Abstract
In a social species like the honey bee (Apis mellifera), changes in foraging strategy require shifts in several groups of specialized workers that are involved in collecting, storing, and processing food. In cases of extreme food shortage, honey bee colonies can switch to a high-risk, high-reward foraging tactic known as honey robbing, which involves stealing mature honey from other colonies. Colonies engaged in honey robbing show a corresponding increase in defensive behaviors displayed by specialist guard bees, presumably because the conditions that provoke robbing also increase the risk of colony invasion. Previous studies suggest aggressive behaviors displayed by robbing forager nestmates modulate guard defensiveness. In the current study, we evaluated which aspects of the robbing experience likely alter forager aggression, and in turn, guard defensiveness. We trained colonies to visit feeders containing either raw honey or a sucrose solution and examined whether food type, experience of conflict at the feeder, or other abiotic cues that reflect the time of the season best explain variation in guard defensiveness. We found little evidence that food type influences forager interactions with guards. Rather, conflict at the feeder is the best predictor of increased aggressive interactions, even when accounting for the effects of seasonal change. Thus, intraspecific conflict at the food resource during robbing may drive shifts in individual forager aggression, activating guard defensiveness as one component of a syndrome of colony-level changes required to accommodate the robbing foraging tactic.
Significance statement
Honey bees possess an extreme foraging tactic that they employ under conditions of resource scarcity. This tactic, honey robbing, requires coordinated changes among worker bees to accommodate enhanced food collection, processing, and storing, as well as nest defense. In a previous study, we showed that robbing foragers show unusually high aggression, and that this shift may trigger greater defensiveness from nestmate guards once foragers return home. Here, we explored the cues that coordinate the change in defensive effort from guards and find that forager conflict at the food resource is a strong predictor of guard defensiveness. These results suggest that guards use behavioral cues from their own foragers to estimate their risk of attack and increase their defensiveness accordingly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foraging for food carries a variety of risks, including time and energy loss, predation, and competition for resources (Reader et al. 2005; Verdolin 2006; Berger and Gotthard 2008). Many animals change how they negotiate the risks and rewards of foraging depending on context, i.e., variation in individual state due to personal experience, body condition, or hunger level (van Alphen et al. 2003; Barnett et al. 2007; Skelhorn and Rowe 2007; Roitberg et al. 2010) or variation in environmental factors like food availability, predation threat, or perceived competition level (Bloxham et al. 2014; Couvillon et al. 2014b; Charalabidis et al. 2017). In social insects like the honey bee (Apis mellifera), colonies forage collectively, so foraging strategies reflect complex, context-dependent decision-making processes (Schmid-Hempel et al. 1985; Seeley 1989; Shafir et al. 2002; Higginson and Barnard 2004; Sasaki and Pratt 2018). For example, foragers save energy by exploiting high-value flowers close to the nest (Couvillon et al. 2014a), but when resources are scarce, they travel farther and accept lower quality food (Seeley 1989; Couvillon et al. 2014b). Foragers are also sensitive to predation risk, using a “stop signal” to discourage nestmates from visiting food resources where they have experienced a predator attack (Nieh 2010), but only when the benefits of risk avoidance outweigh the costs (Borofsky et al. 2020; Bell et al. 2021).
Honey robbing is a distinctive example of a high cost, context-dependent honey bee foraging strategy that becomes prevalent under conditions of food shortage (Free 1954; Downs and Ratnieks 2000; Garbuzov et al. 2020). During honey robbing, foragers switch from collecting nectar from flowers to invading neighboring honey bee colonies to steal mature honey (Free 1954). Robbing has unusual benefits because victim colonies are a consistent and reliable source of many kilograms of processed and concentrated nectar (i.e., honey). However, there are also extreme risks, including forager mortality and increased disease and parasite exposure (Couvillon et al. 2008; Peck and Seeley 2019). As is typical for a honey bee foraging strategy (Seeley and Tovey 1994; Seeley 1997), robbing does not just involve a shift in activity for foraging specialists. Rather, it is best characterized as a colony-level syndrome associated with a variety of changes that allow the workforce to collect and store high volumes of food while still providing adequate service for other tasks, including defense against potential attack from neighboring colonies (Rittschof and Nieh 2021). Sufficient nest defense is particularly critical, because the conditions that promote honey robbing simultaneously increase the risk of invasion by neighboring colonies (Garbuzov et al. 2020).
