Abstract
Key message
Soybean quantitative trait loci for ozone response.
Abstract
Ground-level ozone reduces yield in crops such as soybean (Glycine max (L.) Merr.). Phenotypic variation has been observed for this trait in multiple species; however, breeding for ozone tolerance has been limited. A recombinant inbred population was developed from soybean genotypes differing in tolerance to ozone: tolerant Fiskeby III and sensitive Mandarin (Ottawa). Plants were exposed to ozone treatment for 5 days in greenhouse chambers followed by visual scoring for foliar injury. Mean injury score in the mid-canopy was 16 % for Fiskeby III, and 81 % for Mandarin (Ottawa). Injury scores were lower in younger leaves for both parents and progeny, compared to scores in the older leaves. Segregation was consistent with multigenic inheritance. Correlation coefficients for injury between leaf positions ranged from 0.34 to 0.81, with the closer leaf positions showing the greater correlation. Narrow sense heritability within an ozone treatment chamber was 0.59, 0.40, 0.29, 0.30, 0.19, and 0.35 for the 2nd, 3rd, 4th, 5th, 6th, and combined 3rd–5th main stem leaf positions (numbered acropetally), respectively, based on genotypic means over three independent replications. Quantitative trait loci (QTL) analysis showed that loci were associated with distinct leaf developmental stages. QTL were identified on Chromosome 17 for the 2nd and 3rd leaf positions, and on Chromosome 4 for the 5th and 6th leaf positions. Additional loci were identified on Chromosomes 6, 18, 19, and 20. Interacting loci were identified on Chromosomes 5 and 15 for injury on trifoliate 4. The ozone sensitive parent contributed one favorable allele for ozone response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropospheric ozone (O3) concentration has nearly doubled since the Industrial Revolution, and is predicted to continue to increase in the twenty first century (Ainsworth et al. 2012; Fowler 2008). Ozone is an air pollutant and a greenhouse gas, thereby having both direct and indirect effects on plants, animals and the environment (Ainsworth et al. 2012). It forms in the troposphere from reactions between oxygen and primary air pollutants such as carbon monoxide, nitrogen oxide, and various volatile organic compounds (Prather et al. 2001).
Ozone-mediated cell damage affects processes that directly support yield in crop plants (Ainsworth et al. 2012; Salvatori et al. 2013). Ozone negatively affects both wild and cultivated plants by interfering with important physiological processes, such as photosynthesis and carbon assimilation (Lobell and Gourdji 2012; Reich 1987). Ozone exposure causes an oxidative stress response in which reactive oxygen species form in the leaf mesophyll (Langebartels et al. 2002). Reactive oxygen species may damage cellular constituents, reduce the concentrations of primary metabolites, or they may cause cell death. Ozone exposure leads to physical damage of Photosystem II and reductions in RuBisCO concentration (Ainsworth et al. 2012; Fiscus et al. 2005). Ozone-mediated cell death appears as bronze stippling on the adaxial leaf surface, but damage may also manifest as chlorosis, necrosis, senescence, and decreased leaf area and biomass (Ainsworth et al. 2012; Frei et al. 2008, 2010). Ozone-exposed plants have reduced photosynthetic surface area due to such damage, with concomitant reductions in photosynthetic efficiency and carbon assimilation (Fiscus et al. 2005; Fuhrer 2003).
Among abiotic stress factors, O3 has received inadequate attention in spite of its ability to reduce yields in a wide variety of crop species. Major row crops including soybean are especially sensitive to ozone exposure (Ainsworth et al. 2012). Seasonal average ozone concentrations of 40–50 nmol mol−1 are sufficient to cause phytotoxic symptoms and yield losses of 10–20 % in soybean (Fowler 2008; Heagle et al. 1998). Though it has a reputation as an urban problem, ground-level O3 regularly exceeds phytotoxic thresholds in rural agricultural areas (Ainsworth et al. 2012). Episodic O3 spikes in excess of 100 nmol mol−1 are routinely observed in rural regions of the Northern Hemisphere (Fowler 2008). Furthermore, concentrations of ground-level O3 typically peak during warmer weather, coinciding with yield-determining periods of crop development. Both chronic and acute O3 exposures are phytotoxic, though the degree and type of damage are influenced by numerous factors such as ontogeny, leaf age, genetic-based tolerance, and taxa (Fiscus et al. 2005; Salvatori et al. 2013). Leaf age appears to be particularly influential in the degree of foliar O3 injury observed in multiple species, with older leaves in the lower canopy being more susceptible than younger leaves in the upper canopy (Cheng et al. 2007; Ciompi et al. 1997; Karlsson et al. 1995; Lee et al. 1984; Leitao et al. 2007; Saitanis et al. 2001). However, the effect of leaf age on genetic comparisons or QTL analysis is largely unknown, because related work has focused on small physiological studies rather than large-scale phenotypic screening.
In spite of its general sensitivity to O3, phenotypic variation has been observed among genotypes for this trait in soybean (Burkey and Carter 2009; Damicone et al. 1987; Heagle and Letchworth 1982; Mulchi et al. 1988; Taylor et al. 1982). Understanding the range of genetic variation and identification of associated loci are critical steps in incorporating O3 tolerance into modern soybean cultivars. Quantitative trait loci associated with ozone response have been identified in arabidopsis (Arabidopsis thaliana), rice (Ozyra sativa), and poplar (Populus trichocarpa, Populus deltoides) (Brosche et al. 2010; Frei et al. 2008; Street et al. 2011). Prior to the present study, there have been no published reports for QTL related to O3 response in soybean.
