Abstract
Key message
Greatest potential, QTLs for hypoxia and waterlogging tolerance in soybean roots were detected using a new phenotypic evaluation method.
Abstract
Waterlogging is a major environmental stress limiting soybean yield in wet parts of the world. Root development is an important indicator of hypoxia tolerance in soybean. However, little is known about the genetic control of root development under hypoxia. This study was conducted to identify quantitative trait loci (QTLs) responsible for root development under hypoxia. Recombinant inbred lines (RILs) developed from a cross between a hypoxia-sensitive cultivar, Tachinagaha, and a tolerant landrace, Iyodaizu, were used. Seedlings were subjected to hypoxia, and root development was evaluated with the value change in root traits between after and before treatments. We found 230 polymorphic markers spanning 2519.2 cM distributed on all 20 chromosomes (Chrs.). Using these, we found 11 QTLs for root length (RL), root length development (RLD), root surface area (RSA), root surface area development (RSAD), root diameter (RD), and change in average root diameter (CARD) on Chrs. 11, 12, 13 and 14, and 7 QTLs for hypoxia tolerance of these root traits. These included QTLs for RLD and RSAD between markers Satt052 and Satt302 on Chr. 12, which are important markers of hypoxia tolerance in soybean; those QTLs were stable between 2 years. To validate the QTLs, we developed a near-isogenic line with the QTL region derived from Iyodaizu. The line performed well under both hypoxia and waterlogging, suggesting that the region contains one or more genes with large effects on root development. These findings may be useful for fine mapping and positional cloning of gene responsible for root development under hypoxia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Waterlogging is a major environmental stress limiting soybean yield in the monsoonal areas of Asia (Kokubun 2013), as well as in other parts of the world, such as the Mississippi Delta of the USA (Linkemer et al. 1998; VanToai et al. 2010) and the lowlands of Brazil’s Rio Grande (Pedó et al. 2015). Soybean is vulnerable to waterlogging, which decreases photosynthesis (Oosterhuis et al. 1990; VanToai et al. 1994; Cho and Yamakawa 2006), reduces nitrogen fixation (VanToai et al. 1994; Shimamura et al. 2003; Henshaw et al. 2007a), and lessens biomass and grain yield (Bacanamwo and Purcell 1999; Henshaw et al. 2007b; Rhine et al. 2010). Waterlogging stress can happen during any growing stage, but waterlogging in the early stage results in substantial reduction of grain yield (Kokubun 2013). Therefore, soybean cultivars with strong waterlogging tolerance are desired in regions where rainfall is heavy during early plant growth.
Soil hypoxia (Hossain and Uddin 2011) is a major problem associated with waterlogging because of the slow diffusion of oxygen in water (Armstrong 1980; Wiengweera et al. 1997; Armstrong and Drew 2002). Hypoxia promotes root respiration (Drew 1992). The root system of plants usually responds to waterlogging by staying shallow (Sallam and Scott 1987). As the root system determines aboveground growth, both root and shoot growth are, therefore, inhibited by waterlogging (Sallam and Scott 1987; Shimamura et al. 2003; Araki et al. 2012; Sauter 2013). Therefore, the ability of roots to develop is an important indicator of hypoxia tolerance.
A common response of plants to a lack of oxygen is the production of adventitious roots (Colmer and Voesenek 2009), which can contribute to plant growth under waterlogging stress (Rich et al. 2012). Adventitious roots develop in wheat (Mano and Omori 2007), maize (Zaidi et al. 2003), tomato (McNamara and Mitchell 1990), and soybean (Thomas et al. 2005; Bacanamwo and Purcell 1999; Shimamura et al. 2003; Henshaw et al. 2007a) under waterlogging. However, another crucial aspect is the ability to maintain root development under hypoxia, as seen in seedlings of waterlogging-tolerant soybeans which were able to maintain root elongation and root branching under hypoxia (Sakazono et al. 2014).
Previous studies have measured root morphological traits under waterlogging (Bacanamwo and Purcell 1999; Shimamura et al. 2003; Henshaw et al. 2007a; Sakazono et al. 2014), but measurements at the seedling stage were not accurate because of differences in seed weight, which varies among soybean genotypes (Kaga et al. 2012), and in plant size, which is significantly influenced by seed weight (Vandamme et al. 2016). Therefore, to assess the effects of hypoxia on soybean root traits correctly, we evaluated changes in root traits between before and after treatments.
