Abstract
Many animals deposit odours in their environment, either intentionally or unintentionally, that remain at a site after the animal itself has left. These odours may be exploited by other species as social information, and thus have a significant role in structuring species interactions, even where the species involved rarely interact directly. Here we show that three species of Australian social stingless bees (Meliponini) not only detect the odours left behind by conspecifics, but also those of an abundant introduced competitor, the honey bee Apis mellifera (Apini). Foraging bees deposit pheromones that assist nestmates in locating profitable food sources (signals) and/or involuntary olfactory “footprints” (cues), both of which are vulnerable to exploitation by the foragers of other colonies. Using choice trials, we find that foragers of Tetragonula carbonaria, Tetragonula clypearis and Austroplebeia australis were more attracted to feeders recently used by, and thus carrying the odours of, their own species (both nestmates and non-nestmates) or honey bees, than to clean unused feeders. Australia’s stingless bees may learn to associate honey bee odours with food and exploit this to their advantage, or they may mistake honey bee odours for some other attractant and be misdirected. Our results suggest that introduced social insects could have a previously overlooked impact on resident communities, by modifying the olfactory landscape of shared resources in ways that alter native species’ foraging behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many animals use the social information provided by heterospecifics to make decisions on when, where and how to forage, and thus improve their foraging success (Danchin et al. 2004). In a common scenario, predators benefit from social information to detect prey, and prey do likewise to ensure they forage in safety from predators (Seppänen et al. 2007). Such use of social information may also occur, however, within a trophic level, where it can play a key role in shaping the competitive interactions between species and, in turn, the structure of ecological communities (Goodale et al. 2010). Social insects have proved valuable model systems for understanding these within-trophic interactions because they often deposit signals (pheromones) and cues (e.g. chemical “footprints”) at food sources, both of which are vulnerable to detection and use by competing species (Goodale et al. 2010; Lichtenberg et al. 2011; Menzel et al. 2010; Nieh et al. 2004; Slaa and Hughes 2009; Stout and Goulson 2001).
The use of heterospecific odours by foraging social insects has to date been documented only in systems where the species involved have a long-standing history of sympatry. For example, some arboreal ants form close nesting associations in which one species locates food by following the other’s pheromone trails (Vantaux et al. 2007), some Neotropical stingless bees reduce conflict with congeners by detecting and avoiding their food-marking pheromones (Lichtenberg et al. 2011), and honey bees use the odour cues deposited at flowers by bumble bees to avoid flowers recently depleted of nectar (Stout and Goulson 2001). Yet many ecosystems today include introduced species whose ecological niches overlap with those of resident taxa. The ability of native species to recognise and respond to the odours of aliens, or vice versa, will presumably influence how they interact, and thus the impact of new species on an ecosystem. Such interactions are invisible to the human observer, yet may be a significant component of interspecific competition or facilitation, even where the species are rarely seen to interact directly.
Here, we assess the ability of three species of Australian stingless bees (Meliponini) to detect the odour marks left at food sources by both their own species, and an abundant introduced species, the honey bee, Apis mellifera (Apini). Foraging bees may recruit nestmates to high quality food sources or other resources by actively depositing pheromones at, or leading to, the resource site (e.g. stingless bees: Barth et al. 2008; Alavez-Rosas et al. 2017; honey bees: Free and Williams 1983; Williams et al. 1981) and/or they may leave behind involuntary olfactory footprints at food sources (e.g. stingless bees: Schmidt et al. 2005; Roselino et al. 2016; honey bees: Ferguson and Free, 1979; Bortolotti and Costa 2014; bumble bees: Saleh et al. 2007). Foragers from other colonies may then detect and use these odours to increase their own foraging efficiency, either by exploiting the resource themselves (Nieh et al. 2004), or by avoiding a resource that has been recently depleted (Giurfa and Núñez 1992; Stout and Goulson 2001). The Meliponini have a pan-tropical distribution, comprising an estimated 600 species across two clades: Neotropical and Afro-Australasian (Rasmussen and Cameron 2010). Several species of Neotropical stingless bee use nestmate-deposited odours to identify food sources and some also lay trail pheromones directing nestmates towards food (Barth et al. 2008; Jarau 2009), but whether the use of nestmate-deposited odours in foraging is widespread among Meliponini remains unclear. In one Australian species, T. carbonaria, nestmate-deposited odours have been proposed to aid their ability to rapidly recruit to a food source (Bartareau 1996; Nieh et al. 1999). If Australian stingless bees use the odours deposited by nestmates to locate profitable food sources, and are also capable of exploiting the odours of neighbouring nests’ foragers, then we expected to observe attraction to food sources previously used by both nestmate and non-nestmate conspecifics.