Here we present QTL and phenotypic variation of genotypic means for foliar O3 response in a RIL population of soybean. This population was developed from a cross between the ozone-tolerant Fiskeby III and the ozone-sensitive Mandarin (Ottawa). Fiskeby III is an extremely early maturing cultivar from Sweden that has demonstrated tolerance to multiple abiotic stresses including: ozone (Burkey and Carter 2009), drought, iron deficiency, salinity, and aluminum (Carter, Orf 2013, personal comm.). Closely related Fiskeby materials (Fiskeby V, 870-7-3) have demonstrated tolerance to cold (Holmberg 1973; Hume and Jackson 1981). The Fiskeby materials have been used as a source of cold tolerance in Canadian breeding programs, but otherwise have not been utilized extensively by plant breeders in other regions (Gizlice et al. 1994). The occurrence of multiple abiotic stress resistances in a single cultivar is rare in soybean and suggests that genetic control may be, in part, pleiotropic. This present study examining O3 tolerance is a first step in the genetic elucidation of the stress tolerance complex in Fiskeby III soybean.
Materials and methods
Population development
An F5:6 population of 240 RILs of soybean was developed from a cross between the ozone-tolerant Fiskeby III (PI 438471, maturity group 00) and the ozone-sensitive Mandarin (Ottawa) (PI 548379 maturity group 0). Fiskeby III was released in 1949 by Sven Holmberg as a determinate edamame cultivar (Holmberg 1968, 1973). It is derived from the hybridization of ‘Typ xx stam 9’ × ‘Namikawa (Sachalin)’. Typ xx stam 9 is a European variety or breeding line of unknown origin. Namikawa (Sachalin) is a Japanese landrace from Hokkaido or adjacent islands. Fiskeby III has a coefficient of parentage (CP) or pedigree relationship with the other materials developed by Holmberg that ranges from the equivalent of half sib to full sib (a CP of 0.25–0.50): Fiskeby V (PI 360955A, 0.52), Traff (PI 470930, 0.44), and Fiskeby 840-7-3 (PI 438476, 0.39). Mandarin (Ottawa) is a maturity group 0 selection from a Chinese landrace known simply as Mandarin. Mandarin (Ottawa) was selected in Ottawa, Ontario in 1934. Mandarin (Ottawa) contributes approximtely 17 % of the genetic base to Midwestern and Candian cultivars, based on pedigree analysis (Gizlice et al. 1994) and is the immediate parent of 12 U.S. cultivars. Fiskeby III contributes approximately 1 % of the genetic base of Midwestern and Canadian cultivars and appears as an antecedent in the pedigree of at least 13 early maturing cultivars. Fiskeby III also contributes 50 % of the pedigree in the Swiss cultivar Alvia.
Fiskeby III was hybridized with Mandarin (Ottawa) in the greenhouse in Raleigh, NC in the summer of 2006. The F1 plants were grown in the winter nursery in Chile in 2006–2007. Seed of the resulting population were advanced and subjected to inbreeding by the single seed descent breeding method (Brim 1966). Plants were grown in the summers in Minnesota and in the winters in Chile during 2007–2009. In the winter of 2010, F5 plants were grown and harvested individually in USDA-ARS greenhouses at Raleigh, NC. Plants were grown under a 20 h photoperiod for 2 weeks after emergence, and then under natural daylength thereafter. We recorded pubescence and pod-wall color, and growth habit (determinancy vs. indeterminancy) on an individual plant basis and verified that segregation patterns were consistent with random segregation associated with the single seed descent breeding method (Brim 1966). In the summer of 2010, F5-derived progeny rows from 278 RILs were grown at St. Paul, MN. Plants were harvested by hand and threshed with a stationary thresher as bulks, with all plants in a progeny row harvested together. These rows became the seed source for the 240 RILs employed in the present study.
Greenhouse experimental protocol
Plants were grown in a greenhouse supplied with charcoal filtered (CF) air located on the campus of North Carolina State University in Raleigh, NC, USA (35°78′N, 78°41′W). Three seed per line were direct-sown in 6 L pots containing Fafard #2 Pro Mix (Fafard, Anderson, SC, USA), supplemented with 15 g slow release fertilizer (Osmocote Plus, Scotts-Sierra Horticultural Products, Marysville, OH, USA). Plants were maintained in a vegetative state throughout the experiment using supplemental lighting to produce a 20-h photoperiod. At 14 days after planting, seedlings were thinned to one plant per pot, and treated with Marathon systemic insecticide according to the product label (OHP, Inc., Mainland, PA, USA).
At 21 days after planting, one pot of each line was randomly selected for treatment with ozone (68–72 nmol mol−1 O3 chamber mean, across reps) while another pot containing the same line remained in the CF greenhouse. Ozone-treated plants were exposed for 5 days from 0900 to 1600 h in continuous stirred tank reactors (CSTRs) located in the greenhouse. A CSTR is a cylindrical exposure chamber covered with Teflon film, designed for rapid mixing of gases (Heck et al. 1978; Rogers et al. 1977). Supplementary O3 was generated by electrostatic discharge in dry O2 (O3 generator model GTC-1A, Ozonia North America, Elmwood Park, NJ, USA). The O3 concentration was independently controlled in each CSTR. Relative humidity in the CSTRs was maintained above 50 % using humidity sensors to control solenoid valves that injected moisture into the airstream just prior to the inlet of each chamber. Moisture was provided during the 2011 studies by an electric steam boiler and during the 2012 study with nozzles that used pressurized air and water to create an atomized mist. Chamber temperature, photosynthetic photon flux density (PPFD) and relative humidity averaged 29.7 °C, 339 µmol m−2 s−1, and 62 %, respectively, during the 7-h period O3 exposure period. After the 5-day exposure, plants were then returned to the CF greenhouse for 2 days to allow complete development of foliar injury symptoms.
Data collection
Foliar injury was assessed on the seven oldest main stem trifoliate leaves of ozone-treated plants (T1, T2, T3, T4, T5, T6, and T7, where T1 represents the most basal trifoliate leaf in the canopy and T7 identifies the most apical trifoliate leaf). Ratings were defined as estimates of the percentage of adaxial leaf area that exhibited any type of foliar symptom including chlorosis, necrosis, and/or stipple following the approach established by Heagle (1979, 1982). Three independent raters scored the injury for each leaf over a 3-day period following exposure and symptom development.