In a previous study, we found that colonies in the act of robbing simultaneously increase their defensiveness: guard bees (defensive specialists responsible for denying non-nestmates entry to the colony) become more aggressive towards their own foragers when the foragers return from a robbing trip (Grume et al. 2021). This surprising pattern could reflect the fact that the benefits of hypervigilance against potential robbing invaders sometimes outweigh the costs of nestmate rejection. However, the cues guards use to adjust their behaviors towards robbing nestmates remain unclear. Increased guard defensiveness towards robbing foragers could be a direct or indirect response to a cue acquired or experienced by the robbing forager herself. Honey bees use odors acquired from honeycomb to identify nestmates (Breed et al. 1995), a possible source of sensory confusion between guards and returning foragers during robbing. However, in our previous study (Grume et al. 2021), we found that odor cues acquired from foreign honeycomb do not seem to explain increased guard defensiveness; instead, robbing foragers have a brain molecular profile that resembles a high-aggression bee, suggesting the robbing experience induces a shift in forager aggression that may trigger guard defensiveness. In the current study, we investigate potential causes of increased forager aggression and guard defensiveness during robbing.
One conspicuous feature of robbing that could influence forager behavior and guard response is the type of food collected (stored honey versus floral nectar). There are many aspects of these two resources that differ. For example, high sugar concentration in honey, or the consistent and reliable source of sugar presented by a victim colony, could incentivize robbing activity during the fall when collecting and processing nectar into mature honey is highly costly (Garbuzov et al. 2020). Another critical difference between honey and nectar is the type and concentration of plant phytochemicals and plant volatiles (Gao et al. 2010; Mao et al. 2013; Carlesso et al. 2021; Njoroge et al. 2021), chemicals that could alter forager behavior and thus guard response. We designed the current study to evaluate whether the experience of foraging on honey may directly or indirectly provoke guard defensiveness once foragers return home. However, we also assessed two alternative hypotheses to explain shifts in guard defensiveness, based on previous studies on aggression modulation and robbing patterns in honey bees. The first alternative is that an experience of conflict at the food resource modulates guard defensiveness by enhancing forager aggression. This hypothesis is based on extensive previous studies showing that aggressive encounters with conspecifics alter, and often increase, individual aggression (Alaux and Robinson 2007; Alaux et al. 2009; Rittschof and Robinson 2013; Rittschof et al. 2015; Rittschof 2017), and that foragers sometimes act aggressively towards one another while robbing (Free 1954). The second alternative is that guards use external abiotic cues like day length or temperature variation to modulate their defensiveness, regardless of the cues perceived from the robbing foragers themselves. This hypothesis is based on previous studies showing that robbing activity reliably increases during the fall as the quantity of floral resources declines and the cost of producing honey increases (Garbuzov et al. 2020). This study represents an important step in determining how a robbing colony coordinates a multifaceted shift in foraging strategy to exploit a high-risk, high-value food resource.
Methods
Overview
To assess our primary hypothesis, that foraging on honey directly or indirectly alters guard defensive behaviors towards returning foragers, we trained experimental colonies to forage at a feeder that was filled with raw honey (~ 82% sugars) or an unscented sucrose solution (50% sugars) and monitored guard defensiveness towards foragers when they returned to their home colony. We assessed general guarding activity as well as behavior directed towards individually marked foragers that we confirmed were returning from the trained feeder. The unscented sucrose solution is a high-value reward, but one that is not as concentrated as honey, and devoid of the typical phytochemical compounds that characterize honey. Notably, both reward types are in high quantities in a feeder context compared to natural flowers, and so this design does not account for the differences in absolute food quantity and reliability that distinguish robbing from floral resource foraging. To assess our first alternative hypothesis, that aggressive interactions at the feeder shift forager aggression and thus guard defensiveness, we monitored foraging traffic at the feeder throughout the experiment and assessed whether feeder traffic experienced by foragers predicted guard defensiveness. To assess our second alternative, that abiotic seasonal cues influence guard behavior regardless of forage type and forager experience, we evaluated how guard defensiveness changed for experimental colonies over the course of the summer and fall, and how well seasonality explained variation in guard defensiveness compared to other factors.
