Abstract
Anthropic activities generate contaminants, as pesticides and other pollutants, in the aquatic environment which present a real threat to ecosystems and human health. Thus, monitoring tools become essential for water managers to detect these chemicals before the occurrence of adverse effects. In this aim, algal cell biosensors, based on photosystem II activity measurement, have been designed for several years in previous studies. In this work, we study a new immobilization technique of algal cells in the aim of improving the performance of these biosensors. Immobilization was here achieved by encapsulation in a hybrid alginate/silica translucid hydrogel. The feasibility of this process was here assessed, and the biosensor designed was tested on the detection of chemicals in urban rainwaters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The sealing of the surfaces of urban environments reduces the infiltration of rainwater to underground aquifers and increases runoff in heavily anthropized areas. The leaching of urban soils results in the transfer of a cocktail of chemical compounds comprising a large proportion of pesticides to the aquatic environment (Aalderink et al. 1990; Bertrand-Krajewski et al. 2008; Aryal et al. 2010). These cocktails stem from the maintenance of gardens and green spaces. Thus, a large number of molecules, such as atrazine and diuron whose use has nonetheless been forbidden for several years, are regularly detected in aquatic environments (Dubois and Lacouture 2011). These compounds have harmful effects on the functioning of ecosystems as they degrade habitats (Angerville 2009; Armstrong et al. 1980). The presence of carcinogenic and neurotoxic substances, as well as endocrine disruptors, has often been demonstrated in animals, and their potential impacts on human health also lead to great concern (Aubertot 2005). This observation has led the French and European authorities to strengthen legislation in order to organize actions aimed at reversing this process, notably through the adoption at the end of 2009 of the “Pesticide Package”, composed of four texts: two directives and two regulations in force since June 2011 (Directive 2009/128/CE). Their objective is to reduce risk by requiring member countries to set up action plans that involve reinforced monitoring of the quality of aquatic environments. In this context, biosensors capable of continuous measurement in situ are indispensable tools for decision-makers. In previous works, algal biosensors based on metabolism measurements have been developed by Chouteau et al. (2004, 2005) for heavy metal and pesticide detection in freshwater.
A biosensor can be considered as a combination of a bioreceptor, the biological component, and a transducer, the detection method. The total effect of a biosensor is to transform a biological event into an electrical signal. The first link of a biosensor is the bioreceptor, which has a particularly selective site that identifies the analyte. The bioreceptor ensures molecular recognition and may transform the analyte in some way detectable by the transducer (Tran-Minh 1993; Ciucu et al. 2001; Dzyadevych et al. 2003).
Hereafter, we present a unicellular algal biosensor based on photosystem II activity measurement to evaluate levels of photosynthesis. The algae were chosen for their high sensitivity to pollutants and the position they occupy at the base of trophic chains. They are essential in aquatic ecosystems since they provide oxygen and organic substances to other life forms. Chemical action of pollutants can affect their photosynthetic activity, resulting in oxygen depletion and a decrease in aquatic biodiversity. Therefore, algae can serve as biological monitors of water quality and as biological indicators in the assessment of environmental impact of pollutants. Thus, the tool developed is of considerable ecological pertinence. Photosystem II was reported to be able to detect herbicides in the environment (Giardi et al. 2001). About 30 % of herbicides are targeting the vegetal photosystem II (PSII) (Moreland 1980; Draber et al. 1991). They include derivatives of phenylurea, triazine, and phenolic herbicides. These substances inhibit photosynthetic electron flow by blocking the PSII quinone binding site and thus modify chlorophyll fluorescence. So, PSII activity has so far been used because of its higher sensitivity (Samson and Popovic 1988; Fai et al. 2007).