Genotyping
For each RIL, DNA was isolated from bulked primary root tips of 15–20 F5:6 plants using Qiagen DNeasy Plant Mini Kits in a QiaCube workstation (Qiagen, Hilden, Germany). Genotyping was performed using the Illumina GoldenGate assay on 1536 SNP markers (Hyten et al. 2010), followed by analysis on the Illumina BeadStation 500G (Illumina, Inc, San Diego, CA, USA) using the protocol described by Fan et al. (2006). The resulting allele calls were checked manually in Illumina’s GenomeStudio software, version 2011.1. During the development of the genetic map, markers were excluded if the associated calls satisfied any of the following criteria: call frequency (<95 %), GenTrain score (<0.25), cluster separation (<0.20) with AB_T Mean (<0.20 or 0.80–1.00), or monomorphism (100 % AA or BB). One genotype was removed due to a poor GenCall score (≤0.4). The quality of the map was evaluated in R/qtl (R Development Core Team 2012) for duplicate and missing markers, marker order, similar individuals, segregation distortion, and agreement with the USDA consensus map (Hyten et al. 2010). Following checking of allele calls and quality evaluation, 366 polymorphic markers were retained for QTL analysis.
Experimental design and analysis
The experimental design was an augmented incomplete block (Federer 1956). All genotypes were evaluated in three greenhouse replications: May–July 2011, September–October 2011, and May–July 2012. Due to space constraints in the CSTRs, each replication consisted of 3 runs, planted 7 days apart. Runs were treated as incomplete blocks, and chambers were treated as sub-blocks. Each of the 20 CSTRs contained a total of seven plants: five randomly selected RILs and one individual of each of the two parents. Five extra individuals of each of the two parents were randomly assigned within each statistical replication to act as internal checks. Control plants of each genotype were grown in the charcoal-filtered greenhouse to provide visual comparison during scoring, but were not themselves scored.
Statistical analyses were performed using R Studio, version 0.98.1091 (R Development Core Team 2012) in the Stats, Graphics, Hmisc, and LME4 packages. For O3 treated plants, the mean score across raters was treated as the score for a particular leaf. Data from the 1st and 7th trifoliates were discarded due to confounding factors such as shade-induced senescence and differences in leaf development, respectively. A combined score for the mid-canopy was calculated as the mean across trifoliates T3, T4 and T5 (TriMid), where the greatest degree of injury was observed. Data were checked for homogeneity of variance using Levene’s test, and for normality using Q–Q plots and tests of kurtosis and skewness. Levene’s test showed that data for each variable were homoscedastic. The Q–Q plots indicated normality of the injury data, with some skew. Data transformations were not necessary.
Data for the entire population (RILs plus parents) were fitted to a linear mixed effects model using restricted maximum likelihood estimation in the LME4 package of R. Raw data (i.e. across biological replicates) was used in this analysis. Trifoliate leaf node was treated as a fixed effect, and genotype, chamber, run, rater, and replication were treated as random factors. Best linear unbiased predictions (BLUPs) were calculated for the random effect of Genotype (Piepho et al. 2003), and included with mid-canopy phenotypic means for each line as supplemental material (Supplemental Table 1). Narrow sense heritability was calculated after Nyquist (1991), pooling over 50 cohorts of five RILs each, which were maintained throughout the study. Pearson correlation coefficients and associated p values were calculated among leaf positions using the Hmisc package in R.
QTL analysis was performed on the phenotypic means using composite interval mapping in the R/qtl package, with a window size of 10 and 3 marker covariates (Broman et al. 2003). Prior to QTL analysis, mean values were calculated for each genotype for trifoliates T2 through T6 across raters and biological replications. The Scantwo function was used to detect interacting loci. LOD thresholds were determined for each trait from 1000 permutation tests using significance thresholds of 0.05 and 0.10. Haley-Knott regression was used to further resolve the position and effect on variability of significant QTL. The parental origin of favorable alleles was identified by calculating mean values for the marker classes (A or B) at each significant marker. The marker class with the lower injury score was considered to be the favorable allele, and therefore, the parent with the same genotype was considered to be the origin of a more favorable ozone response. The estimated effect of each QTL was calculated as the difference in mean percent injury between the two allele classes (A and B), divided by two.
Results
Phenotypic variation among RILs and parents for foliar leaf injury
Mandarin (Ottawa), the previously identified susceptible parent, showed greater injury than did Fiskeby III at each leaf node in this study (Table 1). Mean injury scores in Fiskeby III did not exceed 26 % at any leaf node, whereas mean injury in Mandarin (Ottawa) was greater than 70 % in all but one leaf node. Mean mid-canopy (TriMid) injury scores were 16 and 81 % for Fiskeby III and Mandarin (Ottawa), respectively. The magnitude of the difference between the parents was sufficiently great that maximum scores observed for trifoliates 2 and 3 in Fiskeby III were similar to mean scores for Mandarin (Ottawa) at the same leaf nodes. Similarly, in trifoliates 2 through 4, minimum scores for Mandarin (Ottawa) were greater than mean injury scores for Fiskeby III. Injury expression differed for the two parental types in that mean injury scores declined for Fiskeby III acropetally within the canopy, whereas this trend was not observed for Mandarin (Ottawa) (Table 1).