We completed four rounds of the experiment (each round lasted 10 total days, see Fig S1 for timeline), using a unique colony for each experimental round. Colonies were healthy (not undergoing active treatment for Varroa mites) and full-sized. We selected a site for the experiment that was isolated from other colonies to minimize interference from other colonies at the feeder. We placed experimental colonies on-site four days before the experiment began (days 1–4) so foragers had plenty of time to acclimate to the new location.
On day 5 of the experiment, we trained foragers to visit an artificial feeder (detailed below). Over the next four days (days 6–9), we filled the feeder with honey or sucrose on alternating days and monitored colony foraging activity, the number of bees visiting the feeder, colony defensiveness displayed towards returning foragers, and the foraging activity and defensive attacks experienced by individually marked bees (all measures are detailed below). This design mimicked the opportunistic day-to-day switching between robbing and nectar foraging that promoted guard defensiveness in our previous study (Grume et al. 2021). Providing only one type of food at a time allowed us to use general measures of guard defensiveness and colony foraging activity, as well as measures for individually marked foragers. It also ensured that adequate numbers of foragers visited each food type.
After 4 days of testing (day 10), we moved the experimental colony to an outlying apiary 1 km away and introduced a new colony for the subsequent round of the experiment. Our rationale for the choice of 1 km is that a similar approach did not lead to interference from prior experimental colonies in a previous study (Grume et al. 2021). However, in that study, we did not train the bees to visit the feeder. In the current study, some foragers may return to the learned feeder site even after their colony has been moved, which could in part explain the presence of interfering bees in the study (see “Results”). We observed a single case of a marked bee returning to the feeder once a colony was relocated, but there were likely more unmarked bees that did so as well. We explicitly evaluate the possibility that conflict at the feeder (whether driven by the presence of non-nestmates or nestmates) influences guard defensiveness (see “Results”). It is also possible that some of these past foragers drifted into our present experimental colony; however, we never observed large numbers of bees or clusters of bees returning en masse to the new experimental colony, suggesting the move was far enough for the former experimental colony to adjust to its new location.
We conducted this experiment from July 15 to September 18, 2020, on the University of Kentucky North Research Farm (Lexington, KY). The experimental timeframe (July–September) captures the time of the season that transitions from relatively abundant floral resources in mid-summer (low honey robbing risk) to very low floral resource availability coupled with reduced time to process collected food prior to the onset of winter (very high honey robbing risk). We used colony order to explore the extent to which guard defensiveness increased across the rounds of the study, which might suggest a role for abiotic factors in modulating guard behavior. However, with only one experimental colony per round, we cannot rule out the possibility that colony-level variation in demography or genetic background, and perhaps some activity from neighboring or former colonies (see above), are responsible for the seasonal variation we observe. Due to the number of personnel involved in data collection and other logistical constraints, we were also unable to test multiple colonies simultaneously, which would have allowed us to assess any seasonal effects more confidently. For these reasons, our results should be interpreted cautiously.
Honey and sucrose feeder design
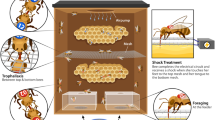
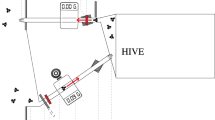
We built a gravity feeder to continuously supply honey or sucrose during training and testing (see below). The feeder consisted of a 0.5-L glass jar mounted on a round plastic base that was approximately 20 cm in diameter. The base had 6 troughs that were approximately 1 cm deep and 5 cm long extending out radially from the jar. There was another circular trough that connected the radial troughs (Fig S2). This design allowed several hundred bees to feed at once and made it relatively easy to paint individual bees on their thoraces as they fed. At the beginning of each experimental day, we filled the jar with either unscented sucrose (50% w/w) or raw honey derived from a bulk extraction of ~ 20 colonies during the previous year. This concentration of sucrose has been used in numerous field studies of honey bee foraging behavior (see Balderrama et al. 1992; Blatt and Roces 2001; Mujagic and Erber 2009; Eiri and Nieh 2016 among others). It is a ~ 10% higher concentration of sugar than is found in most floral nectars (Pamminger et al. 2019), thus making it possible to train the bees to the feeder in the presence of natural food sources, but it is much lower than the total concentration of sugars in honey (~ 82% w/w of sugars to water, with a distribution of sugars around 40/30/5 w/w/w glucose, fructose, and sucrose; Doner 1977; Aljohar et al. 2018). The feeder was placed on a small wooden table similar in height to the entrance of the hive (~ 20 cm high).