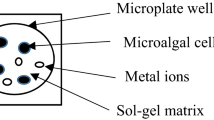
Since the sensitivity of biosensors is directly linked to the method of bioreceptor immobilization, we present in this paper a method of tool improvement by encapsulating the algae in a hybrid alginate/silica material. The design of biosensors based on direct silica encapsulation of algal cells is intrinsically limited by the restricted viability and high level of cellular stress developed during and after direct entrapment in the silica host. Recent work has demonstrated the possibility of cell division inside inorganic matrices by means of a two-step encapsulation procedure based on sol–gel chemistry (Perullini et al. 2005). This strategy expands the range of possible applications as cells can not only be entrapped within silica hydrogels, but plant and algal cells are also able to grow inside, even for periods of months (Perullini et al. 2007; Sicard et al. 2011). In addition, the mesoporous texture of the silica hydrogel provides a shield preventing the release of entrapped cells, as well as the contamination of the inner culture by exogenous strains. Moreover, in a previous work, translucent alginate–silica matrices have been investigated for the entrapment of three algal strains in order to develop a prototype of biosensor (Ferro et al. 2012). In the mentioned work, after a biocompatibility assay of the inorganic host material with the biological guests (Chlorella vulgaris, Chlamydomonas reinhardtii, and Pseudokirchneriella subcapitata), the detection and quantification of pure herbicides in artificial samples were evaluated, as a proof of concept for the development of a biosensor. DCMU (3-(3,4-dichlorophenyl)-1,1-dimethyl urea) and atrazine, as examples of herbicides that are known to inhibit the algal PSII, were evaluated, and a limit of detection of 0.1 μM of atrazine was established for the most sensitive algal species (C. reinhardtii).
The goal of the present work is to evaluate the performance of the developed biosensor on rainwater from urban catchments contaminated by pesticides. Hereafter, we present the analysis of samples of rainwater from urban catchments contaminated by pesticides compared with bioassays on non-immobilized algae. The results obtained lead to considering the application of the biosensor developed to detect pesticides in the environment.
Material and methods
Three algal strains were used: C. vulgaris (CV), P. subcapitata (PS), and C. reinhardtii (CR). They were purchased from the Culture Collection of Algae and Protozoa (Cumbria, UK).
C. vulgaris and P. subcapitata were grown in Lefebvre–Czarda medium (AFNOR 1980), whereas C. reinhardtii was grown in Tris–acetate–phosphate (TAP) medium (Gorman and Levine 1965), and these were transplanted weekly under sterile conditions (autoclaving 20 min, 130 °C, 1.3 bars). Algae were maintained in a nycthemeral cycle of 16 h of illumination at 5000 lx and 8 h of darkness.
Photosystem II activity was measured by chlorophyll fluorescence emission which was done using a fluorometer for an excitation wavelength of 460 nm and an emission wavelength of 680 nm. The transducer is an optical fibre placed in contact with the immobilized algae.
Algal cell immobilization was carried out in two steps. The algae were encapsulated beforehand in a calcium alginate matrix which in turn was immobilized in an inorganic silica matrix, thus minimizing the contact of algae with soluble silica precursors. A mixture of 50 μL of cells suspended with 50 μL of Tris–HCl buffer (10 mM, pH 7.5) and 100 μL 2 % Na(I) alginate (104 cells/mL) was introduced in a multiwell plate and further immersed in 0.1 M CaCl2 for 10 min in order to achieve alginate crosslinking. After that, a 1:3 mixture (pH = 6.5) of sodium silicate (Riedel-de Haën; NaOH 10 %, SiO2 27 %) and pre-formed silica particles (LUDOX HS-40, 40 % in water; Aldrich) was poured on the top, leading to a mesoporous monolithic structure after sol–gel transition. The silica hydrogel thickness of 1.9 mm optimizes the optical quality and mass transport.
Material biocompatibility was assessed in a previous work (Ferro et al. 2012) by comparing the growth of the encapsulated algae with that of free algae, under the same temperature and light conditions. The results obtained showed that the growth of CV was not affected by encapsulation, whereas that of the two other species was slightly inhibited (6 % for PS and 13 % for CR). We considered that these inhibition rates remained acceptable to continue the tests with the three species of algae.
