Abstract
Purpose
Embolization of arteriovenous malformations (AVMs) before radiosurgery has been reported to negatively impact the obliteration rate. This study aims to assess treatment outcomes in a series of 190 patients treated by Gamma Knife radiosurgery (GKRS) for previously embolized AVMs.
Methods
The institutional database of AVMs was retrospectively reviewed between January 2004 and March 2018. The clinical and radiological data of patients treated with GKRS for previously embolized AVMs were analyzed. Predicting factors of obliteration and hemorrhage following GKRS were assessed with univariate and multivariate regression analyses.
Results
The mean AVM size was significantly reduced after embolization (p < 0.001). The obliteration rate was 78.4%. Multivariate analyses showed that a lower Spetzler-Martin grade (p = 0.035) and a higher marginal dose (p = 0.007) were associated with obliteration. Post-GKRS hemorrhages occurred in 14 patients (7.4%). A longer time between diagnosis and GKRS was the only factor associated with post-GKRS hemorrhages in multivariate analysis (p = 0.022). Complications related to the combined treatment were responsible for a new permanent neurological disability in 20 patients (10.5%), and a case of death (0.5%).
Conclusions
This study shows that the embolization of AVMs does not have a negative impact on the obliteration rate after radiosurgery. Embolization reduces the AVM size to a treatable volume by GKRS. However, the combined treatment results in an increased complication rate related to the addition of the risks of each treatment modality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The contemporary management of cerebral arteriovenous malformations (AVMs) has evolved through a multidisciplinary approach that involves multiple interventional options, including microsurgery, embolization, radiosurgery, and the combination of these modalities [1]. Radiosurgery is a well-established treatment that has demonstrated its efficacy and safety in AVMs management [1, 2]. The most favorable outcomes using this modality are obtained in patients with small- and medium-volume lesions (volume < 10 cm3), while the treatment of large volume AVMs is associated with a lower obliteration rate and a higher rate of adverse radiation effects [1, 3]. Endovascular treatment is more commonly used in a multimodal approach as a neoadjuvant therapy preceding either microsurgery or radiosurgery [4]. Embolization of AVMs before radiosurgery reduces the size of the lesion and brings it to a treatable volume by radiosurgery [5]. Furthermore, endovascular treatment may be used to treat high-risk angioarchitectural abnormalities associated with AVMs, such as aneurysms, which reduce the risk of bleeding during the latency phase after radiosurgery [6]. Various embolic agents are currently used in AVMs embolization. N-butyl cyanoacrylate (NBCA) has been used for a long time, showing its efficiency and safety in AVMs embolization [7]. Non-adhesive embolic agents such as Onyx have been developed, and higher rates of AVMs cure were obtained using these agents [7]. However, embolization before radiosurgery exposes patients to an additional risk of complications related to the endovascular procedure [4]. These complications may be hemorrhagic or ischemic, with a commonly reported rate of permanent neurological morbidity between 4 and 9% [8,9,10]. Numerous authors have postulated that embolization has a negative impact on the efficiency of radiosurgery by reducing the obliteration rate of AVMs treated by radiosurgery [2, 3, 11, 12]. However, this notion remains controversial. It has been shown recently by Chen et al. that there is a lack of superiority of treatment by radiosurgery alone compared to treatment by radiosurgery with prior embolization in a matched cohort study comprising 101 patients each, with comparable groups in terms of AVM angioarchitectural characteristics and volume [13]. Given this controversy, we aimed to assess in this study the relevance of the combined approach by describing the clinical and radiological outcomes in terms of obliteration, the occurrence of hemorrhage after treatment, and radiation-related complications in a series of 190 patients treated by Gamma Knife radiosurgery (GKRS) for previously embolized AVMs. Predicting factors of obliteration and post-GKRS hemorrhage, and outcomes according to AVM scoring systems were also analyzed in this study population.
Methods
Patients and population
AVM patients were managed at our institution, since 2004, according to a multidisciplinary protocol with a strategy based on embolization of AVMs with an intent to cure the lesion; and treatment of the residual nidus by GKRS. After obtaining institutional ethics committee approval, this AVM database was retrospectively reviewed to identify all patients treated between January 2004 and March 2018. All newly treated patients with GKRS for previously embolized cerebral AVMs were included in the study. The inclusion required a minimum follow-up of 3 years unless obliteration was reached or hemorrhage occurred within this period. Patients were excluded if they were treated with staged radiosurgery or if they were lost to follow-up.