Feeder training
Although foraging honey bees can spontaneously discover artificial food resources, they will more reliably visit feeders if they are trained to the location and the time of day the feeder is present (Seeley 1997). Feeder training lasted three to four hours (depending on the amount of colony foraging activity) and was conducted in two stages: initiation and orientation.
During initiation, we loaded a 50% sucrose solution (w/w) into the feeder, which was placed on a platform at the same height as the colony entrance and directly in front of the colony. We then waited for foragers to spontaneously investigate the feeder. Once a small population of foragers (~ 6) landed on the feeder and collected food, we ended the initiation phase and moved on to the orientation phase. Because foraging food scouts must spontaneously locate the feeder, initiation is usually the longest stage of feeder training (Van Nest and Moore 2018). Although we did not precisely time the duration of the initiation stage, it was roughly equivalent across all colonies used in this experiment.
During the orientation stage, we progressively moved the feeder away from the colony in roughly 1-m increments. Between each move, we waited approximately five minutes to allow foragers to return to the feeder and collect food. Because foragers can visually locate the nearby feeder and begin to recruit nestmates to the food location over the course of this stage, we eventually began moving the feeder over larger increments (up to 10 m; Van Nest and Moore 2018). The final position of the feeder in this experiment was 20 m from the colony. Once the orientation stage was complete, the feeder was removed, but the platform was left in the same position overnight. At the beginning of each subsequent day of the experiment (days 6–9), we returned the feeder (filled with the appropriate food, see above) and allowed 30 min for the colony to rediscover the feeder before starting data collection. We used a random number generator to determine which food type was used on the first day of data collection, and then we alternated food sources on the following 3 days.
Data collection
Starting on experiment day 6, we collected data over a 1-h period between 1000 and 1200. We chose this time because it was consistently warm enough for flight and not so hot that foraging activity was inhibited. Data collection involved three observers. Two observers monitored the colony entrance for measures of colony foraging activity, defensiveness, return trip timing for individually marked bees, and defensive behaviors directed towards individually marked bees (information on individually marked bees is detailed below). These observers were blinded to the feeder treatment. The other observer was stationed at the feeder, where they painted individual foragers with unique identifying marks, recorded the timing of visits from these foragers to the feeder, and tallied feeder bee traffic. This observer could not be blinded to the feeder treatment. Colony foraging activity, colony defensiveness, and feeder bee traffic measurements were taken every 10 min over the 1-h observation period (N = 6 measures for each experimental day per colony). Colony foraging activity and feeder foraging activity were measured at the same time, while colony defensiveness was staggered 5 min behind. For colony foraging activity and feeder bee traffic, we counted the number of foragers that entered the colony, or the number of foragers present at the feeder over a 1-min period. Colony defensiveness measures followed approaches from previous studies (Grume et al. 2021; detailed below).
Colony defensiveness measurements
Previous studies show that increased colony defensiveness in the robbing context is usually the result of an increased frequency of defensive actions by individual guards, not an increase in the total number of individuals acting as guards (Grume et al. 2021). We thus recorded behaviors displayed by guards as a group rather than track individuals. During each 1-min observation period, we quantified the defensiveness of the guards by recording five different types of behaviors that escalate in severity (Richard et al. 2012; Preston et al. 2019; Grume et al. 2021). The mildest defensive behavior is antennation, in which guard bees rub their antennae on returning foragers and use scent cues to determine if the foragers are nestmates or non-nestmate intruders (Moore et al. 1987). The next behavior is antennation with open mandibles, a threat behavior. The next behavior is biting, where guards bite the legs, antennae, or abdomen of forager bees. Abdomen flexion is the next most severe behavior, where a guard grabs the forager and flexes her abdomen without extruding her stinger. Stinging is the most severe of the guard behaviors, in which the stinger is extruded in an attempt puncture the cuticle of the other bee. We weighted the tallies of these behaviors based on their severity with antennation receiving a one and stinging receiving a five. We then summed all tallies for an overall measure of colony defensiveness (Richard et al. 2012; Li-Byarlay et al. 2014; Rittschof et al. 2015). This weighted sum approach has some drawbacks, namely that it assumes a linear relationship among behaviors in terms of their relative severity. However, because almost all behaviors we observed were the first level, antennation (see “Results”), such an issue will have minimal effects on the inferences of this study.