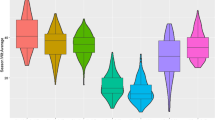
For the RILs, the mean injury score in the mid-canopy (TriMid) was 57 %, which was intermediate to the two parents. Phenotypic variation among the RILs was observed at each leaf node (Fig. 1). Correlation coefficients for injury between individual leaf positions ranged from 0.34 to 0.81 and for RIL means was 0.40–0.87, with the closer leaf positions showing the greater correlation (Table 2). Segregation in the population appeared to be continuous, mound shaped, and consistent with the concept of multigenic inheritance (Fig. 1). Approximately 13, 5, 4, 12, 35, and 4 members of 240 RILs were numerically equivalent to- or better than- the resistant Fiskeby III parent at the second, third, fourth, fifth, sixth trifoliates, and mid-canopy TriMid trait, respectively. The mean frequency of RILs numerically equivalent to- or better than—Fiskeby III was 0.058 over all leaf positions, which is consistent with a four gene model of inheritance (frequency = ~0.54) if one assumes that all resistance alleles are derived from Fiskeby III. If one assumes that one positive allele is from the sensitive Mandarin (Ottawa) parent and that this positive allele is an effective or an equivalent substitute for any positive allele derived from the resistant parent, then binomial analysis suggests inheritance could be controlled by as many as seven loci. For this latter model, it is assumed that any combination of 6 positive alleles, regardless of origin, would be sufficient to recover the resistant phenotype of Fiskeby III. Mean injury scores for the RILs ranged from 0 to 100, or close to 100, for each trifoliate leaf node (a wider range than the parents), suggesting the possibility of transgressive segregation, supporting the notion that at least one resistance allele could be derived from the sensitive Mandarin (Ottawa). Three RILs showed mean injury scores that were lower than those of Fiskeby III at every trifoliate leaf node examined (Supplemental Table 2). Three additional lines had lower mean values than Fiskeby III at 3 of the 5 leaf nodes.
Histogram panel showing phenotypic distribution of percent foliar O3 damage for five trifoliate leaf nodes (Trifoliates 2, 3, 4, 5, 6) and for the mid-canopy (mean of trifoliates 3, 4 and 5) in the soybean recombinant inbred population Fiskeby III × Mandarin (Ottawa). Phenotypic values for the parent lines are identified by arrows (“FIII” and “M(O)”). Injury scores reflect mean values by recombinant inbred line, across biological replications and ratings assigned by multiple raters
Standard deviations based on plant-to-plant (i.e. replication to replication) variation within RILs did not change drastically from the lower to upper canopy, but increased for Mandarin and decreased for Fiskeby III (Table 1). Although the standard deviation for Fiskeby declined acropetally, coefficients of variation were greater in Fiskeby III at every leaf node, compared to Mandarin (Ottawa) or the RILs. Coefficients of variation increased acropetally in the canopy for both parents and for the RILs, with the steepest increases observed between trifoliates 4 and 5 (Table 1).
Genotype accounted for 22 % of the total variance among random effects, with replications over time (11 % of the variation) and random plant-to-plant variation (59 %) accounting for most of the rest (Table 3). Chamber, run, and rater effects did not strongly influence injury scores. Genotypes with strongly negative BLUP values corresponded to more favorable phenotypic values, while positive BLUPs corresponded to unfavorable injury responses (Supplemental Table 1). Heritability within a chamber based on the mean of three replicates was 0.59, 0.40, 0.29, 0.30, 0.19, and 0.35 for the T2, T3, T4, T5, T6, and combined T3–T5 main stem leaf positions (numbered acropetally). The heritability observed over three runs (seasons) is on a par with seed yield of soybean in field studies (Brim 1973). The heritability calculations based on this dataset of O3 response predict that experimental designs with five replications would be required to increase heritability to a point that half of the phenotypic variation among RILs could be attributed to genotype effects (Table 4).
QTL analysis
The QTL analysis of each leaf position showed that QTL were associated with distinct developmental zones within the canopy. QTL were identified on Chromosome 17 for the 2nd and 3rd leaf positions (lower in canopy), and on Chromosome 4 for the 5th and 6th leaf positions (higher in canopy). A total of nine QTL were identified for foliar O3 response, including 2 interacting loci (Fig. 2; Table 5). Four significant loci were identified at p ≤ 0.05 (Chromosomes 1, 4, 17, 18), and three additional loci were identified at p ≤ 0.10 (Chromosomes 6, 19, 20). Loci were identified for each of the trifoliate leaf nodes studied, with some loci associated with multiple leaf nodes (e.g. Chromosome 17 Pos 56 for T2 and T3; Chromosome 4 Pos 45 for T5 and T6) (Fig. 2). Two of the QTL detected for individual leaf nodes in the mid-canopy were also detected for the combined score across trifoliates 3–5 (TriMid). The QTL of greatest effect was associated with a marker on Chromosome 18. This locus accounted for 9.5 % of the observed variation in injury on the 4th trifoliate. The QTL of least effect was associated with the 5th trifoliate leaf, and was identified on Chromosome 19. This locus accounted for 3 % of the variation observed for injury at that leaf node. A pair of interacting loci was identified on Chromosomes 5 and 15 at p ≤ 0.05 for trifoliate 4. LOD values for this pair of loci indicated the possibility of an epistatic interaction (Table 5). Both parents contributed favorable alleles for O3 response (Table 5). Of the seven non-interacting loci identified in this study, one originated from Mandarin (Ottawa) and six originated from Fiskeby III.
Chromosome map showing marker locations (dashes) and significant quantitative trait loci (non-interacting loci: black circles; interacting loci: grey triangles) for O3 response in the soybean recombinant inbred population Fiskeby III × Mandarin (Ottawa). Associated trifoliate leaf nodes associated with each locus are indicated
Discussion
Abiotic stresses are increasingly important in agriculture, due to the unpredictable and varied effects of climate change. Ozone is among the most insidious of abiotic stresses, because it cannot be anticipated on a seasonal basis, and its phytotoxic effects cannot be remediated. Ozone inhibits plant growth in both wild and cultivated species by reducing rates of photosynthesis and carbon assimilation (Fiscus et al. 2005). Ozone exposure has been shown to reduce yields in several crop species, including soybean (McGrath et al. 2015; Heagle et al. 1998), snap bean (Phaseolus vulgaris L.) (Burkey et al. 2005), wheat (Triticum aestivum L.) (Mishra et al. 2013), and rice (Reid and Fiscus 2008).
Understanding phenotypic variation and identification of relevant genetic loci are critical first steps in breeding for O3 tolerance. Genomic analysis has identified QTL for O3 response in several crop species including rice, Arabidopsis, and poplar (Brosche et al. 2010; Frei et al. 2010; Street et al. 2011). However, no previous reports have been published identifying QTL for O3 response in soybean. Fiskeby III soybean is especially tolerant to O3 and, thus, offers a unique opportunity to employ QTL analysis to identify novel genes and alleles for this trait. In this study, we developed and evaluated a RIL population from the hybridization of ozone-tolerant Fiskeby III × ozone-susceptible Mandarin (Ottawa). The parental lines differed greatly from one another, with Fiskeby III’s injury being well below that of Mandarin (Ottawa). These findings support our previous work characterizing ozone response of these cultivars (Burkey and Carter 2009).