Embolization technique
In all patients, endovascular treatment was performed under general anesthesia and without systemic heparinization in the majority of the cases. The goal of each session is to occlude the nidus by injecting Glubran (GEM, Italy), Onyx (ev3, USA), or both into arterial feeders without complete occlusion of draining veins to avoid hemorrhage complications. The concentration of Glubran for each injection was left to the discretion of the operator depending on several parameters linked to the morphology of the AVM. The rate of occlusion in each session depends on the size and morphology of the AVM, and the duration of the procedure. Targeted treatment of high-risk features was also performed in untreatable AVMs. After the treatment, control angiographic series were performed, including working, frontal, and lateral views. All embolization sessions were performed with the objective to have a compact AVM remnant, in order to get only one target for the complementary GKRS.
AVM nidus volume and maximum diameter were measured for each patient on the first pre-embolization angiography using the Pacs Osiris system. The nidus was defined on the cerebral angiography when early draining veins were observed on the arterial phase of the angiogram. The nidus volume was calculated according to the AxBxC/2 method [14]. The post-embolization volumes and maximum diameters of the AVM nidus were measured on the day of GKRS treatment angiography. The post-embolization volumes were also measured according to the AxBxC/2 method. The target volumes measured during GKRS planning were also reported. Post-embolization and target volumes were also reported according to the embolic agent used.
Radiosurgery technique
The Gamma Knife Model C was used between 2004 and 2010, the Perfexion between 2010 and 2017, and the Icon between 2017 and 2018. For all patients, the Leksell Model G stereotactic frame (Elekta AB) was set up using local anesthesia assisted by light sedation, with more rare recourse to general anesthesia. 3D stereotactic MR imaging was performed, followed by CT and cerebral angiography. The target was defined on 3D stereotactic MR imaging and adjusted using anteroposterior and lateral angiography images. Only the non-embolized part of the nidus was included in the treatment target. When Onyx was used, nidus definition was often challenging, and Onyx was usually identified on T1 weighted MRI as a flow void. Planning and dosimetry were carried out by the neurosurgeon in collaboration with the radiation oncologist and the radiophysicist.
Clinical and radiological data and follow-up
For each patient, the medical records were reviewed to collect data related to clinical history, presentation, clinical status, and complications after each embolization session and during the post-GKRS follow-up. Data related to endovascular treatment were reviewed to identify the details of the endovascular procedure, the embolic agent used, and the embolization outcomes. The radiological reports were reviewed to determine for each patient the AVM nidus location, the type of drainage, and the angioarchitectural features. Data related to GKRS planning were reviewed to identify the marginal dose (Gy), isodose prescription line, maximum dose (Gy), volume, and maximum diameter of the nidus measured during radiosurgical planning. Imaging studies (angiography and MRI) performed during the post-GKRS follow-up were reviewed to determine the status of AVM obliteration and post-GKRS complications. MRI was generally performed every 6 months after GKRS during the first 2 years of follow-up and once a year afterward. Angiography was performed when feasible if the MRI showed signs of obliteration. Patients underwent clinical examination after each radiological examination. Obliteration was defined angiographically by the disappearance of the nidus and any arteriovenous shunt. Obliteration on MRI was defined in this study by the disappearance of nidus enhancement as well as any nidus-related venous enhancement. The annual hemorrhage rate was defined by the number of post-GKRS hemorrhagic events divided by the number of patient-years of post-GKRS follow-up without obliteration.
Statistical analysis
Continuous variables were summarized by their means and standard deviation (SD), and qualitative variables were summarized by numbers and percentages. Means of continuous variables were compared between 2 independent groups using classical Student’s t tests or Welch’s t tests in case of variance inequality and with paired t tests in the case of 2 paired samples.
Associations between variables were analyzed with linear univariate and multivariate regressions when appropriate. Statistical significance was considered when p was < 0.05. All statistical tests were performed using IBM-SPSS (version 27.0) software (IBM Corp, Armonk, NY, USA).