Numerous studies have reported QTLs related to waterlogging tolerance in soybean (VanToai et al. 2001; Reyna et al. 2003; Cornelious et al. 2005; Githiri et al. 2006; Sayama et al. 2009; Nguyen et al. 2012), but most focused on indirect measures, such as injury score and tolerance index. For example, a QTL associated with better soybean growth and grain yield under waterlogging was identified on chromosome (Chr.) 18 near marker Sat_064 (VanToai et al. 2001). QTLs for flooding injury score and tolerance index were found and mapped on Chrs. 3, 5, 12, 13, and 16 (Cornelious et al. 2005), Chrs. 2, 6, 7, 10, 11, 14, and 19 (Githiri et al. 2006), and Chrs. 11 and 13 (Nguyen et al. 2012). Although many QTL studies of waterlogging tolerance in soybean have been reported, QTLs specific to root development characteristics under both hypoxia and waterlogging have not yet been reported.
Dr. Kohsei Tabuchi at Hokuriku National Agricultural Research Center evaluated waterlogging tolerance of soybean germplasm, mainly originated from Japan under field conditions (personal communication, 2001). Field-grown plants were waterlogged for 5 weeks beginning at 5 weeks after sowing. Tolerance was evaluated based on seed yield reduction in response to treatments, leaf color under treatments and observation at maturity. Most germplasm classified as tolerant were late-maturing probably because late maturity confers a longer growth period for recovery from flooding stress (Githiri at al. 2006). Among them, Iyodaizu (accession number of Genetic Resources Center, NARO: JP29488) was classified into tolerant group with medium maturity (maturity group IV). Iyodaizu was also hypoxia-tolerant under our capillary watering system without soil medium (Sakazono et al. 2014). Therefore, we conducted this study to identify QTLs for root development using F8:9 recombinant inbred lines (RILs) derived from a cross between Iyodaizu and hypoxia-susceptible cultivars. In addition, we developed near-isogenic lines (NILs) carrying target QTLs from backcrossed lines using marker-assisted selection (MAS) and then examined their root development under both hypoxia and waterlogging.
Materials and methods
Plant materials
For QTL analysis, we used a population of 94 F8:9 RILs derived from a cross between Iyodaizu (100-seed weight 13 g), and Tachinagaha (100-seed weight 36 g), the leading Japanese cultivar in the Kanto region (accession number: JP67666). Iyodaizu is hypoxia-tolerant, and Tachinagaha is hypoxia-susceptible (Sakazono et al. 2014). RILs were developed without any selection by the single-seed descent method from the F1 plant. To develop NILs carrying target QTLs, we conducted recurrent backcrossing to Tachinagaha using MAS. We genotyped the BC5F2, BC6F1, and BC6F2 generations with nine simple sequence repeat markers: Satt192, Satt442, Sat_334, Satt541, Satt469, Satt052, Sat_206, Satt676, and Satt302. A homozygous line designated NIL-9-4-5 (100- seed weight 26 g) carried the fixed target QTLs. The QTLs were validated under hypoxia and waterlogging conditions.
Plant culture and hypoxia treatment
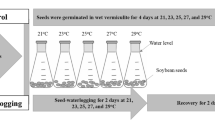
The RILs were tested under hypoxia in 2014 and under both hypoxia and normoxia in 2015. To ensure a uniform rate of germination, we incubated eight seeds of each genotype per Petri dish at 23 °C and 70% RH for 3 days (Fig. 1a). The seeds were then treated with a fungicide (benomyl, ½ of dry seed weight), sown in moist vermiculite (Midorisangyou, Fukuoka, Japan) in plastic cups, and held at 23 °C for 2 days. Uniform seedlings of each genotype were then selected for treatment. The seedlings were inserted through holes punched in a 7-mm-thick polystyrene board and held upright with plugs of silicone rubber. Each board, holding 20 seedlings, was placed over an opaque plastic container (386 mm × 256 mm × 135 mm), with the roots in deionized distilled water continuously aerated at 1.0 L min−1 by air pumps (Fig. 1b). The plants were first grown for 4 days at 23 °C in a growth chamber (220 µmol m2 s−1 light density, 14 h light/10 h dark, 23 °C). Then at 6 days after sowing (DAS), the plants were grown for 7 days under either normoxia (control) or hypoxia. Under normoxia, seedlings were grown in deionized distilled water aerated by two air pumps at opposite ends of the container to maintain a dissolved oxygen (DO) concentration of >7.0 mg L−1 (Fig. 1b). Under hypoxia, seedlings were grown in 0.1% (w/v) agar solution in deionized distilled water that was deoxygenated with a continuous flow of nitrogen to maintain a DO concentration of <1.0 mg L−1 (Fig. 1c). DO was measured with a DO meter (CM-51, Horiba, Kyoto, Japan) at the start (6 DAS) and end of treatment (13 DAS). The experiments were conducted in 3 replications with the randomized complete block method. Five plants from each RIL were used as an experimental unit.