Honey bees are native to Africa and Eurasia but A. mellifera has been widely introduced elsewhere alongside human agriculture (Moritz et al. 2005), including Australia in c.1822 (Hopkins 1886). Their impact on native bees is poorly understood but generally assumed to be neutral, or negative due to competition for food and nest sites, pathogen transfer and the pollination and spread of exotic plants (Goulson 2003; Mallinger et al. 2017; Paini 2004; Stout and Morales 2009; Wojcik et al. 2018). In Australia, honey bees and stingless bees have overlapping ranges and use common floral resources (Fig. 1A, Heard 2016), indicating there is potential for these species to be regularly exposed to each others’ food-marking odours. Stingless bees and honey bees in Australia, therefore, provide an excellent opportunity to assess whether the food-marking odours of introduced species can affect the foraging behaviour of native species. If Australian stingless bees recognise and respond to honey bee odours during foraging, then we expected to observe either attraction to, or avoidance of, food sources previously used by honey bees.
Methods
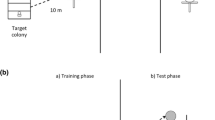
We performed a series of feeder choice trials to determine whether three species of Australian stingless bee (Tetragonula carbonaria, Tetragonula clypearis and Austroplebeia australis) respond to food sources recently used by conspecifics, or by A. mellifera (hereafter: honey bee). Used food sources accumulate the odour marks (pheromones and/or footprints) of previous foragers. Trials took place in Sydney for T. carbonaria (outdoor grassy areas at the University of Sydney’s Crommelin Field Research Station − 33.5496° S, 151.29926° E) and A. australis (a residential property in southern Sydney; 34.0627 S, 151.1193 E) during the summers of 2015, 2016 and 2018. Trials for T. clypearis were conducted in Cairns (in the backyard of a rented house; 16.9256° S, 145.7753° E) during winter 2015. We used two hived colonies per stingless bee species and 1–2 hived colonies of honey bees per location. The two stingless bee colonies per site were separated from each other by at least 10 m. The colonies used per species were not recently propagated from each other (i.e. not close relatives) and, therefore, considered to be independent. All colonies were free-flying.
Training
At each colony, we trained foragers to artificial feeders positioned directly in front of the colony entrance (Fig. 1B). We observed bees travelling between the feeder and the hive to confirm foragers originated from our hives. Feeders were 50 ml specimen jars wrapped in blue-electrical tape to make them opaque, and fitted atop perspex discs (75 mm diameter) with 24 radial grooves (1 mm wide). When the jar was filled with feeding solution, the solution flowed into the grooves where it could be accessed by the bees. We placed a filter paper (90 mm diameter) beneath each feeder to increase the landing area, and the whole feeder was positioned on a platform fixed to a wooden stake such that it was at approximately the same height off the ground as the hive entrance (c. 40 cm). Feeding solution comprised of 50% sugar solution (T. clypearis trials) or 50% commercial honey bee honey solution (T. carbonaria and A. australis trials). The training phase continued until ten or more bees were feeding simultaneously on a feeder.
Choice trials
We conducted four types of choice trials for each stingless bee colony (n = 3 trials per type per colony): nestmates, conspecific non-nestmates, honey bees and site-controls. Each trial had an odour-marking colony and a test colony, excluding the control. In nestmate trials, the same colony performed both roles.
Trials had two phases (Fig. 1B): in phase one, foragers at the marking colony and test colony were given 30 min to use the feeders in the training positions in front of their respective hives; in phase two, the feeder in front of the marking colony (now the marked feeder) was taken and placed alongside a clean unused feeder of identical appearance and food volume in front of the testing colony but at a distance one metre beyond the training feeder. At the same time, the test colony’s training feeder was removed and the trial session began. We recorded the number of test colony bees landing on either the marked or clean feeder for 20 min, catching all bees with an aspirator immediately upon landing to prevent recounting, and to prevent further odour deposition. We counted only bees that approached and landed when no other bees were on the feeders, to minimise the chance that foragers were using the visual cue of a preceding nestmate (a cue used by other stingless bees; Slaa et al. 2003); these uncounted bees were also aspirated at the feeders. We swapped the position of the marked and clean feeders every five minutes during trials to reduce any influence of wind direction, and alternated the starting position (left or right) of marked feeders between trials. Across all trials, the majority of forager visits occurred in the first 10 min of the 20-min test period (mean: 86%), as expected given that trained foragers were progressively captured as time elapsed. In site-control trials, we presented test colonies with a choice of two clean feeders to confirm that there was no arbitrary bias to select left-hand or right-hand feeders in the testing position for each colony. In all trials, we were careful to ensure both feeders were exposed to equal sunlight, and that no spills were made during feeder movements that might affect feeder choices.