Results
A total of 239 consecutive patients were treated with GKRS for previously embolized cerebral AVMs between January 2004 and March 2018. Among them, 35 were lost to follow-up, and 14 others were treated with staged radiosurgery. The final study group was comprised of 190 patients. The mean age at diagnosis was 31 years (range 1–73 years). The initial presentation was hemorrhage in 91 patients (47.9%), seizure in 45 patients (23.7%), headaches in 23 patients (12.1%), focal deficit in 11 patients (5.8%), and incidental in 20 patients (10.5%). The patients’ clinical characteristics and AVM radiologic features regarding the location, angioarchitectural risk factors, and venous drainage type are shown in Table 1.
Embolization outcomes
The mean number of embolization sessions per patient was 2.5 sessions (range 1–11 sessions). The embolic agent used was Glubran in 77.9% of patients, Onyx in 17.9%, and a mix of both in 4.2%. Embolization details are shown in Table 2. The mean pre-embolization nidus volume and maximum diameter were 17.1 cm3 (range 0.1–85 cm3) and 31 mm (range 5–75 mm), respectively. The mean post-embolization volume and maximum diameter of the nidus were 4.2 cm3 (range 0.1–21 cm3) and 17.1 mm (range 1–56 mm), respectively. There was a statistically significant reduction in nidus volume and maximum diameter after embolization (p < 0.001 for both). The mean post-embolization volume was 3.9 cm3 (range 0.1–21 cm3) when Glubran was used, 4.6 cm3 (range 0.1–15.5 cm3) with Onyx, and 8.1 cm3 (range 0.1–16 cm3) when both agents were used. An additional analysis of AVMs whose pre-embolization volume was < 10 cm3 has been performed. In this subgroup, the mean pre-embolization volume was 3.3 cm3 (range 0.1–9.1 cm3) and the mean post-embolization volume was 1.7 cm3 (range 0.1–8.3 cm3). There was a statistically significant volume reduction after embolization in this subgroup (p < 0.001).
Twelve patients (6.3%) had hemorrhages during the embolization or within hours of the procedure. Among them, vessel perforation occurred in 3 patients (1.6%), one patient (0.5%) had a flow-related aneurysm rupture during the embolization, and 8 patients (4.2%) had a delayed post-embolization hemorrhage within hours of the procedure. Seven patients (3.7%) developed a transient neurological deficit after the endovascular intervention with no evidence of hemorrhagic complications on the CT scan. Ischemic complications were identified in 13 patients (6.8%). These were related to inadvertent embolization of normal arteries in 3 patients (1.6%), a retained microcatheter in the nidus in one patient (0.5%), and venous sinus thrombosis in one patient (0.5%).
Radiosurgical parameters
The mean time between diagnosis and GKRS was 27 months (range 0–282 months). The distribution of the study population based on the Pollock & Flickinger AVM score, the modified Pollock & Flickinger AVM score, the Virginia Radiosurgery AVM scale (VRAS), and the Spetzler-Martin grade is shown in Online Resource 1. The mean target volume measured during planning was 5.7 cm3 (range 0.1–31.7 cm3). The mean target volume was 5.2 cm3 (range 0.1–29 cm3) when Glubran was used, 6.6 cm3 (range 0.1–31.7 cm3) with Onyx, and 9.2 cm3 (range 0.1–20.5 cm3) when both agents were used. The mean marginal dose in this study was 20 Gy (range 12–30 Gy), with a mean maximum dose of 43 Gy (range 24–60 Gy) and a mean isodose prescription line of 50%.