Target QTLs were validated under both hypoxia and waterlogging. The hypoxia and normoxia experiments were conducted in a growth chamber as the same method described above. The waterlogging experiments were conducted in a greenhouse at the Experimental Farm of Kyushu University, Fukuoka, Japan (33°37′N, 130°27′E). The seeds of parents and NILs were germinated as above. Seedlings were transplanted into pots containing 0.5 kg of dried soil (20.7% clay, 23.2% silt, and 56.1% sand; sandy clay-loam) on 25 April 2015. The pots were then put in a tray (924 mm × 610 mm × 200 mm) in water maintained at a 2.0-cm depth. At 4 DAS, the seedlings were thinned to one representative seedling per pot. At 6 DAS, seedlings were grown for 7 days under either drained (control) or waterlogging conditions. Under control conditions, the water level was maintained at 2.0 cm depth. Under waterlogging conditions, it was maintained at 2.0 cm above the soil surface. All experiments had five replicates arranged in a randomized complete block design. Single plants were used as an experimental unit.
Measurement of root development traits
Root traits were measured before and after treatments. The root systems of individual plants were scanned with an image scanner (GTX-970 scanner, Epson, Nagano, Japan) under the control of WinRhizo software (Regent Instruments, Inc., Quebec, Canada). According to WinRhizo regent instruments, total root length (RL) of root system was measured on the so-called skeleton images. Root diameter (RD) was measured continuously along the root and classified into ten groups with unequal width (>0 and \(\leqq\)0.5, >0.5 and \(\leqq\)1.0, >1.0 and \(\leqq\)1.5, >1.5 and \(\leqq\)2.0, >2.0 and \(\leqq\)2.5, >2.5 and \(\leqq\)3.0, >3.0 and \(\leqq\)3.5, >3.5 and \(\leqq\)4.0, >4.0 and \(\leqq\)4.5, and >4.5 mm). Root surface area (RSA) was automatically calculated by software based on root length and root diameter of root in each class. Root length development (RLD), root surface area development (RSAD), and the change in average root diameter (CARD) were calculated as the change in value of traits between when treatments were started and when treatments ended. Roots and shoots were dried in oven at 80 °C until constant weight. Then root dry weight (RDW) and shoot dry weight (SDW) were determined. We calculated a “hypoxia tolerance index” (HTI, %) for each trait as hypoxia value/normoxia value × 100.
Linkage map construction
DNA was extracted from fresh soybean leaves by the CTAB method (Murray and Thompson 1980). The simple sequence repeat (SSR) markers developed by USDA (Song et al. 2004) or by the Kazusa DNA Research Institute (Hisano et al. 2007) were used to evaluate polymorphism among parents. Polymorphic markers were used to construct a linkage map from the 94 RILs. The PCR mixture contained 10 ng of genomic DNA, 2.25 pmol of primer, 625 pmol of nucleotides, and 0.125 unit of ExTaq in 1× ExTaq buffer supplied by the manufacturer (Takara Bio) in a total volume of 5 µL. The PCR conditions consisted of an initial 5 min at 95 °C; 40 cycles of 30 s at 95 °C, 30 s at 55 °C, and 30 s at 72 °C; and a final 5 min at 72 °C. The products were stored at 10 °C and then separated in 8% polyacrylamide gels. The linkage map was constructed in MAPMAKER/EXP v.3 software (Lander et al. 1987).
QTL mapping
QTL analysis was performed by composite interval mapping in QTL Cartographer v. 2.5 software (Wang et al. 2007). Background cofactors with a 10-cM window were associated using forward and backward regression (0.05 confidence level). The LOD threshold for each trait was calculated using 1000 permutations corresponding to a genome-wide significance level of 0.05.
Statistical analysis
Statistics were analyzed in SYSTAT 13 software (SYSTAT Software, Inc., San Jose, CA, USA). The effects of genotype and hypoxia treatment on root development traits were analyzed by two-way ANOVA. The t test was used to compare the average values among treatments.
Results
Variance analysis
In 2014, genotype had a significant effect on RD, CARD, RDW, and SDW, but not on RL, RLD, RSA, or RSAD (Table 1). Hypoxia had a significant effect on all traits except SDW. The genotype by hypoxia interaction had a significant effect on all traits except CARD and SDW. In 2015, genotype had a significant effect on all traits except for CARD. Hypoxia and the genotype by hypoxia interaction had a significant effect on all traits except SDW.
Among measured traits, SDW was only affected by genotype but not by hypoxia or the genotype by hypoxia interaction in both years.
