Abstract
The German airway management guidelines are intended to serve as an orientation and decision-making aid and thus contribute to the optimal care of patients undergoing anesthesiologic- and intensive medical care. As part of the pre-anesthesiologic evaluation, anatomical and physiological indications for difficult mask ventilation and intubation shall be evaluated. This includes the assessment of mouth opening, dental status, mandibular protrusion, cervical spine mobility and existing pathologies. The airway shall be secured while maintaining spontaneous breathing if there are predictors or anamnestic indications of difficult or impossible mask ventilation and/or endotracheal intubation. Various techniques can be used here. If there is an unexpectedly difficult airway, a video laryngoscope is recommended after unsuccessful direct laryngoscopy, consequently a video laryngoscope must be available at every anesthesiology workplace. The airway shall primarily be secured with a video laryngoscope in critically ill- and patients at risk of aspiration. Securing the airway using translaryngeal and transtracheal techniques is the “ultima ratio” in airway management. The performance or supervision of airway management in the intensive care unit is the responsibility of experienced physicians and nursing staff. Appropriate education and regular training are essential. Clear communication and interaction between team members are mandatory before every airway management procedure. Once the airway has been secured, the correct position of the endotracheal tube must be verified using capnography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Information on these guidelines
Leading professional association.
German Society for Anesthesiology and Intensive Care Medicine (DGAI).
Special note
Medicine is subject to a continuous development process, therefore all information, particularly on diagnostic and therapeutic procedures, can only ever reflect the state of knowledge at the time of printing of the guidelines. The most excellent possible care has been taken about the therapy recommendations and the medication selection and dosage.
The practitioner remains responsible for any diagnostic, therapeutic application, medication, and dosage.
For better readability, the generic masculine is used.
Procedure for consensus building
This guidelines are an expert consensus. It was approved by the Board of the German Society of Anesthesiology and Intensive Care Medicine. The consensus classification and recommendations were adopted as an expert consensus of the guideline group. The strength of the recommendation results from the wording used (shall/should/can) according to the grading in Table 1.
Introduction
Securing the airway is a core competence of anesthesiologists, intensive care, and emergency physicians, as oxygenation is impossible without an open/secured airway. Introducing new techniques and implementing guidelines and strategies for the care of the difficult airway have significantly reduced morbidity and mortality.
These guidelines are intended to help ensure optimal care for anesthesia and intensive care patients. It is intended to serve as an orientation and decision-making aid for practitioners. For many of the following recommendations on practical procedures or the use of specific techniques in airway management, there are needs to be more high-quality clinical studies. The guidelines presented here, therefore, represents the consensus and resulting strength of the recommendations of a group of experts (corresponds to level “S1” of the classification scheme of the German Association of Scientific Medical Societies—see www.awmf.org). It is, consequently, not feasible or possible to specify a Grade of Recommendation.
For securing the airway in prehospital emergency medicine [1], polytrauma/critically injured treatment [2] and pediatric anesthesia [3] reference is made to the existing German recommendations and guidelines.
1. Definition, predictors and incidence of difficult airway
Definition
“Difficult airway” refers to problems that may occur during airway management. The specialist standard in anesthesiology is assumed as the professional qualification for assessing the “difficult airway.” In the following, all recommendations refer to the standard of a specialist in anesthesiology and sufficient experience in the routine application of the respective technique.
Difficulties arise at various levels: Oxygenation using the face mask or a supraglottic airway device (SGA, for definition, see chapter “Techniques for securing the airway”) is defined as difficult or impossible if the initial level of oxygen saturation cannot be reached due to one or more problems.
The placement of an SGA is considered difficult if more than two attempts are required for correct placement.
An impossible laryngoscopy is present if the glottis cannot be visualized. This corresponds to a laryngoscopy finding according to Cormack & Lehane grade III or IV [4]. Difficult endotracheal intubation is present if more than two attempts are required to place the tube.
A “cannot ventilate, cannot oxygenate” situation exists when neither ventilation nor oxygenation is possible.
Incidences and predictors
Incidence of the difficult airway
The exact determination of complications and the incidence of difficult mask ventilation, laryngoscopy or intubation is unclear because the definition of difficult airway in different studies and the underlying situation (e.g., elective induction in the operating room versus emergency intubation in the intensive care unit) are very different [5]. The incidence of difficult mask ventilation is about 6%, of impossible mask ventilation about 0.04–0.15% [6,7,8,9,10], of which 86–94% were unexpected [7, 11]. The incidence of difficult direct laryngoscopy is 1.5–8.0%, the incidence of difficult intubation is slightly lower [12, 13]. An unexpected “cannot intubate, cannot oxygenate” situation can be expected with a probability of 0.008% (1:13,000) to 0.004% (1:25,000) [8, 10]. Possible inter-individual differences in the practical skills of the practitioner must also be considered. Again, the specialist standard only vaguely describes individual competence. It is therefore likely, that different working groups will arrive at different results with an identical patient population. This challenge must always be considered when interpreting such clinical trials.
Predictors of difficult mask ventilation
In addition to the known factors listed in Table 2, the patient’s medical history shall be considered: Has mask ventilation ever been difficult or is there any evidence of this in the form of an anesthesia record or anesthesia problem card? Are there any anatomical features that may indicate difficult mask ventilation? The more of predictors are present, the greater the likelihood of problems.
Predictors of difficult laryngoscopy and intubation
Problems with endotracheal intubation are often subsumed under the term “difficult intubation” without distinguishing between “laryngoscopy” and “endotracheal intubation”. Since the anatomical and optical axes converge during direct laryngoscopy and successful tube placement can be expected if the laryngoscopy findings are acceptable, this blurring is of secondary importance. However, when indirect laryngoscopy techniques are used (for definition, see Sect. 3: Techniques for airway management) the two procedures must be separated because the incidence of difficult laryngoscopy is always lower than the incidence of difficult or impossible intubation.
Like the anatomical and physiological parameters used to assess mask ventilation (Table 2), these are also defined to predict difficult laryngoscopy and intubation (Table 3). Although the likelihood of a difficult airway increases with the number of predictors, a difficult laryngoscopy can also be found in the presence of only one predictor: Typical examples are a very small mouth opening or an obstructing subglottic tumor.
Various tests and scores are published to assess difficult laryngoscopy/intubation. Mouth opening is particularly important because laryngoscopy is usually performed through the mouth and the instruments used must be placed correctly. Mouth opening is determined by the distance between incisors in the upper and lower jaw and is also known as the “interincisor distance”. There is some disagreement among authors as to when the mouth opening is critically small or at least significantly reduced. An interincisor distand of less than < 3.5 cm is usually interpreted as pathological [14].
None of the described tests or scores alone can reliably predict difficult laryngoscopy or intubation. The upper lip bite test, which has a good predictive value for difficult intubation, is important [15]. The Mallampati test modified by Samsoon and Young is currently the most widely used test [4], along with the Patil thyromental distance of less than 6–7 cm [16], the Wilson risk score [17], the Arne multifactorial risk index [18], and the El Ganzouri score [19]. In a meta-analysis [20], the combination of Mallampati and the thyro-omental distance recommended by El Ganzouri [19] is suggested. All these scores have different sensitivities. In addition, there are known weaknesses in their application by today’s anesthesiologists [21, 22], which further reduces their validity. Therefore, good training in the assessment of the difficult airway is also important.
Recommendation 1
As part of the airway examination, anatomic and physiologic indications for difficult mask ventilation and intubation shall be evaluated: Mouth opening, dental status, mandibular protrusion, cervical spine mobility, pathology (neck, face), known difficult airway, consideration of imaging and ENT findings as appropriate.
Recommendation 2
There shall be an algorithm/standard within each institution that defines the procedure for airway management when predictors for a difficult airway are present. Scores can be used.
Predictors for difficult video laryngoscopy
Compared to direct laryngoscopy, video laryngoscopy often provides better visualization of the vocal cords. However, there are also indicators that predict a more difficult visualization of the glottis or more difficult tube placement using a video laryngoscope (Table 4). The significance of the predictors also depends on the shape of the laryngoscope blade used [23, 24].
Predictors of difficult placement and/or oxygenation with a supraglottic airway device
Several factors can make placement and/or oxygenation difficult or impossible with an SGA [25,26,27,28]. These factors include Table 5.
Difficult airway due to physiological impairment
Typically, a difficult airway is mainly based on anatomical conditions as described above. However, physiologic pathologies should also be considered when planning airway management [29]. For example, hypoxia, hypotension, severe metabolic acidosis, or right ventricular failure carry a high risk of adverse events during airway management. Therefore, these impairments should be critically evaluated even in the absence of anatomic predictors of a difficult airway. The presence of cardiopulmonary decompensation may distract from a difficult airway or further complicate an already difficult airway [29, 30]. Prolonged attempts to secure the airway should be avoided whenever possible. Therefore, the decision to intubate under spontaneous breathing [28, 31] may be useful (see Sect. 4: Strategies for securing the airway).
2. Airway ultrasound
Ultrasound examination of the respiratory tract from the hypopharynx and base of the tongue to the lungs and pleura is increasingly the focus of clinical trials. However, these are mostly studies with a small number of cases and a low level of evidence [32]. Several parameters are available to provide information on airway anatomy during preoperative airway assessment and as potential screening parameters for difficult airways [33].
Recommendation 3
Preanesthetic ultrasound of the airway may be helpful in identifying difficult airway management. To assess the risk of aspiration, point-of-care ultrasound can be used to determine the filling status of the stomach.
The diagnostic accuracy, especially for non-specialist examiners, and the impact of clinical decisions made based on ultrasound examinations require further investigation [34].
After securing the airway, ultrasound examination of both lungs can be performed [35, 36]. It is not an alternative for capnography.
Recommendation 4
Ultrasound examination of the trachea and lungs can provide evidence for correct tube position.
Although inspection and palpation are sufficient in most patients, the inability to identify the cricothyroid membrane is a major contributor to the high failure rate of cricothyrotomy. Airway ultrasound to identify this membrane can double the success rate. After a short but structured training, the cricothyroid membrane can be identified by ultrasound even in difficult anatomic circumstances by anesthesiologists previously inexperienced in airway ultrasound technique and can be helpful when trans laryngeal access is required [37, 38].
Recommendation 5
If the airway is known or expected to be difficult, a preanesthetic ultrasound examination of the upper airway with visualization and marking of the cricothyroid membrane may be helpful in preparation for trans laryngeal airway management.
Recommendation 6
In the case of a difficult airway, ultrasound of the upper airway and neck may be helpful to aid in the initiation of invasive airway management.
3. Techniques for airway management
Preoxygenation
Preoxygenation during induction of general anesthesia is aimed to delay desaturation during the apnea phase [39, 40].
Recommendation 7
Spontaneously breathing patients shall be preoxygenated prior to induction of anesthesia. This should be performed using a tight-fitting face mask with 100% oxygen and a fresh gas flow of 10 L for at least 3–4 min. Alternatively, the patient shall take eight deep breaths at the same oxygen flow for a maximal duration of 60 s.
Preoxygenation can be modified for certain risk groups:
-
Geriatric patients benefit from extending preoxygenation to 5 min [40].
-
Bariatric patients benefit from upper body elevation (25–30 degrees), pressure-supported ventilation during spontaneous breathing, and pressure-controlled ventilation in the apneic phase [41,42,43].
-
The use of noninvasive ventilation (e.g., pressure support: 8 cmH2O, PEEP 5 cmH2O) during preoxygenation in patients at risk for hypoxia can improve apnea tolerance [44, 45].
-
If a rapid preoxygenation is required, pressure support ventilation (e.g., pressure support: 8 cmH2O, PEEP 5 cmH2O) can almost reduce the time needed by halve [45].
Upper body elevation is beneficial in patients with respiratory insufficiency, as it can prolong apnea time without hypoxia.
Apneic oxygenation
The goal of apneic oxygenation is to prolong apnea time without decreasing oxygen saturation by delivering oxygen via standard nasal cannula at a flow rate of 15 L/min. In theory, apneic oxygenation is based on the influx of high dose oxygen into the airway dead space and the washout of carbon dioxide from the alveoli [46].
The acronym “THRIVE” (transnasal humidified rapid insufflation ventilatory exchange) describes a high-flow oxygen therapy of ca. 60 L/min, in which heated and humidified oxygen is delivered via a specific high-flow nasal cannula [47].
An advantage of using a nasal cannula for apneic oxygenation is that they do not obstruct airway access during tracheal intubation. Apneic oxygenation can be particularly beneficial in patients at risk for hypoxemia [48, 49]. A disadvantage is that they may compromise the seal of a face mask if manual ventilation becomes necessary. So far, apneic oxygenation has no proven role as a rescue technique for oxygenation in already desaturated patients [46].
Recommendation 8
The use of apneic oxygenation during laryngoscopy can be considered in patients at risk for hypoxemia.
For intensive care patients, it has been shown that hypoxemia occurs less frequently in acute respiratory failure with THRIVE [50, 51].
Mask ventilation
Mask ventilation is the basic measure of airway management. It can be performed manually (bag) or with pressure-controlled ventilation.
Oro-/nasopharyngeal airway support
If mask ventilation is difficult, oro/nasopharyngeal airway devices shall be used if depth of anesthesia is sufficient [52, 53].
Neuromuscular blockade
Neuromuscular blockade improves mask ventilation in most cases, resulting in higher tidal volumes and less desaturations [54,55,56].
Recommendation 9
In the absence of a difficult airway a planned neuromuscular blockade shall be performed immediately after achieving adequate depth of anesthesia without checking for a sufficient mask ventilation.
Supraglottic airway devices
The term supraglottic airway devices refer to instruments that are located outside the glottis. There are two types of airway devices:
-
Laryngeal mask type (LM type)
-
Laryngeal tube type (LT type)
Within the LM type group, there are sometimes considerable differences due to the design, so that results from publications or recommendations cannot be transferred to every device within the group [57].
The LT type is mainly used in prehospital emergency medicine in Germany.
