Abstract
Background
Intracranial arteriovenous malformations (AVMs) may show a harmful development. AVMs are treated by surgery, embolization, or radiation therapy.
Objective
This study investigated obliteration rates and side effects in patients with AVMs treated by radiation therapy.
Methods
A total of 40 cases treated between 2005 and 2013 were analyzed. Single-dose stereotactic radiosurgery (SRS) was received by 13 patients and 27 received hypofractionated stereotactic radiation therapy (HSRT). In 20 patients, endovascular embolization had been performed prior to irradiation and 24 patients (60 %) had a history of previous intracranial hemorrhage.
Results
Treatment resulted in complete obliteration (CO) in 23/40 cases and partial obliteration in 8/40. CO was achieved in 85 % of patients receiving SRS compared to 44 % of those receiving HSRT. In the HSRT group, a first indication of an influence of AVM volume on obliteration rate was found. Equivalent 2 Gy fraction doses (EQD2) >70 Gy showed an obliteration rate of 50 %. Prior embolization was significantly associated with a higher portion of CO (p = 0.032). Median latency period (24.2 vs. 26 months) until CO was similar in both groups (SRS vs. HSRT). The rate of intracranial hemorrhage in patients with no prior bleeding events was 0 %.
Conclusion
Excellent obliteration rates were achieved by SRS. Consistent with the literature, this data analysis suggests that the results of HSRT are volume-dependent. Furthermore, regimens with EQD2 doses >70 Gy appear more likely to achieve obliteration than schemes with lower doses. The findings indicate that radiation therapy does not increase the risk of bleeding. Prior embolization may have a good prognostic impact.
Zusammenfassung
Hintergrund
Intrakranielle arteriovenöse Malformationen (AVM) können einen komplikationsbehafteten Verlauf zeigen. AVMs sind mittels Operation, Embolisation oder Strahlentherapie behandelbar.
Zielsetzung
Die Studie untersucht Obliterationsraten und Nebenwirkungen bestrahlter AVM-Patienten.
Methoden
Analysiert wurden 40 Fälle, die zwischen 2005 und 2013 behandelt wurden. Insgesamt 13 Patienten erhielten eine Einzeitradiochirurgie (SRS), 27 Patienten wurden hypofraktioniert-stereotaktisch behandelt (HSRT). Eine endovaskuläre Embolisation vor der Strahlentherapie erhielten 20 Patienten. Vor der Strahlentherapie hatten 60 % der Patienten bereits eine intrakranielle Blutung.
Ergebnisse
In 23/40 Fällen wurde eine komplette (CO) und in 8/40 eine partielle Obliteration erreicht. Ein CO wurde in 85 % der SRS-Patienten und in 44 % der HSRT-Patienten erreicht. In der HSRT-Gruppe fanden wir einen ersten Hinweis auf einen Einfluss der AVM-Volumina auf die Obliterationsraten. Eine EQD2-Analyse zeigte eine Obliterationsrate von 25 % bei 58,3 Gy und von 50 % bei Summendosen >70 Gy. Eine vorherige Embolisation war signifikant mit einem höheren CO-Anteil (p = 0,032) assoziiert. Die medianen Latenzzeiten (24,2 vs. 26 Monate) bis zur CO waren in beiden Gruppen (SRS vs. HSRT) ähnlich. Die Rate an intrakraniellen Blutungen bei Patienten ohne vorheriges Blutungsereignis lag bei 0 %.