Root development traits
The root morphology (RL, RSA) and root development traits (RLD, RSAD) of the parents significantly differed between normoxia and hypoxia (Fig. 2; Table 2). Under normoxia, values of these traits of Tachinagaha were much higher than those of Iyodaizu. In contrast, under hypoxia, values of Iyodaizu were higher than those of Tachinagaha except for RSA in 2015. Hypoxia reduced RL and RSA of Tachinagaha by 54–56 and 47–50%, respectively, relative to normoxia, but increased those of Iyodaizu by 18–20 and 25–33%. RD of both tended to increase under hypoxia (HTI >1). Hypoxia reduced RLD and RSAD of Tachinagaha by 74–75 and ~67%, respectively, but increased those of Iyodaizu by 11–21 and ~34% respectively. The frequency distributions of RLD and RSAD showed continuous variation in the RIL population under hypoxia; the values of Iyodaizu were the same as or slightly higher than the means of the RIL population, but those of Tachinagaha were much lower than the mean (Fig. 3).
Genetic linkage map
Of the surveyed SSR markers, 230 showed distinct polymorphism among parents and were used for linkage mapping. Among the 94 RILs, 3 RILs had markers with genotypes different from the parents and were considered to have been the result of outcrossing, so we excluded them from analysis. A genetic linkage map consisting of 20 linkage groups (LGs) spanning 2519.2 cM with an average distance of 10.95 cM between makers was constructed. The order of the markers was in line with the published soybean integrated genetic map (http://soybase.org/).
QTLs for root development traits
We performed QTL analysis for RL, RLD, RSA, RSAD, RD, and CARD in 2014 and 2015. We found a total of 11 QTLs on Chromosomes (Chrs.) 11 (Qcard-11), 12 (Qrl-12, Qrld-12, Qrsad-12, Qrd-12), 13 (Qrl-13, Qrsa-13), and 14 (Qrl-14, Qrd-14, Qcard-14) (Table 3; Fig. 4). Three QTLs were associated with RL under hypoxia; two of these were found on Chrs. 14 (LG B2) and 13 (LG F) in 2014, and the other on Chr. 12 (LG H) in 2015. A QTL for RLD was found on Chr.12 (LG H) in 2014 and 2015. A QTL for RSA was found on Chr. 13 (LG F) in 2014. A QTL for RSAD was found on Chr. 12 (LG H) in 2014 and 2015. Three QTLs for RD were found on Chr. 12 (LG H) and Chr. 14 (LG B2), one of them in 2014 under hypoxia and the other two in 2015 under normoxia (Chr. 14) and hypoxia (Chr. 12). Two QTLs for CARD were found on Chrs. 11 (LG B1) and 14 in 2015.
Molecular linkage map of the RIL population derived from Tachinagaha × Iyodaizu, and summary of QTLs for all traits responsive to hypoxia in the mapping population. Q QTL, rl root length, rld root length development, rsad root surface area development, rd root diameter, card change in average root diameter. In all QTL names, the first number represents the chromosome location; black, QTLs found in 2014; red, QTLs found in 2015. Markers are listed to the right and the distances from the top of each linkage group are listed to the left. Length of vertical bars is equal to the 1-LOD likelihood confidence interval. Horizontal lines in the middle of the bars indicate the positions of the QTL peaks. (Color figure online)
Only the QTLs for RLD and RSAD were detected stably in both years (Table 3; Fig. 4). Both were located in the same marker interval on Chr. 12 (LG H). The single QTL associated with RLD under hypoxia had LOD scores of 3.97 in 2014 and 5.12 in 2015. The Iyodaizu allele had a positive effect at this locus (Table 3). The proportion of phenotypic variation explained by this QTL was 13% in 2014 and 19% in 2015. One QTL was detected for RSAD under hypoxia in the same region as Qrld-12, and the Iyodaizu allele again had a positive effect. The proportion of phenotypic variation it explained was 13% in 2014 and 11% in 2015.
QTLs for HTI of root traits
Seven QTLs were mapped for hypoxia-tolerant index (HTI) of root traits on chromosomes: Chr. 4 (Qhti-4), Chr. 9 (Qhti-9), Chr. 12 (Qhti-12-1 and Qhti-12-2), and Chr. 14 (Qhti-14-1, Qhti-14-2 and Qhti-14-3) in this study (Table 4). These QTL accounted for 10–18% of phenotypic variation. Chr. 12 and Chr.14 were more important than other of these, with greater additive effect and higher explanation of phenotypic variation as compared with other chromosomes detected.
