminated soil. Arch Environ Contam Toxicol 38:439–445. https://doi.org/10.1007/s002449910058
Moreno-Garrido I (2013) Microalgal immobilization methods. In: Guisan JM (ed) Immobilization of enzymes and cells, vol 1051. Humana Press, Totawa, pp 327–347. https://doi.org/10.1007/978-1-62703-550-7_22
Perullini M, Rivero MM, Jobbágy M, Mentaberry A, Bilmes SA (2007) Plant cell proliferation inside an inorganic host. J Biotechnol 127:542–548. https://doi.org/10.1016/j.jbiotec.2006.07.024
Perullini M, Ferro Y, Durrieu C, Jobbágy M, Bilmes SA (2014) Sol–gel silica platforms for microalgae-based optical biosensors. J Biotechnol 179:65–70. https://doi.org/10.1016/j.jbiotec.2014.02.007
Peterson HG, Nyholm N, Ruecker N (2005) Algal microplate toxicity test suitable for heavy metals. Small-scale freshwater toxicity investigations. Springer, Dordrecht, pp 243–270
Pham TPT, Cho CW, Yun YS (2010) Algal biosensor-based measurement system for rapid toxicity detection. In: Sharma MK (ed) Advances in measurement systems. Intech Open, London, pp 273–288
Podola B, Nowack ECM, Melkonian M (2005) The use of multiple-strain algal sensor chips for the detection and identification of volatile organic compounds. Biosens Bioelectron 19:1253–1260. https://doi.org/10.1016/j.bios.2003.11.015
Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T (2019) Effects of microplastics on microalgae populations: a critical review. Sci Total Environ 665:400–405. https://doi.org/10.1016/j.scitotenv.2019.02.132
Rathnayake IVN (2010) Development of whole-cell microbial biosensors to assess the heavy metal bioavailability in the soil environment (Doctoral dissertation)
Rathnayake IVN, Megharaj M, Naidu R (2021) Green fluorescent protein based whole cell bacterial biosensor for the detection of bioavailable heavy metals in soil environment. Environ Technol Innov. https://doi.org/10.1016/j.eti.2021.101785
Roxby DN, Rivy H, Gong C, Gong X, Yuan Z, Chang GE, Chen YC (2020) Microalgae living sensor for metal ion detection with nanocavity-enhanced photoelectrochemistry. Biosens Bioelectron 165:112420. https://doi.org/10.1016/j.bios.2020.112420
Scognamiglio V, Antonacci A, Arduini F, Moscone D, Campos EVR, Fraceto LF, Palleschi G (2019) An eco-designed paper-based algal biosensor for nanoformulated herbicide optical detection. J Hazard Mater 373:483–492. https://doi.org/10.1016/j.jhazmat.2019.03.082
Shitanda I, Takada K, Sakai Y, Tatsuma T (2005) Compact amperometric algal biosensors for the evaluation of water toxicity. Anal Chim Acta 530:191–197. https://doi.org/10.1016/j.aca.2004.09.073
Teo SC, Wong LS (2014) Whole cell-based biosensors for environmental heavy metals detection. Ann Res Rev Biol. https://doi.org/10.9734/ARRB/2014/9472
Tsopela A, Laborde A, Salvagnac L, Ventalon V, Bedel-Pereira E, Séguy I, Temple-Boyer P, Juneau P, Izquierdo R, Launay J (2016) Development of a lab-on-chip electrochemical biosensor for water quality analysis based on microalgal photosynthesis. Biosens Bioelectron 79:568–573. https://doi.org/10.1016/j.bios.2015.12.050
Turemis M, Rodio G, Pezzotti G, Touloupakis E, Johanningmeier U, Bertalan I, Litescu S, Rea G, Giardi MT (2017) A novel optical/electrochemical biosensor for real time measurement of physiological effect of astaxanthin on algal photoprotection. Sens Actuators B 241:993–1001. https://doi.org/10.1016/j.snb.2016.10.115
Żur J, Wojcieszyńska D, Guzik U (2016) Metabolic responses of bacterial cells to immobilization. Molecules 21(7):958. https://doi.org/10.3390%2Fmolecules21070958
Zhao J, Liu Y, Li X, Lu G, You L, Liang X, …, Du Y (2013) Highly sensitive humidity sensor based on high surface area mesoporous LaFeO3 prepared by a nanocasting route. Sens Actuators B 181:802–809. https://doi.org/10.1016/J.SNB.2013.02.077
Acknowledgements
This research was supported by the University of South Australia Presidents Scholarship (UPS) in collaboration with Cooperative Research Centre for Contamination Assessment and Remediation of the Environment (CRC CARE) Australia fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rathnayake, I.V.N., Megharaj, M. & Naidu, R. Sol–Gel Immobilized Optical Microalgal Biosensor for Monitoring Cd, Cu and Zn Bioavailability in Freshwater. Bull Environ Contam Toxicol 110, 73 (2023). https://doi.org/10.1007/s00128-023-03709-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00128-023-03709-5


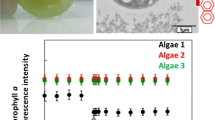


 – 24 h;
– 24 h;  – 48 h;
– 48 h;  – 96 h of incubation)
– 96 h of incubation)