For T. carbonaria and A. australis, we also conducted additional “extra-odour” trials, to assess whether foragers showed a general bias towards feeders with extra, or novel, odours. Such a bias is not expected for social bees, in which responses to flower odours are learnt (Reinhard et al 2004; McCabe and Farina 2009), though Australian stingless bees have not yet been tested in this respect. In “extra-odour” trials, we presented test colonies with a choice between a clean feeder and one in which the feeder site was scented with vanilla by adding four 2 μl drops of diluted vanilla extract onto the filter paper beneath the feeder (1:10 solution; Queen Natural Vanilla Extract, one drop per quarter of the circular paper). Clean feeders in these trials received drops of water only. We were unable to perform this trial type with T. clypearis, due to the time constraints of our fieldwork in this species’ native range of north-eastern Australia.
In all trial types, we handled feeders with gloves and cleaned them with 100% ethanol and soapy water between trials. For T. carbonaria trials, the number of marking bees visiting the feeder during the phase one (“marking phase”) was limited to 20 by using paint-pens to mark the thorax of 20 foragers and allowing only these painted bees to use the feeder (non-painted visitors were removed with an aspirator; see Table S1). Trials with T. clypearis and A. australis allowed an indeterminate number of bees to use feeders during the allocated time for phase one; for these species, typically around ten bees were on the feeder at any one time during this period but the total number of individual bees using the feeder in the marking phase was not recorded.
Analysis
We compared the number of bees landing on the marked and blank feeders in each trial to the number that might be expected by chance if the probability of choosing each feeder was 50%, using the binomial probability function (one tailed; following Nieh et al. 2004). To assess overall responses within trial types per species, we also used binomial tests to compare the number of all trials of a given trial type in which a majority of bees chose the marked feeder to that expected by chance if there were no preference for marked feeders (where chance predicts marked feeders are preferred in three of six trials). Finally, we used Chi-square tests for homogeneity of proportions to assess, per species, if the proportion of foragers choosing marked feeders was similar between the two colonies tested per species, and between all trials within and across colonies of the same type. This allowed us to detect whether colony-level effects were driving preferences in any trial type, and whether forager behaviour was consistent within colony. All analyses were conducted in SPSS ver. 20.
Results
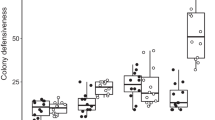
Stingless bee foragers in all three species (T. carbonaria, T. clypearis and A. australis) were attracted in higher numbers to feeders that had previously been used by their nestmates than clean unused feeders in all trials, consistent with these species depositing an odour that aids in recruitment (6 out of 6 trials, binomial test; P < 0.05 per species; Table 1; Fig. 2, Supplementary Tables S1–S3). Foragers were also more attracted to feeders recently used by either conspecifics of another colony, or by honey bees, than they were to clean unused feeders (6 out of 6 trials, P < 0.05 per trial type per species; Table 1; Fig. 2, Supplementary Tables S1–S3). Thus, these stingless bees can detect and use both intraspecific odours, and the odours of an introduced species (A. mellifera), in foraging decisions.
The proportion of foragers (± 95% C.I.) of three species of Australian stingless bee (Tetragonula carbonaria, Austroplebeia australis and Tetragonula clypearis) choosing odour-marked feeders over clean unused feeders in choice trials where marked feeders were those recently used by (i) nestmates, (ii) conspecifics from another colony, (iii) honey bees, or (iv) scented with vanilla. Trials with added vanilla were conducted for T. carbonaria and A. australis only. Numbers above bars indicate the total number of foragers for which choice was recorded across all six trials for a given species and trial type. Asterisks above bars indicate a significant preference for the marked feeder across all trials according to a binomial test
Among trials that presented a choice between two clean feeders, there was no significant bias towards left or right (P > 0.05 for all colonies; Supplementary Table S1–S3). In colonies of T. carbonaria and A. australis, for which “extra-odour” trials were conducted, there was also no preference for vanilla-scented feeders over unscented feeders, indicating foragers did not simply prefer more scented feeders (P = 0.31 per species; Table 1; Fig. 2; Supplementary Table S4).
For almost all trial types, the proportion of foragers choosing odour-marked feeders was homogenous across trials (chi-square tests of homogeneity, P > 0.05; Table 1; Supplementary Tables S1–S4); that is, foragers’ preferences were similar in magnitude between the two colonies tested per species, indicating no colony-level effect, and also similar in magnitude between each of the three trials per colony, indicating consistent responses by the foragers of each colony. The exception was A. australis responding to feeders used by nestmates; in this case, while there was a preference for used feeders in all trials, it varied significantly in magnitude between the six trials (range: 64–95% of foragers per trial chose the feeder recently used by nestmates; Supplementary Table S3). This variation might have arisen from variation in the numbers of foragers marking the feeder during the marking phase, where more marking bees would presumably elicit a stronger preference for used feeders in the test phase than fewer marking bees.