Schlussfolgerung
Die SRS erzielte exzellente Obliterationsraten. Wie zu erwarten und übereinstimmend mit der Literatur, legt unsere Analyse nahe, dass die Ergebnisse der HSRT offenbar volumenabhängig sind. Regime mit EQD2-Dosen >70 Gy scheinen eher zu einer Obliteration zu führen als Konzepte mit EQD2-Dosen von 58 Gy. Zudem erhöht die Strahlentherapie das Blutungsrisiko nicht. Eine vorherige Embolisation hat möglicherweise einen positiven Effekt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cerebral arteriovenous malformations (AVMs) are focal conglomerations of dilated arteries and veins in the brain that are directly connected without an intervening capillary bed. The main risks of brain AVM are intracranial hemorrhage (ICH) and epileptic seizures. The bleeding risk is estimated at 1–2 % per year in general [1, 2], and is as high as 30 % per year in patients with a history of ICH [3, 4]. In addition to neurosurgical and neuroradiological treatments, linear accelerator or CyberKnife-based (Accuray, Sunnyvale, CA, USA) stereotactic radiosurgery (SRS) is an established modality for treatment of intracranial AVM. SRS is often possible in cases where surgical resection is unfeasible due to a high risk of mortality [5, 6]. For small AVMs, SRS is generally delivered as a single dose of radiation. Patients with larger malformations receive hypofractionated stereotactic radiotherapy (HSRT), but the fractionation schemes used and obliteration rates achieved with HSRT vary widely. The probability of obliteration depends on the volume of the AVM nidus and the radiation dose [7, 8]. Obliteration rates for AVMs with volumes smaller than 2 cm³ are higher than 80 %, while those for AVMs with volumes larger than 2 cm³ drop off sharply according to the literature, to between 53 and 17 % after 3 years [9]. Moreover, normal brain tissue surrounding AVMs shows radiation-induced changes following distinct dose–volume relationships, allowing assessment of the risk of edema or brain-barrier breakdown upfront of SRS [10]. Through the use of modern technology, stereotactic irradiation treatments for cerebral AVMs are associated with a low rate of adverse effects [11].
The present study analyzed retrospectively collected data on obliteration rates confirmed by digital subtracted angiography (DSA) or MRI, radiation doses delivered, and AVM volumes treated at the Department of Radiation Oncology of Erlangen University Hospital (UK-Er). The question of which dose regimen is most optimal for the treatment of large cerebral AVMs was explored.
Methods
Patient characteristics
A total of 40 patients (16 men, 24 women; mean age 40.5 years, range 16–67 years) with AVMs of the brain were treated at the UK-Er Department of Radiation Oncology from 2005 to 2013. Of these patients, 13 (32.5 %) received single-dose SRS at doses of 18 Gy (n = 7) or 20 Gy (n = 6), and 27 (67.5 %) received HSRT at the following doses: 35 Gy in fractions of 7 Gy (n = 5) or 5 Gy (n = 1), 40 Gy in fractions of 4 Gy (n = 6), 48 Gy in fractions of 4 Gy (n = 7), or 52 Gy in fractions of 4 Gy (n = 7). At baseline, 8 brain AVMs were classified as grade 1, 16 as grade 2, 13 as grade 3, and 3 as grade 4 according to the Spetzler–Martin grading scale; none of the patients had grade 5. A history of intracranial hemorrhage prior to treatment was recorded for 24 patients (60 %). In 20 patients, endovascular embolization had been performed prior to stereotactic radiation therapy (using Onyx, Medtronic, Dublin, Ireland, in 14/20 patients; unknown n = 3; other agents n = 3) and one of these patients had trimodality treatment consisting of partial resection, embolization, and irradiation. Treatment recommendations were made in weekly multidisciplinary in-house team discussions.
Radiation treatment
Treatment planning consisted of a digital subtraction angiogram (DSA), a planning computed tomography (CT) scan, and a magnetic resonance imaging (MRI) angiography scan with a standard in-house protocol containing arterial time-of-flight (TOF) sequences in each case. The DSA, planning CT scan (1–2 mm slice thickness), and radiation treatment were accomplished using a stereotactic fixation system (BrainLab, Feldkirchen, Germany). The target volume consisted of the complete AVM nidus plus a safety margin of 1 mm. Nidus volume definition was performed by a neuroradiologist and a radiation oncologist together. In all cases, irradiation was performed using a NOVALIS linear accelerator (BrainLab) to deliver 6 MeV photons to the target. Isocenter verification was performed before each treatment session. Dose was prescribed to target volume surrounding the 90 % isodose.
Follow-up
All patients initially received semiannual and subsequently annual neuroimaging follow-up consisting of an MRI scan; DSA was additionally performed to confirm complete occlusion. Median follow-up was 55 months (range 5–103 months).