The RILs in the population exhibited a wide range of response to O3, from 0 to 100 %, or close to 100 % injury, for each trifoliate leaf node examined (Table 1). Inspection of the broad phenotypic variation among the RILs and recovery of parental phenotypes in the population suggested that 4–7 genes could be involved in genetic control of O3 response, depending on the genetic model employed. Apparent transgressive segregation was observed in which six of 240 RILs showed less injury at all or most leaf nodes, compared to Fiskeby III (Fig. 1; Supplemental Table 2). Although the parents displayed drastic phenotypic differences responses to O3, our results show that the sensitive Mandarin (Ottawa) contributed a favorable allele to the progeny population.
Marker analysis revealed that seven QTL loci were associated with an individual trifoliate leaf position leaf node, while two loci were associated with injury on multiple leaves. For loci associated with multiple leaves, the QTL identified were for distinct developmental zones within the canopy. A locus on Chromosome 4 was associated with response in the middle to upper canopy (trifoliates T5 and T6), while a locus on Chromosome 17 was associated with ozone response in the lower canopy (trifoliates T2 and T3). With the exception of the locus on Chromosome 17, associated with the lower canopy, most QTL detected were for injury in the middle to upper canopy (trifoliates T4–T6).
Effects of leaf age, position, and variability on screening methodology and QTL analysis
Leaf maturity affects the severity of O3 injury in various crops, with younger leaves sustaining less damage than mature leaves. The influence of leaf age on O3 injury has been well established in physiological studies employing a small number of genotypes in several species including soybean (Cheng et al. 2007; Lee et al. 1984), pumpkin (Cucurbita maxima) (Ciompi et al. 1997), common bean (Phaseolus vulgaris) (Leitao et al. 2003), maize (Zea mays) (Ahmad et al. 2012; Leitao et al. 2007; Pino et al. 1995), and clover (Trifolium spp.) (Karlsson et al. 1995). These studies support our results in which injury scores decreased acropetally within the canopy. We observed this phenomenon consistently across the entire Fiskeby III × Mandarin (Ottawa) RIL population and in the parents, except in those rare instances in which injury scores were either 0 or 100 % throughout the canopy. Our QTL results (Table 5) and the genotypic and biological correlations of injury at various leaf positions (Table 2) indicate that leaf age may also influence genotypic ranking of O3 tolerance within a given population. This leaf age effect can also be seen in the higher heritability of O3 response in the lower canopy leaves (Table 4).
The variation in QTL location with canopy position provides insight into the long-standing observation that O3 injury is more severe for older leaves than for younger leaves. The QTL analysis suggests a complicated mechanism for O3 response where the genes associated with O3 effects are different depending upon leaf developmental stage. The mechanisms underlying this phenomenon have not been studied directly, but there are potential linkages to redox metabolism. Upon movement from air spaces into the liquid phase inside the leaf, O3 initially interacts with the cell wall components and thus the leaf apoplast represents a critical early site in regulating O3 responses (Fiscus et al. 2005). Apoplast ascorbic acid redox status changes significantly throughout leaf development becoming more oxidized with leaf age (Takahama 1998). In soybean plants of similar age as this study, leaf apoplast ascorbic acid was primarily oxidized in trifoliates T2, T4, and T6, but other unknown compounds with redox properties were found to be differentially distributed throughout the canopy (Cheng et al. 2007). This change in leaf apoplast chemistry across leaves of different ages reflects a shift in metabolism. As leaves transition from expanding (trifoliates T5 and T6 in this study) to mature (trifoliates T2 and T3) tissue, the accompanying shift in metabolism could involve different QTLs for controlling ozone response. Given that leaf development occurs quickly (days) relative to the active period of a mature leaf within the canopy (weeks), the QTLs identified for trifoliates T2, T3, and perhaps T4 in this study are more likely to be associated with genes that contribute significantly to the differential O3 sensitivity reflected in sensitive and tolerant germplasm because mature leaves represent a majority of the total leaf area for plants subjected to O3 episodes that occur randomly throughout the growing season. This may explain why the response to O3 is more active in recently matured leaves, compared to the oldest leaves in the canopy (trifoliate T1 in this study) where senescence becomes a factor. In other plant species, leaf age has been shown to play an important role in how individual leaves respond to O3 injury (Bagard et al. 2008; Karlsson et al. 1995; Zhang et al. 2010).
Patterns of data variability differed for each of the parents and for the progeny lines, as observed with the coefficient of variation (CV) for each of the parents and for the RILs as an isolated set (Table 1). The CV is useful in comparing dispersion in data by adjusting for differences in mean values between groups. Overall, higher CVs showed that data dispersion was greater in Fiskeby at all leaf nodes, compared to Mandarin or the RILs as an isolated set. Although Fiskeby’s mean injury scores were among the lowest observed in this study at each leaf node, injury scores exceeding 50 % were observed in some individuals at each leaf node. An increase in variability of injury scores in the upper canopy was observed for the RILs and both parents (Table 1). Coefficients of variation did not substantially change in trifoliates T2–T4 for Mandarin (Ottawa) and for the RILs, with an increase in variability in trifoliates T5 and T6. Fiskeby plants (12 out of 168 individuals tested in this study) with injury scores exceeding 50 % strongly influenced the standard deviation and coefficient of variation in that parent. Variability in Fiskeby’s injury score increased moderately in the lower canopy, and more steeply from trifoliate T5 to trifoliate T6. The observed increase in variability in the upper canopy is likely linked to variation in leaf age above the 5th main stem node across the population.
