Abstract
Purpose
Primary central nervous system lymphoma (PCNSL) is a rare malignancy of the central nervous system with high invasiveness. There is little consensus on the treatment of PCNSL. This study retrospectively studied data from PCNSL patients in a single center to summarize treatment experience and explore prognostic factors.
Methods
Survival curves were drawn using the Kaplan–Meier method and prognostic factors were analyzed using Cox’s hazards model.
Results
In multivariate analysis, cerebrospinal fluid lactic acid dehydrogenase (CSF LDH; p = 0.005 and p = 0.002), neutrophil to lymphocyte ratio (NLR; p = 0.014 and p = 0.038), and completion of four cycles of induction therapy (p < 0.001and p < 0.001) were significant and independent predictors of overall survival (OS) and progression-free survival (PFS), respectively.
Conclusion
On the basis of this study, we propose that PCNSL patients should receive early induction therapy with sufficient cycles. Subsequent consolidation therapy can prevent relapses and improve survival. In patients with PCNSL, the independent prognostic factors for OS and PFS were CSF LDH level, NLR, and full cycles of induction therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system lymphoma (PCNSL) is a form of extranodal non-Hodgkin lymphoma that involves different structures including the brain, meninges, spinal cord, and/or eyes, without evidence of systemic disease. Characterized by rarity but high invasiveness, the overall incidence of PCNSL is 0.47 per 100,000 people per year [1]. Patients have a limited survival time and the recurrence rate is high.
The symptoms of PCNSL lack specificity. Moreover, the tumor grows at different sites, so the presentation of PCNSL may vary. Compared with other intracranial tumors, PCNSL tends to grow in the periventricular region. Most of the clinical manifestations are caused by intracranial occupying lesions, including intracranial hypertension and neurological damage. For instance, headache, vomiting, consciousness and/or cognitive impairment, and limb movement or sensory dysfunction are common symptoms of the disease [2]. A combination of physical examination, imaging examinations, and pathology are required to confirm the diagnosis. The gold standard for diagnosing central nervous system (CNS) lymphoma is stereotactic biopsy, and 95% of cases have a histology of diffuse large B‑cell lymphoma (DLBCL) [3]. DLBCL in the brain reveals highly proliferative lymphoma cells that diffusely infiltrate the brain parenchyma in a typical angiocentric growth pattern [4]. In accordance with the 2016 revision of the World Health Organization classification of lymphoid neoplasms, the term primary CNS lymphoma is defined as primary CNS DLBCLs. The major cell-of-origin classification is the non-germinal center B‑cell-like (NGCB) type, which has a typical immunohistochemical profile of CD10−BCL6−MUM1+/− or CD10−BCL6+MUM1+ [5]. It is thought that PCNSL arises from B cells that arrest at exit stages of B‑cell germinal centers. Various pathogenetic mechanisms in PCNSL have been described, including dysregulations by genetic alterations in signaling pathways of NF-kB, JAK/STAT, Toll-like receptors, and B‑cell receptors. Mutations in specific genes, including MYD88, PIM1, TBL1XR1, TRDM1, BTG2, and PRDM1, contribute to disease pathogenesis [6]. Analyzing mutated genes, detecting Epstein-Barr virus (EBV)-EBER, and genetically testing MYC translocation and BCL2, BCL6, and IgH rearrangement, as well as testing mutations in MYD88 and CD79B, will be helpful for differential pathologic diagnosis [7]. Patients were risk stratified according to the International Extranodal Lymphoma Study Group (IELSG) classification and the Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic model. The IELSG included age, the Eastern Cooperative Oncology Group (ECOG) performance score, serum lactic acid dehydrogenase (LDH) levels, cerebrospinal fluid (CSF) protein levels, and involvement of deep regions of the brain (periventricular, basal ganglia, brainstem, and/or cerebellum). The MSKCC was simpler and included three classes: class 1 (patients < 50 years), class 2 (patients ≥ 50 years; Karnofsky performance score [KPS] ≥ 70), and class 3 (patients ≥ 50; KPS < 70) [8, 9]. In recent years, a number of studies have suggested that complete blood cell count (CBC)-derived inflammatory biomarkers such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) can be used as simple prognostic markers for a variety of cancers [10,11,12]. Many studies have suggested that the NLR is a prognostic indicator in aggressive NHL, especially DLBCL and peripheral T‑cell lymphoma (PTCL) [13, 14]. The occurrence and development of PCNSL is affected by the systemic inflammatory response, so CBC-derived inflammatory biomarkers such as NLR and PLR are expected to play an important role in predicting the efficacy of treatment and the survival rate of PCNSL patients [15].