Individually marked forager behavioral measurements
In addition to our general measures of colony foraging activity and defensiveness, we marked and followed individual foragers to assess how feeding experience impacted the likelihood of a guard response. We also used individual data to calculate the time elapsed between feeder visits, which we predicted would increase with decreasing resource value and increasing resource competition (Seeley 1994).
We tracked five individual bees per colony because we could successfully mark and keep track of this number consistently, even on days with heavy foraging activity. On the first day of the experiment, we paint-marked five foragers with model paint (The Testor Corporation, Rockford, IL, USA) while they collected food at the feeder. We selected foragers that appeared healthy but experienced (no obvious deformities or behavioral signs of illness, but with mild wing wear); we reasoned that these foragers were most likely to make repeated trips to the feeder (Prado et al. 2020). Once marked, we recorded the time of day associated with each subsequent feeder visit and colony return visit, as well as whether the individual experienced any defensive behaviors from guards during each return visit. We scored guard behaviors as described for colony-level measurements. However, because defensive behaviors were relatively rare and always low level antennation or antennation with open mandibles, we analyzed defensiveness as a binomial (“yes” or “no”) regardless of the number and type of defensive behaviors encountered. We did not observe any clear instances of aggressive behaviors displayed by individually marked bees towards guards, nor did we observe casting behaviors typical of robbing bees (Free 1954).
Data processing
Although feeder bee traffic and colony foraging activity measures were taken simultaneously, other behavioral measures at the individual and colony levels were not always perfectly matched temporally. To evaluate relationships among colony defensiveness, colony foraging activity, and feeder bee traffic, as well as colony foraging activity, colony defensiveness, and guarding behaviors directed towards individually marked bees, we matched up the temporally closest recordings of each factor. Colony defensiveness measures were 5 min apart from colony foraging measures and feeder traffic measures (see above), and the timing of these measurements relative to individual bee observations varied with individual trip timing. Overall, there was an average of a 5-min difference between colony foraging activity measurements and observations of individual foragers at the colony entrance, and an average of a 4-min difference between colony defensive measures and observations of individual foragers returning to the colony and interacting with guards. Because we hypothesized that feeder bee traffic may lead to aggressive interactions among foragers, altering the response of guards once the foragers returned home, we analyzed feeder bee traffic measures that directly preceded the marked individual’s return home. We worked with two separate datasets and built different models for analyses of colony-level and individual patterns because the measurement frequency was greater when tracking individuals. These results are discussed separately. Notably, the colony-level measures that contribute to both datasets are the same.
Statistics
Data analyses were performed in R version 4.1.1. Statistical tests and data transformations, where relevant, are listed in the appropriate location in the “Results” section. Mixed model analyses, including linear mixed models (LMM) and generalized linear mixed models (GLMM) were performed using lme4::lmer and lme4::glmer, respectively. We assessed overall model fits by examining residual distributions using qqplots and histograms (DHARMa::simulateResiduals or the residuals function in base R). For analyses of colony-level factors (colony defensiveness, colony foraging activity) we used GLMMs with a Poisson distribution including experimental day as a random effect because measurements for the same treatments were repeated across multiple days for each colony. For analyses of individually marked foragers, we used binomial GLMMs and the unique forager identity as a random effect. Where possible, we included experiment day as an additional random effect, as some individuals were assessed over multiple days. However, due to the relatively small number of individuals tracked (and variation as to whether they were followed over one or multiple days), a model with experiment day often resulted in a singular fit warning, and this factor was eliminated in these cases. To assess seasonal effects on behavior, we treated colony identity (1–4) as an ordinal predictor of variation in our response factors above. We made figures using the R package ggplot2.
Results
Hypothesis 1: Returning foragers fed honey provoke guards
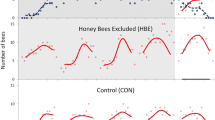
Similar to previous studies (Harrison et al. 2019; Grume et al. 2021), antennation made up over 90% of observed guard defensive behaviors, followed by 4% each for antennation with mandibles open and biting, and less than 1% each for abdomen flexion and stinging. We found no evidence that foraging on honey is sufficient to induce changes in guard defensiveness (Fig. 1). An analysis of feeder treatment (honey versus sucrose) on a per colony basis showed significant treatment differences for colony 4 (LMM, X21 = 10.5, P = 0.001), a non-significant trend for colony 2 (LMM, X21 = 3.6, P = 0.06) and no differences for colony 1 (LMM, X21 = 0.01, P = 0.9) and colony 3 (LMM, X21 = 0.01, P = 0.9). In both cases of significant (or nearly significant) treatment differences, colony defensiveness was higher for the sucrose treatment, opposite of the prediction if honey is the cue that elevates forager aggression and/or provokes guards during robbing. We found similar results when we assessed whether individual foragers were attacked upon return to the colony following a feeder visit (Fig. 2, colony 1: X21 = 0.3, P = 0.6, colony 2: X21 = 0.7, P = 0.4, colony 3: X21 = 0.3, P = 0.6, colony 4: LMM, X21 = 9.7, P = 0.002).