Multiple stress tolerance
Fiskeby III, developed through classical hybridization and selection breeding efforts in Sweden (Holmberg 1973), is unique in that it has tolerance to multiple abiotic stresses in addition to O3 that include cold (Holmberg 1973; Hume and Jackson 1981), salinity (Lenis et al. 2011) and sulphentrazone herbicide (Hulting et al. 2001). This discovery offers a rare opportunity for dissecting and understanding the underlying physiological and genetic basis for this phenomenon and evaluating the benefit of tolerance mechanisms acting in concert, when challenged with co-occurring stresses. We have taken the first step in this process through dissection of O3 tolerance derived from Fiskeby III. Since elevated O3 is episodic, its effects often combine in commercial settings with those of chronic stresses, such as drought, extreme temperature, nutrient deficiencies or toxicities, and biotic stresses. Understanding combined stresses is challenging because plant response to multiple stresses is thought to be interactive and not well predicted by responses to the individual stresses (Mittler 2006). Although crop plants are routinely exposed to combined stress in field situations, research in this area has been limited.
Implications to plant breeding
As a factor that significantly reduces crop yields (McGrath et al. 2015), O3 stress is an issue in agriculture that merits additional attention by plant breeders. Although short term exposures to evaluate foliar injury do not assess O3 impacts on seed yield, there are studies showing yield loss in soybean is associated with foliar symptoms. Greater foliar injury reported for cultivar Forrest relative to cultivar Essex (Cheng et al. 2007) was associated with a reduction in seed yield when these cultivars were exposed to elevated O3 throughout the growing season (Robinson and Britz 2000). In an earlier study, yield loss was associated with foliar injury in three of four soybean cultivars tested by Heagle and Letchworth (1982). This study upholds previous results of genetic resistance to O3 injury for Fiskeby III (Burkey and Carter 2009). The QTL analysis showed that resistance is controlled by multiple loci and suggests that soybean breeders should consider using relatively large RIL populations (more than 200 members) to recover progeny as resistant as the resistant parent. The multiple QTL loci identified in this study require validation and refinement to ensure their best use in marker assisted selection programs. However, they offer the clear promise of aiding breeders in manipulating the O3 resistance trait, which is currently difficult and expensive to measure. Phenotyping is widely recognized as a critical bottleneck in the discovery of gene function, and its subsequent application to plant breeding efforts. Development of phenotyping rubrics that are accurate, rapid, and inexpensive is an ongoing challenge in crop science. However, results of the present study provide some insight into the refinement of screening approaches for detecting O3 resistance. For example, independent observers assigned scores that were highly correlated and, thus, we did not gain much precision from using multiple scorers. Good assessment of foliar injury was obtained using trifoliate leaves in the mid-canopy of soybean, where accumulated injury was great and genotypic differences were easily distinguished. Young leaves were less useful for rating. Injury was assessed separately for each trifoliate leaf in the current study. However, plant breeders actively involved in plant selection can likely evaluate O3 foliar injury accurately by assigning a single overall visual score to the leaves in the middle canopy of a plant. The optimum time to phenotype for O3 response in our chambers occurred when plants had 3–8 fully expanded trifoliate leaves. Prior to this, plants did not have sufficient mature leaf area to evaluate injury. Heritability estimates suggest that five replications over time are sufficient to raise precision of ratings to the point that half the variability among RILs can be attributed to genetics rather than genotype × environment and/or experimental error. Our study did not permit an examination of the efficiency of dropping replications over time in favor of growing additional replications of plants and chambers simultaneously. However, it is likely that more simultaneous replications will have some utility in screening efforts. These observations may help those interested in breeding or studying O3 resistance to avoid factors that may complicate scoring of foliar injury, such as shade-induced senescence in older plants, immature leaves, and nutrient issues in the upper and lower canopy.
Author contribution statement
All authors contributed to the review and editing of the manuscript. AB participated in experimental design, carried out collection, analysis, and interpretation of data, and drafted the manuscript. KB participated in the design and implementation of phenotyping experiments, data interpretation, as well as oversight of the CSTR exposure system. TC participated in the development of the RIL population, experimental design, and data interpretation. JO participated in the development of the RIL population including phenotyping for agronomic traits and maintained the seed for phenotyping and genotyping. PC conducted the Illumina GoldenGate SNP genotyping assays.
Abbreviations
- CF:
-
Charcoal filtered
- CP:
-
Coefficient of parentage
- CSTR:
-
Continuously stirred tank reactors
- LOD:
-
Logarithm of odds
- O3 :
-
Ozone
- PPFD:
-
Photosynthetic photon flux density
- RIL:
-
Recombinant inbred line
- SNP:
-
Single nucleotide polymorphism
- TriMid:
-
Mid-canopy trifoliate leaves, trifoliates 3, 4 and 5
- T1, T2, etc.:
-
First trifoliate leaf to expand, second trifoliate leaf to expand, etc.