Clinical and radiological outcomes post-GKRS
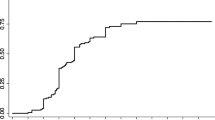
The mean follow-up after GNRS was 6 years (range 0.1–16 years). The obliteration rate based on angiography or MRI was 78.4%. Angiographically proven obliteration was found in 47.3% of patients. Obliteration based on MRI alone was found in 31% of patients. The AVM obliteration rate over time represented by the modified Kaplan–Meier graph is shown in Fig. 1. The obliteration rate was 83.5% in patients with hemorrhagic presentation and 73.7% in patients with a non-hemorrhagic presentation. Regarding the embolic agent used, the obliteration rate was 78.4% in the AVMs embolized with Glubran and 82.4% when Onyx was used. In patients with pre-embolization AVM volume < 10 cm3, the obliteration rate was 86.2%. In patients with pre-embolization AVM volume > 10 cm3, the obliteration rate was 70.8%. The mean time to obliteration was 2.5 years. The mean Pollock & Flickinger AVM score and modified Pollock & Flickinger AVM score in the case of obliteration were significantly lower than those in the case of non-obliteration (p = 0.004 and p = 0.005, respectively). The distribution of VRAS scores and Spetzler-Martin grades were significantly different in cases of obliteration or not (p = 0.042 and p = 0.001, respectively), showing different patterns in VRAS scores according to obliteration incidence and showing lower Spetzler-Martin grades in cases of obliteration (Online Resource 2). Predictors of post-GKRS obliteration in univariate regression analysis were decreased post-embolization volume, lower Spetzler-Martin grade, lower Pollock & Flickinger AVM score, lower modified Pollock & Flickinger AVM score, higher marginal dose, and higher maximum dose. By multivariate regression analysis, lower Spetzler-Martin and high marginal dose were the only factors independently associated with obliteration (p = 0.035 and p = 0.007, respectively). The details of univariate and multivariate regression analysis of predictors of obliteration are shown in Table 3.
Among the forty-one patients (21.6%) with non-obliterated AVMs, 3 patients (1.6%) had a new endovascular treatment, 5 patients (2.6%) had a new GKRS session, 1 patient (0.5%) underwent an embolization and GKRS, and 31 patients (16.3%) were managed with observation.
Post-GKRS hemorrhage
Post-GKRS hemorrhage occurred in 14 patients (7.4%), with an annual post-GKRS hemorrhage rate of 2.3%. Among them, one patient had an AVM considered to be angiographically obliterated with signs of recanalization on post-hemorrhagic angiography. Bleeding occurred in 71% of cases during the first 3 years post-GKRS. The initial presentation among patients with post-GKRS bleeding was hemorrhage in 7 patients (50%), and 7 others (50%) had unruptured AVMs. In 50% of the cases, the hemorrhages were without any clinical consequences. There were 5 patients (2.6%) who had a permanent neurological deficit and 2 deaths (1.1%). A larger post-embolization volume and longer time between diagnosis and GKRS were associated with post-GKRS hemorrhage in univariate regression analysis. A longer time between diagnosis and GKRS was the only factor associated with post-GKRS hemorrhage in multivariate regression analysis (p = 0.022). The details of univariate and multivariate regression analysis of predictors of post-GKRS hemorrhage are reported in Table 4.
Complications of combined treatment and clinical status
Complications related to embolization with or without clinical consequences were observed in 36 patients (18.9%). Thirty patients (15.8%) had newly developed neurological abnormalities after an embolization session. Embolization-related complications are detailed in Table 2. Radiation-induced changes, defined as T2 signal changes typically noted 6 to 18 months after GKRS, were observed in 65 patients (34.2%), including 7 (3.7%) who developed neurological disorders. Seven patients (3.7%) presented post-GKRS radionecrosis. Among them, two patients underwent surgical resection, and one patient died because of severe mass effect. Six patients (3.2%) developed a cyst during the follow-up, including 3 (1.6%) who required surgical drainage. Details of post-GKRS complications are shown in Online Resource 3. In total, complications related to this combined strategy were responsible for a new transient neurological disorder in 26 patients (13.7%), a new permanent neurological disability in 20 patients (10.5%), and a case of death (0.5%). Among patients with a pre-embolization AVM volume < 10 cm3, 7 (7.4%) had permanent neurological complications that were all related to embolization. Among patients with a pre-embolization AVM volume > 10 cm3, 14 (14.6%) had permanent neurological complications. Regarding the clinical presentation, permanent neurological abnormalities were observed in 9 patients (9.8%) with hemorrhagic AVM presentation and 11 (11.2%) with a non-hemorrhagic presentation. In patients with AVMs embolized with Glubran, there were 16 permanent neurological complications (10.8%). When Onyx was used, 5 patients had permanent neurological complications (14.7%). The clinical status at last follow-up in the entire study population was represented as follows: 86 patients (45.3%) without any neurological abnormality, 43 patients (22.6%) who had a focal neurological deficit, 51 patients (26.8%) with epilepsy, 19 patients (10%) who had a chronic headache, 8 patients (4.2%) with cognitive impairment, one patient (0.5%) who suffered from chronic vertigo, and 6 patients (3.2%) who died. Among the patients who died, the cause of death was not related to AVM in 2 patients (1.1%), and it was unknown in one patient (0.5%). Two patients (1.1%) died after post-GKRS AVM rupture, and one patient (0.5%) died due to radionecrosis, as mentioned above.