By setting the cuff pressure to a maximum of 60 cm H2O, the incidence of airway injury and swelling can be reduced [58, 59].
Recommendation 10
Cuff pressure for supraglottic airways shall be measured after placement and shall not exceed 60 cmH2O.
Indications
The use of the LM-type for primary airway management in elective surgery offers numerous advantages over ventilation with a face mask and, in certain procedures, over ventilation with an endotracheal tube (Table 6). Therefore, its use in elective surgery is indicated when there are no limitations (Table 7).
Second generation SGA
While the first generation of SGA’s allow only ventilation, the second generation also allows safe position control. Various tests can be used to ensure safe position control with the tip in the postcricoid region. In addition, gastric pressure and volume can be reduced by inserting a gastric tube. This results in a potentially lower risk of regurgitation and aspiration. Because of these advantages, it makes sense to always use second-generation airway devices. Positioning tests include visual inspection for sufficient insertion depth, gastric leakage test (“bubble” test), and resistance-free insertion of a gastric tube [61]. The positive jugulum test (supra-sternal notch test) can also provide information on the correct position. These tests have only been described for second-generation LM-type SGA.
Recommendation 11
When using second-generation LM-type airway devices, tests shall be performed to verify the position.
Extended indications
The term “extended indications” is used to describe procedures that require increased leak-tightness for ventilation and protection against gastric insufflation or regurgitation.
These include, but are not limited to, use in planned prolonged procedures, laparoscopic procedures, bariatric patients, and non-supine procedures. These procedures can also be performed with SGA when
-
second-generation LM type is used,
-
position tests have been successfully passed,
-
the application remains under 8 h [62],
-
and the practicioner has sufficient experience.
Recommendation 12
Based on current evidence, an LM-type SGA can be used as an alternative to an endotracheal tube for the extended indications after a risk-benefit assessment. In these cases, a second-generation LM-type SGA should be used, and a gastric tube advanced through the gastric lumen. After placement, position and leakage shall be checked.
Intubation via the supraglottic airway
LM-type SGAs designed for “blind” or endoscopically guided intubation have a special position in airway management. These devices have the potential advantage that, in addition to the benefits of LM-type use, endotracheal intubation can also be performed. It should be noted that the probability of success of non-endoscopically guided (so-called “blind”) intubation is highly dependent on the type of LM used and the expertise of the operator and has been reported to vary from 15–98% [63].
Recommendation 13
Intubation shall only be attempted without a flexible intubation endoscope if the operator has the necessary expertise and is using an SGA that has a high success rate when used correctly.
Indications for SGA of the laryngeal tube type
Experience and recommendations for the use of laryngeal tube types of SGA are mainly available in prehospital emergency medicine [1]. Sufficient experience is required for safe use [64].
The indication for LT-type SGA is as an alternative for difficult airway management when mask ventilation, insertion of an LM-type SGA or intubation fails because of the different way the devices works and its position in the esophagus/pharyngeal area.
Recommendation 14
Emergency use should be reserved for those with sufficient experience in patient use.
Direct laryngoscopy
Conventional intubation can be performed with various shaped blades. The often recommended “sniffing position” for head positioning during intubation may improve or at least not worsen the view of the glottis [65, 66].
Recommendation 15
For direct laryngoscopy, positioning in the sniffing position can be helpful for a better view.
If laryngoscopy or intubation is difficult, guide rods, stylets or tube insertion devices can be used to place the tube. Tube insertion introducers are long, semi-flexible plastic rods placed primarily in the trachea to facilitate tube placement [67]. Nomenclature is not standardized. They are often referred to as bougie, stylet, or intubation catheter.
Indirect laryngoscopy
The term “indirect laryngoscope” refers to instruments that do not require or allow direct visualization of the glottic plane. In principle, this can be achieved in two ways: Firstly, by using small camera chips whose image is transmitted to a monitor (e.g. video laryngoscopes, flexible and rigid intubation endoscopes). On the other hand, by using an optical system that provides a view through an eyepiece via optical fibers (e.g. classical fiber optics) or prisms.
Video laryngoscopy
The view of the glottic plane can often be improved with video laryngoscopy after direct laryngoscopy is difficult or impossible [66, 68, 69]. The primary use of video laryngoscopy increases the likelihood of successful intubation on the first attempt during anesthesia [70,71,72].
Recommendation 16
When direct laryngoscopy is expected to be difficult or in emergencies, video laryngoscopy should be preferred over direct laryngoscopy.
Today, the term video laryngoscopy encompasses a wide variety of devices, some of which differ fundamentally in shape, technology, and handling. An important distinguishing feature of video laryngoscopes is the shape of the blade [68, 73]. At present, no clear recommendation can be made regarding the use of a specific video laryngoscope. The use of disposable blades may have a negative impact on visualization and intubation success compared to metal or reusable blades from the same manufacturer [74]. In less experienced users, the use of a video laryngoscope may lead to increased primary intubation success. However, despite good visualization, intubation may be prolonged and unsuccessful, especially when a hyper angulated blade is used [75, 76].
Videolaryngoscopes with Macintosh blades.
If direct laryngoscopy is unexpectedly difficult, indirect visualization of the glottic plane can often be achieved with this type of blade [77]. These instruments can be used to optimize and facilitate training in the technique of endotracheal intubation [78]. There is no advantage to using a rigid stylet over a flexible to facilitate tube placement. Direct laryngoscopy offers no advantages over video laryngoscopy in elective patients in the operating room and is associated with a significantly lower success rate in the first intubation attempt [79].
Recommendation 17
In anesthesia, a video laryngoscope with a Macintosh blade can be used for standard intubation.
Video laryngoscopes with hyper angulated blade.
With more curved blades, it is not necessary to adjust the oro-pharyngo-laryngeal axis, so the intubation procedure is performed indirectly with the obligatory video laryngoscopic view. When using hyper angulated blades from different manufacturers, the differences in success and visibility appeared to be marginal [80]. The real difficulty of intubation with a video laryngoscope with a hyper angulated blade arises during the intubation procedure, despite optimal visualization: The tube must be advanced at a steep angle corresponding to the curvature of the blade, and the tip of the tube must be lowered towards the trachea after passing the vocal cords.
Recommendation 18
A stylet shall be used when using a hyper angulated blade with sufficient practical experience to use these devices successfully in emergency situations. The tube with the stylet should be bent to follow the shape of the blade all the way to the tip.
Avoiding excessive head tilt and carefully loading the epiglottis may be helpful during the intubation procedure. However, retraction of the blade and a less than optimal view of the glottis may also facilitate tube placement [81].
Video laryngoscopes with endotracheal tube guide.
These systems have a guide channel on the laryngoscope blade to guide the endotracheal tube to the glottis. Due to the hyper angulated blade, all systems of this type are mandatory indirect laryngoscopes. It is currently unclear whether the use of a guide channel has advantages or disadvantages compared to other blade types.
Rigid intubation endoscopes
Rigid intubation endoscopes are an alternative for intubating unexpectedly difficult airways [82, 83]. Sufficient experience in patients with normal airways is required to use these devices successfully in emergency situations [84].
Flexible intubation endoscopes
The flexible endoscopic approach is a common technique for endotracheal intubation in both unexpected and expected difficult airways. The classic instrument is a fiberoptic scope with glass fibers for visualization through an eyepiece. Increasingly, endoscopes are available with a camera chip at the tip of the instrument that transmits the image as an electrical signal to a monitor.
Translaryngeal/transtracheal techniques
Securing the airway with translaryngeal and transtracheal techniques is the “ultima ratio” in airway management. They may be required both primarily, for example, in the event of supraglottic airway obstruction, and secondarily in the event of impending asphyxia after unsuccessful attempts to secure the airway using less invasive techniques. Regular training in these maneuvers is critical to success, as is timely indication for their use, preferably under controlled conditions rather than after the onset of asphyxia.
Cricothyrotomy
A cricothyrotomy (synonym: coniotomy, cricothyroidotomy) involves cutting the cricothyroid ligament and inserting a cannula or endotracheal tube into the airway below the level of the glottis. There are three different techniques, two of which are percutaneous: In the catheter-over-needle technique, the puncture of the airway is like the placement of an indwelling venous cannula. In the Seldinger technique, after puncturing the trachea, a guide wire is inserted and then the cannula is placed over it. Surgical cricothyrotomy involves cutting the cricothyroid ligament with a scalpel, pushing the thyroid and cricoid cartilages apart, and placing a small endotracheal tube or tracheal cannula.
Techniques
In recent years, several studies have shown that the surgical technique is superior to percutaneous cricothyrotomy techniques in terms of speed, success rate, and incidence of complications [85,86,87,88,89,90]. However, an evidence-based recommendation for the optimal cricothyrotomy technique cannot be derived. The complication and failure rates for emergency cricothyrotomy have been reported to range from just under 30 to over 50% [91, 92]. However, training in realistic scenarios on high-quality simulators and in a dedicated team can significantly increase the success rate of cricothyrotomy and reduce the time required to perform it [93].
Tracheostomy
Tracheostomy can be performed electively under local anesthesia while maintaining spontaneous breathing. Typical indications are stenosing tumors of the larynx and hypopharynx.
Surgical tracheostomy by an experienced surgeon may also be an alternative to cricothyrotomy in individual cases (keyword: tracheostomy readiness) in the context of an airway emergency [94]. Prerequisites are immediate availability of the material, good environmental conditions (e.g. in the operating room) and excellent routine to perform this procedure technically safe and, above all, very quickly even in an emergency under high time pressure.
Trans laryngeal/transtracheal oxygenation and ventilation
After emergency placement of thin cannulas through the cricothyroid ligament, tracheal access is available, but ventilation is very limited due to the small lumen. Diffusional oxygenation can be achieved by insufflating high flow oxygen into the trachea [95]. Problems associated with this method of trans laryngeal/transtracheal oxygenation are often inadequate expiration or ventilation with subsequent hypercarbia, as well as the risk of barotrauma and hemodynamic compromise, especially in the setting of supraglottic airway obstruction.
With appropriate systems that allow controlled, active expiration, even a 2.0 mm lumen in an otherwise completely obstructed upper airway can provide adequate ventilation and avoid complications [96, 97]. These systems can also be used after placement of tube change devices or intubation catheters with the possibility of oxygenation.
4. Strategies for securing the airway
Levels of airway managing
There are four levels of airway managing with possible access points for oxygenation or ventilation of the patient:
-
Level 1: Spontaneous breathing, assisted or controlled ventilation with a face mask
-
Level 2: Use of a supraglottic airway device
-
Level 3: Placement of an endotracheal tube
-
Level 4: Trans laryngeal/transtracheal access
Recommendation 19
Devices for each level shall be available during elective and emergency airway management.
Planning phase, communication
Each airway management event must be carefully planned. Predictors of a difficult airway must be considered, available equipment and personnel must be evaluated, and options for obtaining assistance and additional equipment in the event of difficulty must be determined, considering the situation (e.g., location of the airway management, time of day).
Poor communication often leads to errors and adverse events [98, 99].
Recommendation 20
Prior to induction of general anesthesia, the team shall communicate the planned procedure and any special features. If difficulties are anticipated, the course of action and possible alternatives shall be specified.
Airway management in a difficult airway
The procedure for securing the airway is based on whether an anticipated difficult airway is present: If there are no predictors of difficulty with mask ventilation, laryngoscopy, and endotracheal tube placement, the airway is secured after induction of general anesthesia after an adequate depth of anesthesia is achieved.
Recommendation 21
There shall be an institution specific algorithm for difficult airway management available. It is known to all involved and includes available and familiar devices and airway techniques.
Technical aspects
Recommendation 22
The ventilator shall be checked before starting preoxygenation. If an anesthesia ventilator is used, the “KURZ” check shall be performed.
“KURZ” check: Set a fresh gas flow of ≥ 2 L/min when the face mask is fitted. Check that an adequate inspiratory oxygen concentration is measured and that the capnograph shows plausible values and readings. The KURZ check is the responsibility of the anesthesiologist and cannot be delegated [100].
Procedure for expected difficult airway
An anticipated difficult airway may be present at all levels of airway management and may be current or related to injury or illness. Problems with the two techniques of “mask ventilation” and “endotracheal intubation” are particularly important when assessing the airway.
Recommendation 23
If a difficult airway is anticipated, regional anesthesia shall be considered first.
However, if regional anesthesia is not effective or if the procedure takes longer than expected, it may still be necessary to secure the airway.
Recommendation 24
If a difficult airway is expected and primary regional anesthesia is performed, a plan for securing the airway shall be communicated within the team and the necessary supplies shall be readily available.
Recommendation 25
If regional anesthesia is not possible and general anesthesia is required, the airway shall be secured while maintaining spontaneous breathing if there are predictors for difficult or impossible mask ventilation and/or endotracheal intubation.
In principle, several techniques can be used if the airway is expected to be difficult:
Recommendation 26
The use of a flexible intubation endoscope is the gold standard in an expected difficult airway. Consequently, it shall be readily available, and the anesthesiologist shall be trained in its use.
A nasal or oral approach may be chosen depending on the patient’s circumstances.
Recommendation 27
Spontaneous breathing shall be maintained until the endotracheal tube is securely placed in the trachea. In awake patients, topical anesthesia of the airway shall be performed. Sedative drugs shall be administered in the lowest dose possible to prevent apnea and/or airway obstruction. During the procedure, the patient shall receive supplemental oxygen.
Awake endoscopic intubation is associated with a very high success rate [101, 102]. Several options for topical anesthesia and patient sedation have been described [103, 104]. There is currently no evidence that one strategy is superior to another. The approach to an awake intubation depends on the experience and preferences of the performing anesthesiologist.