Discussion
We show that foragers of the Australian stingless bees T. carbonaria, T. clypearis and A. australis are attracted to the odours left behind at artificial food sources by the foragers of their own, or other conspecific, colonies. These species, therefore, join the cohort of Neotropical stingless bee species that have been shown to respond to the odours left behind by conspecific visitors under similar experimental conditions (Alavez-Rosas et al. 2017; Boogert et al. 2006; Hrncir et al. 2004; Koethe et al. 2020; Lichtenberg et al. 2009; Nieh et al. 2004, 2003; Schmidt et al. 2005; Villa and Weiss, 1990), consistent with this behaviour being ancestral to all meliponines, and perhaps all corbiculate bees (Saleh and Chittka 2006). Forager-deposited odours may be pheromone signals, chemical footprints or a combination of both (Jarau 2009). Whichever the case for our study species, the ability of foragers to detect both nestmate and non-nestmate odours confirms that their foraging decisions are responsive to the social information provided by conspecifics in their environment, and that colonies can influence the foraging behaviour of their neighbours indirectly via odours left at food sources.
In addition to being attracted to conspecific odours, we found that Australian stingless bees were also attracted to feeders recently used by, and thus carrying the odours of, an introduced species: the honey bee, A. mellifera. Although honey bees have been reported to use the nestmate-attracting pheromone Nasonov to mark artificial feeders or water sources (Free and Williams 1983; Williams et al. 1981), we did not observe honey bees in the marking phase of our trials performing characteristic Nasonov-scenting behaviour (raised and pulsating abdomen). The odours left on feeders by honey bees in our experiment were more likely therefore to be a footprint odour, such as a tarsal gland secretion (Bortolotti and Costa 2014). Indeed, previous studies have shown honey bees themselves are attracted to artificial feeders that have been recently walked on by their nestmates (Williams and Poppy 1997; Koethe et al. 2020). The ability to detect and respond to heterospecific foragers’ odours has been well documented among social insect species that have coevolved in a shared ecosystem (e.g. Lichtenberg et al. 2011; Stout and Goulson 2001; Vantaux et al. 2007; Menzel et al. 2010). In contrast, Australian stingless bees and honey bees have come into sympatry in only the last 200 years. While evolution can of course act on such timescales, our view is that an evolved receptivity to honey bee odours is unlikely to account for the behaviour of all three Australian stingless bee species studied here.
We consider three possible mechanisms to be candidate explanations for Australian stingless bees’ observed attraction to honey bee odours at food sources. First, stingless bees may learn to associate honey bee odours with food. Honey bees were present and abundant at all sites where our trials took place, thus stingless bee foragers in our trials may have learnt to associate honey bees’ food-marking odours with profitable resources during their natural foraging experiences gained prior to, or outside of, our experimental set-up. Social bees are adept at associative learning of odours, which is central to their foraging strategy (Hammer and Menzel 1995) and the odours of non-native heterospecifics are presumably no more challenging to learn than those of non-native flowers, which are also abundant in Australian cities and regularly visited by native bees. Both stingless bees (Roselino et al. 2016) and bumble bees (Ballantyne and Willmer 2012; Saleh and Chittka 2006; Witjes and Eltz 2006), as well as some ants (Menzel et al. 2010), have been shown to be capable of learning and predicting food reward levels from volatile food markings, and such fine-tuned responses to both conspecific and heterospecific forager odours is probably a widespread feature of social insect foraging. Learning would also be consistent with the ambivalence of foragers to vanilla-scented feeders in the “added-odour” trials with two of our study species (T. carbonaria and A. australis). Our test colonies had no opportunity for prior experience with vanilla and, therefore, did not associate it with a food reward. Importantly, under a learning scenario, stingless bees might equally learn to avoid honey bee odours if they were associated with poor resources, such as depleted flowers. That is, if learning explains the behaviour of stingless bees in our study, then we predict the effect of honey bee odours on stingless bee behaviour will vary depending on the learnt relationships between odour and food rewards in different contexts. Similarly, responses to honey bee odours may vary depending on whether or not they are accompanied by a scent that indicates an ongoing resource (e.g. honey) or a depletable resource (e.g. a flower).
A second possible explanation is that stingless bees’ attraction to honey bee odours arises from recognition error, because honey bee odours are similar to their own, or to those of other native biota for which their sensory systems and behaviour are adapted. In the case of recruitment pheromones, very high similarity between those of Australia’s stingless bees and honey bees is unlikely. Pheromone chemistry in bees varies significantly within genera (Lichtenberg et al. 2011), and even between colonies within species (John et al. 2012; Jarau et al. 2010), and behavioural studies indicate that bees can readily detect and respond to this intraspecific variation. Honey bees and stingless bees, meanwhile, diverged from a common ancestor 80 million years ago (Rasmussen and Cameron 2010) and excrete their recruitment pheromones from different glands (labial glands for many stingless bees, Jarau 2009; Nasonov glands for honey bees, Williams et al. 1981). The extent to which foragers could mistake own species’ footprint odours for those of other species, however, is less clear. Some chemical components of footprint cues are certain to be shared between all social bees; the footprints of bumblebees, honey bees and stingless bees are in all cases comprised mostly of long-chain alkanes and alkenes (Cassier and Lensky 1997; Jarau et al. 2004; Schmitt et al. 1991). Yet, as for pheromones, even small differences in footprint chemistry may be enough to elicit species-specific responses in foragers. A next step will be to assess the chemical profile of both honey bee and Australian stingless bees food-marking odours, in combination with behavioural studies of foragers’ responses to candidate compounds. Ideally, characterisation of stingless bee recruitment odours would include food sources placed at greater distances from the hive than those of our study (i.e. > 2 m), to account for the possibility that bees deploy different recruitment odours at larger foraging distances. Notably, even if foragers are not making recognition errors per se, similarity between the chemical profiles of odours of native and non-native species might facilitate more rapid learning, such that both chemistry and learning contribute to odour detection.