- QTL:
-
Quantitative trait loci
References
Ahmad R, Zuily-Fodil Y, Passaquet C, Bethenod O, Roche R, Repellin A (2012) Ozone and aging up-regulate type II metacaspase gene expression and global metacaspase activity in the leaves of field-grown maize (Zea mays L.) plants. Chemosphere 87:789–795. doi:10.1016/j.chemosphere.2011.12.081
Ainsworth EA, Yendrek CR, Sitch S, Collins WJ, Emberson LD (2012) The effects of tropospheric ozone on net primary productivity and implications for climate change. In: Merchant SS (ed) Annual review of plant biology, vol 63. Annual Reviews, Palo Alto, California, pp 637–661
Bagard M, Le Thiec D, Delacote E, Hasenfratz-Sauder MP, Banvoy J, Gerard J, Dizengremel P, Jolivet Y (2008) Ozone-induced changes in photosynthesis and photorespiration of hybrid poplar in relation to the developmental stage of the leaves. Physiol Plant 134:559–574. doi:10.1111/j.1399-3054.2008.01160.x
Brim CA (1966) A modified pedigree method of selection in soybean. Crop Sci 6:220
Brim CA (1973) Quantitative genetics. In: Caldwell BE, Howell RW (eds) Soybeans: improvement, production, and uses. American Society of Agronomy. Madison, WI, pp 155–186
Broman KW, Wu H, Sen S, Churchill GA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19:889–890. doi:10.1093/bioinformatics/btg112
Brosche M, Merilo E, Mayer F, Pechter P, Puzorjova I, Brader G, Kangasjarvi J, Kollist H (2010) Natural variation in ozone sensitivity among Arabidopsis thaliana accessions and its relation to stomatal conductance. Plant Cell Environ 33:914–925. doi:10.1111/j.1365-3040.2010.02116.x
Burkey KO, Carter TE (2009) Foliar resistance to ozone injury in the genetic base of US and Canadian soybean and prediction of resistance in descendent cultivars using coefficient of parentage. Field Crops Res 111:207–217. doi:10.1016/j.fcr.2008.12.005
Burkey KO, Miller JE, Fiscus EL (2005) Assessment of ambient ozone effects on vegetation using snap bean as a bioindicator species. J Environ Qual 34:1081–1086. doi:10.2134/jeq2004.0008
Cheng FY, Burkey KO, Robinson JM, Booker FL (2007) Leaf extracellular ascorbate in relation to O(3) tolerance of two soybean cultivars. Environ Pollut 150:355–362. doi:10.1016/j.envpol.2007.01.022
Ciompi S, Castagna A, Ranieri A, Nali C, Lorenzini G, Soldatini GF (1997) CO2 assimilation, xanthophyll cycle pigments and PSII efficiency in pumpkin plants as affected by ozone fumigation. Physiol Plant 101:881–889. doi:10.1034/j.1399-3054.1997.1010428.x
Damicone JP, Manning WJ, Herbert SJ, Feder WA (1987) Foliar sensitivity of soybeans from early maturity groups to ozone and inheritance of injury response. Plant Dis 71:332–336. doi:10.1094/pd-71-0332
Fan JB, Gunderson KL, Bibikova M, Yeakley JM, Chen J, Garcia EW, Lebruska LL, Laurent M, Shen R, Barker D (2006) Illumina universal bead arrays. In: Kimmel A, Oliver B (eds) DNA microarrays part A: array platforms and wet-bench protocols. Elsevier Academic Press Inc, San Diego, pp 57–73
Federer WT (1956) Augmented (or hoonuiaku) designs. Hawaii Plant Rec LV(2):191–208
Fiscus EL, Booker FL, Burkey KO (2005) Crop responses to ozone: uptake, modes of action, carbon assimilation and partitioning. Plant Cell Environ 28:997–1011. doi:10.1111/j.1365-3040.2005.01349.x
Fowler D (2008) Ground-level ozone in the 21st century: future trends, impacts and policy implications. The Royal Society, London
Frei M, Tanaka JP, Wissuwa M (2008) Genotypic variation in tolerance to elevated ozone in rice: dissection of distinct genetic factors linked to tolerance mechanisms. J Exp Bot 59:3741–3752. doi:10.1093/jxb/ern222
Frei M, Tanaka JP, Chen CP, Wissuwa M (2010) Mechanisms of ozone tolerance in rice: characterization of two QTLs affecting leaf bronzing by gene expression profiling and biochemical analyses. J Exp Bot 61:1405–1417. doi:10.1093/jxb/erq007
Fuhrer J (2003) Agroecosystern responses to combinations of elevated CO2, ozone, and global climate change. Agric Ecosyst Environ 97:1–20. doi:10.1016/s0167-8809(03)00125-7
Gizlice Z, Carter TE Jr, Burton JW (1994) The genetic base for North American public soybean cultivars released between 1947 and 1988. Crop Sci 34:1143–1151
Heagle AS (1979) Ranking of soybean cultivars for resistance to ozone using different ozone doses and response measures. Environ Pollut 19:1–10. doi:10.1016/0013-9327(79)90108-3
Heagle AS, Letchworth MB (1982) Relationships among injury, growth, and yield responses of soybean cultivars exposed to ozone at different light intensities. J Environ Qual 11:690–694
Heagle AS, Miller JE, Pursley WA (1998) Influence of ozone stress on soybean response to carbon dioxide enrichment: III. Yield and seed quality. Crop Sci 38:128–134
Heck WW, Philbeck RB, Dunning JA (1978) A continuous stirred tank reactor (CSTR) system for exposing plants to gaseous air contaminants: principles, specifications, construction, and operation. USDA Agricultural Research Service, Washington, DC, USA, pp 32
Holmberg SA (1968) Research on soybeans at Fiskeby. Sweden's Rapeseed Growers' Anniversary Paper, Special Edition:190–199
Holmberg SA (1973) Soybeans for cool temperate climates. Agric Hort Genetica 31:1–20
Hulting AG, Wax LM, Nelson RL, Simmons FW (2001) Soybean (Glycine max (L.) Merr.) cultivar tolerance to sulfentrazone. Crop Prot 20:679–683
Hume DJ, Jackson AKH (1981) Pod formation in soybeans at low-temperatures. Crop Sci 21:933–937
Hyten DL, Choi IY, Song QJ, Specht JE, Carter TE, Shoemaker RC, Hwang EY, Matukumalli LK, Cregan PB (2010) A high density integrated genetic linkage map of soybean and the development of a 1536 universal soy linkage panel for quantitative trait locus mapping. Crop Sci 50:960–968. doi:10.2135/cropsci2009.06.0360
Karlsson GP, Sellden G, Skarby L, Pleijel H (1995) Clover as an indicator plant for phytotoxic ozone concentrations-Visible injury in relation to species, leaf afge, and exposure dynamics. New Phytol 129:355–365. doi:10.1111/j.1469-8137.1995.tb04306.x