Other techniques described after adequate topical mucosal or local anesthesia include the use of video laryngoscopy [105, 106], tracheotomy [107], establishment of a trans laryngeal/transtracheal approach, and placement of a supraglottic laryngeal mask airway device [108, 109] in the awake, spontaneously breathing patient. With good expertise and in selected cases, these techniques can be used as an alternative to flexible endoscopic intubation. In special situations (e.g., obstructive tumors in the larynx/pharynx/trachea; glottic or tracheal stenosis), it may be appropriate to choose an interdisciplinary approach, e.g., with representatives from the ENT department and specialized techniques.
Decision-making to secure the airway during spontaneous breathing
Failure to secure the airway with spontaneous breathing when the airway is expected to be difficult is often the cause of serious complications [110]. However, the disadvantage of this procedure is the increased time required. It also requires a cooperative patient. In critically ill or injured patients with severe respiratory decompensation, there may be neither the cooperation nor the time to successfully secure the airway with spontaneous breathing. There may be an indication to induce general anesthesia with a subsequent intubation attempt. In this case, a cricothyrotomy should be prepared in parallel.
Recommendation 28
If a difficult airway is anticipated and there is no time or opportunity to secure the airway under spontaneous breathing, the technique with the greatest chance of success shall be selected and the possibility of cricothyrotomy shall be prepared as part of the airway management plan.
In non-time-critical situations and in sufficiently cooperative patients with significant and/or obvious pathological anatomical changes in the head and neck area, intubation under spontaneous breathing is often indicated. In this group of patients, there is usually no prospect of placing a (video) laryngoscope blade or an SGA after induction of anesthesia. Mask ventilation can also often be challenging or impossible.
Recommendation 29
If predictors for difficult mask ventilation and placement of a supraglottic airway device are present, the airway should be secured while maintaining spontaneous breathing.
If there are predictors of difficult intubation by direct laryngoscopy, answering four questions may help determine whether intubation under spontaneous breathing is indicated or whether airway protection can be provided after induction of general anesthesia.
1. Is intubation possible with a video laryngoscope?
Although video laryngoscopy is of great importance in securing a difficult airway, there is a risk of failure of endotracheal intubation with video laryngoscopy when difficult intubation is anticipated. The likelihood of success should be assessed considering patient-related factors, predictors, and the experience of the team in the use of video laryngoscopy. If there is little chance of success, the airway should be secured while maintaining spontaneous breathing.
2. Are there no predictors for difficult mask ventilation and placement of an SGA?
If difficult intubation is anticipated and there are predictors of difficult mask ventilation or difficult SGA placement, intubation should be performed with spontaneous breathing. Failed intubation may be associated with difficult or impossible mask ventilation and vice versa [11, 111, 112].
Similarly, a failed attempt to place an SGA is most likely associated with difficult/impossible mask ventilation [25, 26].
3. Is the patient cardiopulmonary compensated?
The presence of cardiopulmonary decompensation (e.g., severe hypoxia, low apnea tolerance, cardiovascular instability) may distract from a difficult airway or further complicate an already difficult airway [29, 30]. In addition, induction of general anesthesia may further exacerbate decompensation [113]. Separation of airway management and induction of general anesthesia by intubation under spontaneous breathing may therefore be beneficial, improving both patient safety and operator cognitive load.
4. Are there unfavorable ambient conditions?
Unfavorable environmental conditions include lack of staff backup, inadequate airway management equipment, inexperienced airway management team, or a location other than the usual airway management/anesthesia induction site. Using an intubation procedure with spontaneous breathing in an anticipated difficult airway can potentially prevent serious complications if moving the patient to a better location is not an option.
If the answer to any of these questions is “yes”, a spontaneous breathing procedure is indicated.
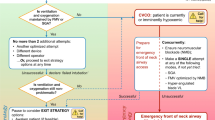
The “Expected Difficult Airway” algorithm (Fig. 1) graphically illustrates these recommendations.
Procedure for unexpectedly difficult airway
Mask ventilation.
Mask ventilation, as the first level of airway management, is the basic measure for ventilating the patient and is an important fallback option in the event of an unexpectedly difficult airway. It is used to deliver oxygen to the patient while additional supplies or help is being brought in.
Recommendation 30
When mask ventilation is difficult in adequately anesthetized patients, measures shall be taken to lift the base of the tongue to open the upper airway.
Simple measures include the use of appropriately sized nasopharyngeal- (e.g., Wendl) or oropharyngeal tubes (e.g., Guedel). Holding the mask with both hands and lifting the lower jaw may allow ventilation [114,115,116].
Recommendation 31
In the absence of contraindications, the head shall be reclined and an Esmarch maneuver shall be performed. The appropriately sized ventilation mask shall be held with both hands to achieve the best possible seal. Lifting the mandible is helpful.
Ventilation is provided by a second assistant or by an appropriately set ventilator using pressure-controlled ventilation at < 15 cmH2O [117]. Neuromuscular blockade improves mask ventilation in many cases [118].
Recommendation 32
If neuromuscular blockade has not yet occurred, it should be considered with a fast-acting muscle relaxant (succinylcholine, rocuronium) at an appropriate dose.
If airway resistance or leakage is abnormally high, technical causes must be ruled out. If these are likely due to the circumstances of the specific situation, but cannot be found and corrected immediately, the “separate manual bag” fall-back option should be used. If mask ventilation is possible with the manual bag, a systematic search for the cause of the malfunction can be undertaken or a replacement device can be used.
Direct laryngoscopy
If an adequate view of the glottis is not possible during direct laryngoscopy, simple maneuvers can improve the view. OELM (optimal external laryngeal manipulation) and BURP (backward upward rightward pressure) have been described as laryngeal manipulation maneuvers. In the absence of contraindications, optimal head positioning by elevation and/or reclination may be helpful [119, 120]. If the view of the level of the vocal cords is limited, a tube insertion device with a soft, atraumatic tip may be helpful [121, 122].
Recommendation 33
If the view of the glottis is limited despite appropriate manipulation, the use of special tube insertion devices may be considered.
Recommendation 34
The number of direct laryngoscopy attempts shall be limited to a maximum of two. Alternative methods shall be used early.
The risk of serious, life-threatening complications such as severe hypoxia, regurgitation, aspiration, hypotension, bradycardia, and cardiac arrest increases, especially in emergency situations, after two intubation attempts and with each subsequent unsuccessful intubation attempt [123, 124].
Recommendation 35
If the airway is unexpectedly difficult after unsuccessful direct laryngoscopy, measures to improve the airway shall be considered first, and another attempt at direct laryngoscopy shall be made only if these measures can increase the chances of success.
Recommendation 36
If direct laryngoscopy is unsuccessful, a video laryngoscope shall be used.
These measures include improved patient positioning (head), suctioning secretions to improve visibility, and the use of tube insertion devices and alternative blades. This also applies to situations where mask ventilation is possible. Repeated manipulation and intubation attempts may cause swelling. Even if mask ventilation is possible, it may become inadequate.
Indirect laryngoscopy
In field of indirect laryngoscopy, video laryngoscopy is now the most important technique for managing the unexpectedly difficult airway, often allowing correct tube placement after a primarily unsuccessful direct laryngoscopy intubation attempt [125].
Recommendation 37
A video laryngoscope shall be available at every anesthesia workstation.
Rigid or flexible endoscopic endotracheal intubation is also possible in anesthetized patients with an unexpectedly difficult airway. However, the use of flexible intubation endoscopes is often more difficult and time-consuming than for an unexpected difficult airway [126].
Supraglottic airway devices
Supraglottic airway devices are central to the management of the unexpectedly difficult airway, as successful positioning may be possible even with difficult mask ventilation and/or frustrating laryngoscopy. If intubation is necessary after successful placement of an SGA, it can be performed blind or with a flexible intubation endoscope, depending on the SGA used.
Recommendation 38
If the airway is unexpectedly difficult, a second-generation SGA should be used.
In adequately anesthetized patients who have difficulty with placement or oxygenation, the head may be tilted for placement of the SGA, taking contraindications into account. Lower jaw lift or an Esmach maneuvre may be helpful. Ventilation may also be achieved by neuromuscular blockade [127, 128] or modification of the SGA model used [129].
Recommendation 39
If problems persist, a change to a different size or a different SGA model shall be considered.
It should be noted that multiple placement attempts can also potentially lead to trauma and swelling of the airway [130, 131].
Recommendation 40
Neuromuscular blockade may be considered if problems persist.
Return to spontaneous breathing
Recommendation 41
If securing the airway with adequate oxygenation (e.g., mask ventilation possible) remains frustrating, the possibility to return to spontaneous breathing shall be evaluated. Depending on the drugs used to induce general anesthesia, antagonism or reversal shall also be considered.
This procedure is not a reliable option if oxygenation is impossible, even if the neuromuscular blockade is reversed immediately (sugammadex after rocuronium), because the actual time of return of spontaneous breathing cannot be predicted [128, 132].
Translaryngeal/transtracheal access
Recommendation 42
If airway management fails at the first three levels, oxygenation shall be provided via a translaryngeal or transtracheal approach if asphyxia is imminent.
Under no circumstances should the invasiveness of this procedure or its uncertain success be interpreted that an indicated cricothyrotomy should be omitted for supposed safety reasons, as this would have even more fatal consequences (hypoxic brain damage or death).
Unexpected airway difficulty algorithm
Considering the above recommendations, the procedures are shown graphically in the algorithm “unexpected difficult airway” (Fig. 2).
The algorithm starts with the “Failed Airway Management” situation. This allows immediate access to the algorithm without a long orientation phase. This is followed by the important “mask ventilation” fork. If adequate oxygenation is possible with mask ventilation, there is no acute risk to the patient. If endotracheal intubation is unsuccessful or placement of an SGA is frustrating, other promising techniques and instruments can be used to secure the airway and muscle relaxation at a sufficient depth of anesthesia should be considered.
Recommendation 43
If mask ventilation is adequate, an alternative device shall be used that has the greatest chance of success in the situation, is readily available, and the practitioner is experienced with.
Recommendation 44
If mask ventilation is adequate and there is no prospect of success or a risk of airway obstruction, no further attempts to secure the airway shall be made and a return to spontaneous breathing shall be considered. Once spontaneous breathing and/or arousal has been achieved, an alternative procedure can be selected, like that for an anticipated difficult airway.
If mask ventilation fails as a fallback option after an initial intubation attempt or becomes inadequate during the procedure, a “can’t ventilate, can’t oxygenate” situation is present. The patient is at a high risk of hypoxia.
Recommendation 45
In the “cannot ventilate, cannot oxygenate” situation, help shall be called immediately.
Depending on the situation and institutional agreements, the following people may be called in to help in this crisis: Nurses, members of the code team, but also individuals from other disciplines (e.g. ENT). Materials for further levels of airway management must be brought to the patient side and prepared for use as needed.
Recommendation 46
If neuromuscular blockade has not yet been initated, it should be performed with a fast-acting muscle relaxant at an appropriate dose.
Recommendation 47
The instrument/aid that has the best chance of success in the given situation, is readily available, and is mastered by the practitioner shall be used.
Recommendation 48
If an SGA is used, it should be a 2nd generation SGA. If a primary SGA has already failed, a different size or alternative SGA model should be used.
Video laryngoscopy plays an important role in this situation. A repeat attempt at mask ventilation may be useful if neuromuscular blockade has been delayed.
Recommendation 49
In a “cannot ventilate, cannot oxygenate” situation, a cricothyrotomy shall be prepared in parallel.
Recommendation 50
Further attempts to secure the airway can be made only with maintained, sufficient oxygenation.
Although this algorithm can be used to manage most unexpectedly difficult airway situations, it is not fully applicable to every conceivable situation.
Recommendation 51
An assessment of the airway (why is the airway management failing?) and a check of vital signs shall be performed at each step of the algorithm (re-evaluation). In addition, the staffing and resource situation shall be critically reviewed at each step.
Every successful attempt to secure the airway ends with a check for successful ventilation using capnography check. However, further measures may be required: For example, if the acutely threatening situation is controlled with an SGA, the subsequent placement of an endotracheal tube may still be necessary for various reasons.
5. Airway management for patients at risk of aspiration
The deviation from “standard” induction of anesthesia in the sense of “rapid sequence induction and intubation” (RSI) is intended to prevent regurgitation and aspiration of gastric contents during induction of anesthesia in patients at risk of aspiration. Consideration of various practical measures in patients at risk for aspiration represents an effective prevention of pulmonary aspiration during induction of anesthesia and endotracheal intubation. The previously described recommendations for the expected difficult airway apply without restriction.
The following groups of patients are considered at risk for aspiration and for whom RSI is recommended [133, 134]:
-
Acute abdomen
-
Vomiting
-
Ileus
-
Emergency (fasting status unclear or delayed gastric emptying due to “stress”)
-
Patient who has consumed more than 50 ml of tea or water < 2 h ago, < 4 h of breast milk or other liquids or < 6 h of animal milk (products) or solid food
-
Use of drugs delaying gastric emptying
-
Patients with symptomatic esophageal reflux disease (e.g. hiatal hernia)
-
Patients with esophageal fistula or diverticulum
-
Patients with intrathoracic gastroesophagostomy (gastric pull-up)
-
Pylorospasm
-
Pregnancya
-
Post bariatric surgery, post Billroth II resection
aIn everyday clinical practice, the period around the 20th week of pregnancy is often established as the limit. However, there is no evidence for this.
Recommendation 52
In obese patients with a BMI > 30 kg/m2 and no other factors that increase the risk of aspiration, an RSI can be omitted, and a “normal” induction sequence can be performed.
Medication preparation
Recommendation 53
10 min before induction, 30 ml of 0.3% sodium citrate solution can be taken orally or administered by gastric tube.
Practical procedure
Recommendation 54
A powerful suction device (preferably motorized) with a large lumen suction tube (e.g., Yankauer suction kit) shall be available on the patient during airway management.
All general anesthesia is induced intravenously. No recommendation can be made regarding patient positioning (upper body neutral, up, or down), however upper body up is associated with multiple benefits.
Preoxygenation is performed as described. After induction of general anesthesia, neuromuscular blockade is performed with a fast-acting muscle relaxant. Rapid endotracheal intubation of the patient at risk for aspiration is performed without prior mask ventilation.