A third possible explanation for our data is that Australian stingless bees show a general preference for more odour over less odour when foraging, thereby preferring recently used feeders in our feeder trials. We consider this explanation the least likely, given that the use of floral odour in foraging decisions in other social bees is well documented to be acquired via previous exposure to the odour, either during foraging or in the nest (e.g. Reinhard et al. 2004; McCabe and Farina 2009). Furthermore, it is difficult to imagine that such a broad rule-of-thumb preference could be adaptive for social bees, given the vast array of flower and other odours present in the environment. Also consistent with this view, neither T. carbonaria nor A. australis showed a preference for feeders scented with vanilla over unscented feeders, though we cannot rule out that certain odours might elicit a general preference relative to no-odour feeders.
Will the ability of stingless bees to detect and respond to the odours of honey bees influence their foraging ecology in natural contexts? Honey bees are abundant throughout Australia and use the same broad range of floral resources as stingless bees (Heard 2016; Elliot et al. 2021). Stingless bees would, therefore, encounter their odours at flowers frequently. If the attraction to honey bee signals stems from simple recognition error, then the outcome for stingless bees is likely variable: they would sometimes benefit from recruitment to mutually profitable food, but they might also risk being diverted away from optimal forage, in cases where the needs of each species differed. Assuming that the attraction has been learnt from positive reinforcement, however, then it follows that stingless bees can benefit from this use of novel heterospecific social information through increased foraging efficiency. Relative to Australia’s stingless bees, honey bees fly earlier in the day and at cooler temperatures, have more foragers, and larger foraging ranges (Heard 2016; Smith et al. 2016). Provided stingless bees are not competitively excluded from shared resources, they might, thus, exploit honey bees’ superior search behaviour to find food quickly. Both species are often seen at the same flowers without obvious antagonism (Fig. 1A, Heard 2016), but whether forager-deposited odours play a role in these shared foraging decisions remains unclear. And as a learnt behaviour, stingless bees might equally benefit in some contexts from learning to avoid honey bee odours at natural forage, perhaps to avoid competition or depleted resources (Roselino et al. 2016; Saleh and Chittka 2006).
Ultimately, the influence of honey bee odours on stingless bee natural foraging success will depend on the interaction of many factors, including the relative preferences for own and heterospecific odours, additional cue and odour preferences (e.g. responses to other species in the environment), the volatility of food marks, food availability and the relative density of each species. These diverse factors remain to be investigated in future studies. For example, a relative preference for own species’ odours may ensure that intraspecific interactions dominate over interspecific ones. Assuming the food-marking odours of each species can be characterised in the future, such preferences could be best tested via trials that offer foragers a choice between feeders with equal doses of species-specific odours. The relative volatility of odours of each species will also affect their importance in foraging ecology. Footprints may be shorter range than some pheromones, and thus more likely to affect behaviour only on close approach to a food source. Likewise, relative dosages of food-marking odours depend on the abundance of foragers using food sources. In our trials, we show that for T. carbonaria, visits by twenty foragers was sufficient to produce an odour at the feeder detectable by subsequent foragers under our experimental conditions. Further work is needed, however, to determine exactly how intensively a food source must be marked before it influences forager preferences in natural conditions. Finally, whether honey bees show a reciprocal ability to detect and respond to stingless bee odour marks is also an open question, and will determine the extent to which social information at shared resources influences the interaction of these species.
The responses of native animals to the odours of introduced animals have been well studied in predator–prey contexts, particularly for vertebrates (Anton et al. 2020; Steindler et al 2018; Webster et al. 2018). For example, the failure of prey to detect or avoid the odour cues of novel mammalian predators is thought to be a key driver of native species population decline (Spencer 2002; Anton et al 2020). In light of our results, we propose that important interactions between native species and the odours of introduced species could also occur within a trophic level. That is, changes in the invisible landscape of foraging odours might be a key component of the impact of some introduced species on their adopted communities. Bees and ants are obvious candidates for such interactions, given their widespread use of odours to mark food. Even solitary bees are reported to odour-mark food sources (Frankie and Vinson 1977; Yokoi and Fujisaki 2007, 2008), and bees can detect the odours left at flowers by other nectar-feeding insects, including hoverflies (Reader et al. 2005) and ants (Ballantyne and Willmer 2012). In the case of bees, native species’ responses to heterospecific odours at flowers also have the potential for knock-on effects on pollination and thus plant communities, by changing insect flower visitation behaviour (Brittain et al. 2013).