Validation of root development QTLs under hypoxia and waterlogging
To verify the effects of root development QTLs (Qrld-12 and Qrsad-12), we compared root development traits of NIL-9-4-5 with those of the recurrent parent Tachinagaha by t test (Fig. 5). Under hypoxia, RLD and RSAD of Tachinagaha were greatly decreased, but those of Iyodaizu and NIL-9-4-5 showed no decrease. Under waterlogging, RLD and RSAD of Tachinagaha were reduced by more than those of Iyodaizu. NIL-9-4-5 maintained root development under waterlogging.
Discussion
Waterlogging hampers both shoot and root growth (Sallam and Scott 1987; Araki et al. 2012), reducing root growth first (Shimamura et al. 2003; Sauter 2013). Bacanamwo and Purcell (1999) concluded that the shoot biomass of a soybean plant was unaltered during the first 7 days of waterlogging, but decreased relative to the control by 21 days. Sakazono et al. (2014) reported the same trend in shoot and root responses to hypoxia. Our results show that the effects of hypoxia on RDW were significant, whereas the effects of hypoxia and the genotype by hypoxia interaction on SDW were not (Table 1). These results indicate that root responses to hypoxia might reflect hypoxia tolerance in soybean under short-term stress.
There are some main parallels between hypoxia under agar solution and soil waterlogged environment. Wiengweera et al. (1997) used 0.1% agar solution to simulate the low diffusion of oxygen in waterlogged soil. The results showed that oxygen concentration rapidly decreased below 1.0 mg L− 1, while ethylene increased during treatment; such changes were similar to those described for waterlogged soil in a field environment by Trought and Drew (1980). This suggests that hypoxia tolerance of plants under hydroponic culture reflected hypoxia tolerance under waterlogged soil in a field environment. Under waterlogging, RL and RDW of soybean plant are inhibited (Sallam and Scott 1987; Shimamura et al. 2003; Henshaw et al. 2007a; Yamane and Iijima 2016). RL in maize also reflected its waterlogging tolerance under field conditions (Souza et al. 2012). These symptoms were also observed in other species (Visser et al. 2000; Colmer 2003; Yamauchi et al. 2014). Under hypoxia, root traits of soybean, especially RL and RSA, were dramatically inhibited and RD increased (Sakazono et al. 2014). Tachinagaha and Iydaizu were used in both of hypoxia and waterlogging studies. Iyodaizu was reported with tolerance (Sakazono et al. 2014; Jitsuyama 2015) and Tachinagaha was a moderately/susceptible cultivar (Sayama et al. 2009; Sakazono et al. 2014; Jitsuyama 2015). Furthermore, the relationship between cultivar-specific hypoxia response and waterlogging tolerance was reported by Jitsuyama (2015).The results showed that the strong positive correlation between RL and field waterlogging-tolerant index was found among 12 studied genotypes including Tachinagaha and Iydaizu, while other traits, such as dry weight, root diameter, number of root tips were not observed (Jitsuyama 2015). In this paper, our results show that RD and CARD of the parents tended to increase under hypoxia. Root development (RLD and RSAD) of Tachinagaha decreased by 59–74%; in contrast, root traits and their development of Iyodaizu tended to increase under hypoxia (Table 2). The effects of hypoxia on the RIL population were similar to those on the parents: RLD and RSAD showed the same responses to hypoxia in the parents and the RIL population, indicating that root development is a mechanism of hypoxia tolerance.
Size of soybean seed depends on cultivars, and seedling growth is positively correlated with seed size (Kaga et al. 2012; Vandamme et al. 2016). To avoid this autocorrelation, we measured the changes in root traits between before and after treatments. This method showed that under hypoxia, RLD and RSAD of Tachinagaha were reduced by 67–75%, but RL and RSA were reduced by 47–56% (Table 2). Whereas QTLs for root traits at the end of hypoxia treatment were unstable between years or showed positive effects of the intolerant parent, detected QTLs for root development traits (Qrld-12, Qrsad-12) contributed by the hypoxia-tolerant parent were stable between years (Table 3).
QTLs for waterlogging injury score and tolerance index were found and mapped on Chrs. 2, 3, 5, 6, 7, 10, 11, 12, 13, 16 and 19 (Cornelious et al. 2005; Githiri et al. 2006; Nguyen et al. 2012). These indexes were proven to be strongly correlated with RL (Jitsuyama 2015). In this study, QTLs were mapped for HTI of root traits on Chrs. 4, 9, 12, and 14; two new of chromosomes 4 and 9 were detected (Table 4). Among these, Qhti-12-1 and Qhti-12-2 were co-localized with Qrld-12 and Qrsad-12, and contributed by Iyodaizu (Tables 3, 4), indicating that root development QTLs could explain for hypoxia tolerance index in soybean.