Recommendation 55
Endotracheal intubation shall be performed with a Macintosh blade video laryngoscope.
The procedure for unexpected difficult intubation is analogous to the procedure for unexpected difficult airway.
Recommendation 56
In patients with a significantly increased risk of aspiration (e.g., ileus, trauma with impaired consciousness), gastric contents shall be aspirated through an existing gastric tube or a large lumen (e.g., 16 Ch) gastric tube placed prior to anesthesia induction, unless contraindicated.
Recommendation 57
The gastric tube may be removed prior to induction or left in place for induction.
Cricoid pressure is not important to prevent aspiration in RSI [135].
Securing the airway in obstetrics
In the AWMF guideline “Obstetric Analgesia and Anesthesia”, the recommended procedure for general anesthesia and after failed intubation in obstetrics is presented using 3 algorithms: 1. safe general anesthesia in obstetrics; 2. procedure for a frustrated endotracheal intubation attempt in obstetrics; 3. procedure for the can’t intubate, can’t oxygenate situation in obstetrics [136]. The anesthetic standard for securing the airway in obstetric anesthesia is RSI. After induction of general anesthesia and adequate neuromuscular blockade without mechanical ventilation, endotracheal intubation should be performed. In patients at risk of acute hypoxia, intermediate pressure-limited ventilation or mechanical pressure-controlled ventilation can ensure oxygenation; if endotracheal intubation is unsuccessful during rapid sequence induction, the unexpectedly difficult airway procedure described above should be used.
6. Single lung ventilation
In patients with requiring single lung ventilation, in addition to difficult mask ventilation and laryngoscopy, correct placement of airway devices for single lung ventilation may be difficult. Therefore, in addition to upper airway anatomy (difficult upper airway), pathology of the deeper tracheobronchial anatomy that makes placement of the double-lumen tube or other airway devices difficult (difficult lower airway; difficult side separation) must be included in the definition of a difficult airway.
The risk and incidence of hypoxia during endotracheal placement of a double-lumen tube is significantly higher (10%) than during intubation with a conventional tube [137].
Recommendation 58
To identify possible predictors of difficult one lung ventilation, clinical examination should be supplemented by the inclusion of radiologic imaging and bronchoscopic findings.
Indicators of possible difficult single lung ventilation are any subglottic or tracheobronchial pathology that prevents or complicates atraumatic endotracheal intubation with a double-lumen tube or bronchial blocker (Table 8).
Procedure for the expected difficult airway
Despite numerous publications describing video-assisted thoracoscopic lung resections in non-intubated patients under regional anesthesia and/or sedation, this concept is limited to a few centers due to the potential need for thoracotomy and a variety of possible anesthetic complications [139, 140].
Recommendation 59
If there are predictors or indications of difficult or impossible mask ventilation and/or endotracheal intubation in patients for one lung ventilation, the airway shall be secured while maintaining spontaneous breathing.
The advantage of video laryngoscopy over flexible endoscopic for awake intubation is that a double lumen tube can be used directly in the absence of anatomic constriction [141,142,143]. Flexible endoscopic awake intubation with a double lumen tube has been published in case reports, but can be technically challenging. Therefore, a standard tube should be placed in the first attempt. After verification of correct placement and induction of general anesthesia, a bronchial blocker can be placed. Alternatively, the standard tube can be removed, and a double lumen tube can be placed using a tube exchange device. However, it should be noted that both the placement of the double-lumen tube and the reinsertion of the single-lumen tube can be frustrating.
Tube change devices are plastic rods that are longer than tube insertion devices and have often to be used to deliver oxygen via a lumen.
Recommendation 60
After placement of the bronchial blocker or transfer to a double-lumen tube, bronchoscopy shall be used to verify correct positioning.
Recommendation 61
In addition to positional monitoring with a flexible endoscope, lung ultrasound may be used to monitor tube position (change from lung sliding to lung pulse on the unventilated side of the lung).
Unexpectedly difficult airway
If the airway is unexpectedly difficult, the patient’s oxygenation and ventilation have top priority. The procedure corresponds to the previous recommendations [144, 145].
Procedure for unsuccessful placement of a double lumen tube
Bronchus blocker
After correct orotracheal placement of a standard tube in patients with a difficult airway, side-separated ventilation can be achieved with a bronchus blocker.
Supraglottic airway devices
Supraglottic airway devices can be used to place an endotracheal tube in difficult airways [146, 147]. In a two-step procedure, the tube or an intubation catheter can be placed in the trachea, or a bronchial blocker can be placed directly for lateral ventilation using a flexible intubation endoscope in SGAs that allow intubation [148,149,150].
Extubation after single lung ventilation
Recommendation 62
In the absence of anesthetic or surgical contraindications return to spontaneous breathing and extubation shall be attempted immediately after surgery, especially in patients undergoing thoracic surgery.
This allows more rapid minimization of existing parenchymal fistulas and prevention of respiratory complications. However, if extubation is not initially indicated, bronchial blockers in a standard tube can be deflated and removed while the tube remains located endotracheal. In the case of a double-lumen tube, the tube should be changed to a standard tube using a tube exchange device. In individual cases, a double-lumen tube may be left in place for a very short period in order to plan extubation practically and without time pressure.
7. Outpatient/ambulatory anesthesia
The proportion of procedures performed on an outpatient basis is increasing worldwide due to several factors. The word “outpatient” or “ambulatory” often leads people to undervalue the anesthesia component—a misconception that can lead to substandard care. In principle, ambulatory anesthesia must meet the same DGAI standards for workplace equipment [151], quality of performance, and staff training [152] as for inpatient procedures in a hospital. The patient must not be exposed to a higher risk of perioperative complications simply because anesthesia is performed on an outpatient basis, regardless of whether the anesthesia workstation is permanently equipped or whether the anesthesiologist must bring and set up all necessary material.
Preoperative evaluation in outpatient anesthesia
In general, patients in ASA classes I–III who do not differ significantly in their risk profile from patients for elective inpatient surgery are suitable for outpatient surgery. However, preoperative evaluation is of particular importance for outpatient surgery. Accordingly, preoperative assessment of the airway for predictors of difficult airway must be performed conscientiously to avoid potential complications. This should not be done on the day of surgery with the patient in a recumbent position, as not all tests can be performed in this way or will give incorrect results, nor should it be based solely on a telephone interview.
Recommendation 63
Preoperative evaluation of the airway for predictors of difficult airway shall also be performed in the outpatient setting.
Recommendation 64
Patients with a predicted difficult airway, especially those with anatomic (postoperative) airway changes, syndromes, or tumors, shall not be managed as outpatients without access to an inpatient facility.
To date, there are no valid threshold values up to which outpatient anesthesia can be performed safely based on the patient’s body weight alone. However, obese patients should be screened for the presence of obstructive sleep apnea (OSAS), which is increasingly associated with higher body mass indexes. Scoring systems (e.g., STOP BANG) have proven useful [153]. OSA has particularly important implications for ambulatory anesthesia, as the presence of OSAS is associated with up to a 4-fold increase in the frequency of difficult mask ventilation, difficult laryngoscopy, and endotracheal intubation [154, 155]. Such an association with the use of an SGA has not been demonstrated. However, it should be noted that the use of an SGA generally fails in 1–1.5% of cases. Because of the association between airway obstruction and OSAS in morbidly obese patients, current evidence does not recommend airway surgery in ambulatory patients with OSAS [156, 157].
Recommendation 65
Postoperatively, a recovery room shall be available that allows for oxygenation and monitoring equipment, as well as the use of (the patient’s own) CPAP device.
In addition, appropriate staffing levels must be ensured until the patient is discharged home or transferred to an inpatient unit.
Equipment in outpatient anesthesia
The anesthesiologist is responsible for ensuring that an adequate supply of oxygen is always available during ambulatory anesthesia. If a central gas supply is not available, a sufficient amount of oxygen (measured against the calculated requirement plus a safety reserve) must be available in gas cylinders.
If no patients with an anticipated difficult airway are being anesthetized, the provision of a flexible intubation endoscope for airway management in ambulatory anesthesia is not mandatory. However, the British NAP4 report showed that even in healthy patients undergoing minor surgery, unexpected airway catastrophes can contribute to mortality, whether due to failed endotracheal intubation or inadequate oxygenation via an inserted SGA [110]. Although the majority of procedures are performed without the need for endotracheal intubation, the ambulatory anesthesiologist should expect to encounter an unexpectedly difficult airway and have both a plan and the necessary equipment to manage it [158, 159].
Recommendation 66
Materials for all levels of airway management shall be available for ambulatory anesthesia outside of dedicated operating rooms.
Recommendation 67
A video laryngoscope shall be available as an alternative to the direct laryngoscope.
Supraglottic airway devices in outpatient anesthesia
In outpatient anesthesia, the use of SGA is very common due to the predictability of the often shorter and minor procedures, the shorter induction and discharge time as well as the lower patient morbidity. First generation SGAs are often used. SGA are also used in the outpatient setting for so-called extended indications (e.g. special positioning, beach chair positioning, prone positioning, laparoscopies, ENT procedures, longer operating times). In this case, second-generation SGAs with the option of gastric decompression should be used to reduce the risk of aspiration and to perform position control tests [160, 161]. In addition to appropriate patient selection, this requires a high level of clinical user expertise, enough personnel and material for possible repositioning of the patient (prone position), and alternative equipment for airway management.
Recommendation 68
Since optimal positioning of the SGA is particularly important for the so-called extended indications, a second-generation SGA with the option of gastric decompression should also be used for outpatient anesthesia to reduce the risk of aspiration and to be able to perform position control tests.
Recommendation 69
For shorter interventions the SGA cuff pressure shall be measured and adjusted as needed.
Capnography
Apnea can be detected early in patients under sedation using continuous capnography [159, 162].
Recommendation 70
All patients undergoing general anesthesia and moderate or deep sedation, regardless of the length of the procedure and the site of anesthesia, shall be monitored by continuous capnography.
8. Airway management for critically ill patients
Compared to elective airway management during anesthesia, airway management in the critically ill patient population is generally more urgent and performed under more difficult conditions in the intensive care unit. Complications are also more commonly reported: Difficult intubation (≥ 2 attempts required) occurs in 4.6% of cases, false esophageal intubation in 5.6% of cases [163, 164]. A drop in oxygen saturation is observed in up to 70% of intubations, with hypoxia (SpO2 80%) in 9.3% of patients [163]. A drop in oxygen saturation is one of the most common reasons for abandoning an intubation attempt in the intensive care unit [163,164,165,166,167]. In addition, a primarily easy airway may become a difficult airway due to physiologic changes in typical ICU courses (e.g., generalized edema in sepsis).
Indications for airway management in the intensive care unit
The correct indication for securing the airway in critically ill patients can be difficult.
Recommendation 71
The benefits and risks shall be carefully weighed when determining the indication for intubation in critically ill patients in the ICU. Indication for intubation may be more liberal to avoid hypoxia.
The incidence of complications such as hypoxemia, aspiration, or cardiovascular arrest during airway management increases with the duration and number of intubation attempts [163, 166, 167]. Successful intubation at the first attempt is therefore of paramount importance. Several aspects must be considered to achieve this goal:
Preparation in the intensive care unit
Recommendation 72
All patients should be assessed in a structured manner on admission to the ICU for the likelihood and presence of predictors of difficult airway management, and the outcome should be visibly documented.
Staff in the intensive care unit
The level of education and training of airway management staff in the ICU influences the complication rate [110, 168, 169]. The presence of a second physician during airway management reduces the complication rate. The consultant level of care, especially in anesthesiology, is associated with a higher success rate and improved hemodynamic optimization [163,164,165].
Recommendation 73
Airway management in the ICU shall be performed or supervised by physicians and nursing staff experienced in airway management.
Equipment in the intensive care unit
Patient-related factors (69%) were identified as the main reason for difficult airway management; however, equipment and resource deficiencies were common (36%) [110, 170].
Recommendation 74
Considering local practices, a standardized and clearly arranged airway trolley shall be available for each airway securing procedure. Analogous to the OR containing materials and devices shall be available for all levels of airway management.
Recommendation 75
There shall be clear communication and interaction between team members prior to any airway management.
Expected difficult airway in the intensive care unit
Recommendation 76
Intubation with spontaneous breathing shall also be performed in the ICU when a difficult airway is anticipated.
The “expected difficult airway” algorithm (Fig. 1) also applies to critical care.
Recommendation 77
During intubation with spontaneous breathing, oxygen should be delivered through a high-flow nasal cannula.
Preoxygenation in the intensive care unit
Recommendation 78
CPAP therapy, noninvasive ventilation, or nasal high-flow may be used to preoxygenate with oxygen, especially in patients with respiratory compromise.
Nasal high-flow reduces hypoxemia in intensive care patients [50, 51].
In patients with respiratory insufficiency, elevation of the upper body is beneficial because it allows apnea to be tolerated longer and reduces the overall complication rate [171,172,173,174].
Recommendation 79
The airway of the critically ill patient shall be secured in the upper body elevated position (≥ 20°).
Laryngoscopy in the intensive care unit
Difficult direct laryngoscopy is frequently observed in critically ill patients and is associated with multiple intubation attempts and incorrect endotracheal tube positioning [123, 163, 164, 166, 167, 175].
The goal of laryngoscopy as the first choice for securing the airway should be prompt, atraumatic tracheal intubation during the first intubation attempt. Compared to conventional laryngoscopy, video laryngoscopy has clear advantages: improved visualization of laryngeal structures, higher rate of successful intubation at the first attempt, lower incidence of esophageal tube placement, and less force required [71, 163, 164, 176,177,178,179,180,181].
Recommendation 80
A video laryngoscope with a Macintosh-like blade shall be used primarily to secure the airway.
Recommendation 81
Hyper angulated blades should be available for difficult airways.
Alternatives to laryngoscopy in the intensive care unit
Recommendation 82
If intubation fails, reoxygenation by SGA or mask ventilation shall be performed immediately.