Treatment and season related changes in colony defensiveness. We assessed colony defensiveness every 10 min over a 1-h observation period for 2 days per treatment per colony. We tallied all defensive behaviors displayed towards incoming foragers regardless of forager identity. We report a weighted measure of colony defensiveness that considers the severity of various defensive behaviors (see “Methods”). Pairwise analyses show treatment differences in defensiveness for colony 4 only, and no evidence overall that feeding on honey increased colony defensiveness towards returning bees. An LMM analysis showed main effects of treatment, colony number (a measure of season, see “Methods”), and their interaction on colony defensiveness, suggesting that colony defensiveness increased significantly over the course of the season, but with some variation as a function of treatment
Variation in the frequency with which an individually marked forager provoked a guard by treatment and across colonies. We found that returning foragers were more likely to provoke aggressive behaviors from guards as the season progressed, but there was little evidence that an experience of foraging on honey increased the probability of provoking a guard
Hypothesis 2: Aggressive interactions at a food source predict elevated guard defensiveness
We recorded tallies of the number of bees present at the feeder (“feeder bee traffic”) which provides a measure of competition at the food resource. We evaluated whether feeder bee traffic was correlated with the degree of colony defensiveness or the probability that an individual forager would provoke a guard response upon return to the colony.
All colony-level analyses used GLMMs. Because we expected that the main source of bees at the feeder was our trained focal experimental colony, and because foraging traffic at the colony entrance predicts guard defensiveness to some extent (Grume et al. 2021), we first evaluated the relationship between feeder bee traffic and colony foraging activity. Treatment (honey versus sucrose) predicted significant variation in both colony foraging activity (X21 = 13.6, P = 0.0002) and feeder bee traffic (X21 = 7.5, P = 0.006), so we analyzed the relationship between colony foraging activity and feeder bee traffic separately for each treatment level. We found that feeder bee traffic and colony foraging activity were uncorrelated for honey (honey: X21 = 1.2, P = 0.28) and weakly correlated for sucrose (X21 = 4.0, P = 0.04), suggesting that feeder traffic was influenced by nearby colonies in addition to our focal colony. We then evaluated how colony foraging traffic, feeder bee traffic, and their interaction impacted colony defensiveness in a single model, separated by treatment.
For sucrose, where there was a greater range of feeder bee traffic, we found significant positive effects of feeder bee traffic and colony foraging traffic on colony defensiveness (colony foraging traffic: X21 = 4.5, P = 0.03, feeder bee traffic: X21 = 33.9, P < 0.00001, interaction: X21 = 2.6, P = 0.11). We assessed variance inflation factors to verify they were low enough (~ 1) to minimize impacts of collinearity between colony foraging traffic and feeder bee traffic in this analysis (see above). A plot of colony foraging activity, feeder bee traffic, and colony defensiveness (Fig. 3) suggests feeder bee traffic is a consistent positive predictor of colony defensiveness across different levels of colony foraging traffic. In contrast, the relationship between colony foraging traffic and colony defensiveness is weaker and somewhat inconsistent. Specifically, there are high levels of colony defensiveness at intermediate levels of colony foraging traffic, corresponding to high levels of feeder bee traffic. This pattern, indicative of feeder visitors coming from other nearby colonies, was largely driven by the last experimental period (Sept. 15, Col. 4, Fig S3). Colony 4 (Sept. 15) also showed higher defensiveness at intermediate levels of feeder bee traffic (Fig S3), which could indicate a greater forager aggressive response to non-nestmates at the feeder (prompting a greater reaction from guards upon return). Another possibility is that there is some periodic intrusion to the home colony by foreign honey bees that is not captured by our data collection methods, although in a prior study we showed that increased colony defensiveness with robbing activity occurs even without the presence of intruders (Grume et al. 2021). The same analysis of colony defensiveness for honey showed no effect of any factor, possibly because feeder bee traffic was substantially lower for this treatment (colony foraging traffic: X21 = 0.46, P = 0.50, feeder bee traffic: X21 = 2.1, P = 0.15, interaction: X21 = 2.8, P = 0.09).