Discussion
In this retrospective study, we analyze the role of the embolization of AVMs before radiosurgery. Using this combined approach, our series’ obliteration rate was 78.4%. This rate is comparable to the most reported recent radiosurgical series, which reported an obliteration rate of 64–86% [2, 15, 16]. In our treatment strategy, the intensified embolization of AVMs led to a significant reduction in the volume treated by radiosurgery. This increases the benefit of radiosurgery as the treated volume with GKRS is smaller. It has been demonstrated that the risk of radiation-related complications is higher when the treated volume is large. [17, 18]. However, the complication rate in our combined strategy is relatively high as we noted 10.5% of permanent neurological complications and one case of death. We also found a permanent neurological complication rate of 7.4% in patients with a pre-embolization AVM volume < 10 cm3. This rate is two times lower than AVM > 10 cm3. Nevertheless, a standalone treatment could be considered in this group of patients with small AVM suitable for GKRS treatment, avoiding the exposition of patients to an additional risk of complications.
The controversy around the role of embolization in combination with radiosurgery has arisen since several studies have suggested that embolization may have a negative impact on the efficiency of radiosurgery in the treatment of AVMs [3, 18, 19]. This was supported by two meta-analyses of 10 and 16 studies that compared treatment by radiosurgery alone and embolization followed by radiosurgery. The conclusion was the superiority of radiosurgery alone in terms of obliteration rate [12, 19]. Several hypotheses have been proposed to explain this effect. The embolization could stimulate angiogenesis through hypoxia, hemodynamic modification, and inflammatory phenomena [20]. The impact of the type of embolic agent on radiosurgical outcomes was also studied. High recanalization rates have been reported with NBCA, while Onyx, which has become the most widely used embolic agent in neuro-interventional procedures, would cause less recanalization than previous embolic agents [21, 22]. Liquid embolization materials have been reported to act as a barrier reducing radiation delivery [23]. Furthermore, they alter the definition of the radiosurgical target, inducing the fragmentation of a compact nidus [24]. However, these hypotheses remain controversial, and the exact mechanism of this effect remains unclear [25, 26]. It must be emphasized that the main comparative studies that have demonstrated the superiority of treatment by radiosurgery alone compared to combined embolization and radiosurgery were composed of heterogeneous groups, with larger AVMs and more complex angioarchitecture in the combined embolization and radiosurgery groups [19]. In addition, it has been shown that the AVM angioarchitecture could influence the efficiency of radiosurgery [27]. Moreover, embolization does not appear to have a negative impact on the radiosurgical effect if AVM pre-embolization characteristics are considered when comparing groups treated by embolization followed by radiosurgery and others treated by radiosurgery alone, with no superiority of radiosurgery alone in terms of obliteration [13].
In our study cohort, 99 patients (52.1%) had unruptured AVMs. The rate of treatment-related permanent neurological impairment in this group was 10.2%. The role of interventional treatment of unruptured AVMs became highly controversial since the A Randomized trial of Unruptured Brain Arteriovenous malformations (ARUBA) trial and The Scottish Audit of Intracranial Vascular Malformations (SAIVM) study showed a better clinical outcome of conservative management of unruptured AVMs.[28, 29]. While these studies have shown that the morbidity of the treatment is higher than expected, several criticisms have been made particularly concerning the treatment modality used, their relatively low success rates, and their high complication rate. In ARUBA, 26% of patients were treated by embolization alone [28]. Furthermore, a recent meta-analysis showed a high complication rate with a low success rate of standalone embolization of AVMs with the intent to cure [30]. Several retrospective analyses, including studies on patients eligible for ARUBA and treated by surgery or radiosurgery, have shown higher success rates and fewer complications [31, 32]. Thus, it would be inappropriate to universally define non-interventional treatment as the standard treatment for unruptured AVMs. Optimizing the outcome goes through a careful patient selection in a multidisciplinary and experienced center.