Recommendation 83
As an alternative to manual mask ventilation, pressure-controlled ventilation with a respirator can be used to avoid gastric insufflation.
The selection of the SGA and the recommendations for translaryngeal airway management are analogous to the recommendations in Sect. 3: Techniques for airway management.
Monitoring the success of airway management in the intensive care unit
Capnographic monitoring is mandatory in order to be able to verify the correct tube position reliably and promptly [163, 164, 182, 183].
Recommendation 84
Capnography shall be used to verify correct tube position reliably and promptly.
Reintubation in the intensive care unit
Complications with the inserted tube or tracheostomy tube occur in up to 80% of critically ill patients [163, 184, 185]. Tracheal reintubation may be necessary due to cuff leakage, intraluminal tube obstruction, or if the tube is initially too small. Several techniques have been published [184, 185]. Possible techniques include video laryngoscopic glottic visualization to assess the current intubation anatomy and splinting of the already secured airway with a tube exchange device [185].
Recommendation 85
For tracheal tube exchange, video-laryngoscopic visualization of the glottis should be performed to assess the current intubation anatomy. A tube changing device should be used.
Tracheal tube replacement is a high-risk procedure and requires the same preparation as initial airway protection. Some tube exchange devices allow oxygen to be delivered through the device via a connector. However, there is a risk of lung barotrauma if these are used when the airway is not fully open. Ventilation is possible with specific devices that allow controlled, active expiration [96].
Recommendation 86
If sufficient expertise is available, emergency ventilation may be performed with the aid a tube exchange device, that allow controlled, active expiration even if the airway is obstructed.
Extubation in the intensive care unit
Extubation can be as critical as initial endotracheal intubation. Extubation failure with the need for reintubation within 24–72 h occurs in 6–25% of all surgical and ICU patients [186]. Table 9 shows risk factors associated with extubation failure in the ICU.
The procedure for extubation is described in Sect. 12: Extubation after difficult airway management.
9. Special considerations for patients with highly contagious respiratory pathogens
In patients with highly contagious diseases, protecting the team is also a top priority during airway management. Diseases with high primary viral replication in the upper respiratory tract and secondary replication in the lower respiratory tract result in high contagiousness and infectivity.
Recommendation 87
When a highly contagious disease is suspected, medical personnel shall use all forms of respiratory protection in addition to the usual hygiene and protection measures.
Airway management in these patients requires specific, communicated, and trained protocols for preparation, performance, and safety. Intubation should be planned and performed electively whenever possible.
10. Anesthesia consult, evaluation and documentation
Recommendation 88
If there is a positive history, appropriate predictors, or other indications of an anticipated difficult laryngoscopy, difficult intubation, or difficult mask ventilation, the patient shall be informed about airway management while maintaining spontaneous breathing as part of the anesthesia consultation.
Recommendation 89
Each airway management procedure shall be documented, with detailed information on any difficulties encountered.
Documentation should include the devices and instruments used for mask ventilation, securing the airway, and intubation, the best view of the glottis obtained according to Cormack and Lehane, if applicable, and the number of attempts until the airway is definitively secured [187].
Recommendation 90
The Cormack and Lehane classification shall also be used for video laryngoscopy and documented in the anesthesia record.
To avoid misinterpretation during subsequent procedures, the record should be kept in such a way that it is possible to reconstruct which laryngoscopic findings were possible with which instrument [188]. It should also be documented whether external manipulation (OELM [189] or BURP [190]) was performed and whether a stylet or other device was required for successful intubation.
Recommendation 91
Tube size, cuff pressure, depth of intubation, placement of a throat pack, and special head positions should be documented to ensure retrospective and unambiguous understanding of the chosen procedure.
Recommendation 92
After difficult airway management, the circumstances and procedures used to resolve the problem shall be documented in the anesthesia record.
Recommendation 93
After difficult airway management, the timing, clinical presentation, and nature and resolution of the problems shall be reported in writing. This should be done even if the problems have been resolved after the procedure and are unlikely to recur. The anesthesia problem card issued by the DGAI shall be kept available for this purpose and given to the patient with the relevant information.
11. Actions after airway management
Recommendation 94
Once the airway is secured, the correct position of the endotracheal tube or SGA shall be verified by capnography.
Capnography plays a very important role in all airway management [183]. Another safe procedure when using an endotracheal tube is intubation under direct or indirect visualization of the glottis.
Recommendation 95
Auscultation of the chest shall be performed to rule out a tube position that is too low. In a noisy environment or when auscultation is not possible, pleural sliding can be detected by sonography.
Once an SGA has been advanced to a sufficient depth, a typical capnography and flow curve, sufficient leak pressure during ventilation, and bilateral breath sounds are signs of correct positioning. Various tests to verify the position of SGAs with a gastric drainage channel are described in the chapter “Airway Management Techniques”.
Recommendation 96
After placement of both an endotracheal tube and an SGA, the cuff pressure shall be checked and adjusted if necessary.
There is no clear evidence for the use of corticosteroids after difficult airway management or prolonged laryngeal manipulation to prevent laryngeal edema. Giving a single dose one hour before planned extubation is not effective [191,192,193]. In contrast, multiple single doses given 12–24 h before extubation reduce the incidence of post-extubation stridor [193,194,195]—especially in high-risk patients with a bypass air volume of less than 110 ml on the bypass air test [196] (see next Sect. 12: Extubation after difficult airway management).
12. Extubation after difficult airway management
In contrast to intubation, planned extubation is always highly elective, allowing sufficient time to identify risk factors and plan extubation in time to prevent serious complications by optimizing conditions [197]. Nevertheless, 0.1–0.5% of patients under general anesthesia require reintubation after surgery [198], which is associated with both more severe complications and increased mortality [110]. Common causes of airway problems after extubation are listed in Table 10.
Recommendations for extubation
Although extubation is elective, the environment and conditions are often not as optimized as for intubation. An extubation strategy should also be in place prior to induction of anesthesia in the event of accidental extubation [199], so that not only predictors of difficult intubation, but also possible difficult reintubation, can be identified at an early stage. In particular, the necessary conditions for extubation or continuation of ventilation can then be planned in good time: These include readiness for cricotyhrotomy and/or tracheotomy, adequate qualifications for care during and after anesthesia, special devices and equipment for difficult reintubation (e.g., tube insertion aids, video laryngoscopes and/or bronchoscopes).
Recommendation 97
Predictors of difficult reintubation shall be identified before extubation. A clear strategy for potential problems should be defined.
Depending on the patient and procedure, it may be useful to evaluate the supraglottic area prior to extubation in an anesthetized patient. This can be done with video laryngoscopy.
Recommendation 98
The secondary air leak test can be performed to detect significant obstructing laryngeal edema.
This is done by deflating the cuff and measuring the difference between inspiratory and expiratory tidal volumes during the first 6 breaths. There is a low risk of clinically relevant laryngeal edema with an average leak greater than 110 ml per breath [200].
The expertise of an ear, nose and throat or oral and maxillofacial surgeon may be required immediately. If necessary, a “cricothyrotomy/tracheotomy readiness” is required.
Recommendation 99
In special situations, an airway catheter (e.g., tube change aid or extubating kit) may be placed endotracheally prior to extubation to allow for oxygen insufflation or flow-controlled ventilation and to serve as a guide for reintubation, if necessary.
Most patients can speak despite the catheter. Length of placement up to several days has been described [201]. Nasal placement seems to be better tolerated than oral placement. A dislodgement rate of 4% has been reported regardless of position [202, 203].
In patients who are expected to have difficulty after extubation due to a hyperresponsive tracheobronchial system, the endotracheal tube may be removed early and an SGA placed in special situations for assisted ventilation and to avoid a cough stimulus.
Recommendation 100
Monitoring of the patient after extubation shall be performed by qualified personnel. New symptoms that may indicate the development of an airway complication (e.g., hoarseness, (increasing) swelling, difficulty swallowing, chest pain, and emphysema formation) shall be recognized early.
The “Procedure for planned extubation” algorithm illustrates the procedure graphically (Fig. 3).
13. Education and training
Education and training for clinical skills in airway management cannot be taught exclusively on patients. Simulation training is now recommended by many professional societies [133, 145, 204,205,206].
The core technical skills that need to be used frequently, even in unpredictable and time-critical situations, should be developed and trained.
Recommendation 101
To successfully secure the unexpected and expected difficult airway, sound education and regular training, including simulation, shall be provided.
In the event of airway problems, only those instruments and techniques that are regularly used and mastered in elective patients are likely to be successful.
Recommendation 102
Regular training should be provided on airway trainers for translaryngeal/transtracheal techniques.
Education and training must focus on optimizing oxygen delivery to difficult airways using techniques such as noninvasive positive pressure ventilation for pre-oxygenation, apneic oxygen delivery, and high-flow oxygen via nasal cannula. In addition, non-technical skills such as decision-making and team communication are essential for all involved and at least as important for preventing errors [204, 207].
Recommendation 103
The individual techniques should generally be learned in four steps:
1. acquisition of theoretical knowledge.
Airway anatomy, physiology, and assessment.
Theory and practical use of airway equipment.
Basic and advanced airway management techniques.
Evidence-based guidelines for difficult airway management.
Local and institutional airway management guidelines.
Complications associated with the difficult airway and their management.
2. Practice techniques and skills on airway phantoms and airway simulators.
3. Practice techniques under supervision on patients with normal airways. This should be done until safe handling is assured, including in emergency situations.
4. use the techniques under supervision on patients with difficult airways and regular use in routine clinical practice.
Non-technical skills are also required: Situational awareness and vigilance to recognize a crisis, leadership and coordination, effective professional communication.
14. Checklists and human factors
Human factors are the major cause of medical errors and have been identified as an independent factor in respiratory complications [208,209,210]. Deficiencies such as lack of patient preparation, lack of control over equipment, and deviations from existing standards or protocols occur in half of critical incidents in the ICU [208, 211, 212]. Thus, factors such as team composition/work, patient status, environment, and the need for immediate decisions influence the effectiveness of airway management in critically ill patients [208, 210,211,212]. Checklists and algorithms can improve team effectiveness in stressful situations, help make well-considered decisions, avoid unnecessary interventions, and optimize the use of available resources [211, 212]. Checklists and algorithms can improve complex work structures and avoid (routine) periinterventional errors.
Recommendation 104
Checklists and algorithms should be always available and accessible to all team members.
References
Timmermann A, Bt B, Byhahn C (2019) Dörges V, Eich C, Gräsner JT et al. S1Guideline: Prehospital airway management. Anesthesiology. Intensive Care Med 60:316–336
Dan B (2022) guideline polytrauma / treatment of severely injured patients. AWMF, online, p 3
F. Hoffmann JK, B. Urban, P. Jung, C. Eich, F. Hoffmann, A. Schiele, A. Parsch, B. Matsche, C. Eich, K. Becke, B. Landsleitner, S.G. Russo, M. Bernhard, T. Nicolai. Interdisciplinary consensus statement: Airway management with supraglottic airway devices in pediatric emergency medicine—laryngeal mask is state-of-the-art. Anästh Intensivmed 2016;57:377–86.
Samsoon GL, Young JR (1987) Difficult tracheal intubation: a retrospective study. Anaesthesia 42:487–490
Heidegger T (2021) Management of the Difficult Airway. N Engl J Med 384:1836–1847
Roth D, Pace NL, Lee A, Hovhannisyan K, Warenits AM, Arrich J et al (2019) Bedside tests for predicting difficult airways: an abridged Cochrane diagnostic test accuracy systematic review. Anaesthesia 74:915–928
Nørskov AK, Wetterslev J, Rosenstock CV, Afshari A, Astrup G, Jakobsen JC et al (2017) Prediction of difficult mask ventilation using a systematic assessment of risk factors vs. existing practice—a cluster randomized clinical trial in 94,006 patients. Anaesthesia 72:296–308
Kheterpal S, Han R, Tremper KK, Shanks A, Tait AR, O’Reilly M et al (2006) Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology 105:885–891
Langeron O, Masso E, Huraux C, Guggiari M, Bianchi A, Coriat P et al (2000) Prediction of difficult mask ventilation. Anesthesiology 92:1229–1236
Kheterpal S, Healy D, Aziz MF, Shanks AM, Freundlich RE, Linton F et al (2013) Incidence, predictors, and outcome of difficult mask ventilation combined with difficult laryngoscopy: a report from the multicenter perioperative outcomes group. Anesthesiology 119:1360–1369
Norskov AK, Rosenstock CV, Wetterslev J, Astrup G, Afshari A, Lundstrom LH (2015) Diagnostic accuracy of anaesthesiologists’ prediction of difficult airway management in daily clinical practice: a cohort study of 188 064 patients registered in the Danish Anaesthesia Database. Anaesthesia 70:272–281
Lavery GG, McCloskey BV (2008) The difficult airway in adult critical care. Crit Care Med 36:2163–2173
Adnet F, Racine SX, Borron SW, Clemessy JL, Fournier JL, Lapostolle F et al (2001) A survey of tracheal intubation difficulty in the operating room: a prospective observational study. Acta Anaesthesiol Scand 45:327–332
Savva D (1994) Prediction of difficult tracheal intubation. Br J Anaesth 73:149–153
Detsky ME, Jivraj N, Adhikari NK, Friedrich JO, Pinto R, Simel DL et al (2019) Will This Patient Be Difficult to Intubate? The Rational Clinical Examination Systematic Review. JAMA 321:493–503
Patil V (1983) Predicting the difficulty of intubation utilizing an intubation gauge. Anesth Rev 10:32–33
Wilson ME, Spiegelhalter D, Robertson JA, Lesser P (1988) Predicting difficult intubation. Br J Anaesth 61:211–216
Arne J, Descoins P, Fusciardi J, Ingrand P, Ferrier B, Boudigues D et al (1998) Preoperative assessment for difficult intubation in general and ENT surgery: predictive value of a clinical multivariate risk index. Br J Anaesth 80:140–146
el-Ganzouri AR, McCarthy RJ, Tuman KJ, Tanck EN, Ivankovich AD (1996) Preoperative airway assessment: predictive value of a multivariate risk index. Anesth Analg 82:1197–1204
Shiga T, Wajima Z, Inoue T, Sakamoto A (2005) Predicting difficult intubation in apparently normal patients: a meta-analysis of bedside screening test performance. Anesthesiology 103:429–437
Ilper H, Franz-Jager C, Byhahn C, Klages M, Ackermann HH, Zacharowski K et al (2018) Mallampati. Anaesthesiologist. Update 67:738–744
Ilper H, Grossbach A, Franz-Jäger C, Byhahn C, Klages M, Ackermann HH et al (2018) Thyromental distance. Anaesthesist 67:198–203
Kleine-Brueggeney M, Greif R, Schoettker P, Savoldelli GL, Nabecker S, Theiler LG (2016) Evaluation of six videolaryngoscopes in 720 patients with a simulated difficult airway: a multicentre randomized controlled trial. Br J Anaesth 116:670–679
Kohse EK, Siebert HK, Sasu PB, Loock K, Dohrmann T, Breitfeld P et al (2022) A model to predict difficult airway alerts after videolaryngoscopy in adults with anticipated difficult airways—the VIDIAC score. Anaesthesia 77:1089–1096
Ramachandran SK, Mathis MR, Tremper KK, Shanks AM, Kheterpal S (2012) Predictors and clinical outcomes from failed Laryngeal Mask Airway Unique: a study of 15,795 patients. Anesthesiology 116:1217–1226
Vannucci A, Rossi IT, Prifti K, Kallogjeri D, Rangrass G, DeCresce D et al (2018) Modifiable and Nonmodifiable Factors Associated With Perioperative Failure of Extraglottic Airway Devices. Anesth Analg 126:1959–1967
Katsiampoura AD, Killoran PV, Corso RM, Cai C, Hagberg CA, Cattano D (2015) Laryngeal mask placement in a teaching institution: analysis of difficult placements. F1000Res 4:102
Law JA, Duggan LV, Asselin M, Baker P, Crosby E, Downey A, et al. Canadian Airway Focus Group updated consensus-based recommendations for management of the difficult airway: part 2. Planning and implementing safe management of the patient with an anticipated difficult airway. Canadian journal of anaesthesia = Journal canadien d’anesthesie 2021;68:1405–36.