Data availability
Raw data provided in Supplementary Material tables.
Code availability
Not applicable.
References
Alavez-Rosas D, Malo EA, Guzmán MA, Sánchez-Guillén D, Villanueva-Gutiérrez R, Cruz-López L (2017) The stingless bee Melipona solani deposits a signature mixture and methyl oleate to mark valuable food sources. J Chem Ecol 43:945–954. https://doi.org/10.1007/s10886-017-0886-0
Anton A, Geraldi NR, Ricciardi A, Dick JTA (2020) Global determinants of prey naivete to exotic predators. Proc R Soc B. https://doi.org/10.1098/rspb.2019.2978
Ballantyne G, Willmer P (2012) Floral visitors and ant scent marks: noticed but not used? Ecol Entomol 37:402–409. https://doi.org/10.1111/j.1365-2311.2012.01378.x
Bartareau T (1996) Foraging behaviour of Trigona carbonaria (Hymenoptera: Apidae) at multiple-choice feeding stations. Aust J Zool 44:143–153
Barth FG, Hrncir M, Jarau S (2008) Signals and cues in the recruitment behavior of stingless bees (Meliponini). J Comp Phys A 194:313–327. https://doi.org/10.1007/s00359-008-0321-7
Boogert NJ, Hofstede FE, Aguilar Monge I (2006) The use of food source scent marks by the stingless bee Trigona corvina (Hymenoptera: Apidae): the importance of the depositor’s identity. Apidologie 37:366–375. https://doi.org/10.1051/apido:2006001
Bortolotti L, Costa C (2014) Chemical communication in the honey bee. In: Mucignat-Caretta C (ed) Neurobiology of chemical communication. CRC Press/Taylor&Francis, Boca Raton
Brittain C, Williams N, Kremen C, Klein AM (2013) Synergistic effects of non-Apis bees and honey bees for pollination services. Proc R Soc B 280:20122767. https://doi.org/10.1098/rspb.2012.2767
Cassier P, Lensky Y (1997) The exocrine glands of the honey bees. In: Mizrahi A, Lensky Y (eds) Bee products. Springer, Boston. https://doi.org/10.1007/978-1-4757-9371-0
Danchin É, Giraldeau L-A, Valone TJ, Wagner RH (2004) Public information: from nosy neighbors to cultural evolution. Science 305:487–491. https://doi.org/10.1126/science.1098254
Elliot B, Wilson R, Shapcott A, Keller A, Newis R, Cannizzaro C, Burwell C, Smith T, Leonhardt SD, Kämper W, Wallace H (2021) Pollen diets and niche overlap of honey bees and native bees in protected areas. Basic Appl Ecol 50:169–180. https://doi.org/10.1016/j.baae.2020.12.002
Ferguson AW, Free JB (1979) Production of a forage-marking pheromone by the honeybee. J Apic Res 18:128–135
Frankie GW, Vinson SB (1977) Scent marking of passion flowers in Texas by females of Xylocopa virginica texana. J Kansas Entomol Soc 50:613–625
Free J, Williams IH (1983) Scent-marking of flowers by honeybees. J Apic Res 22:86–90
Giurfa M, Núñez JA (1992) Honeybees mark with scent and reject recently visited flowers. Oecologia 89:113–117. https://doi.org/10.1007/BF00319022
Goodale E, Beauchamp G, Magrath RD, Nieh JC, Ruxton GD (2010) Interspecific information transfer influences animal community structure. Trends Ecol Evol 25:354–361. https://doi.org/10.1016/j.tree.2010.01.002
Goulson D (2003) Effects of introduced bees on native ecosystems. Ann Rev Ecol Evol Syst 34:1–26. https://doi.org/10.1146/annurev.ecolsys.34.011802.132355
Hammer M, Menzel R (1995) Learning the memory in the honey bee. J Neurosci 15:1617–1630
Heard TA (2016) The Australian native bee book: keeping stingless bee hives for pets, pollination and sugarbag honey. Sugarbag Bees, Brisbane
Hopkins I (1886) Illustrated Australasian bee manual. Gordon & Gotch, Auckland
Hrncir M, Jarau S, Zucchi R, Barth FG (2004) On the origin and properties of scent marks deposited at the food source by a stingless bee, Melipona seminigra. Apidologie 35:3–13. https://doi.org/10.1051/apido:2003069
Jarau S (2009) Chemical communication during food exploitation in stingless bees. In: Jarau S, Hrncir M (eds) Food exploitation by social insects—ecological, behavioral, and theoretical approaches. CRC-Press, Boca Raton, pp 223–249
Jarau S, Hrncir M, Ayasse M, Schulz C, Francke W, Zucchi R, Barth FG (2004) A stingless bee (Melipona seminigra) marks food sources with a pheromone from its claw retractor tendons. J Chem Ecol 30:793–804. https://doi.org/10.1023/B:JOEC.0000028432.29759