The treatment of PCNSL is divided into two phases: induction and consolidation. There is no consistent standard strategy because few phase III randomized clinical trials have been conducted for PCNSL treatment. It is wildly accepted that high-dose methotrexate (HD-MTX)-based polychemotherapy is the backbone of induction treatment for newly diagnosed PCNSL [16]. Rituximab, a monoclonal antibody targeting the B cell surface antigen CD20, is also an important part in common induction regimens. While PCNSLs are sensitive to chemotherapy, the survival outcomes and prognosis of PCNSL remain poor compared with that of systemic lymphoma because of the existence of the blood–brain barrier and some other reasons. Approximately 50% of cases experience relapse in the first 2 years, and fewer than 20% of patients are long-term disease-free survivors; thus, improvement in the efficacy of induction therapy and the consolidation therapy following remission is urgently needed [17, 18]. Two principal consolidation approaches are currently used: whole–brain radiotherapy (WBRT) or high-dose chemotherapy and autologous stem cell transplant (HDC-ASCT) [19]. Clinical strategies vary based on the concrete state of each patient, such as age and response to induction therapy. Additionally, novel agents such as lenalidomide and ibrutinib are currently under investigation as maintenance strategies [20].
In the present study, we collected information from 124 PCNSL patients at our institution from July 2009 to April 2022. Here, we aimed to investigate the basic characteristics of these patients and treatments and further sought underlying prognostic factors of the disease and long-term survival outcomes.
Materials and methods
Patient data
A total of 139 patients were diagnosed with central nervous system lymphoma (CNSL) at our center between July 2009 and April 2022; 7 patients with secondary central nervous system lymphoma (SCNSL) and 8 patients who were lost to follow-up were excluded, leaving 124 patients for study analysis. None of the cases of PCNSL were associated with immunodeficiency. All patients underwent chemotherapy, and some patients also underwent radiotherapy (RT) and ASCT. All eligible patients were followed up from the date of diagnosis until death, loss to follow-up, or study termination on April 22, 2023. The baseline data were evaluated before any treatment, including symptomatic treatment (for example, steroid therapy), surgery, induction, and consolidation therapy. The median length of follow-up was 24.5 months. All patients were followed up by magnetic resonance imaging (MRI) after every 2–3 courses of induction chemotherapy, after consolidation therapy, then at 3‑month intervals during the first 3 years, and every 6 months for the next 2 years. Follow-up information was obtained directly from inpatient and outpatient information systems or from telephone interviews. The clinical data, including demographic characteristics, previous history, signs and symptoms, imaging, pathologic reports, treatment modalities and survival data, were retrospectively reviewed. It is worth mentioning that routine blood results were also collected before induction therapy began. The NLR and PLR were calculated as follows: NLR = neutrophil count/lymphocyte count, PLR = platelet count/lymphocyte count.
Treatment modalities
The different treatments are presented in Table 1. The management of PCNSL is contentious because of the current absence of a uniform consensus on the optimal treatment regimen. Due to the diffusely infiltrative growth of tumors, the effect of surgery is limited. Surgical resection might play a role in significantly improving overall survival (OS) and progression-free survival (PFS) compared with stereotactic biopsy in a subset of patients. The whole cohort of our study received surgery to clarify the diagnosis by pathological and immunohistochemical examination, with 65 patients diagnosed by stereotactic biopsy and 59 patients diagnosed by surgical resection.
All 124 patients received HD-MTX-based chemotherapy as first-line induction treatment, including MATRix (HD-MTX, cytosine arabinoside, thiotepa, and rituximab), MOP (HD-MTX, vincristine, and procarbazine), and R‑MPV (methotrexate, procarbazine, and vincristine). Overall, 23 (18.5%) patients did not complete induction therapy because of tumor progression or intolerance to drugs; thus, these patients received palliative therapy as their sole treatment. Of the remaining 101 patients, 64 had NGCB subtypes and tyrosine kinase inhibitors (TKIs) were part of the treatment regimen of 24 patients, with an increase in use over time.
A total of 30.6% of the patients received consolidation treatment after induction therapy, consisting of WBRT (22.6%), HDC-ASCT (7.2%), and HDC-ASCT+RT. Intensity-modulated radiotherapy was administered in 29 patients (IMRT). Patients were in the supine position and fixed with individualized head, neck, and shoulder thermoplastic masks to scan simulation positioning enhanced computed tomography (CT) and MRI from the top of the head to the lower margin of the fourth cervical vertebra. The positioning images were imported into the Eclipse TPS (Varian Medical Systems, Palo Alto, CA, USA) through the Varian network. All gross tumor volume (GTV) images were contoured by the same radiologist based on MRI and positron-emission tomography (PET), and confirmed by an experienced radiation oncologist. The clinical target volume (CTV) was defined as the potential microscopic tumor including whole brain, the upper two cervical vertebrae, and the posterior part of the orbit. PGTV was expanded by 0.5 cm from the GTV, while the PTV was expanded by 0.3 cm from the CTV. All of the 29 patients underwent whole-brain radiotherapy (WBRT). Sequential boost RT was added to WBRT in 22 patients. The dose of the whole brain ranged from 23.4 to 41.4 Gy and that of gross disease localization was boosted to 36–50.4 Gy. The mean dose of whole brain was 31.8 Gy (95% CI = 29.3–34.4). The mean dose of the boost was 46.7 Gy (95% CI = 45.2–48.2). One patient proceeded to HDC-ASCT following WBRT.