Kornas RL, Owyang CG, Sakles JC, Foley LJ, Mosier JM (2021) Evaluation and Management of the Physiologically Difficult Airway: Consensus Recommendations From Society for Airway Management. Anesth Analg 132:395–405
Mosier JM, Joshi R, Hypes C, Pacheco G, Valenzuela T, Sakles JC (2015) The Physiologically Difficult Airway. West J Emerg Med 16:1109–1117
Apfelbaum JL, Hagberg CA, Connis RT, Abdelmalak BB, Agarkar M, Dutton RP et al (2022) American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology 2022(136):31–81
Kristensen MS, Teoh WH, Graumann O, Laursen CB (2014) Ultrasonography for clinical decision-making and intervention in airway management: from the mouth to the lungs and pleurae. Insights Imaging 5:253–279
Petrisor C, Dîrzu D, Trancă S, Hagău N, Bodolea C (2019) Preoperative difficult airway prediction using suprahyoid and infrahyoid ultrasonography derived measurements in anesthesiology. Med Ultrason 21:83–88
Carsetti A, Sorbello M, Adrario E, Donati A, Falcetta S (2022) Airway Ultrasound as Predictor of Difficult Direct Laryngoscopy: A Systematic Review and Meta-analysis. Anesth Analg 134:740–750
Zechner PM, Breitkreutz R (2011) Ultrasound instead of capnometry for confirming tracheal tube placement in an emergency? Resuscitation 82:1259–1261
Austin DR, Chang MG, Bittner EA (2021) Use of Handheld Point-of-Care Ultrasound in Emergency Airway Management. Chest 159:1155–1165
Kristensen MS, Teoh WH, Rudolph SS. Ultrasonographic identification of the cricothyroid membrane: best evidence, techniques, and clinical impact. British journal of anaesthesia 2016;117 Suppl 1:i39–i48.
Ansari U, Malhas L, Mendonca C (2019) Role of Ultrasound in Emergency Front of Neck Access: A Case Report and Review of Literature. A A Pract 13:382–385
Fudickar AWK, Preoxygenation BT (2022) visualization by a simple mathematical model. Anästh Intensivmed 63:148–154
Nimmagadda U, Salem MR, Preoxygenation CGJ (2017) Physiologic Basis, Benefits, and Potential Risks. Anesth Analg 124:507–517
Bouroche G, Bourgain JL (2015) Preoxygenation and general anesthesia: a review. Minerva Anestesiol 81:910–920
De Jong A, Futier E, Millot A, Coisel Y, Jung B, Chanques G et al (2014) How to preoxygenate in operative room: healthy subjects and situations “at risk”. Ann Fr Anesth Reanim 33:457–461
Georgescu M, Tanoubi I, Fortier LP, Donati F, Drolet P (2012) Efficacy of preoxygenation with non-invasive low positive pressure ventilation in obese patients: crossover physiological study. Ann Fr Anesth Reanim 31:e161–5
Lee JH, Jung H, Jang YE, Kim EH, Song IK, Kim HS et al (2019) Manual vs pressure-controlled facemask ventilation during the induction of general anesthesia in children: A prospective randomized controlled study. Paediatr Anaesth 29:331–337
Hart D, Reardon R, Ward C, Miner J (2013) Face mask ventilation: a comparison of three techniques. J Emerg Med 44:1028–1033
Lyons C, Callaghan M (2019) Uses and mechanisms of apnoeic oxygenation: a narrative review. Anaesthesia 74:497–507
Patel A, Nouraei SA (2015) Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnea time in patients with difficult airways. Anaesthesia 70:323–329
Marshall SD, Pandit JJ (2016) Radical evolution: the 2015 Difficult Airway Society guidelines for managing unanticipated difficult or failed tracheal intubation. Anaesthesia 71:131–137
Rajan S, Joseph N, Tosh P, Kadapamannil D, Paul J, Kumar L (2018) Effectiveness of transnasal humidified rapid-insufflation ventilatory exchange versus traditional preoxygenation followed by apnoeic oxygenation in delaying desaturation during apnoea: A preliminary study. Indian J Anaesth 62:202–207
Ricard JD (2016) Hazards of intubation in the ICU: role of nasal high flow oxygen therapy for preoxygenation and apneic oxygenation to prevent desaturation. Minerva Anestesiol 82:1098–1106
Jaber S, Monnin M, Girard M, Conseil M, Cisse M, Carr J et al (2016) Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomized controlled OPTINIV trial. Intensive Care Med 42:1877–1887
Greenberg RS (2002) Facemask, nasal, and oral airway devices. Anesthesiol Clin North Am 20:833–861
El-Orbany M, Woehlck HJ (2009) Difficult mask ventilation. Anesth Analg 109:1870–1880
Priebe HJ (2019) Another Nail in the Coffin of the Practice of Checking Mask Ventilation Before Administration of a Muscle Relaxant. Anesth Analg 129:e103–e4
Soltész S, Alm P, Mathes A, Hellmich M, Hinkelbein J (2017) The effect of neuromuscular blockade on the efficiency of facemask ventilation in patients difficult to facemask ventilate: a prospective trial. Anaesthesia 72:1484–1490
Min SH, Im H, Kim BR, Yoon S, Bahk JH, Seo JH (2019) Randomized Trial Comparing Early and Late Administration of Rocuronium Before and After Checking Mask Ventilation in Patients With Normal Airways. Anesth Analg 129:380–386
Noppens RR, Piepho T (2015) Laryngeal mask. Off to new shores? Anaesthesist 64:5–6
Kriege M, Alflen C, Eisel J, Ott T, Piepho T, Noppens RR (2017) Evaluation of the optimal cuff volume and cuff pressure of the revised laryngeal tube “LTS-D” in surgical patients. BMC Anesthesiol 17:19
Schalk R, Seeger FH, Mutlak H, Schweigkofler U, Zacharowski K, Peter N et al (2014) Complications associated with the prehospital use of laryngeal tubes—a systematic analysis of risk factors and strategies for prevention. Resuscitation 85:1629–1632
Timmermann A, Nickel EA, Puhringer F (2015) Second-generation laryngeal masks. Anaesthesist 64:7–15
Mahajan R, Batra YK (2009) Water bubble test to detect malposition of PLMA. J Anesth 23:634–635
Goldmann K, Dieterich J, Roessler M. Laryngopharyngeal mucosal injury after prolonged use of the ProSeal LMA in a porcine model: a pilot study. Canadian journal of anaesthesia = Journal canadien d’anesthesie 2007;54:822–8.
Timmermann A (2011) Supraglottic airways in difficult airway management: successes, failures, use and misuse. Anaesthesia 66(Suppl 2):45–56
Mohr S, Weigand MA, Hofer S, Martin E, Gries A, Walther A et al (2013) Developing the skill of laryngeal mask insertion: prospective single center study. Anaesthesist 62:447–452
El-Orbany MI, Getachew YB, Joseph NJ, Salem MR, Friedman M (2015) Head elevation improves laryngeal exposure with direct laryngoscopy. J Clin Anesth 27:153–158
Akihisa Y, Hoshijima H, Maruyama K, Koyama Y, Andoh T (2015) Effects of sniffing position for tracheal intubation: a meta-analysis of randomized controlled trials. Am J Emerg Med 33:1606–1611
Driver BE, Semler MW, Self WH, Ginde AA, Trent SA, Gandotra S et al (2021) Effect of Use of a Bougie vs Endotracheal Tube With Stylet on Successful Intubation on the First Attempt Among Critically Ill Patients Undergoing Tracheal Intubation: A Randomized Clinical Trial. JAMA 326:2488–2497
Aziz MF, Brambrink AM, Healy DW, Willett AW, Shanks A, Tremper T et al (2016) Success of Intubation Rescue Techniques after Failed Direct Laryngoscopy in Adults: A Retrospective Comparative Analysis from the Multicenter Perioperative Outcomes Group. Anesthesiology 125:656–666
Gu Y, Robert J, Kovacs G, Milne AD, Morris I, Hung O, et al. A deliberately restricted laryngeal view with the GlideScope® video laryngoscope is associated with faster and easier tracheal intubation when compared with a full glottic view: a randomized clinical trial. Canadian journal of anaesthesia = Journal canadien d’anesthesie 2016;63:928–37.
Aziz MF, Healy D, Kheterpal S, Fu RF, Dillman D, Brambrink AM (2011) Routine clinical practice effectiveness of the Glidescope in difficult airway management: an analysis of 2,004 Glidescope intubations, complications, and failures from two institutions. Anesthesiology 114:34–41
Pieters BMA, Maas EHA, Knape JTA, van Zundert AAJ (2017) Videolaryngoscopy vs. direct laryngoscopy use by experienced anaesthetists in patients with known difficult airways: a systematic review and meta-analysis. Anaesthesia 72:1532–1541
Lewis SR, Butler AR, Parker J, Cook TM, Schofield-Robinson OJ, Smith AF (2017) Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation: a Cochrane Systematic Review. Br J Anaesth 119:369–383
Cavus E, Bein B, Dörges V (2011) Airway management—video-assisted procedures. Anasthesiology, intensive care medicine, emergency medicine, pain therapy. Anästhesiol Intensivmed Notfallmed Schmerzther 46:588–596
Noppens RR, Werner C, Piepho T (2010) Indirect laryngoscopy: alternatives to airway protection. Anaesthesiologist 59:149–161
Sakles JC, Patanwala AE, Mosier J, Dicken J, Holman N (2014) Comparison of the reusable standard GlideScope(R) video laryngoscope and the disposable cobalt GlideScope(R) video laryngoscope for tracheal intubation in an academic emergency department: a retrospective review. Acad Emerg Med 21:408–415
Andersen LH, Rovsing L, Olsen KS (2011) GlideScope videolaryngoscope vs. Macintosh direct laryngoscope for intubation of morbidly obese patients: a randomized trial. Acta Anaesthesiol Scand 55:1090–1097
Hansel J, Rogers AM, Lewis SR, Cook TM, Smith AF (2022) Videolaryngoscopy versus direct laryngoscopy for adults undergoing tracheal intubation. Cochrane Database Syst Rev 4:Cd11136
Noppens RR, Möbus S, Heid F, Schmidtmann I, Werner C, Piepho T. Evaluation of the McGrath Series 5 videolaryngoscope after failed direct laryngoscopy. Anaesthesia2010.