Statistical analysis
IBM SPSS version 21 (IBM, Armonk NY, USA) was used for statistical analyses, including univariate analysis (log-rank test) with Kaplan–Meier curves for graphic representation of the results, non-parametric tests (Kruskal–Wallis and Mann–Whitney U tests), and the Chi-square test for frequency distributions. Multivariate analysis was not possible due to the exploratory nature and small sample size of this retrospective analysis. The investigated parameters were age, gender, previous intracranial hemorrhage, target volume in cm³, and radiation dose. These parameters were tested for correlation with the outcomes “complete obliteration”, which was defined as complete disappearance of the AVM nidus in DSA or, if DSA was refused by the patient, MRI; “partial obliteration” (which was defined as any volume reduction in MRI); and “no change in AVM volume” in response to treatment, which was similar to work done by others [11, 19]. Side effects (e. g., bleeding recurrence and treatment-related disorders) were also analyzed.
Results
Treatment resulted in complete obliteration in 23 (57.5 %) of 40 cases, partial obliteration (volume reduction) in 8/40 (20 %), and no change in AVM volume without bleeding in 7/40 cases, as demonstrated by MRI or DSA. AVM bleeding with no change was detected in 2/40 patients. The median time to complete obliteration was 24 months (95 % confidence interval 17.78 to 31.21 months), with a range of 1 to 63 months. In 13/23 patients, complete occlusion was confirmed by DSA and 10/23 were confirmed by MRI only (because of patients refusal of DSA) (Table 1).
Side effects
The most severe side effect was ICH, which occurred in 4/40 patients (10 %; Spetzler–Martin grade II n = 2, III n = 2), all with a history of ICH before radiation treatment. None of these patients had been treated by embolization or surgery before (p = 0.035).
This corresponds to a bleeding recurrence rate of 16 % (4/24) in patients with previous hemorrhages. The rate of intracranial hemorrhage in patients with no bleeding events prior to irradiation was 0 %. Of the 4 ICH patients, 1 died from a massive ICH. Three out of four ICHs occurred after HSRT (35 Gy in 7 Gy fractions, n = 2; or 40 Gy in 4 Gy fractions, n = 1), and the fourth after single-dose SRS at a dose of 20 Gy. Complete obliteration of the nidus after ICH was ultimately shown in 2 patients and 3 of the 4 patients developed no further side effects after their ICH; the patients were treated only by medication. The time from radiation therapy until the onset of bleeding ranged from 5 to 19 months.
All other adverse events were mild. No patients developed radionecrosis or cysts. 8/40 patients (20 %) reported intermittent headaches or sensitivity to changes in weather, which had also been present before treatment in most cases. Epileptic seizures occurred in four cases (one after antiepileptic drug discontinuation), but all 4 patients had a history of epileptic seizures prior to radiation treatment. However, an increase in seizure frequency was observed in one case. No correlation of adverse events to Spetzler–Martin grade could be found.
Prognostic factors for complete obliteration
Complete obliteration was achieved in 85 % (11/13) of patients receiving single-dose SRS compared to only 44 % of those receiving HSRT (p = 0.11). However, the median target volume in the single-dose group (0.76 cm³) was significantly smaller than that in the hypofractionated group (6.02 cm³; p < 0.001). Interestingly, patients with a history of embolization did more often show a complete obliteration (16/20, p = 0.032) and a tendency to be treated more often by SRS (p = 0.091). Volume of PTV did not differ significantly between these two groups (p = 0.28), nor did the applied radiation dose (p = 0.15) or Spetzler–Martin grade (p = 0.56).
In the single-dose SRS group, there was no association between the applied radiation dose (18–20 Gy) and the incidence of complete obliteration. This is, however, based on a small number of samples.
In the HSRT group, the findings suggest an influence of AVM volume on the obliteration rate, which was 50 % for volumes <12 cm³ compared to only 16 % for volumes >12 cm³ (p = 0.751). To assess the influence of radiation dose on obliteration rate, radiation doses were converted to 2 Gy equivalent dose fractions (EQD2), assuming an α/β ratio of 3. The patients were thus dichotomized into two groups: those receiving a radiation dose of 58.3 Gy versus those with doses of 70–75 Gy. An obliteration rate of 25 % (2/8) was achieved in the former group (58.3 Gy), and 50 % (10/19) in the latter (70–75 Gy). However, this difference was not significant (p = 0.464) due to the small sample size.