Statistical analysis
The primary endpoints were OS and PFS. OS was defined as the time from diagnosis to death from any cause. PFS was defined as the time from diagnosis to the first instance of disease progression, recurrence, or death from any cause. Survival curves were estimated using the Kaplan–Meier method and were analyzed with the log-rank test. Univariate and multivariate analyses were carried out by using Cox proportional hazards regression models to determine independent prognostic factors. Multivariate Cox analysis, performed to determine the independent prognostic factors based on the statistically significant factors selected by univariate analyses, were presented as hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs). A p-value of < 0.05 was considered statistically significant. All statistical analyses were conducted using R software (version 4.1.0, 18.05.2021; Posit, PBc, Vienna, Austria).
Results
Optimal cut-off values for NLR and PLR
Receiver operating characteristic (ROC) curves were analyzed by comparing the area under the curve (AUC) to calculate the optimal cutoff values for NLR and PLR. The optimal cutoff values for NLR and PLR for OS were 2.77 (sensitivity: 0.783; specificity: 0.655) and 173 (sensitivity: 0.500; specificity: 0.779), respectively. The optimal cut-off values for PFS were 4 (sensitivity: 0.776; specificity: 0.708) and 170.59 (sensitivity: 0.647; specificity: 0.713), respectively.
Patient characteristics
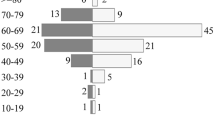
A total of 124 patients with a confirmed diagnosis of PCNSL at Xiangya Hospital, Central South University, China, between July 2009 and April 2022 were retrospectively analyzed. Patients without a positive histologic diagnosis or follow-up visits were excluded. The basic clinical characteristics of the patients are summarized in Table 2. Among 124 PCNSL patients in our cohort, 75 were males and the remaining 49 were females. The mean age at diagnosis was 53.9 years (range 20–79). According to Eastern Cooperative Oncology Group performance status (ECOG-PS), which was evaluated before any treatment, including symptomatic treatment (for example, glucocorticoid), surgery, induction, and consolidation therapy, the number of patients with ECOG < 2 was the same as that with ECOG ≥ 2. The majority of the tumors had a unilateral location, and there was deep lesion involvement in 80 cases. For the IELSG risk score, most patients (60.5%) were classified in the intermediate-risk group, with 21 (16.9%) and 18 (14.5%) patients in the low- and high-risk groups, respectively. A total of 43 (34.7%), 53 (42.7%) and 28 (22.6%) patients were stratified into the low, intermediate, and high MSKCC risk groups, respectively. Regarding cell origination, 118 patients could be clearly identified by pathological and immunohistochemical methods: 36 (29.0%) patients had germinal center B cell-like (GCB), and 82 (66.1%) had NGCB.
Survival according to patient baseline characteristics
The median duration of follow-up for the whole cohort was 24.5 months. The median OS was 34 months (95% CI = 26–57 months), with 1‑year, 2‑year, 3‑year, and 5‑year OS rates of 79.0%, 61.2%, 48.1%, and 35.8%, respectively. The median PFS was 21 months (95% CI=13–34 months), with 1‑year, 2‑year, 3‑year, and 5‑year PFS rates of 59.7%, 44.6%, 37.9%, and 28.9%, respectively. As illustrated by the survival curves (Figs. 1, 2, and 3), no statistically significant difference was observed in OS (p = 0.41) or PFS (p = 0.14) between patients older than 60 years and patients aged 60 years or younger. The median OS (40 months vs. 33 months, p = 0.49) and PFS (30 months vs. 10 months, p = 0.14) of male and female patients were not significantly different. The median OS of patients with ECOG < 2 and ≥ 2 were 40 months and 33 months, respectively, with no statistically significant difference observed (p = 0.084). The median PFS of patients with ECOG < 2 and ≥ 2 was 23 months and 16 months, respectively, with no statistically significant difference observed (p = 0.096). Patients with normal CSF LDH levels had better OS (p = 0.013) and PFS (p = 0.0096) than those with elevated CSF LDH levels. Regarding CBC-derived inflammatory biomarkers, higher NLR (p < 0.0001) and PLR (p = 0.01) were significantly associated with lower OS. Similarly, higher NLR (p < 0.0001) and PLR (p = 0.013) were significantly associated with lower PFS.
Survival in association with treatment modality
The pathological diagnosis was established by biopsy in 65 patients and by resection in 59 patients. No significant difference was observed in the survival outcomes between the biopsy and resection groups (p = 0.1; p = 0.33). The median OS was higher in the induction therapy group than in the salvage therapy group (47 months vs. 7 months, p < 0.0001), and induction therapy was shown to have a significant impact on PFS (p < 0.0001). Among 64 patients with the NGCB subtype who received induction therapy, the chemotherapy plus TKI group showed higher 2‑year OS and PFS rates when compared with those of the chemotherapy group (91.7% vs. 52.5%, p = 0.00031; 86.5% vs. 35.7%, p = 0.00017). The 2‑year OS rates for the RT+ASCT, ASCT, and RT groups were 100, 88.9, and 77.9% (p = 0.44), respectively. The 2‑year PFS rates for the RT+ASCT, ASCT, and RT groups were 100, 88.9, and 69.4% (p = 0.26), respectively.