RL was strongly correlated with RSA under hypoxia (Henshaw et al. 2007a). In our results, RLD and RSAD showed the same responses to treatments (Fig. 5) in agreement with the mapping of the QTLs for these traits together on Chr. 12. These QTLs, contributed by Iyodaizu, were located between markers between Satt052 and Satt302, and did not appear under normoxia (Table 3; Fig. 4). Many QTLs for soybean seed weight, including seed weight 23-2, 35-4, 13-8, 36-4, 41-1, and 34-4 were found in our introgression region of Qrld-12 and Qrsad-12 on chr. 12 (http://soybase.org/). In this study, seed weight of NIL-9-4-5 was smaller than recurrent parent Tachinagaha. Thus, a negative effect of target QTLs on root development under normoxia conditions may be relevant to seed weight QTLs. Sayama et al. (2009) detected fours QTLs for flooding tolerance of soybean plant at germination stage which were named Sft1, Sft2, Sft3 and Sft4. Interestingly, one of these QTLs (Sft1) was identified in the marker interval containing Qrld-12 and Qrsad-12. Effective targeting of QTL alleles in breeding programs requires QTLs to be stably expressed among years and environments. NIL-9-4-5, carrying both QTLs, showed stable root performance under both hypoxia and waterlogging (Fig. 5). These results indicate that QTLs for root development have the greatest potential for breeding for both hypoxia and waterlogging tolerance in soybean.
In conclusion, we identified QTLs that contribute to root development under hypoxia conditions. QTLs for RLD and RSAD (Qrld-12, Qrsad-12) on Chr.12 (LG H) were stable across years. NIL9-4-5, carrying the target QTLs, performed well under both hypoxia and waterlogging conditions, confirming the effect of these QTLs. Markers proximal to the QTLs may be useful for the development of cultivars tolerant to waterlogging. Fine mapping and positional cloning may reveal gene(s) responsible for root development under hypoxia conditions and help elucidate the mechanism of waterlogging tolerance in soybean. Effects of the QTLs should be verified under field conditions.
Author contribution statement
TM, RT, KH and LVN designed the methodology. TM, RT, LVN, TOR, SK and RT developed experimental materials. TM, RT and TA oversaw and guided experiments, data analysis, writing and editing. LVN, NT, SK, TOR, SMG, TS and MI and KS conducted the experiments and analyzed the data. LVN wrote the manuscript. All authors read and approved the final manuscript.
Abbreviations
- CARD:
-
Change in average root diameter
- Chr.:
-
Chromosome
- DAS:
-
Days after sowing
- HTI:
-
Hypoxia tolerance index
- LG:
-
Linkage group
- NIL:
-
Near-isogenic line
- QTL:
-
Quantitative trait loci
- RD:
-
Root diameter
- RDW:
-
Root dry weight
- RIL:
-
Recombinant inbred lines
- RL:
-
Root length
- RLD:
-
Root length development
- RSA:
-
Root surface area
- RSAD:
-
Root surface area development
- SDW:
-
Shoot dry weight
References
Araki H, Hossain MA, Takahashi A (2012) Waterlogging and hypoxia have permanent effects on wheat root growth and respiration. J Agron Crop Sci 198:264–275. doi:10.1111/j.1439-037X.2012.00510.x
Armstrong W (1980) Aeration in higher plants. Adv Bot Res 7:225–332
Armstrong W, Drew MC (2002) Root growth and metabolism under oxygen deficiency. In: Yoav W, Amram E, Uzi K (ed) Plant root: the hidden half 3rd edn. Marcel Dekker Inc., New York, pp 729–761
Bacanamwo M, Purcell LC (1999) Soybean dry matter and N accumulation responses to flooding stress, N sources and hypoxia. J Exp Bot 50:689–696. doi:10.1093/jxb/50.334.689
Cho JW, Yamakawa T (2006) Effects on growth and seed yield of small soy bean cultivars of flooding conditions in paddy field. J Fac Agr Kyu Univ 52:189–193
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36. doi:10.1046/j.1365-3040.2003.00846.x
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681. doi:10.1071/FP09144
Cornelious BP, Chen P, Chen N, de Leon N, Shannon JG, Wang D (2005) Identification of QTLs underlying waterlogging tolerance in soybean. Mol Breed 16:103–112. doi:10.1007/s11032-005-5911-2