Colony defensiveness significantly increases with the number of bees encountered at the treatment feeder. Plots show data for the sucrose treatment only, where there were significant main effects of feeder bee traffic (top) and colony foraging traffic (bottom) on colony defensiveness. The unusual elevation in colony defensiveness at intermediate levels of colony foraging traffic was largely attributable to colony 4 (Fig S3). This pattern could indicate the presence of bees from other colonies, as data were collected during the most competitive time of year for food resources
We also assessed how feeder bee traffic and colony foraging traffic impacted guard defensive behaviors directed towards individually marked bees (Fig. 4). For the individual forager dataset (see “Methods”), we again assessed the correlation between feeder bee traffic and colony foraging traffic on a per treatment basis. These factors were uncorrelated for honey (LMM, X21 = 0.43, P = 0.51) but strongly correlated for sucrose (LMM, X21 = 38.8, P < 0.0001). GLMM analysis for honey with feeder bee traffic, colony foraging traffic, and their interaction as fixed effects showed that feeder bee traffic significantly increased the frequency of defensive behavior by guards (GLMM, honey: X21 = 4.0, P < 0.05), with no impact of colony foraging activity (X21 = 1.9, P = 0.2) or the interaction term (X21 = 3.4, P = 0.052). For sucrose (where feeder bee traffic and colony foraging traffic were strongly correlated), we performed two GLMMs and found a significant positive effect of feeder bee traffic on the frequency of defensive behaviors by guards (X21 = 15.4, P < 0.0001) and no effect of colony foraging traffic (X21 = 0.9, P = 0.35). Taking our colony and individual responses together, our results support the hypothesis that robbing foragers may alter their behavior and become more likely to provoke guards due to their interactions with other bees at the food source.
Returning foragers were more likely to provoke guards as feeder bee traffic increased. Plots show whether a forager provoked a guard defensive response as a function of the bee traffic at the feeder experienced just prior to returning to the colony. Each dot represents an individual foraging trip, and dots are colored by the level of foraging traffic at the colony entrance nearest the time at which the forager returned. Trend lines indicate the sigmoidal function of a logistic regression, while shading indicates the standard error around this regression line
Finally, we evaluated whether feeder bee traffic (log transformed to optimize model fit) predicted the latency for individual foragers to return to the feeder, which could be evidence of suppressed foraging at the individual level. We found no significant relationship between feeder bee traffic and the latency to return to the feeder (GLMM, honey: X21 = 2.5, P = 0.11, sucrose: X21 = 3.8, P = 0.051). Feeder bee traffic was generally inversely correlated with the latency to return to the feeder (Fig S4), which could suggest foragers are responding positively to the quality of the food resource as opposed to negatively to the risk of attack.
Hypothesis 3: Guards use seasonal abiotic cues to tune their defensiveness to robbing risk
Because we assessed behavior for four different colonies serially between mid-July and mid-September, we used colony number as an ordinal variable that corresponds to time of the season. An LMM for colony defensiveness with colony number, treatment, and their interaction as fixed effects showed that all fixed effects significantly predicted variation in colony defensiveness (LMM, colony number: X21 = 11.4, P = 0.0007, treatment: X21 = 5.5, P = 0.02, colony number × treatment: X21 = 8.8, P = 0.003; Fig. 1). We performed a similar analysis where we used the actual week of the experiment (1–9.9) as opposed to colony number as an indicator of season (to account for the fact that there were variable time gaps between rounds of the experiment). This analysis gave very similar results (data not shown).
An assessment of defensive behaviors displayed towards marked individual bees gave similar results to the colony-level data but with no significant effect of treatment (GLMM, colony number: X21 = 5.2, P = 0.02, treatment: X21 = 0.004, P = 0.95, colony × treatment: X21 = 5.4, P = 0.02, Fig. 2). The significant interaction effect is a result of colony 4, where no individuals experienced defensiveness after foraging on honey. Notably, feeder bee traffic is also significantly correlated with colony number and thus season (LMM with experiment day as a random effect, X21 = 6.6, P = 0.01, Fig S3), suggesting competitive interactions at the food source may drive what appears to be a seasonal effect.