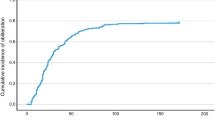
The median latency period (24.2 vs. 26 months) from treatment until complete obliteration was independent of the administered dose in the HSRT group or type of radiation treatment (SRS vs. HSRT [Fig. 1]).
Patients with lower-grade Spetzler–Martin AVMs received radiosurgery more frequently that those with higher-grade malformations (p = 0.004); however, there was no correlation between Spetzler–Martin grade and the rate of complete obliteration (p = 0.461). The same applies to the latency period before complete obliteration (p = 0.81).
Discussion
Radiation treatment of large intracranial AVMs is challenging. Single-dose SRS is generally used to treat brain AVMs smaller than 2 to 5 cm³, while a variety of different approaches are used for larger malformations. Obliteration rates achieved by single-dose SRS of small brain AVMs range from 60 % to more than 80 %, depending on the size of the malformation [12–15]. The results obtained in the current small sample are consistent with those described in the literature. Radiation doses administered in single-dose SRS currently fall within the range of 18 to 22 Gy [11, 16, 17].
HSRT dose regimens vary between different institutions as well as within a single institution. In a review by Wang et al., the total HSRT doses ranged from 26 to 42 Gy and were delivered in fractions of 4 to 7 Gy. The volumes of the treated AVMs also vary greatly, with sizes ranging from 2.2 cm³ to 46 cm³ [9, 18]. Consequently, the obliteration rates published in the literature range from 17 % after 3 years [19] to 53 % after 3 years [14]. In the present sample, HSRT achieved an overall obliteration rate of 44 %. These retrospective volume and dose analyses showed at least initial indications that obliteration may be volume- and dose-dependent: an obliteration rate of 50 % was achieved in patients with AVM volumes <12 cm³ compared to only 16 % in patients with larger AVMs. Conversion of the irradiated dose to EQD2 also showed an obliteration rate of 50 % at doses >70 Gy compared to only 25 % at doses <70 Gy. This largely corresponds to the data in the literature. For example, Cetin et al. described a target volume <2 cm³ as an independent prognostic factor for obliteration, as determined by multivariate analysis, while Veznedaroglu et al. found a 7-fold greater likelihood of obliteration in a cohort of patients treated with 7 Gy fractions than in those treated with 5 Gy fractions [17, 20]. Other authors found a better response in patients treated with a single-dose of 6 Gy compared to a fractionated dose of 5 Gy [21]. A fractionated 35 Gy schedule did show a significantly shorter time until obliteration than a treatment with 30–32.5 Gy, but also a significantly higher rate of radionecrosis [22]. Because higher doses are needed to achieve favorable obliteration rates, some institutions did introduce a staged radiosurgery approach as an alternative to HSRT. Large volumes are split into two to three subvolumes of equal size, and then treated with radiosurgery with a 2–9 months interval. Kano et al. treated 47 patients with a median AVM volume of 22 cm³ using two-step SRS and achieved obliteration rates of 9 % after 3 years and 32 % after 5 years. No cyst formation was seen during follow-up, and a higher margin dose was a significant factor for obliteration [23]. The need for smaller volumes and higher doses when using staged SRS was also reported by Seymour et al: a good response could be achieved after 5 years in 68 % and a dose ≥17 Gy was a strong predictor of response [24].
The latency period from treatment until the onset of complete obliteration was independent of the radiation dose or the kind of radiation therapy (single-dose versus hypofractionated RT) in the current analysis. The observed latency periods of 24 and 26 months, respectively, largely correspond to the figures reported in the literature [11]. Contrary to the present results, Zabel-du Bois et al. found a difference between the latency periods from single-dose and hypofractionated SRS until complete obliteration in a series of 48 patients (15 HSRT), with a median 46.5 months after hypofractionated compared to a median 29.2 months after single-dose radiation therapy. The fact that the HRST target volumes (median 27 cm³) in their study were very large compared to the relatively small volumes (median 6 cm³) treated in the present analysis may explain this discrepancy [19].