Drew MC (1992) Soil aeration and plant root metabolism. Soil Sci 154:259–268
Githiri SM, WananabeS, HaradaK, Takahashi R (2006) QTL analysis of flooding tolerance in soybean at an early vegetative growth stage. Plant Breed 125:613–618. doi:10.1111/j.1439-0523.2006.01291.x
Henshaw TL, Gilbert RA, Scholberg JMS, Sinclair TR (2007a) Soya bean (Glycine max L. Merr.) genotype response to early-season flooding: I. root and nodule development. J Agron Crop Sci 193:177–188. doi:10.1111/j.1439-037X.2007.00257.x
Henshaw TL, Gilbert RA, Scholberg JMS, Sinclair TR (2007b) Soya bean (Glycine max L. Merr.) genotype response to early-season flooding: II. Aboveground growth and biomass. J Agron Crop Sci 193:189–197. doi:10.1111/j.1439-037X.2007.00258.x
Hisano H, Sato S, Isobe S, Sasamoto S, Wada T, Matsuo A, Fujishiro T, Yamada M, Nakayama S, Nakamura Y, Watanabe S, Harada K, Tabata S (2007) Characterization of the soybean genome using EST-derived microsatellite markers. DNA Res 14:271–281. doi:10.1093/dnares/dsm025
Hossain MA, Uddin SN (2011) Mechanism of waterlogging tolerance in wheat: morphological and metabolic adaptations under hypoxia or anoxia. Aust J Crop Sci 5:1094–1101
Jitsuyama Y (2015) Morphological root responses of soybean to rhizosphere hypoxia reflect waterlogging tolerance. Can J Plant Sci 95:999–1005. doi:10.4141/CJPS-2014-370
Kaga A, Shimizu T, Watanabe S, Tsubokura Y, Katayose Y, Harada K, Vaughan DA, Tomooka N (2012) Evaluation of soybean germplasm conserved in NIAS genebank and development of mini core collections. Breed Sci 61:566–592. doi:10.1270/jsbbs.61.566
Kokubun M (2013) Genetic and cultural improvement of soybean for waterlogged conditions in Asia. Field Crops Res 152:3–7. doi:10.1016/j.fcr.2012.09.022
Lander ES, Green P, Abrahamson J, Barlow, Daly MJ, Lincoln, Newberg LA (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181. doi:10.1016/0888-7543(87)90010-3
Linkemer G, Board JE, Musgrave ME (1998) Waterlogging effects on growth and yield components in late-planted soybean. Crop Sci 38:1576–1584. doi:10.2135/cropsci1998.0011183X003800060028x
Mano Y, Omori F (2007) Breeding for flooding tolerant maize using “teosinte” as a germplasm resource. Plant Root 1: 17–21. doi:10.3117/plantroot.1.17
McNamara ST, Mitchell CA (1990) Adaptive stem and adventitious root responses of two tomato genotypes to flooding. Hort Sci 25(1):100–103
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Nguyen VT, Vuong TD, VanToai T, Lee JD, Wu X, Rouf Mian MA, Dorrance AE, Shannon JG, Nguyen HT (2012) Mapping of quantitative trait loci associated with resistance to Phytophthora sojae and flooding tolerance in soybean. Crop Sci 52:2481–2493. doi:10.2135/cropsci2011.09.0466
Oosterhuis DM, Scott HD, Hampton RE, Wullschleger SD (1990) Physiological responses of two soybean [Glycine max (L.) Merr] cultivars to short-term flooding. Environ Exp Bot 30:85–92. doi:10.1016/0098-8472(90)90012-S
Pedó T, Koch F, Martinazzo EG, Villela FA, Aumonde TZ (2015) Physiological attributes, growth and expression of vigor in soybean seeds under soil waterlogging. Afr J Agric Res 10: 3791–3797. doi:10.5897/AJAR2015.9661
Reyna N, Cornelious B, Shannon JG, Sneller CH (2003) Evaluation of a QTL for waterlogging tolerance in southern soybean germplasm. Crop Sci 43 :2077–2082 .doi:10.2135/cropsci2003.2077
Rhine MD, Stevens G, Shannon G, Wrather A, Sleper D (2010) Yield and nutritional responses to waterlogging of soybean cultivars. Irrig Sci 28:135–142. doi:10.1007/s00271-009-0168-x
Rich S, Ludwig M, Colmer T (2012) Aquatic adventitious root development in partially and completely submerged wetland plants Cotula coronopifolia and Meionectes brownii. Ann Bot 110:405–414. doi:10.1093/aob/mcs051
Sakazono S, Nagata T, Matsuo R, Kajihara S, Watanabe M, Ishimoto M, Shimamura S, Harada K, Takahashi R, Mochizuki T (2014) Variation in root development response to flooding among 92 soybean lines during early growth stages. Plant Pro Sci 17:228–236. doi:10.1626/pps.17.228
Sallam A, Scott HD (1987) Effects of prolonged flooding on soybeans during early vegetative growth. Soil Sci 144:61–66