The optical quality of the hybrid alginate/silica material was compared to that obtained from free algae in the presence of sodium alginate and encapsulated in calcium alginate. To do this, chlorophyll fluorescence emission measurements were compared under the different conditions described. The results revealed a slight loss of fluorescence intensity in the presence of sodium alginate that was not increased by gelification in the calcium alginate or by inclusion in the hybrid material. These losses were considered acceptable for the experiments to continue.
Determination of photosynthetic activity was evaluated using the method described by (Ban-Dar et al. 1989) based on measuring chlorophyll fluorescence emission which is inversely proportional to the level of photosynthesis. Thus, photosynthetic activity can be determined by measuring fluorescence before and after contamination by dichloromethyl urea (DCMU) at 4 mg/L, as it leads to the total blocking of photosynthesis and thus maximum fluorescence. Fluorescence enhancement after DCMU treatment is expressed as percentage of fluorescence after DCMU exposure reported to the fluorescence before exposure. The degradation of photosynthetic activity was tested at first with atrazine, as a pesticide model, and after with rainwater. Photosynthetic inhibition was expressed as residual activity:
with ∆F = Fu − Fo (Fu is the fluorescence after DCMU (at 4mg/L) exposure and Fo the fluorescence before DCMU exposure.
The rainwater samples were taken from two retention basins (Chassieu and Ecully) in the suburb of Lyon in which water is stored before re-infiltration into the groundwater.
Results and discussion
Atrazine, whose effect is well known on photosynthesis, is used in this study as a pesticide model to test the impact of the encapsulation on the sensor’s efficiency.
Ferro et al. (2012) have shown in a previous study that DCMU at 4 mg/L (concentration which leads to the total blocking of photosynthesis) can produce the enhancement of chlorophyll fluorescence on free algae, in the presence of sodium alginate, encapsulated in calcium alginate or immobilized in two steps in an alginate/silica gel. This study was carried out on three algal species (CV, PS, and CR) and showed that the increase of fluorescence was not significantly different between the free algae and the algae immobilized in one or two steps for the PS and CR strains. However, the increase of fluorescence was much lower for the CV strain once the cells had been immobilized. It is difficult to explain this phenomenon: it is not possible to know at this stage if algae are damaged by immobilization or if encapsulation reduces the DCMU effect.
In this paper, a study of photosynthetic activity after exposure to different concentrations of atrazine was carried out in the aim to give more information about the behaviour of algae after encapsulation in one or two steps. The results in Table 1 express the residual photosynthetic activity, A res (%), as described in the “Material and methods” section, after a 40 min exposure at different atrazine concentrations ranging from 10−3 to 10 μM. Experiments were conducted on the three algal species with three replicates for each measurement.
Results show that photosynthetic activity of free algae decreases, in a dose-dependent manner, with the concentration of atrazine in the same way for the three species. This decrease is also observed for algae encapsulated in one or two steps; however, the decrease stops in alginate above 1 μM.
Thus, the limit of detection (LOD) was not observed on free algae; it seems lower than 10−3 μM. A res increases with the number of immobilization steps (higher in calcium alginate than on free algae, higher in alginate/SiO2 than in alginate). This means that the encapsulation material provides a barrier to diffusion of atrazine towards algal cells. This can explain why atrazine at 10−3 μM has no effect on CV and CR immobilized in two steps; in this case, the LOD is between 10−3 and 10−2 μM. These results show, however, the feasibility of detecting atrazine with algae immobilized in one or two steps. On the other hand, the optical biosensors designed with a double encapsulation of algal cells could present the advantage to protect algae from external damages. Photosynthetic activity seems to be preserved after the two immobilization steps for the three strains studied here.
Assays after rainwater exposure
In the aim to assess the performance of encapsulated algae for detection of chemicals in the environment, photosynthetic activity was measured in the three strains during the different steps of encapsulation after a 24-h exposure of rainwater collected during several rainfalls inside two basins.