We found in this study population that outcomes were congruent with AVM prognostic scores relevant to radiosurgery, namely, the Pollock & Flickinger AVM score and the modified Pollock & Flickinger AVM score, which were validated as predictors of favorable outcomes in AVM patients undergoing radiosurgery [2, 33,34,35]. However, these outcomes were inconsistent with the VRAS score, as we found a significantly higher rate of non-obliteration in VRAS score 1. In multivariate analysis, the Spetzler-Martin grading system was an independent predictor of outcomes in our study population. Even if it has already been reported that this score could have a predictive effect on radiosurgical outcomes, this grading system was mainly validated for surgical series and is generally not considered an adequate system in the prediction of outcomes in patients treated with other modalities [33, 36, 37]. In this study, a high marginal dose was independently associated with obliteration, as shown in multivariate analysis. We did not find a predictive effect of the lesion volume on obliteration in this study, contrary to what has been reported in other series [2, 11].
The late therapeutic effect of radiosurgery treatment exposes patients to a risk of hemorrhage during the latency period preceding obliteration. The annual post-GKRS hemorrhagic risk reported in the literature varies roughly between 1 and 4.8% [2, 15, 36, 38]. In our series, the hemorrhage rate was 7.4% with an annual rate of 2.3%, which is comparable to the rates reported in the radiosurgical literature with or without embolization. Post-GKRS hemorrhage occurred in the majority of cases during the period in which AVMs were still patent. This finding suggests that embolization does not appear to have a protective effect during this period.
Our study has limitations due to the retrospective nature of the analysis. In addition, obliteration was assessed in a portion of the study population by MRI only without angiographic examination. Moreover, even the ABC method has been used for pre-embolization and post-embolization AVMs volume measurement, it has not been validated in this indication and is therefore subject to bias [39].
Conclusion
In summary, this study showed that in combined approach using endovascular treatment followed by radiosurgery for AVMs, the embolization does not have a negative impact on the obliteration rate after radiosurgery. The main advantage of embolization is to reduce the volume of the AVM, thus making it possible to treat large volume AVMs by radiosurgery. However, the combined treatment results in a relatively increased complication rate related to the addition of the risks of each treatment modality.
References
Chen CJ, Ding D, Derdeyn CP et al (2020) Brain arteriovenous malformations: a review of natural history, pathobiology, and interventions. Neurology 95:917–927
Starke RM, Kano H, Ding D et al (2017) Stereotactic radiosurgery for cerebral arteriovenous malformations: evaluation of long-term outcomes in a multicenter cohort. J Neurosurg 126:36–44. https://doi.org/10.3171/2015.9.JNS151311
Pollock BE, Flickinger JC, Lunsford LD et al (1998) Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery 42:1239–1244. https://doi.org/10.1097/00006123-199806000-00020
Zaki Ghali MG, Kan P, Britz GW (2019) Curative embolization of arteriovenous malformations. World Neurosurg 129:467–486. https://doi.org/10.1016/j.wneu.2019.01.166
Blackburn SL, Ashley WW, Rich KM et al (2011) Combined endovascular embolization and stereotactic radiosurgery in the treatment of large arteriovenous malformations: clinical article. J Neurosurg 114:1758–1767. https://doi.org/10.3171/2011.1.JNS10571
Kano H, Kondziolka D, Flickinger JC et al (2012) Aneurysms increase the risk of rebleeding after stereotactic radiosurgery for hemorrhagic arteriovenous malformations. Stroke 43:2586–2591. https://doi.org/10.1161/STROKEAHA.112.664045