Langebartels C, Wohlgemuth H, Kschieschan S, Grun S, Sandermann H (2002) Oxidative burst and cell death in ozone-exposed plants. Plant Physiol Biochem 40:567–575. doi:10.1016/s0981-9428(02)01416-x
Lee EH, Jersey JA, Gifford C, Bennett J (1984) Differential ozone tolerance in soybean and snapbeans—analysis of ascorbic-acid in O3-susceptible and O3-resistant cultivars by high-performance liquid chromotography. Environ Exp Bot 24:331. doi:10.1016/0098-8472(84)90030-3
Leitao L, Goulas P, Biolley JP (2003) Time-course of Rubisco oxidation in beans (Phaseolus vulgaris L.) subjected to a long-term ozone stress. Plant Sci 165:613–620. doi:10.1016/s0168-9452(03)00230-9
Leitao L, Delacote E, Dizengremel P, Le Thiec D, Biolley JP (2007) Assessment of the impact of increasing concentrations of ozone on photosynthetic components of maize (Zea mays L.), a C-4 plant. Environ Pollut 146:5–8. doi:10.1016/j.envpol.2006.05.019
Lenis JM, Ellersieck M, Blevins DG, Sleper DA, Nguyen HT, Dunn D, Lee JD, Shannon JG (2011) Differences in ion accumulation and salt tolerance among Glycine accessions. J Agron Crop Sci 197:302–310
Lobell DB, Gourdji SM (2012) The influence of climate change on global crop productivity. Plant Physiol 160:1686–1697. doi:10.1104/pp.112.208298
McGrath JM, Betzelberger AM, Wang S, Shook E, Zhu X-G, Long SP, Ainsworth EA (2015) An analysis of ozone damage to historical maize and soybean yields in the United States. Proc Natl Acad Sci USA. doi:10.1073/pnas.1509777112
Mishra AK, Rai R, Agrawal SB (2013) Differential response of dwarf and tall tropical wheat cultivars to elevated ozone with and without carbon dioxide enrichment: growth, yield and grain quality. Field Crops Res 145:21–32. doi:10.1016/j.fcr.2013.02.007
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19. doi:10.1016/j.tplants.2005.11.002
Mulchi CL, Lee E, Tuthill K, Olinick EV (1988) Influence of ozone stress on growth processes, yields and grain quality characteristics among soybean cultivars. Environ Pollut 53:151–169. doi:10.1016/0269-7491(88)90031-0
Piepho HP, Buchse A, Emrich K (2003) A hitchhiker’s guide to mixed models for randomized experiments. J Agron Crop Sci 189:310–322
Pino ME, Mudd JB, Baileyserres J (1995) Ozone-induced alterations in the accumulation of newly synthesized proteins in the leaves of maize. Plant Physiol 108:777–785
Prather M, Ehhalt D, Dentener F, Derwent R, Dlugokencky E, Holland EA, Isaksen I, Katima J, Kirchhoff V, Matson P, Midgley P, Wang M (2001) Atmospheric chemistry and greenhouse gases. In: Houghton JT et al (eds) Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge, UK, pp 239–287
R Development CoreTeam (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reich PB (1987) Quantifying plant response to ozone: a unifying theory. Tree Physiol 3:63–91
Reid CD, Fiscus EL (2008) Ozone and density affect the response of biomass and seed yield to elevated CO2 in rice. Glob Change Biol 14:60–76. doi:10.1111/j.1365-2486.2007.01472.x
Robinson JM, Britz SJ (2000) Tolerance of a field grown soybean to elevated ozone level is concurrent with higher leaflet ascorbic acid level, higher ascorbate-dehydroascorbate redox status, and long term photosynthetic productivity. Photosynth Res 64:77–87
Rogers HH, Jeffries HE, Stahel EP, Heck WW, Ripperton LA, Witherspoon AM (1977) Measureing air pollutant uptake by plants: direct kinetic technique. J Air Pollut Control Assoc 27:1192–1197
Saitanis CJ, Riga-Karandinos AN, Karandinos MG (2001) Effects of ozone on chlorophyll and quantum yield of tobacco (Nicotiana tabacum L.) varieties. Chemosphere 42:945–953. doi:10.1016/s0045-6535(00)00158-2
Salvatori E, Fusaro L, Mereu S, Bernardini A, Puppi G, Manes F (2013) Different O3 response of sensitive and resistant snap bean genotypes (Phaseolus vulgaris L.): the key role of growth stage, stomatal conductance, and PSI activity. Environ Exp Bot 87:79–91. doi:10.1016/j.envexpbot.2012.09.008
Street NR, Tallis MJ, Tucker J, Brosche M, Kangasjarvi J, Broadmeadow M, Taylor G (2011) The physiological, transcriptional and genetic responses of an ozone- sensitive and an ozone tolerant poplar and selected extremes of their F2 progeny. Environ Pollut 159:45–54. doi:10.1016/j.envpol.2010.09.027
Takahama U (1998) Ascorbic acid-dependent regulation of redox levels of chlorogenic acid and its isomers in the apoplast of leaves of Nicotiana tabacum L. Plant Cell Physiol 39:681–689
Taylor GE, Tingey DT, Ratsch HC (1982) Ozone flux in Glycine max (L) Merr.—Sites of regularion and relationship to leaf injury. Oecologia 53:179–186. doi:10.1007/bf00545661
Zhang JW, Schaub M, Ferdinand JA, Skelly JM, Steiner KC, Savage JE (2010) Leaf age affects the responses of foliar injury and gas exchange to tropospheric ozone in Prunus serotina seedlings. Environ Pollut 158:2627–2634. doi:10.1016/j.envpol.2010.05.003
Acknowledgments
The authors recognize the excellent technical assistance of Jeff Barton for maintenance and operation of the CSTR exposure systems and for evaluation of foliar injury and Renee Tucker for plant management. We also thank Dr. Costas Saitanis for his valuable input and discussion.
Funding
This work was supported by Grants #0286 and #1320-532-5610 from the United Soybean Board.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The research described in this paper complies with the current laws of the country in which it was performed.
Additional information
Communicated by V. Hahn.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Burton, A.L., Burkey, K.O., Carter, T.E. et al. Phenotypic variation and identification of quantitative trait loci for ozone tolerance in a Fiskeby III × Mandarin (Ottawa) soybean population. Theor Appl Genet 129, 1113–1125 (2016). https://doi.org/10.1007/s00122-016-2687-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-016-2687-1