In the current cohort, patients who had a history of embolization prior to irradiation showed complete obliteration significantly more often (p = 0.032). Other groups described that embolization preceding SRS may have a negative impact on obliteration [25–28]. In some studies, the group treated with a combined approach showed a higher percentage of patients with Spetzler–Martin grades III and IV [26, 28], and Schwyzer et al. concluded that the lower obliteration rate may be caused by the fact that combined treatment is applied to higher-grade AVMs. Oermann et al. found lower obliteration rates in an embolized cohort compared to nonembolized patients; however, in contrast to angioarchitectural complexity, embolization was no longer a significant factor after multivariate analysis. The authors concluded that this reflects the use of upfront embolization in more complex AVM nidi, which may create bias in the results [29]. Other authors found combining embolization and radiation therapy to be effective in treating AVMs [30]. Embolization may not only reduce the volume of the AVM but also the vascular density, and good response rates after HSRT were reported [31]. In a recent report, Nataraj et al. found 67 % of patients treated by SRS only to be cured, compared to 70 % of those treated by embolization and SRS [32]. Recent reports using Onyx embolization and SRS found promising results [33, 34]. Interestingly, in none of the studies investigating this bimodality treatment could a higher rate of side effects be found when combining embolization and radiation therapy. The case is the same in the current analysis, where recurrent bleeding occurred in only patients treated by irradiation alone. According to the literature, using Onyx prior to radiation therapy seems to be safe, and according to the present analysis, it also seems to be effective without adding toxicity. It can be used for decreasing the volume of the nidus, although attention has to be paid to target volume definition afterwards, because of the changes in radiologic imaging or fragmentation of the nidus.
ICH is a complication that may occur during the course of AVM treatment; another complication is epileptic seizures. While a recent randomized study showed a negative prognostic impact of treating unbled AVMs during a 33-month follow-up period, other data show excess mortality in untreated patients after more than 10 years and the lowest rate in patients with totally occluded AVMs [2, 35].
In the present study, bleeding after radiation treatment occurred in 4 patients (10 %). At least one bleeding event prior to radiation therapy had been suffered by 60 % of the patients, corresponding to a rate of 16 % in this subgroup. Other investigators estimate that up to 30 % of patients with a history of hemorrhage can be expected to incur subsequent bleeding during the first year [4]. The 16 % rate of hemorrhage in this study is lower and the authors believe that this relatively low rate was an effect of AVM therapy. This is largely consistent with the literature data. For example, Yen et al. did not observe a single ICH in 155 patients with subtotally obliterated AVMs [36] and Karlson et al. found that the observed number of ICHs during the latency period until AVM obliteration was significantly lower than expected [37].
Radionecrosis is reported to occur in 0 to 7 % of radiosurgically treated cerebral AVM cases in the literature [11, 13, 15]. In contrast, no case of radionecrosis was observed in the current sample. The patients had mostly mild adverse events (e. g., headache and sensitivity to changes in weather), which were often present before irradiation. An increase in the frequency of epileptic seizures was reported by 1 patient. All patients who developed epileptic seizures after radiation therapy had a history of seizures prior to treatment.
Conclusion
SRS achieves excellent rates of control of small-volume AVMs, whereas the treatment of large AVMs remains a challenge. As expected and consistent with the published literature, the presented data analysis suggests that the results of HSRT are volume- and dose-dependent. Staged SRS may be an alternative to HSRT. The clear advantage of radiation therapy is the possibility of its application in regions where surgery and embolization may be harmful, such as the basal ganglia. Interestingly, preceding embolization with Onyx may have a good prognostic impact on the obliteration rates of irradiated AVMs. Further validation in a randomized study is needed.