Vollherbst DF, Chapot R, Bendszus M, Möhlenbruch MA (2022) Glue, onyx, squid or PHIL? Liquid embolic agents for the embolization of cerebral arteriovenous malformations and dural arteriovenous fistulas. Clin Neuroradiol 32:25–38. https://doi.org/10.1007/s00062-021-01066-6
Taylor CL, Dutton K, Rappard G et al (2004) Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg 100:810–812. https://doi.org/10.3171/jns.2004.100.5.0810
van Beijnum J, van der Worp HB, Buis DR et al (2011) Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA 306:2011–2019. https://doi.org/10.1001/jama.2011.1632
Panagiotopoulos V, Gizewski E, Asgari S et al (2009) Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (Onyx). Am J Neuroradiol 30:99–106. https://doi.org/10.3174/ajnr.A1314
Huo X, Jiang Y, Lv X et al (2016) Gamma Knife surgical treatment for partially embolized cerebral arteriovenous malformations. J Neurosurg 124:767–776. https://doi.org/10.3171/2015.1.JNS142711
Xu F, Zhong J, Ray A et al (2014) Stereotactic radiosurgery with and without embolization for intracranial arteriovenous malformations: a systematic review and meta-analysis. Neurosurg Focus 37:E16. https://doi.org/10.3171/2014.6.FOCUS14178
Chen C-J, Ding D, Lee C-C et al (2021) Stereotactic radiosurgery with versus without embolization for brain arteriovenous malformations. Neurosurgery 88:313–321. https://doi.org/10.1093/neuros/nyaa418
Kashanian A, Sparks H, Kaprealian T, Pouratian N (2019) Assessing the volume of large cerebral arteriovenous malformations: can the ABC/2 formula reliably predict true volume? J Clin Neurosci 65:1–5. https://doi.org/10.1016/j.jocn.2019.04.038
Pollock BE, Link MJ, Stafford SL et al (2016) Stereotactic radiosurgery for arteriovenous malformations: the effect of treatment period on patient outcomes. Neurosurgery 78:499–509. https://doi.org/10.1227/NEU.0000000000001085
Starke RM, Yen C-P, Ding D, Sheehan JP (2013) A practical grading scale for predicting outcome after radiosurgery for arteriovenous malformations: analysis of 1012 treated patients. J Neurosurg 119:981–987. https://doi.org/10.3171/2013.5.JNS1311
Yamamoto M, Kawabe T, Barfod BE (2013) Long-term side effects of radiosurgery for arteriovenous malformations. In: Niranjan A, Kano H, Lunsford LD (eds) Progress in Neurological Surgery. S. Karger AG, pp 97–106
Kano H, Kondziolka D, Flickinger JC et al (2012) Stereotactic radiosurgery for arteriovenous malformations after embolization: a case-control study. J Neurosurg 117:265–275. https://doi.org/10.3171/2012.4.JNS111935
Russell D, Peck T, Ding D et al (2018) Stereotactic radiosurgery alone or combined with embolization for brain arteriovenous malformations: a systematic review and meta-analysis. J Neurosurg 128:1338–1348. https://doi.org/10.3171/2016.11.JNS162382
Buell TJ, Ding D, Starke RM et al (2014) Embolization-induced angiogenesis in cerebral arteriovenous malformations. J Clin Neurosci Off J Neurosurg Soc Australas 21:1866–1871. https://doi.org/10.1016/j.jocn.2014.04.010
Natarajan SK, Ghodke B, Britz GW et al (2008) Multimodality treatment of brain arteriovenous malformations with microsurgery after embolization with onyx: single-center experience and technical nuances. Neurosurgery 62:1213–1226. https://doi.org/10.1227/01.neu.0000333293.74986.e5
Rao VR, Mandalam KR, Gupta AK et al (1989) Dissolution of isobutyl 2-cyanoacrylate on long-term follow-up. AJNR Am J Neuroradiol 10:135–141
Andrade-Souza YM, Ramani M, Beachey DJ et al (2008) Liquid embolisation material reduces the delivered radiation dose: a physical experiment. Acta Neurochir (Wien) 150:161–164. https://doi.org/10.1007/s00701-007-1482-9 (discussion 164)
Kwon Y, Jeon SR, Kim JH et al (2000) Analysis of the causes of treatment failure in gamma knife radiosurgery for intracranial arteriovenous malformations. J Neurosurg 93(Suppl 3):104–106. https://doi.org/10.3171/jns.2000.93.supplement
Bing F, Doucet R, Lacroix F et al (2012) Liquid embolization material reduces the delivered radiation dose: clinical myth or reality? AJNR Am J Neuroradiol 33:320–322. https://doi.org/10.3174/ajnr.A2943