Survival in association with various clinicopathological factors
There were significant differences in terms of median PFS (48 months vs. 29 months, p = 0.0092) between the GCB and NGCB groups but not in terms of OS (40 months vs. 14 months, p = 0.07) between the GCB and NGCB groups. For patients with Ki-67 ≥ 90 and < 90, no statistically significant differences were observed in terms of median OS (33 months vs. 46 months, p = 0.36) and PFS (19 months vs. 23 months, p = 0.73). Patients with and without deep lesions showed no significant differences in OS (p = 0.62) or PFS (p = 0.43). No statistically significant difference was observed in terms of OS (p = 0.53) or PFS (p = 0.24) between patients with unilateral and bilateral lesions. The 2‑year OS rates of the MSKCC low-, intermediate-, and high-risk groups were 64.5%, 67.1%, and 45.6%, respectively, with no statistically significant difference observed (p = 0.19). The 2‑year PFS rates of the MSKCC low-, intermediate-, and high-risk groups were 50.4%, 48.9%, and 27.8%, respectively, with no statistically significant difference observed (p = 0.11). The 2‑year OS rates of the IELSG low-, intermediate-, and high-risk group were 76.2%, 64.7%, and 41.2%, respectively (p = 0.082). The 2‑year PFS rates of the IELSG low-, intermediate-, and high-risk groups were 61.9%, 43.9%, and 38.1%, respectively, with no statistically significant difference observed (p = 0.33).
Prognostic analysis
As shown in Fig. 4, elevated CSF LDH (HR = 2.16, 95% CI = 1.26–3.71, p = 0.005), higher NLR (HR = 2.38, 95% CI = 1.19–4.78, p = 0.014), and the noncompletion of four cycles of induction therapy (HR = 0.21, 95% CI = 0.11–0.39, p < 0.001) were independent prognostic factors for poor OS in the multivariate analysis. Normal CSF LDH, lower NLR, and the completeness of four cycles of induction therapy were statistically significant independent prognostic factors for better PFS (HR = 0.002, 95% CI = 1.36–3.74, p = 0.002; HR = 1.82, 95% CI = 1.03–3.22, p = 0.038; HR = 0.22, 95% CI = 0.11–0.41, p < 0.001; respectively), while higher PLR and NGCB lost their significance.
Failure patterns and treatments
During the follow-up period, the death toll was 72, whereby 41 deaths were caused by recurrence, 23 by tumor progression, 3 by treatment-related adverse events, and the remaining 5 due to other causes. In our study cohort, 51 of 124 patients (41.1%) experienced recurrence during follow-up. The median time to recurrence was 14 months (range 2–129). For the patients who did not finish full cycles of induction therapy, the median time to recurrence was 4 months (range 2–21). The relapse of 49 patients occurred in the central nervous system: 16 patients had in situ recurrence and 33 had other sites of CNS recurrence. Among them, multifocal recurrences were noted in 14 patients. Two patients showed extra-CNS relapse.
After recurrence, 3 patients with a single lesion were treated by stereotactic radiosurgery (SRS), 19 received chemotherapy, 7 received radiotherapy, and 7 received chemotherapy plus radiotherapy. Among them, 3 patients received immunotherapy in addition to chemotherapy or radiotherapy. The remaining 13 patients died within days of detection of recurrence and therefore did not have a chance to receive treatment. The 2 patients with extra-CNS relapse underwent a systemic lymphoma chemotherapy regimen.
Discussion
In our study, the 1‑year, 2‑year, 3‑year, and 5‑year OS rates were 79.0%, 61.2%, 48.1%, and 35.8%, respectively, and the 1‑year, 2‑year, 3‑year, and 5‑year PFS rates were 59.7%, 44.6%, 37.9%, and 28.9%, respectively. The survival outcomes of PCNSL patients in other reports are shown in Table 3. The OS of our study was similar to the results of previous studies, while the outcomes of PFS showed considerable variance among the current studies. The reason for this disparity is probably the number of patients included and the difference in consolidation therapy. Yuan, X.G. et al.’s study represented the largest sample of PCNSL patients in a single institution, with 3‑year OS and PFS rates of 50% and 33%, respectively. The 3‑year PFS rates of our study were higher than those of Yuan, X.G., et al.’s study, which may be because no patients received ASCT in their study cohort [21]. Even so, a considerable percentage of progression was observed in our study. Close follow-up and salvage techniques will be needed in these patients.