References
Stapf C, Mast H, Sciacca RR, Choi JH, Khaw AV, Connolly ES et al (2006) Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 66:1350–1355
Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR et al (2014) Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 383:614–621
Hernesniemi JA, Dashti R, Juvela S, Vaart K, Niemela M, Laakso A (2008) Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery 63:823–831
Mast H, Young WL, Koennecke HC, Sciacca RR, Osipov A, Pile-Spellman J et al (1997) Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet 350:1065–1068
Treuer H, Hoevels M, Luyken K, Visser-Vandewalle V, Wirths J, Kocher M et al (2015) Intracranial stereotactic radiosurgery with an adapted linear accelerator vs. robotic radiosurgery: Comparison of dosimetric treatment plan quality. Strahlenther Onkol 191:470–476
Fuetsch M, El Majdoub F, Hoevels M, Muller RP, Sturm V, Maarouf M (2012) Stereotactic LINAC radiosurgery for the treatment of brainstem cavernomas. Strahlenther Onkol 188:311–316
Flickinger JC, Pollock BE, Kondziolka D, Lunsford LD (1996) A dose-response analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys 36:873–879
Yamamoto Y, Coffey RJ, Nichols DA, Shaw EG (1995) Interim report on the radiosurgical treatment of cerebral arteriovenous malformations. The influence of size, dose, time, and technical factors on obliteration rate. J Neurosurg 83:832–837
Wang H‑C, Chang RJ, Xiao F (2012) Hypofractionated stereotactic radiotherapy for large arteriovenous malformations. Surg Neurol Int 3:S105–S110
Levegrun S, Hof H, Essig M, Schlegel W, Debus J (2004) Radiation-induced changes of brain tissue after radiosurgery in patients with arteriovenous malformations: dose/volume-response relations. Strahlenther Onkol 180:758–767
Fokas E, Henzel M, Wittig A, Grund S, Engenhart-Cabillic R (2013) Stereotactic radiosurgery of cerebral arteriovenous malformations: long-term follow-up in 164 patients of a single institution. J Neurol 260:2156–2162
Kano H, Lunsford LD, Flickinger JC, Yang H‑C, Flannery TJ, Awan NR et al (2012) Stereotactic radiosurgery for arteriovenous malformations, Part 1: management of Spetzler-Martin Grade I and II arteriovenous malformations. J Neurosurg 116:11–20
Chang T‑C, Shirato H, Aoyama H, Ushikoshi S, Kato N, Kuroda S et al (2004) Stereotactic irradiation for intracranial arteriovenous malformation using stereotactic radiosurgery or hypofractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 60:861–870
Aoyama H, Shirato H, Nishioka T, Kagei K, Onimaru R, Suzuki K et al (2001) Treatment outcome of single or hypofractionated single-isocentric stereotactic irradiation (STI) using a linear accelerator for intracranial arteriovenous malformation. Radiother Oncol 59:323–328
Pollock BE, Link MJ, Stafford SL, Garces YI, Foote RL (2016) Stereotactic Radiosurgery for Arteriovenous Malformations: The Effect of Treatment Period on Patient Outcomes. Neurosurgery 78(4):499–509. doi:10.1227/neu.0000000000001085
Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD (2002) An analysis of the dose-response for arteriovenous malformation radiosurgery and other factors affecting obliteration. Radiother Oncol 63:347–354
Cetin IA, Ates R, Dhaens J, Storme G (2012) Retrospective analysis of linac-based radiosurgery for arteriovenous malformations and testing of the Flickinger formula in predicting radiation injury. Strahlenther Onkol 188:1133–1138
Karlsson B, Lindqvist M, Blomgren H, Wan-Yeo G, Soderman M, Lax I et al (2005) Long-term results after fractionated radiation therapy for large brain arteriovenous malformations. Neurosurgery 57:42–49
Zabel-du Bois A, Milker-Zabel S, Huber P, Schlegel W, Debus J (2006) Linac-based radiosurgery or hypofractionated stereotactic radiotherapy in the treatment of large cerebral arteriovenous malformations. Int J Radiat Oncol Biol Phys 64:1049–1054
Veznedaroglu E, Andrews DW, Benitez RP, Downes MB, Werner-Wasik M, Rosenstock J et al (2004) Fractionated stereotactic radiotherapy for the treatment of large arteriovenous malformations with or without previous partial embolization. Neurosurgery 55:519–511