Sauter M (2013) Root responses to flooding. Curr Opin Plant Bol 16:282–286. doi:10.1016/j.pbi.2013.03.013
Sayama T, Nakazaki T, Ishikawa G, Yagasaki K, Yamada N, Hirota N, Hirat K, Yoshikawa T, Saito H, Teraishi M, Okumoto Y, Tsukiyama T, Tanisaka T (2009) QTL analysis of seed-flooding tolerance in soybean (Glycine max [L.] Merr.). Plant Sci 176:514–521. doi:10.1016/j.plantsci.2009.01.007
Shimamura S, Mochizuki T, Nada Y, Fukuyama M (2003) Formation and function of secondary aerenchyma in hypocotyl, roots and nodules of soybean (Glycine max) under flooded conditions. Plant Soil 251:351–359. doi:10.1023/A:1023036720537
Song QJ, Marek LF, Shoemaker RC, Lark KG, Concibido VC, Delannay X, Specht JE, Cregan PB (2004) A new integrated genetic linkage map of the soybean. Theor Appl Genet 109:122–128. doi:10.1007/s00122-004-1602-3
Souza TC, Castro EM, Magalhães PC, Alves ET, Pereira FJ (2012) Early characterization of maize plants in selection cycles under soil flooding. Plant Breed 131:439–501. doi:10.1111/j.1439-0523.2012.01973.x
Thomas AL, Guerreiro SMC, Sodek L (2005) Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Ann Bot 96:1191–1198. doi:10.1093/aob/mci272
Trought MCT, Drew MC (1980) The development of waterlogging damage in wheat seedlings (Triticum aestivum L.) I. Shoot and root growth in relation to changes in the concentrations of dissolved gases and solutes in the soil. Plant Soil 54:77–94. doi:10.1007/BF02182001
Vandamme E, Pypers P, Smolders E, Merckx R (2016) Seed weight affects shoot and root growth among and within soybean genotypes beyond the seedling stage: implications for low P tolerance screening. Plant Soil 401:65–78. doi:10.1007/s11104-015-2564-8
VanToai TT, Beuerlein JE, Schmitthenner AF, Martin SKSt (1994) Genetic variability for flooding tolerance in soybeans. Crop Sci 34:1112–1115. doi:10.2135/cropsci1994.0011183X003400040051x
VanToai TT, Martin SKSt, Chase K, Boru G, Schnipke V, Schmitthennr AF, Lark KG (2001) Identification of a QTL associated with tolerance of soybean to soil waterlogging. Crop Sci 41:1247–1252. doi:10.2135/cropsci2001.4141247x
VanToai TT, Hoa TTC, Hue NTN, Nguyen HT, Shannon JG, Rahman MA (2010) Flooding tolerance of soybean [Glycine max (L.) Merr.] germplasm from Southeast Asia under field and screen-house environments. Open Agric J 4:38–46. doi:10.2174/1874331501004010038
Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ (2000) Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ 23:1237–1245. doi:10.1046/j.1365-3040.2000.00628.x
Wang S, Baston CJ, Zeng ZB (2007) Windows QTL cartographer 2.5. Department of Statistics. North Carolina State University, Raleigh
Wiengweera A, Greenway H, Thomson CJ (1997) The use of agar nutrient solution to simulate lack of convection in waterlogging soils. Ann Bot 80:115–123. doi:10.1006/anbo.1996.0405
Yamane K, Iijima M (2016) Nodulation control of crack fertilization technique reduced the growth inhibition of soybean caused by short-term waterlogging at early vegetative stage. Plant Prot Sci 19(3):438–448. doi:10.1080/1343943X.2016.1164573
Yamauchi T, Abe F, Kawaguchi K, Oyanagi A, Nakazono M (2014) Adventitious roots of wheat seedlings that emerge in oxygen-deficient conditions have increased root diameters with highly developed lysigenous aerenchyma. Plant Signal Behav 9:e28506
Zaidi PH, Rafique S, Singh NN (2003) Response of maize (Zea mays L.) genotypes to excess soil moisture stress: morpho-physiological effects and basis of tolerance. Eur J Agron 19: 383–399
Acknowledgements
This work was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics-based Technology for Agricultural Improvement, SFC-1005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Brian Diers.
Rights and permissions
About this article
Cite this article
Van Nguyen, L., Takahashi, R., Githiri, S.M. et al. Mapping quantitative trait loci for root development under hypoxia conditions in soybean (Glycine max L. Merr.). Theor Appl Genet 130, 743–755 (2017). https://doi.org/10.1007/s00122-016-2847-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-016-2847-3