Water collected in Chassieu comes from an industrial area and those from Ecully come from agricultural land. Organic compounds were measured in the samples collected; results are presented in Table 2, and they show that there are more pesticides in the Ecully basin (diuron particularly) and more hydrocarbons in the Chassieu basin.
Figures 1 and 2 show the results of A res after exposure to a sample of rainwater pooled after several events. The results show an impact of leachates on the photosynthetic activity of free algae for the three species (residual photosynthetic activity <100 %) after exposure to rainwaters collected in the two basins.
If we compare A res of algae encapsulated in alginate with that of free algae, we can see that A res is >100 % for CV and CR and near 100 % for PS after exposure to Chassieu water, whereas the results are different with Ecully water: in this case, A res is <100 % excepted for PS in the presence of alginate and CR after encapsulation. After encapsulation in two steps, A res is <100 % for CV, which means that photosynthetic activity is inhibited. In this last case, photosynthetic activity is inhibited for PS with Chassieu water and enhanced with Ecully water. At last, A res is <100 % with the two types of water.
These results show a difference of behaviour not easy to understand between Chassieu and Ecully waters. It is not very surprising because these waters have different organic compositions. The enhancement of photosynthetic activity in the presence of alginate could perhaps be explained by a synergetic effect between the alginate and some molecules present in the leachates. Rainwater contains a complex cocktail of molecules and measurements could reflect several effects with synergism or antagonisms.
Conclusion
In spite of the different uncertainties and doubts identified in this work, the feasibility of using a translucid inorganic hydrogel to build an optical biosensor using immobilized algal cells appears to have been validated. According to our results and in agreement with previous studies, the immobilization of green algae in a sol–gel silica matrix by means of the two-step procedure presents high biocompatibility. This encapsulation method is easy to carry out and a matrix with good optical and mechanical performances is obtained. In addition, the precursors of silica and polymer particles used in this synthesis are inexpensive reagents, which is interesting from the standpoint of possible future applications. The transparency and structure of the algal layer permit direct contact with optical fibres to produce a fluorescence emission that can be detected by a fluorometer. This whole-cell biosensor functioning without reagent is of great interest economically for monitoring different chemical levels in the environment. Studies on the choice of the algal species best adapted as a function of the molecules to be detected remain to be performed.
References
Aalderink RH, Van Duin EHS, Peels CE, Scholten MJM (1990) Some characteristics of run-off quality from a separated sewer system in Leleystad, the Netherlands. Proceedings of the 5th International Conference on Urban Storm Drainage, Osaka, Japan, pp 427–432
AFNOR (1980) Détermination de l’inhibition de croissance de Scenedesmus subspicatus par une substance. Norme experimentale NT90-304; Association Française de Normalisation, Paris, France
Angerville R (2009) Evaluation des risques écotoxicologiques liés au déversement de Rejets Urbains par Temps de Pluie (RUTP) dans les cours d’eau: Application à une ville française et à une ville haïtienne. Thèse de doctorat, Institut National des Sciences Appliquées de Lyon, 479 p
Armstrong JW, Thom RM, Chew KK (1980) Impact of a combined sewer overflow on the abundance, distribution and community structure of subtidal benthos. Mar Environ Res 4:3–23