Jarau S, Dambacher J, Twele R, Aguilar I, Francke W, Ayasse M (2010) The trail pheromone of a stingless bee, Trigona corvina (Hymenoptera, Apidae, Meliponini), varies between populations. Chem Senses 35:593–601. https://doi.org/10.1093/chemse/bjq057
John L, Aguilar I, Ayasse M, Jarau S (2012) Nest-specific composition of the trail pheromone of the stingless bee Trigona corvina within populations. Insectes Soc 59:527–532. https://doi.org/10.1007/s00040-012-0247-5
Koethe S, Fischbach V, Banysch S, Rejnartz S, Hrncir M, Lunau K (2020) A comparative study of food source selection in stingless bees and honeybees: scent marks, location, or color. Front Plant Sci 11:516. https://doi.org/10.3389/fpls.2020.00516
Lichtenberg EM, Hrncir M, Nieh JC (2009) A scientific note: foragers deposit attractive scent marks in a stingless bee that does not communicate food location. Apidologie 40:1–2. https://doi.org/10.1051/apido:2008073
Lichtenberg EM, Hrncir M, Turatti IC, Nieh JC (2011) Olfactory eavesdropping between two competing stingless bee species. Behav Ecol Sociobiol 65:763–774. https://doi.org/10.1007/s00265-010-1080-3
Mallinger RE, Gaines-Day HR, Gratton C (2017) Do managed bees have negative effects on wild bees? A systematic review of the literature. PLoS ONE 12:e0189268. https://doi.org/10.1371/journal.pone.0189268
McCabe SI, Farina WM (2009) Odor information transfer in the stingless bee Melipona quadrifasciata: effect of in-hive experiences on classical conditioning of proboscis extension. J Comp Phys A 195:113–122. https://doi.org/10.1007/s00359-008-0391-6
Menzel F, Pokorny T, Blüthgen N, Schmitt T (2010) Trail sharing among tropical ants: interspecific use of trail pheromones? Ecol Entomol 35:495–503. https://doi.org/10.1111/j.1365-2311.2010.01206.x
Moritz RFA, Hartel S, Neumann P (2005) Global invasions of the western honeybee (Apis mellifera) and the consequences for biodiversity. Ecoscience 12:289–301. https://doi.org/10.2980/i1195-6860-12-3-289.1
Nieh JC, Tautz J, Spaethe J, Bartareau T (1999) The communication of food location by a primitive stingless bee, Trigona carbonaria. Zoology 102:238–246
Nieh JC, Ramirez S, Nogueira-Neto P (2003) Multi-source odor-marking of food by a stingless bee, Melipona mandacaia. Behav Ecol Sociobiol 54:578–586. https://doi.org/10.1007/s00265-003-0658-4
Nieh JC, Barreto LS, Contrera FA, Imperatriz-Fonseca VL (2004) Olfactory eavesdropping by a competitively foraging stingless bee, Trigona spinipes. Proc R Soc B 271:1633–1640. https://doi.org/10.1098/rspb.2004.2717
Paini D (2004) Impact of the introduced honey bee (Apis mellifera) (Hymenoptera: Apidae) on native bees: a review. Austral Ecol 29:399–407. https://doi.org/10.1111/j.1442-9993.2004.01376.x
Rasmussen C, Cameron EC (2010) Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal. Biol J Linn Soc 99:206–232. https://doi.org/10.1111/j.1095-8312.2009.01341.x
Reader T, MacLeod I, Elliott PT, Robinson OJ, Manica A (2005) Inter-order interactions between flower-visiting insects: foraging bees avoid flowers previously visited by hoverflies. J Insect Behav 18:51–57. https://doi.org/10.1007/s10905-005-9346-8
Reinhard J, Srinivasan M, Zhang S (2004) Scent-triggered navigation in honeybees. Nature 427:411. https://doi.org/10.1038/427411a
Roselino AC, Rodrigues AV, Hrncir M (2016) Stingless bees (Melipona scutellaris) learn to associate footprint cues at food sources with a specific reward context. J Comp Phys A 202:657–666. https://doi.org/10.1007/s00359-016-1104-1
Saleh N, Chittka L (2006) The importance of experience in the interpretation of conspecific chemical signals. Behav Ecol Sociobiol 61:215–220. https://doi.org/10.1007/s00265-006-0252-7
Saleh N, Scott A, Bryning G, Chittka L (2007) Distinguishing signals and cues: bumblebees use general footprints to generate adaptive behaviour at flowers and nest. Arthropod-Plant Interact 1:119–127. https://doi.org/10.1007/s11829-007-9011-6
Schmidt VM, Zucchi R, Barth FG (2005) Scent marks left by Nannotrigona testaceicornis at the feeding site: cues rather than signals. Apidologie 36:285–291. https://doi.org/10.1051/apido:2005002
Schmitt U, Lübke G, Francke W (1991) Tarsal secretion marks food sources in bumblebees (Hymenoptera: Apidae). Chemoecology 2:35–40. https://doi.org/10.1007/BF01240664