There are some controversies remaining regarding the current treatment methods for PCNSL. Previous studies have reported that resection had no benefit for improving survival and may even lead to higher mortality rates [22, 23]. In such infiltrative tumors within the surrounding brain structures, aggressive surgery might lead to poor clinical outcomes. However, different views have emerged in recent years. Rae, A.I., et al.’s study found that craniotomy is associated with a survival benefit over biopsy; moreover, patients with better prognostic factors had an even longer survival benefit with craniotomy [24]. Similarly, some studies suggest that surgical resection might be a better choice for some patients [25,26,27]. Overall, the role of surgical resection for PCNSL is still unclear, and there is no guideline or consensus as yet. In our study, OS was better in patients who received resection than in those who received biopsy; however, the results were not statistically significant (p = 0.1). No discernible differential PFS trend was observed between the two surgical techniques. Biopsy has the main advantage of less aggressiveness and a shorter recovery period before induction therapy, while resection could relieve cranial hypertension and mass effect immediately, as well as alleviating tumor load, thus elevating the efficacy of subsequent treatment. The surgical method should be selected according to the specific case on the premise of ensuring safety.
HD-MTX-based chemotherapy is currently the recognized induction therapy. The main controversy is the combination of agents. Based on the results of the phase II IELSG32 trial, MATRix (HD-MTX, cytarabine, thiotepa, and rituximab) is one of the most preferred options. However, high hematological toxicity was reported in subsequent results [17, 28, 29]. Furthermore, rituximab was considered to be the chief component of treatment since more than 90% of patients were CD20+. Nevertheless, it is difficult to penetrate the blood–brain barrier (BBB) because of its macromolecular characteristics. There have been only two randomized studies regarding the efficacy of rituximab in PCNSL, and the studies reported conflicting results [17, 30]. In addition, novel drugs against PCNSL, such as monoclonal antibodies, immunomodulatory drugs (IMiDs), Bruton’s tyrosine kinase (BTK) inhibitors, and PD-1 inhibitors, with more rapid penetration of the BBB and less toxicity, have shown excellent results in several clinical trials [31,32,33]. Alterations in B‑cell antigen receptor (BCR) signaling, which is involved in PCNSL pathogenesis and regulation of development, participate in the differentiation and survival of B lymphocytes. BTK is the central signaling node of the BCR signaling pathway [34]. A prospective study in 52 patients with relapsed/refractory (r/r) PCNSL demonstrated a response rate of 52% with ibrutinib, and even patients without obvious genomic alterations in the BCR pathway demonstrated a response to ibrutinib [33]. In our study, all patients were treated with HD-MTX-containing induction chemotherapy. In our study, 64 of the 101 patients were NGCB subtype, 40 received HD-MTX-based chemotherapy, and 24 patients added TKIs to the induction regimens. It is obvious that the addition of TKIs significantly improved OS (91.7% vs. 52.5%, p = 0.00031) and PFS (86.5% vs. 35.7%, p = 0.00017). It is worth mentioning that earlier use of TKIs led to better survival for the NGCB subtype.
ASCT and WBRT were effective in PCNSL as consolidation therapy. In the largest randomized phase III trial to date, 551 patients received high-dose MTX-based chemotherapy with or without WBRT (SD 1.8 Gy, ED 45 Gy). There was no difference in median OS between those who received WBRT and those who did not, while those who received WBRT had a longer median PFS (15.4 vs. 9.9 months, p = 0.034) [35]. However, compliance was far from satisfactory because only 318 of 551 patients in the trial received treatment. In addition, population imbalances after induction chemotherapy were another flaw that could affect the results. The dose-dependent toxicity of WBRT is an important factor limiting its application. With whole-brain doses greater than 30 Gy, the incidence of neurocognitive decline after WBRT is relatively high. According to a meta-analysis published in 2015, WBRT is independently associated with an increased risk of neurologic side effects, which is approximately 5.23 times as high as among those who did not receive WBRT [36]. The survival benefit of WBRT should be balanced against the increased risk of neurotoxicity. In the PRECIS study, 140 patients aged 18 to 60 were randomized to receive WBRT (40 Gy) or ASCT after induction therapy. No significant differences were observed in 8‑year OS between the ASCT and WBRT groups (69% vs. 65%, respectively, p = 0.90). There were 3 cases of recurrence in the ASCT group and 24 in the WBRT group (HR = 0.13, p < 0.001) [37]. A meta-analysis in an evidence-based expert consensus in China showed that higher grade 3/4 hematologic adverse events (AEs) occurred in the ASCT group than in the WBRT group [38]. HDC-ASCT was only suitable for young patients (< 70 years) with no related complications and intact neurocognitive function [39, 40]. The high incidence of hematologic adverse events and the possibility of treatment-related death should also be noted. The components of HDC before ASCT need to be explored in the future. Considering the dose-related neurotoxicity of radiotherapy, reduced-dose whole-brain radiotherapy (rd-WBRT), used initially in the particularly fragile patient population of children with medulloblastoma, has been proposed for PCNSL treatment [41]. Those achieving a CR received 23.4 Gy and those with a PR received 30 Gy ± simultaneous integrated boost up to 40 Gy. The efficacy and safety of this strategy have been demonstrated in several studies in recent years [42, 43]. However, no study has directly compared the curative effect and side effects of ASCT and rd-WBRT. Among 38 patients who received consolidation therapy in our cohort, the OS and PFS of the ASCT group were better than those of the RT group; however, the results were not statistically significant (p = 0.26 and p = 0.14, respectively). This might be related to our small sample size and the younger age composition of the consolidation treatment group. Further prospective trials are needed, on the one hand to optimize HDC regimens of ASCT and on the other hand to reduce long-term neurotoxicity via improved radiotherapy techniques and dose adjustment.