Xiao F, Gorgulho AA, Lin C‑S, Chen C‑H, Agazaryan N, Vinuela F et al (2010) Treatment of giant cerebral arteriovenous malformation: hypofractionated stereotactic radiation as the first stage. Neurosurgery 67:1253–1259
Lindvall P, Bergstrom P, Blomquist M, Bergenheim AT (2010) Radiation schedules in relation to obliteration and complications in hypofractionated conformal stereotactic radiotherapy of arteriovenous malformations. Stereotact Funct Neurosurg 88:24–28
Kano H, Kondziolka D, Flickinger JC, Park K‑J, Parry PV, Yang H‑C et al (2013) Multistaged volumetric management of large arteriovenous malformations. Prog Neurol Surg 27:73–80
Seymour ZA, Sneed PK, Gupta N, Lawton MT, Molinaro AM, Young W et al (2016) Volume-staged radiosurgery for large arteriovenous malformations: an evolving paradigm. J Neurosurg 124:163–174
Andrade-Souza YM, Ramani M, Scora D, Tsao MN, terBrugge K, Schwartz ML (2007) Embolization before radiosurgery reduces the obliteration rate of arteriovenous malformations. Neurosurgery 60:443–442
Kano H, Kondziolka D, Flickinger JC, Park K‑J, Iyer A, Yang H‑C et al (2013) Stereotactic radiosurgery after embolization for arteriovenous malformations. Prog Neurol Surg 27:89–96
Back AG, Vollmer D, Zeck O, Shkedy C, Shedden PM (2008) Retrospective analysis of unstaged and staged Gamma Knife surgery with and without preceding embolization for the treatment of arteriovenous malformations. J Neurosurg 109(Suppl):57–64
Schwyzer L, Yen C‑P, Evans A, Zavoian S, Steiner L (2012) Long-term results of gamma knife surgery for partially embolized arteriovenous malformations. Neurosurgery 71:1139–1138
Oermann EK, Ding D, Yen C‑P, Starke RM, Bederson JB, Kondziolka D et al (2015) Effect of prior embolization on cerebral arteriovenous malformation radiosurgery outcomes: A case-control study. Neurosurgery 77:406–417
Izawa M, Chernov M, Hayashi M, Iseki H, Hori T, Takakura K (2009) Combined management of intracranial arteriovenous malformations with embolization and gamma knife radiosurgery: comparative evaluation of the long-term results. Surg Neurol 71:43–43
Lindvall P, Wikholm G, Bergstrom P, Lofroth P, Bergenheim AT (2005) Combined effects of embolization and hypofractionated conformal stereotactic radiotherapy in arteriovenous malformations of the brain. Interv Neuroradiol 11:223–229
Nataraj A, Mohamed MB, Gholkar A, Vivar R, Watkins L, Aspoas R et al (2014) Multimodality treatment of cerebral arteriovenous malformations. World Neurosurg 82:149–159
Pierot L, Kadziolka K, Litre F, Rousseaux P (2013) Combined treatment of brain AVMs with use of Onyx embolization followed by radiosurgery. AJNR Am J Neuroradiol 34:1395–1400
Lee C‑C, Chen C‑J, Ball B, Schlesinger D, Xu Z, Yen C‑P et al (2015) Stereotactic radiosurgery for arteriovenous malformations after Onyx embolization: a case-control study. J Neurosurg 123:126–135
Laakso A, Dashti R, Seppanen J, Juvela S, Vaart K, Niemela M et al (2008) Long-term excess mortality in 623 patients with brain arteriovenous malformations. Neurosurgery 63:244–245
Yen CP, Varady P, Sheehan J, Steiner M, Steiner L (2007) Subtotal obliteration of cerebral arteriovenous malformations after gamma knife surgery. J Neurosurg 106:361–369
Karlsson B, Lindquist C, Steiner L (1996) Effect of Gamma Knife surgery on the risk of rupture prior to AVM obliteration. Minim Invasive Neurosurg 39:21–27
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Knippen, F. Putz, S. Semrau, U. Lambrecht, A. Knippen, M. Buchfelder, S. Schlaffer, T. Struffert, and R. Fietkau declare that they have no competing interests.
Ethical standards
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. For this retrospective study, formal consent was not required.
Rights and permissions
About this article
Cite this article
Knippen, S., Putz, F., Semrau, S. et al. Predictors for occlusion of cerebral AVMs following radiation therapy. Strahlenther Onkol 193, 185–191 (2017). https://doi.org/10.1007/s00066-016-1056-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-016-1056-y