Seppänen J-T, Forsman JT, Mönkkönen M, Thomson RL (2007) Social information use is a process across time, space, and ecology, reaching heterospecifics. Ecology 88:1622–1633. https://doi.org/10.1890/06-1757.1
Slaa EJ, Hughes WOH (2009) Local enhancement, local inhibition, eavesdropping, and the parasitism of social insect communication. In: Jarau S, Hrncir M (eds) Food exploitation by social insects: ecological, behavioura and theoretical approaches. CRC Press, Boca Raton, pp 147–164
Slaa JE, Wassenberg J, Biesmeijer JC (2003) The use of field-based social information on eusocial foragers: local enhancement among nestmates and heterospecifics in stingless bees. Ecol Entomol 28:369–379. https://doi.org/10.1046/j.1365-2311.2003.00512.x
Smith JP, Heard TA, Beekman M, Gloag R (2016) Flight range of the Australian stingless bee Tetragonula carbonaria (Hymenoptera: Apidae). Austral Entomol. https://doi.org/10.1111/aen.12206
Spencer RJ (2002) Experimentally testing nest site selection: fitness trade-offs and predation risk in turtles. Ecology 83:2136–2144
Steindler LA, Blumstein DT, West R, Moseby KE, Letnic M (2018) Discrimination of introduced predators by ontogenetically naïve prey scales with duration of shared evolutionary history. Anim Behav 137:133–139. https://doi.org/10.1016/j.anbehav.2018.01.013
Stout JC, Goulson D (2001) The use of conspecific and interspecific scent marks by foraging bumblebees and honeybees. Anim Behav 62:183–189. https://doi.org/10.1006/anbe.2001.1729
Stout JC, Morales CL (2009) Ecological impacts of invasive alien species on bees. Apidologie 40:388–409. https://doi.org/10.1051/apido/2009023
Vantaux A, Dejean A, Dor A, Orivel J (2007) Parasitism versus mutualism in the ant-garden parabiosis between Camponotus femoratus and Crematogaster levior. Insectes Soc 54:95–99. https://doi.org/10.1007/s00040-007-0914-0
Villa JD, Weiss MR (1990) Observations on the use of visual and olfactory cues by Trigona spp. foragers. Apidologie 21:541–545
Webster C, Massaro M, Michael DR, Bambrick D, Riley JL, Nimmo DG (2018) Native reptiles alter their foraging in the presence of the olfactory cues of invasive mammalian predators. R Soc Open Sci 5:180136. https://doi.org/10.1098/rsos.180136
Williams CS, Poppy GM (1997) Responses of individual honey bees to artificial feeders visited by themselves and to feeders visited by hivemates. J Apic Res 36:105–108. https://doi.org/10.1080/00218839.1997.11100935
Williams IH, Pickett JA, Martin AP (1981) The Nasanov pheromone of the honeybee Apis mellifera L. (Hymenoptera, Apidae). Part II. Bioassay of the components using foragers. J Chem Ecol 7:225–237
Witjes S, Eltz T (2006) Influence of scent deposits on flower choice: experiments in an artificial flower array with bumblebees. Apidologie 38:12–18. https://doi.org/10.1051/apido:2006048
Wojcik VA, Morandin LA, Adams LD, Rourke KE (2018) Floral resource competition between honey bees and wild bees: Is there clear evidence and can we guide management and conservation? Environ Entomol 47:822–833. https://doi.org/10.1093/ee/nvy077
Yokoi T, Fujisaki K (2007) Repellent scent-marking behaviour of the sweat bee Halictus (Seladonia) aerarius during flower foraging. Apidolgie 38:474–481
Yokoi T, Fujisaki K (2008) Recognition of scent marks in solitary bees to avoid previously visited flowers. Ecol Res 24:803–809. https://doi.org/10.1007/s11284-008-0551-8
Acknowledgements
We thank W. Forno, M. Damon and J. Groening for their assistance.
Funding
RG was supported by a University of Sydney Postdoctoral Fellowship. MB was supported by the ARC (FT120100120).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RG, JS and RS. The first draft of the manuscript was written by RG and JS and all authors commented on versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gloag, R., Smith, J.P., Stephens, R.E. et al. Australian stingless bees detect odours left at food sources by nestmates, conspecifics and honey bees. Insect. Soc. 68, 151–159 (2021). https://doi.org/10.1007/s00040-021-00823-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-021-00823-7