Pathologic review of PCNSL cells appeared as round or oval, medium to large cells, with vacuole nuclei and prominent nucleoli. They were consistent with centroblasts or immunoblasts morphologically. Lymphoma cells grew in a typical perivascular pattern, diffusely infiltrated the brain parenchyma, and were highly proliferative with Ki-67 levels up to 90% [44]. Approximately 90% of PCNSLs were diffuse large B‑cell lymphomas and were further subdivided into GCB and non-GCB groups, as proposed by Hans et al. [45]. The GCB phenotype was defined as CD10+BCL6+/−MUM1+/− or CD10−BCL6+MUM1−, while the non-GCB phenotype was defined as CD10−BCL6+MUM1+ or CD10−BCL6−MUM1+/−. The latter subtype was generally correlated with inferior clinical outcomes [46]. The pathological features of PCNSL are shown in Fig. 5.
The hematoxylin–eosin stain and immunohistochemical characteristics of primary central nervous system lymphoma (PCNSL). PCNSL cells diffusely infiltrated the brain parenchyma and grew in a typical perivascular pattern (a, b). Lymphoma cells appeared as round or oval, medium to large cells, with vacuole nuclei and prominent nucleoli. They usually showed a high Ki-67 levels (c). CD20, a classical B‑cell surface marker, showed intact cell membrane positive staining (d). The most frequently used immunohistochemical markers are CD10 (e), BCL6 (f), MUM1 (g), and BCL2 (h)
In numerous studies, age and performance status were confirmed as independent prognostic factors in PCNSL [47,48,49]. However, there is no consensus on the specific definition of old age. Most prognostic studies of PCNSL defined agedness as older than 60 years [50,51,52]. In fact, this threshold should be based primarily on the ability to tolerate more aggressive treatments such as ASCT. Other prognostic factors reported in studies were race, sex, marital status, NLR, serum LDH level, PD-L1, and so on [53,54,55]. The IELSG and MSKCC prognostic systems are currently widely used. However, questions arose about the availability of variables and the reproducibility of verification in some studies, which limits their application in clinical routine [56]. Our prognostic analysis showed that elevated CSF LDH, higher NLR, and the completeness of four cycles of induction therapy are independent prognostic factors for better OS in PCNSL patients. Our multivariate Cox models identified CSF LDH, NLR, and four cycles of induction therapy as independent factors affecting PFS. The results suggested that CSF LDH and NLR might be valuable for predicting the prognosis of PCNSL, which serves as a basis for further study in combination with clinical radiology and molecular databases, which can be used to refine, optimize, and develop a prognostic model of higher clinical value.
Some limitations of our study are noteworthy. This was a retrospective analysis in a single center; therefore, we failed to analyze specific agents used in induction therapy and before ASCT; thus, we could not determine the superior regimens. Because few patients received ASCT or rd-WBRT as consolidation therapy, we could not compare the efficacy and toxicity of these two treatments. However, the present retrospective cohort is one of the largest single-institutional published reports. This study also unveiled the major prognostic factors and explored the potential benefit gained from adding TKIs to induction therapy.