Chen C-J, Ding D, Lee C-C et al (2021) Embolization of brain arteriovenous malformations with versus without onyx before stereotactic radiosurgery. Neurosurgery 88:366–374. https://doi.org/10.1093/neuros/nyaa370
Oermann EK, Ding D, Yen C-P et al (2015) Effect of prior embolization on cerebral arteriovenous malformation radiosurgery outcomes: a case-control study. Neurosurgery 77:406–417. https://doi.org/10.1227/NEU.0000000000000772 (discussion 417)
Mohr JP, Parides MK, Stapf C et al (2014) Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 383:614–621. https://doi.org/10.1016/S0140-6736(13)62302-8
Al-Shahi Salman R, White PM, Counsell CE et al (2014) Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA 311:1661. https://doi.org/10.1001/jama.2014.3200
Wu EM, El Ahmadieh TY, McDougall CM et al (2020) Embolization of brain arteriovenous malformations with intent to cure: a systematic review. J Neurosurg 132:388–399. https://doi.org/10.3171/2018.10.JNS181791
Nerva JD, Mantovani A, Barber J et al (2015) Treatment outcomes of unruptured arteriovenous malformations with a subgroup analysis of ARUBA (A Randomized Trial of Unruptured Brain Arteriovenous Malformations)-eligible patients. Neurosurgery 76:563–570. https://doi.org/10.1227/NEU.0000000000000663
Rutledge WC, Abla AA, Nelson J et al (2014) Treatment and outcomes of ARUBA-eligible patients with unruptured brain arteriovenous malformations at a single institution. Neurosurg Focus 37:E8. https://doi.org/10.3171/2014.7.FOCUS14242
Pollock BE, Flickinger JC (2008) Modification of the radiosurgery-based arteriovenous malformation grading system. Neurosurgery 63:239–243. https://doi.org/10.1227/01.NEU.0000315861.24920.92 (discussion 243)
Hartmann A, Stapf C, Hofmeister C et al (2000) Determinants of neurological outcome after surgery for brain arteriovenous malformation. Stroke 31:2361–2364. https://doi.org/10.1161/01.str.31.10.2361
Friedman WA, Bova FJ, Bollampally S, Bradshaw P (2003) Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery 52:296–307. https://doi.org/10.1227/01.neu.0000043692.51385.91 (discussion 307-308)
Hirschmann D, Goebl P, Witte FH et al (2020) Evaluation of the radiosurgical treatment of cerebral arteriovenous malformations: a retrospective single-center analysis of three decades. J Neurointerventional Surg 12:401–406. https://doi.org/10.1136/neurintsurg-2019-015332
Saatci I, Geyik S, Yavuz K, Cekirge HS (2011) Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: long-term results in 350 consecutive patients with completed endovascular treatment course. J Neurosurg 115:78–88. https://doi.org/10.3171/2011.2.JNS09830
Koltz MT, Polifka AJ, Saltos A et al (2013) Long-term outcome of Gamma Knife stereotactic radiosurgery for arteriovenous malformations graded by the Spetzler-Martin classification. J Neurosurg 118:74–83. https://doi.org/10.3171/2012.9.JNS112329
Roark C, Vadlamudi V, Chaudhary N et al (2018) ABC/2 Method Does not Accurately Predict Cerebral Arteriovenous Malformation Volume. Neurosurgery 82:220–225. https://doi.org/10.1093/neuros/nyx139
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mehdi Yahia-Cherif, Chifra Fenton, Florence Lefranc and Boris Lubicz. The first draft of themanuscript was written by Mehdi Yahia-Cherif and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study obtained institutional ethics committee approval. Ethics committee ULB-Erasme.
Number: P2022/078.
Consent to participate
This retrospective study was approved by the institutional ethics committee approval, and patients’ consents were waived.
Consent for publication
This retrospective study was approved by the institutional ethics committee approval, and patients’ consents were waived.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yahia-Cherif, M., Fenton, C., Bonnet, T. et al. Embolization before Gamma Knife radiosurgery for cerebral arteriovenous malformations does not negatively impact its obliteration rate: a series of 190 patients. Neuroradiology 65, 391–399 (2023). https://doi.org/10.1007/s00234-022-03066-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03066-w