Kriege M, Noppens RR, Turkstra T, Payne S, Kunitz O, Tzanova I et al (2023) A multicentre randomized controlled trial of the McGrath Mac videolaryngoscope versus conventional laryngoscopy. Anaesthesia
Herbstreit F, Fassbender P, Haberl H, Kehren C, Peters J (2011) Learning endotracheal intubation using a novel videolaryngoscope improves intubation skills of medical students. Anesth Analg 113:586–590
Aziz MF, Abrons RO, Cattano D, Bayman EO, Swanson DE, Hagberg CA et al (2016) First-Attempt Intubation Success of Video Laryngoscopy in Patients with Anticipated Difficult Direct Laryngoscopy: A Multicenter Randomized Controlled Trial Comparing the C‑MAC D‑Blade Versus the GlideScope in a Mixed Provider and Diverse Patient Population. Anesth Analg 122:740–750
Bein B, Yan M, Tonner PH, Scholz J, Steinfath M, Dörges V (2004) Tracheal intubation using the Bonfils intubation fibrescope after failed direct laryngoscopy. Anaesthesia 59:1207–1209
Halligan M, Charters P (2003) A clinical evaluation of the Bonfils Intubation Fibrescope. Anaesthesia 58:1087–1091
Falcetta S, Pecora L, Orsetti G, Gentili P, Rossi A, Gabbanelli V et al (2012) The Bonfils fiberscope: a clinical evaluation of its learning curve and efficacy in difficult airway management. Minerva Anestesiol 78:176–184
Heymans F, Feigl G, Graber S, Courvoisier DS, Weber KM, Dulguerov P (2016) Emergency Cricothyrotomy Performed by Surgical Airway-naive Medical Personnel: A Randomized Crossover Study in Cadavers Comparing Three Commonly Used Techniques. Anesthesiology 125:295–303
King W, Teare J, Vandrevala T, Cartwright S, Mohammed KB, Patel B (2016) Evaluation of a novel Surgicric® cricothyroidotomy device for emergency tracheal access in a porcine model. Anaesthesia 71:177–184
Chrisman L, King W, Wimble K, Cartwright S, Mohammed KB, Patel B (2016) Surgicric 2: A comparative bench study with two established emergency cricothyroidotomy techniques in a porcine model. Br J Anaesth 117:236–242
Poole O, Vargo M, Zhang J, Hung O (2017) A comparison of three techniques for cricothyrotomy on a manikin. Can J Respir Ther 53:29–32
Nakstad AR, Bredmose PP, Sandberg M (2013) Comparison of a percutaneous device and the bougie-assisted surgical technique for emergency cricothyrotomy: an experimental study on a porcine model performed by air ambulance anaesthesiologists. Scand J Trauma Resusc Emerg Med 21:59
Andresen ÅEL, Kramer-Johansen J, Kristiansen T (2019) Percutaneous vs surgical emergency cricothyroidotomy: An experimental randomized crossover study on an animal-larynx model. Acta Anaesthesiol Scand 63:1306–1312
Bair AE, Panacek EA, Wisner DH, Bales R, Cricothyrotomy SJC (2003) a 5-year experience at one institution. J Emerg Med 24:151–156
Kwon YS, Lee CA, Park S, Ha SO, Sim YS, Baek MS. Incidence and outcomes of cricothyrotomy in the “cannot intubate, cannot oxygenate” situation. Medicine (Baltimore) 2019;98:e17713.
Andresen ÅEL, Kramer-Johansen J, Kristiansen T (2022) Emergency cricothyroidotomy in difficult airway simulation—a national observational study of Air Ambulance crew performance. BMC Emerg Med 22:64
Dillon JK, Christensen B, Fairbanks T, Jurkovich G, Moe KS (2013) The emergent surgical airway: cricothyrotomy vs. tracheotomy. Int J Oral Maxillofac Surg 42:204–208
Preussler NP, Schreiber T, Hüter L, Gottschall R, Schubert H, Rek H et al (2003) Percutaneous transtracheal ventilation: effects of a new oxygen flow modulator on oxygenation and ventilation in pigs compared with a hand triggered emergency jet injector. Resuscitation 56:329–333
de Wolf MWP, van der Beek T, Hamaekers AE, Theunissen M, Enk D (2018) A prototype small-bore ventilation catheter with a cuff: cuff inflation optimizes ventilation with the Ventrain. Acta Anaesthesiol Scand 62:328–335
de Wolf MW, Gottschall R, Preussler NP, Paxian M, Enk D. Emergency ventilation with the Ventrain(®) through an airway exchange catheter in a porcine model of complete upper airway obstruction. Canadian journal of anaesthesia = Journal canadien d’anesthesie 2017;64:37–44.
Catchpole K, Mishra A, Handa A, McCulloch P (2008) Teamwork and error in the operating room: analysis of skills and roles. Ann Surg 247:699–706
Catchpole KR, Giddings AE, de Leval MR, Peek GJ, Godden PJ, Utley M et al (2006) Identification of systems failures in successful pediatric cardiac surgery. Ergonomics 49:567–588
Prien THBM (2019) Czaplik, M. Hölzl, Ch. Hönemann, J. Grensemann, Th. Muders, R. Sattler, D. Schädler, T. Krauß. Functional testing of the anaesthesia machine to ensure patient safety—Recommendation of the Commission for Standardization and Technical Safety of the DGAI. Anesth. Intensivmed 60:75–83
Law JA, Morris IR, Brousseau PA, de la Ronde S, Milne AD. The incidence, success rate, and complications of awake tracheal intubation in 1,554 patients over 12 years: a historical cohort study. Canadian journal of anaesthesia = Journal canadien d’anesthesie 2015;62:736–44.
El-Boghdadly K, Onwochei DN, Cuddihy J, Ahmad I (2017) A prospective cohort study of awake fiberoptic intubation practice at a tertiary center. Anaesthesia 72:694–703
Johnston KD, Rai MR. Conscious sedation for awake fiberoptic intubation: a review of the literature. Canadian journal of anaesthesia = Journal canadien d’anesthesie 2013;60:584–99.
He XY, Cao JP, He Q, Shi XY (2014) Dexmedetomidine for the management of awake fiberoptic intubation. Cochrane Database Syst Rev 2014:Cd9798
Alhomary M, Ramadan E, Curran E, Walsh SR (2018) Videolaryngoscopy vs. fiberoptic bronchoscopy for awake tracheal intubation: a systematic review and meta-analysis. Anaesthesia 73:1151–1161
Jiang J, Ma DX, Li B, Wu AS, Xue FS (2018) Videolaryngoscopy versus fiberoptic bronchoscope for awake intubation—a systematic review and meta-analysis of randomized controlled trials. Ther Clin Risk Manag 14:1955–1963
Thomson S (1905) Tracheotomy under local anaesthesia. Br Med J 2:922–923
Lee MC, Absalom AR, Menon DK, Smith HL (2006) Awake insertion of the laryngeal mask airway using topical lidocaine and intravenous remifentanil. Anaesthesia 61:32–35
Lim WY, Wong P (2019) Awake supraglottic airway guided flexible bronchoscopic intubation in patients with anticipated difficult airways: a case series and narrative review. Korean J Anesthesiol 72:548–557
Cook TM, Woodall N, Frerk C (2011) Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth 106:617–631
Cook TM, MacDougall-Davis SR. Complications and failure of airway management. British journal of anaesthesia 2012;109 Suppl 1:i68–i85.
Williamson JA, Webb RK, Szekely S, Gillies ER, Dreosti AV (1993) The Australian Incident Monitoring Study. Difficult intubation: an analysis of 2000 incident reports. Anaesth Intensive Care Med 21:602–607
Mosier JM (2020) Physiologically difficult airway in critically ill patients: winning the race between haemoglobin desaturation and tracheal intubation. Br J Anaesth 125:e1–e4
Fei M, Blair JL, Rice MJ, Edwards DA, Liang Y, Pilla MA et al (2017) Comparison of effectiveness of two commonly used two-handed mask ventilation techniques on unconscious apnoeic obese adults. Br J Anaesth 118:618–624
Sato S, Hasegawa M, Okuyama M, Okazaki J, Kitamura Y, Sato Y et al (2017) Mask Ventilation during Induction of General Anesthesia: Influences of Obstructive Sleep Apnea. Anesthesiology 126:28–38
Joffe AM, Hetzel S, Liew EC (2010) A two-handed jaw-thrust technique is superior to the one-handed “EC-clamp” technique for mask ventilation in the apneic unconscious person. Anesthesiology 113:873–879
Bouvet L, Albert ML, Augris C, Boselli E, Ecochard R, Rabilloud M et al (2014) Real-time detection of gastric insufflation related to facemask pressure-controlled ventilation using ultrasonography of the antrum and epigastric auscultation in nonparalyzed patients: a prospective, randomized, double-blind study. Anesthesiology 120:326–334
Ikeda A, Isono S, Sato Y, Yogo H, Sato J, Ishikawa T et al (2012) Effects of muscle relaxants on mask ventilation in anesthetized persons with normal upper airway anatomy. Anesthesiology 117:487–493
Levitan RM, Mechem CC, Ochroch EA, Shofer FS, Hollander JE (2003) Head-elevated laryngoscopy position: improving laryngeal exposure during laryngoscopy by increasing head elevation. Ann Emerg Med 41:322–330
Schmitt HJ, Mang H (2002) Head and neck elevation beyond the sniffing position improves laryngeal view in cases of difficult direct laryngoscopy. J Clin Anesth 14:335–338
Gataure PS, Vaughan RS, Latto IP (1996) Simulated difficult intubation. Comparison of the gum elastic bougie and the stylet. Anaesthesia 51:935–938
Jabre P, Combes X, Leroux B, Aaron E, Auger H, Margenet A et al (2005) Use of gum elastic bougie for prehospital difficult intubation. Am J Emerg Med 23:552–555
Martin LD, Mhyre JM, Shanks AM, Tremper KK, Kheterpal S (2011) 3,423 emergency tracheal intubations at a university hospital: airway outcomes and complications. Anesthesiology 114:42–48
Hasegawa K, Shigemitsu K, Hagiwara Y, Chiba T, Watase H, Brown CA 3rd et al (2012) Association between repeated intubation attempts and adverse events in emergency departments: an analysis of a multicenter prospective observational study. Ann Emerg Med 60:749–54:e2
Paolini JB, Donati F, Drolet P. Review article: video-laryngoscopy: another tool for difficult intubation or a new paradigm in airway management? Canadian journal of anaesthesia = Journal canadien d’anesthesie 2013;60:184–91.
Delaney KA, Hessler R (1988) Emergency flexible fiberoptic nasotracheal intubation: a report of 60 cases. Ann Emerg Med 17:919–926
Hattori K, Komasawa N, Miyazaki Y, Kido H, Deguchi S, Minami T (2016) Muscle relaxant facilitates i‑gel insertion by novice doctors: A prospective randomized controlled trial. J Clin Anesth 33:218–222
Fujiwara A, Komasawa N, Nishihara I, Miyazaki S, Tatsumi S, Nishimura W et al (2015) Muscle relaxant effects on insertion efficacy of the laryngeal mask ProSeal(®) in anesthetized patients: a prospective randomized controlled trial. J Anesth 29:580–584
Van Zundert AA, Kumar CM, Van Zundert TC (2016) Malpositioning of supraglottic airway devices: preventive and corrective strategies. Br J Anaesth 116:579–582
Engelhardt T, Virag K, Veyckemans F, Habre W (2018) Airway management in paediatric anaesthesia in Europe—insights from APRICOT (Anaesthesia Practice In Children Observational Trial): a prospective multicentre observational study in 261 hospitals in Europe. Br J Anaesth 121:66–75
Stinson HR, Srinivasan V, Topjian AA, Sutton RM, Nadkarni VM, Berg RA, et al. Failure of Invasive Airway Placement on the First Attempt Is Associated With Progression to Cardiac Arrest in Pediatric Acute Respiratory Compromise. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2018;19:9–16.
Ortiz-Gómez JR, Palacio-Abizanda FJ, Fornet-Ruiz I (2014) Failure of sugammadex to reverse rocuronium-induced neuromuscular blockade: a case report. Eur J Anaesthesiol 31:708–709
Kennedy CC, Cannon EK, Warner DO, Cook DA (2014) Advanced airway management simulation training in medical education: a systematic review and meta-analysis. Crit Care Med 42:169–178
Ng A, Smith G (2001) Gastroesophageal reflux and aspiration of gastric contents in anesthetic practice. Anesth Analg 93:494–513
Algie CM, Mahar RK, Tan HB, Wilson G, Mahar PD, Wasiak J (2015) Effectiveness and risks of cricoid pressure during rapid sequence induction for endotracheal intubation. Cochrane Database Syst Rev 2015:Cd11656
Bremerich DAT, Chappell D, Hanß R, Kaufner L (2020) Kehl F S1 guideline: Obstetric analgesia and anesthesia. Anästh Intensivmed 61:300–339
Ehrenfeld JM, Mulvoy W, Sandberg WS (2010) Performance comparison of right- and left-sided double-lumen tubes among infrequent users. J Cardiothorac Vasc Anesth 24:598–601
Loop T, Spaeth J (2018) Airway management in thoracic anesthesia with the double lumen tube. Anästhesiol Intensivmed Notfallmed Schmerzther 53:174–185
Wang ML, Galvez C, Chen JS, Navarro-Martinez J, Bolufer S, Hung MH et al (2017) Non-intubated single-incision video-assisted thoracic surgery: a two-center cohort of 188 patients. J Thorac Dis 9:2587–2598
Pompeo E, Dauri M (2013) Is there any benefit in using awake anesthesia with thoracic epidural in thoracoscopic talc pleurodesis? J Thorac Cardiovasc Surg 146:495–7:e1
Russell T, Slinger P, Roscoe A, McRae K, Van Rensburg A (2013) A randomized controlled trial comparing the GlideScope(®) and the Macintosh laryngoscope for double-lumen endobronchial intubation. Anaesthesia 68:1253–1258
Lin W, Li H, Liu W, Cao L, Tan H, Zhong Z (2012) A randomized trial comparing the CEL-100 videolaryngoscope(TM) with the Macintosh laryngoscope blade for insertion of double-lumen tubes. Anaesthesia 67:771–776
Hsu HT, Chou SH, Wu PJ, Tseng KY, Kuo YW, Chou CY et al (2012) Comparison of the GlideScope® videolaryngoscope and the Macintosh laryngoscope for double-lumen tube intubation. Anaesthesia 67:411–415
Piepho T, Cavus E, Noppens R, Byhahn C, Dorges V, Zwissler B, et al. S1 guidelines on airway management : Guideline of the German Society of Anesthesiology and Intensive Care Medicine. Anaesthesiologist 2015;64:27–40.
Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A et al (2015) Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth 115:827–848
Heard AM, Lacquiere DA, Riley RH (2010) Manikin study of fiberoptic-guided intubation through the classic laryngeal mask airway with the Aintree intubating catheter vs the intubating laryngeal mask airway in the simulated difficult airway. Anaesthesia 65:841–847
Cook TM, Silsby J, Simpson TP (2005) Airway rescue in acute upper airway obstruction using a ProSeal Laryngeal mask airway and an Aintree catheter: a review of the ProSeal Laryngeal mask airway in the management of the difficult airway. Anaesthesia 60:1129–1136
Wong DT, Yang JJ, Mak HY, Jagannathan N (2012) Use of intubation introducers through a supraglottic airway to facilitate tracheal intubation: a brief review. Can J Anaesth 59:704–715
Huitink JM, Koopman EM, Bouwman RA, Craenen A, Verwoert M, Krage R et al (2013) Tracheal intubation with a camera embedded in the tube tip (Vivasight() ). Anaesthesia 68:74–78
Sawasdiwipachai P, Boonsri S, Suksompong S, Prowpan P (2015) The uses of laryngeal mask airway ProSeal and endobronchial blocker for one lung anesthesia. J Anesth 29:660–665
Beck GKBEBMD (2013) H. Hofer TI, H. Komar, E. Mertens,, T. Prien AS, H. Sorgatz, J. Strauß, H. Van Aken and F. Vescia. Minimum requirements for the anesthetic workplace. Anesth. Intensivmed 54:39–42
Coburn MRRH (2015) Bause, J. Biscoping, M. Fries, D. Henzler, T.-M. Iber, J. Karst, P. Meybohm, B. Mierke, F. Pabst, G. Schälte, JH. Schiff, A. Stevanovic, M. Winterhalter. Quality indicators in anesthesiology. Anästh Intensivmed 2016(57):219–230
Chung F, Abdullah HR, STOP-Bang Questionnaire LP (2016) A Practical Approach to Screen for Obstructive Sleep Apnea. Chest 149:631–638
Leong SM, Tiwari A, Chung F, Wong DT (2018) Obstructive sleep apnea as a risk factor associated with difficult airway management—A narrative review. J Clin Anesth 45:63–68
Nagappa M, Wong DT, Cozowicz C, Ramachandran SK, Memtsoudis SG, Chung F (2018) Is obstructive sleep apnea associated with difficult airway? Evidence from a systematic review and meta-analysis of prospective and retrospective cohort studies. PLoS ONE 13:e204904
Saur P, Roggenbach J, Meinl S, Klinger A, Stasche N, Martin E et al (2012) Outpatient anesthesia for patients with obstructive sleep apnea: results of a national survey. Anaesthesist 61:14–7:20–24
Rosero EB, Joshi GP (2021) Outcomes of Sleep Apnea Surgery in Outpatient and Inpatient Settings. Anesth Analg 132:1215–1222
Tibble R CL. The Difficult Airway and Videolaryngoscope use in Day Surgery. The Journal of One Day Surgery 2015;25:48–50.
(2023) Royal College of Anaesthetists. Guidelines for the Provision of Anaesthesia Services in the Non-theatre Environment. Chapter 7:
Timmermann A, Bergner UA, Russo SG (2015) Laryngeal mask airway indications: new frontiers for second-generation supraglottic airways. Curr Opin Anaesthesiol 28:717–726
Pierre MSME, Schaffartzik W, Schleppers A (2013) Laryngeal mask placement in the prone position. An online-survey among German anaesthetists in private practice. Anästh Intensivmed 54:181–191
Kodali BS (2013) Capnography outside the operating rooms. Anesthesiology 118:192–201
Russotto V, Myatra SN, Laffey JG, Tassistro E, Antolini L, Bauer P et al (2021) Intubation Practices and Adverse Peri-intubation Events in Critically Ill Patients From 29 Countries. JAMA 325:1164–1172
Lascarrou JB, Boisrame-Helms J, Bailly A, Le Thuaut A, Kamel T, Mercier E et al (2017) Video Laryngoscopy vs Direct Laryngoscopy on Successful First-Pass Orotracheal Intubation Among ICU Patients: A Randomized Clinical Trial. JAMA 317:483–493
Jaber S, Amraoui J, Lefrant JY, Arich C, Cohendy R, Landreau L et al (2006) Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med 34:2355–2361
Griesdale DE, Bosma TL, Kurth T, Isac G, Chittock DR (2008) Complications of endotracheal intubation in the critically ill. Intensive Care Med 34:1835–1842
Mort TC (2004) The incidence and risk factors for cardiac arrest during emergency tracheal intubation: a justification for incorporating the ASA Guidelines in the remote location. J Clin Anesth 16:508–516
Toolis M, Tiruvoipati R, Botha J, Green C, Subramaniam A (2019) A practice survey of airway management in Australian and New Zealand intensive care units. Crit Care Resusc 21:139–147
Higgs A, Cook TM, McGrath BA. Airway management in the critically ill: the same, but different. British journal of anaesthesia 2016;117 Suppl 1:i5–i9.
Thomas AN, Panchagnula U, Taylor RJ (2009) Review of patient safety incidents submitted from Critical Care Units in England & Wales to the UK National Patient Safety Agency. Anaesthesia 64:1178–1185
Khandelwal N, Khorsand S, Mitchell SH, Joffe AM (2016) Head-Elevated Patient Positioning Decreases Complications of Emergent Tracheal Intubation in the Ward and Intensive Care Unit. Anesth Analg 122:1101–1107
Ramkumar V, Umesh G, Philip FA (2011) Preoxygenation with 20° head-up tilt provides longer duration of non-hypoxic apnea than conventional preoxygenation in non-obese healthy adults. J Anesth 25:189–194
Lane S, Saunders D, Schofield A, Padmanabhan R, Hildreth A, Laws D (2005) A prospective, randomized controlled trial comparing the efficacy of pre-oxygenation in the 20 degrees head-up vs supine position. Anaesthesia 60:1064–1067
Miguel-Montanes R, Hajage D, Messika J, Bertrand F, Gaudry S, Rafat C et al (2015) Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med 43:574–583
Schwartz DE, Matthay MA, Cohen NH (1995) Death and other complications of emergency airway management in critically ill adults. A prospective investigation of 297 tracheal intubations. Anesthesiology 82:367–376
De Jong A, Molinari N, Terzi N, Mongardon N, Arnal JM, Guitton C et al (2013) Early identification of patients at risk for difficult intubation in the intensive care unit: development and validation of the MACOCHA score in a multicenter cohort study. Am J Respir Crit Care Med 187:832–839
Rombey T, Schieren M, Pieper D (2018) Video Versus Direct Laryngoscopy for Inpatient Emergency Intubation in Adults. Dtsch Ärztebl Int 115:437–444
Bhattacharjee S, Maitra S, Baidya DK (2018) A comparison between video laryngoscopy and direct laryngoscopy for endotracheal intubation in the emergency department: A meta-analysis of randomized controlled trials. J Clin Anesth 47:21–26
Arulkumaran N, Lowe J, Ions R, Mendoza M, Bennett V, Dunser MW (2018) Videolaryngoscopy versus direct laryngoscopy for emergency orotracheal intubation outside the operating room: a systematic review and meta-analysis. Br J Anaesth 120:712–724
Hypes CD, Stolz U, Sakles JC, Joshi RR, Natt B, Malo J et al (2016) Video Laryngoscopy Improves Odds of First-Attempt Success at Intubation in the Intensive Care Unit. A Propensity-matched Analysis. Annals ATS 13:382–390
Mosier JM, Stolz U, Chiu S, Sakles JC (2012) Difficult airway management in the emergency department: GlideScope videolaryngoscopy compared to direct laryngoscopy. J Emerg Med 42:629–634
Ward KR, Yealy DM (1998) End-tidal carbon dioxide monitoring in emergency medicine, Part 2: Clinical applications. Acad Emerg Med 5:637–646
Linko K, Paloheimo M, Tammisto T (1983) Capnography for detection of accidental oesophageal intubation. Acta Anaesthesiol Scand 27:199–202
Mort TC (2009) Tracheal tube exchange: feasibility of continuous glottic viewing with advanced laryngoscopy assistance. Anesth Analg 108:1228–1231
Mort TC, Braffett BH (2015) Conventional Versus Video Laryngoscopy for Tracheal Tube Exchange: Glottic Visualization, Success Rates, Complications, and Rescue Alternatives in the High-Risk Difficult Airway Patient. Anesth Analg 121:440–448
Rothaar RC, Epstein SK (2003) Extubation failure: magnitude of the problem, impact on outcomes, and prevention. Curr Opin Crit Care 9:59–66
Cormack RS, Lehane J (1984) Difficult tracheal intubation in obstetrics. Anaesthesia 39:1105–1111
Cavus E (2014) Glottic visualization with videolaryngoscopy: Proposal for a modified, indexed Cormack-Lehane Score. Bja: Br J Anaesth 113:
Benumof JL, Cooper SD (1996) Quantitative improvement in laryngoscopic view by optimal external laryngeal manipulation. J Clin Anesth 8:136–140
Takahata O, Kubota M, Mamiya K, Akama Y, Nozaka T, Matsumoto H et al (1997) The efficacy of the “BURP” maneuver during a difficult laryngoscopy. Anesth Analg 84:419–421
Gaussorgues P, Boyer F, Piperno D, Gerard M, Leger P, Robert D (1988) Do corticosteroids prevent postextubation laryngeal edema? Prospective study of 276 adults. Crit Care Med 16:649
Ho LI, Harn HJ, Lien TC, Hu PY, Wang JH (1996) Postextubation laryngeal edema in adults. Risk factor evaluation and prevention by hydrocortisone. Intensive Care Med 22:933–936
Fan T, Wang G, Mao B, Xiong Z, Zhang Y, Liu X et al (2008) Prophylactic administration of parenteral steroids for preventing airway complications after extubation in adults: meta-analysis of randomized placebo controlled trials. BMJ 337:a1841
Lee CH, Peng MJ, Wu CL (2007) Dexamethasone to prevent postextubation airway obstruction in adults: a prospective, randomized, double-blind, placebo-controlled study. Crit Care 11:R72
François B, Bellissant E, Gissot V, Desachy A, Normand S, Boulain T et al (2007) 12‑h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomized double-blind trial. Lancet 369:1083–1089
Jaber S, Jung B, Chanques G, Bonnet F, Marret E (2009) Effects of steroids on reintubation and post-extubation stridor in adults: meta-analysis of randomized controlled trials. Crit Care 13:R49
Peterson GN, Domino KB, Caplan RA, Posner KL, Lee LA, Cheney FW (2005) Management of the difficult airway: a closed claims analysis. Anesthesiology 103:33–39
Cavallone LF, Vannucci A (2013) Review article: Extubation of the difficult airway and extubation failure. Anesth Analg 116:368–383
Popat M, Mitchell V, Dravid R, Patel A, Swampillai C, Higgs A (2012) Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia 67:318–340
Miller RL, Cole RP (1996) Association between reduced cuff leak volume and postextubation stridor. Chest 110:1035–1040
Mort TC. Continuous airway access for the difficult extubation: the efficacy of the airway exchange catheter. Anesthesia and analgesia 2007;105:1357–62, table of contents.
Roten FM, Steffen R, Kleine-Brueggeney M, Greif R, Wipfli M, Arnold A et al (2019) Dislocation rates of postoperative airway exchange catheters—a prospective case series of 200 patients. BMC Anesthesiol 19:52
McManus S, Jones L, Anstey C, Senthuran S (2018) An assessment of the tolerability of the Cook staged extubation wire in patients with known or suspected difficult airways extubated in intensive care. Anaesthesia 73:587–593
Rehak A, Watterson LM (2020) Institutional preparedness to prevent and manage anaesthesia-related ‘can’t intubate, can’t oxygenate’ events in Australian and New Zealand teaching hospitals. Anaesthesia 75:767–774
Baker PA, Weller JM, Greenland KB, Riley RH, Merry AF (2011) Education in airway management. Anaesthesia 66(Suppl 2):101–111
Sun Y, Pan C, Li T, Gan TJ (2017) Airway management education: simulation based training versus non-simulation based training—A systematic review and meta-analyses. BMC Anesthesiol 17:17
Links MJ, Watterson L, Martin P, O’Regan S, Molloy E (2020) Finding common ground: meta-synthesis of communication frameworks found in patient communication, supervision and simulation literature. BMC Med Educ 20:45
Flin R, Fioratou E, Frerk C, Trotter C, Cook TM (2013) Human factors in the development of complications of airway management: preliminary evaluation of an interview tool. Anaesthesia 68:817–825
Reader T, Flin R, Lauche K, Cuthbertson BH (2006) Non-technical skills in the intensive care unit. Br J Anaesth 96:551–559
Haerkens MH, Jenkins DH, van der Hoeven JG (2012) Crew resource management in the ICU: the need for culture change. Ann Intensive Care 2:39
Harvey R, Foulds L, Housden T, Bennett KA, Falzon D, McNarry AF et al (2017) The impact of didactic read-aloud action cards on the performance of cannula cricothyroidotomy in a simulated ‘can’t intubate can’t oxygenate’ scenario. Anaesthesia 72:343–349
Marshall SD, Mehra R (2014) The effects of a displayed cognitive aid on non-technical skills in a simulated ‘can’t intubate, can’t oxygenate’ crisis. Anaesthesia 69:669–677
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
See detailed information: https://register.awmf.org/assets/guidelines/001_Anaesthesiologie_und_Intensivmedizin/001-028i_S1_Atemwegsmanagement_2023-09.pdf
This article does not contain any studies with human participants or animals performed by any of the authors.
The supplement containing this article is not sponsored by industry.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Die deutsche Zusammenfassung der Leitlinie finden Sie unter Piepho, T., Kriege, M., Byhahn C. et al. (2024) Empfehlungen zur neuen S1 Leitlinie Atemwegsmanagement Die Anaesthesiologie https://doi.org/10.1007/s00101-024-01414-4
Last revision: 21.08.2023
Period of validity: 5 years
Translation from German to English by R. Noppens, MD, PhD, FEAMS, FRCPC

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Piepho, T., Kriege, M., Byhahn, C. et al. German guidelines for airway management 2023. Anaesthesiologie (2024). https://doi.org/10.1007/s00101-024-01413-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s00101-024-01413-5