Conclusion
PCNSL is a rare tumor. Our study retrospectively analyzed the clinical characteristics of patients with PCNSL in our center from July 2009 to April 2022, summarized the treatment experience, and analyzed the prognostic factors. PCNSL is highly aggressive. Hence, we emphasize the importance of early treatment and compliance with induction therapy. Further consolidation therapy is also necessary because of the high recurrence rate of PCNSL. The value and side effects of WBRT compared with those of ASCT require further investigation.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
References
Villano JL et al (2011) Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer 105(9):1414–1418
Shao L et al (2021) Recent Progress on Primary Central Nervous System Lymphoma-From Bench to Bedside. Front Oncol 11:689843
Liu J et al (2015) Immunochemotherapy for primary central nervous system lymphoma with rituximab, methotrexate, cytarabine and dexamethasone: Retrospective analysis of 18 cases. Mol Clin Oncol 3(4):949–953
Schaff LR, Grommes C (2021) Update on Novel Therapeutics for Primary CNS Lymphoma. Cancers (basel) 13(21)
Swerdlow SH et al (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127(20):2375–2390
Baraniskin A, Schroers R (2021) Liquid Biopsy and Other Non-Invasive Diagnostic Measures in PCNSL. Cancers (basel) 13(11)
Yuan Y et al (2021) Current and emerging therapies for primary central nervous system lymphoma. Biomark Res 9(1):32
Ferreri AJ et al (2003) Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol 21(2):266–272
Abrey LE et al (2006) Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 24(36):5711–5715
Pine JK et al (2015) Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: the relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br J Cancer 113(2):204–211
Krenn-Pilko S et al (2014) The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer 110(10):2524–2530
Shoji F et al (2020) Complete Blood Cell Count-Derived Inflammatory Biomarkers in Early-Stage Non-Small-Cell Lung Cancer. Ann Thorac Cardiovasc Surg 26(5):248–255
Keam B et al (2015) Neutrophil to lymphocyte ratio improves prognostic prediction of International Prognostic Index for patients with diffuse large B‑cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma 56(7):2032–2038
Troppan K et al (2014) The derived neutrophil to lymphocyte ratio is an independent prognostic factor in patients with diffuse large B‑cell lymphoma. Br J Cancer 110(2):369–374
You H, Wei L, Kaminska B (2021) Emerging insights into origin and pathobiology of primary central nervous system lymphoma. Cancer Lett 509:121–129
Young PA et al (2020) Durable Survival Outcomes in Primary and Secondary Central Nervous System Lymphoma After High-dose Chemotherapy and Autologous Stem Cell Transplantation Using a Thiotepa, Busulfan, and Cyclophosphamide Conditioning Regimen. Clin Lymphoma Myeloma Leuk 20(7):468–479
Ferreri AJ et al (2016) Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 3(5):e217–27
Cote GM et al (2012) Autologous stem cell transplantation with thiotepa, busulfan, and cyclophosphamide (TBC) conditioning in patients with CNS involvement by non-Hodgkin lymphoma. Biol Blood Marrow Transplant 18(1):76–83
Sarid N et al (2021) Impact of contemporary regimens on the outcomes and toxicity of primary CNS lymphoma: a single-center retrospective analysis of 73 patients. J Neurooncol 151(2):211–220
Wirsching HG et al (2021) Targeted Therapies and Immune Checkpoint Inhibitors in Primary CNS Lymphoma. Cancers (basel) 13(12)
Yuan XG et al (2020) Primary central nervous system lymphoma in China: a single-center retrospective analysis of 167 cases. Ann Hematol 99(1):93–104
Citterio G et al (2017) Primary central nervous system lymphoma. Crit Rev Oncol Hematol 113:97–110
Schlegel U (2009) Primary CNS lymphoma. Ther Adv Neurol Disord 2(2):93–104
Rae AI et al (2019) Craniotomy and Survival for Primary Central Nervous System Lymphoma. Neurosurgery 84(4):935–944
Schellekes N et al (2021) Resection of primary central nervous system lymphoma: impact of patient selection on overall survival. J Neurosurg 135(4):1016–1025
Wu S et al (2021) The role of surgical resection in primary central nervous system lymphoma: a single-center retrospective analysis of 70 patients. BMC Neurol 21(1):190
Zhang Q, Wang DW, Shu HS (2022) Outcome of Primary Central Nervous System Lymphoma Treated with Combined Surgical Resection and High-Dose Methotrexate Chemotherapy: A Single-Institution Retrospective Study. Turk Neurosurg 32(1):1–5
Ferreri AJM et al (2022) Long-term efficacy, safety and neurotolerability of MATRix regimen followed by autologous transplant in primary CNS lymphoma: 7‑year results of the IELSG32 randomized trial. Leukemia 36(7):1870–1878
Ferreri AJM et al (2017) Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol 4(11):e510–e523
Bromberg JEC et al (2019) Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 20(2):216–228
Ferreri AJM et al (2020) Improving the antitumor activity of R‑CHOP with NGR-hTNF in primary CNS lymphoma: final results of a phase 2 trial. Blood Adv 4(15):3648–3658
Ghesquieres H et al (2019) Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective ‘proof of concept’ phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA). Ann Oncol 30(4):621–628
Soussain C et al (2019) Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer 117:121–130
Rawlings DJ et al (2017) Altered B cell signalling in autoimmunity. Nat Rev Immunol 17(7):421–436
Korfel A et al (2015) Randomized phase III study of whole-brain radiotherapy for primary CNS lymphoma. Neurology 84(12):1242–1248