Aryal R, Vigneswaran S, Kandasamy J, Naidu R (2010) Urban stormwater quality and treatment. Korean J Chem Eng 27:1343–1359
Aubertot JN (2005) Pesticides, agriculture et environment. Rapport, INRA et Cemagref, France, p 64
Ban-Dar H, Yuh-Shein L, Yuh-Ren J (1989) A method for analysis of fluorescence curve from DCMU-poisoned chloroplasts. Biochim Biophys Acta Bioenerg 975:44–49
Bertrand-Krajewski JL, Becouze C, Dembélé A, Coquery M, Cren-Olivé C, Barillon B, Dauthuille P, Chapgier J, Grenier-Loustalot MF, Marin P (2008) Priority pollutants in stormwater: the ESPRIT project. Proceedings of the 11th International Conference on Urban Drainage, Edinburgh, UK, 31 augt-5 sept 2008
Chouteau C, Dzyadevych S, Chovelon JM, Durrieu C (2004) Development of novel conductometric biosensors based on immobilised whole cell Chlorella vulgaris microalgae. Biosens Bioelectron 19(9):1089–1096
Chouteau C, Dzyadevych S, Durrieu C, Chovelon JM (2005) A bi-enzymatic whole cell conductometric biosensor for heavy metal ions and pesticides detection in water sample. Biosens Bioelectron 21(2):273–281
Ciucu A, Lupu A, Pirvutoi S, Palleschi G (2001) Biosensors for heavy metals determination based on enzyme inhibition. Chem Mater Sci 63:33–44
Directive 2009/128/CE, Règlement (CE) n°1107/2009, Directive 2009/127/CE et Règlement (CE n°1185/2009)
Draber W, Tietjen K, Kluth JF, Trebst A (1991) Herbicides in photosynthesis research. Angew Chem Int Ed Engl 30:1621–1633
Dubois A, Lacouture L (2011) Rapport du commissariat général du développement durable, MEDDTL
Dzyadevych SV, Soldatkin AP, Korpan YI, Arkhypova VN, EL’skaya AV, Chovelon JM, Martelet C, Jaffrezic-Renault N (2003) Biosensors based on enzyme field-effect transistors for determination of some substrates and inhibitors. Anal BioanalChem 377:496–506
Fai PB, Grant A, Reid B (2007) Chlorophyll-a fluorescence as a biomarker for rapid toxicity assessment. Environ Toxicol Chem 26(7):1520–1531
Ferro Y, Perulloni M, Jobbagy M, Bilmes SA, Durrieu C (2012) Development of a biosensor for environmental monitoring based on microalgae immobilized in silica hydrogel. Sensors 12:16879–16891
Giardi MT, Koblizek M, Masojidek J (2001) Photosystem II based biosensors for the detection of pollutants. Biosens Bioelectron 16:1027–1033
Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A 54:1665–1669
Moreland DE (1980) Mechanisms of action of herbicides. Annu Rev Plant Physiol 31:597–638
Perullini M, Jobbágy M, Soler-Illia GJAA, Bilmes SA (2005) Cell growth at cavities created inside silica monoliths synthesized by sol-gel. Chem Mater 78:3806–3808
Perullini M, Rivero MM, Jobbágy M, Mentaberry A, Bilmes SA (2007) Plant cell proliferation inside an inorganic host. J Biotechnol 127:542–548
Samson G, Popovic R (1988) Use of algal fluorescence for determination of phytotoxicity of heavy metals and pesticides as environmental pollutants. Ecotoxicol Environ Saf 16:272–278
Sicard C, Perullini M, Spedalieri C, Coradin T, Brayner R, Livage J, Jobbagy M, Bilmes SA (2011) CeO2 nanoparticles for the protection of photosynthetic organisms immobilized in silica gels. Chem Mater 23:1374–1378
Tran-Minh C (1993) Biosensors. Chapman & Hall, London
Acknowledgments
This work has been financially supported by ECOS SUD (University of Paris 13), MINCYT (Argentina), L’Office national de l’eau et des milieux aquatiques (France), and CONICET (GI-PIP 11220110101020, UBACyT 20020130100048BA, ANPCyT-PICT 2012-1167 and 2013-2045) from Argentina.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Durrieu, C., Ferro, Y., Perullini, M. et al. Feasibility of using a translucid inorganic hydrogel to build a biosensor using immobilized algal cells. Environ Sci Pollut Res 23, 9–13 (2016). https://doi.org/10.1007/s11356-015-5023-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5023-4