Kasenda B et al (2015) First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)—a systematic review and individual patient data meta-analysis. Ann Oncol 26(7):1305–1313
Houillier C et al (2022) Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients Age 60 Years and Younger: Long-Term Results of the Randomized Phase II PRECIS Study. J Clin Oncol 40(32):3692–3698
Chen T et al (2022) Evidence-based expert consensus on the management of primary central nervous system lymphoma in China. J Hematol Oncol 15(1):136
Illerhaus G et al (2016) High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol 3(8):e388–97
Ferreri AJ, Illerhaus G (2016) The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood 127(13):1642–1649
Packer RJ et al (1999) Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children’s Cancer Group Study. J Clin Oncol 17(7):2127–2136
Mishima K et al (2023) Randomized phase III study of high-dose methotrexate and whole-brain radiotherapy with/without temozolomide for newly diagnosed primary CNS lymphoma: JCOG1114C. Neuro Oncol 25(4):687–698
Morris PG et al (2013) Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 31(31):3971–3979
Lauw MIS et al (2020) Primary Central Nervous System Lymphomas: A Diagnostic Overview of Key Histomorphologic, Immunophenotypic, and Genetic Features. Diagnostics (basel) 10(12)
Hans CP et al (2004) Confirmation of the molecular classification of diffuse large B‑cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103(1):275–282
Schaff LR, Grommes C (2022) Primary central nervous system lymphoma. Blood 140(9):971–979
Eloranta S et al (2018) Increasing incidence of primary central nervous system lymphoma but no improvement in survival in Sweden 2000–2013. Eur J Haematol 100(1):61–68
Mendez JS et al (2018) The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol 20(5):687–694
Scordo M et al (2021) Outcomes Associated With Thiotepa-Based Conditioning in Patients With Primary Central Nervous System Lymphoma After Autologous Hematopoietic Cell Transplant. JAMA Oncol 7(7):993–1003
Sopittapan T, Tunthanathip T, Kaewborisutsakul A (2020) Outcome and Prognostic Factors of Primary Central Nervous System Lymphoma in Southern Thailand. Asian J Neurosurg 15(3):560–565
Shan Y, Hu Y (2018) Prognostic Factors and Survival in Primary Central Nervous System Lymphoma: A Population-Based Study. Dis Markers p:7860494
Niparuck P et al (2019) Treatment outcome and prognostic factors in PCNSL. Diagn Pathol 14(1):56
Asano K et al (2022) Clinicopathological risk factors for a poor prognosis of primary central nervous system lymphoma in elderly patients in the Tohoku and Niigata area: a multicenter, retrospective, cohort study of the Tohoku Brain Tumor Study Group. Brain Tumor Pathol
Tang D et al (2022) Epidemiologic Characteristics, Prognostic Factors, and Treatment Outcomes in Primary Central Nervous System Lymphoma: A SEER-Based Study. Front Oncol 12:817043
Jung J et al (2017) Prognostic role of the neutrophil-to-lymphocyte ratio in patients with primary central nervous system lymphoma. Oncotarget 8(43):74975–74986
Morales-Martinez A et al (2022) Prognostic factors in primary central nervous system lymphoma. Curr Opin Oncol 34(6):676–684
Zhu T et al (2015) Clinical characteristics and outcome of patients with primary central nervous system lymphoma. Zhonghua Xue Ye Xue Za Zhi 36(10):849–852
Koh, H.K., et al., Role of radiation therapy in primary central nervous system lymphoma : KROG 14–20 Collaborative Study of Brain and Lymphoma Committee. J Neurooncol, 2017. 135(3): p. 629–638.
Patekar, M., et al., Primary CNS Lymphoma in India: A 17-Year Experience From the All India Institute of Medical Sciences. J Glob Oncol, 2019. 5: p. 1–9.
Neuhauser M et al (2019) Increasing use of immunotherapy and prolonged survival among younger patients with primary CNS lymphoma: a population-based study. Acta Oncol 58(7):967–976
She C et al (2015) Clinical and prognostic analysis of 30 cases of primary central nervous system lymphoma. Zhonghua Xue Ye Xue Za Zhi 36(4):282–285
Mao C et al (2019) Characteristics and Outcomes of Primary Central Nervous System Lymphoma: A Retrospective Study of 91 Cases in a Chinese Population. World Neurosurg 123:e15–e24
Niparuck P et al (2019) Treatment outcome and prognostic factors in PCNSL. Diagn Pathol 14(1):56
Funding
This study was supported by grants from the Hunan Provincial Natural Science Foundation of China (no. 2021JJ31104).
Author information
Authors and Affiliations
Contributions
Mrs. Tang and Mrs. Wei had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Tang, Wei; acquisition, analysis, or interpretation of data: Tang, Wu; drafting of the manuscript: Tang, Wu, Tan, Long; critical revision of the manuscript for important intellectual content: Hong, Lyu, Wei; statistical analysis: Tang, Wu; obtained funding: Wei; administrative, technical, or material support: Tang, Wei; supervision: Lyu, Wei.
Corresponding author
Ethics declarations
Conflict of interest
Z. Tang, G. Wu, F. Tan, Y. Long, J. Hong, Z. Lyu and R. Wei declare that they have no competing interests.
Ethical standards
This retrospective study was approved by the ethics committee of Xiangya Hospital.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, Z., Wu, G., Tan, F. et al. Survival outcomes and treatment experience of 124 patients with primary central nervous system lymphoma. Strahlenther Onkol 200, 760–773 (2024). https://doi.org/10.1007/s00066-024-02219-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-024-02219-5