Discussion
Previous work showed that foragers returning from a robbing trip provoked heightened defensiveness from guard bees at their home colony, likely due to changes in forager aggression (Grume et al. 2021). In the current study, we assessed whether feeding on honey or interacting with conspecifics at a food source drive this shift in forager-guard aggression, or if guards are using private information like seasonal cues to modulate their behavior in response to robbing risk. The strongest pattern we observed was that guard bees were more defensive towards returning forager nestmates when there was a high level of bee traffic at the food source the foragers visited. This result, consistent whether we assessed colony-level patterns or defensive behaviors directed towards individually marked foragers, indicates that conflict at a food source gives rise to changes in forager aggression and guard response, possibly explaining the behavioral patterns associated with robbing. We found no evidence that food resource type consistently played a role in these patterns. Moreover, although guard defensiveness increased as the season progressed, feeder bee traffic also increased over this time frame; combined with our limited colony-level replication, we cannot definitively disentangle the role of seasonal cues in the aggression dynamics we observed.
Honey bee colonies use odor cues to identify nestmates, behaving defensively towards individuals they identify as non-nestmates (Breed 1983; Breed et al. 1988). Though some early studies suggested that floral odors acquired during feeding trips alter guard nestmate recognition abilities and thus defensive behaviors (Ribbands et al. 1952; Wilson 1971), more recent work, especially studies investigating relatively long-term exposure to food odors, have not supported this idea (reviewed in Downs et al. 2000, 2001). In our current study, we assessed whether switching from honey to sucrose day to day was sufficient to induce a change in guard behavior (presumably due to a shift in forager aggression). Our findings largely agree with more recent previous work showing that food type alone is not enough to alter guard behavior (only one colony showed differences in defensiveness as a function of food type). In the robbing context, this outcome suggests foraging on honey is not sufficient to cause an increase in aggression in forager bees. It is important to note that in the current study, both the sucrose and honey treatments represent high-value food rewards, and honey and nectar differ in other ways that are not represented in the current study design (discussed below, see also “Methods”). Though we cannot rule out the possibility that honey provides important aggression-modulating information to forager bees, our results suggest that direct competition at the food source may be the more critical cue. Future studies could consider other ways that odor cues, like accumulation of alarm pheromone from aggressive interactions between foragers at a food source, may influence guard behavior.
While honey stores at victim colonies draw in large numbers of foragers during a robbing event, we found that honey is not sufficient to either attract large numbers of foragers or provoke guard defensiveness once those foragers return home. In contrast, one of four experimental colonies showed evidence that foraging on sucrose, rather than honey, elevated guard defensiveness. Higher attraction of bees to the sucrose feeder may be partially responsible for the treatment difference in colony defensiveness (discussed below). However, compared to the other three experimental colonies, this colony also had higher than expected defensiveness in relationship to both feeder bee traffic and colony foraging traffic, which could indicate an additional factor is impacting forager and/or guard behavior. This colony was assessed during the time of the year when robbing is common. Some seasonal increase in invasion threat (Garbuzov et al. 2020) or forager drift, e.g., outside our observation window, could have led to increased defensiveness. Because bees from other colonies were also more likely to visit the feeder at this time of year (indicated by increased feeder bee traffic without a concomitant increase in colony foraging traffic), another possibility is that competing with non-nestmates at the feeder has a greater impact on forager aggression than competing with nestmates.
Our results showing greater traffic at the sucrose versus honey feeder stand in contrast to our previous study showing heavy foraging traffic directed at victim colonies housing unprotected full honeycomb frames, along with very little activity at sucrose feeders placed at the same site on different days (Grume et al. 2021). This discrepancy between current and prior results could be explained by variation in study design, the desired foraging target, and/or forager motivation and resource profitability. In the current study, we trained foragers to the feeder location as opposed to relying on them discovering the food resource spontaneously (Grume et al. 2021). It is possible that the large disparity in bee visitation between the sucrose feeder and full honeycomb frames in our previous work reflected an ability to locate unguarded honey in a natural colony context versus sucrose in an artificial feeder, an ability that training overcame in the current study. Notably, we also trained foragers using sucrose, which may have resulted in a preference for this food type. Finally, training may have enhanced the memory of the highly profitable feeder, regardless of treatment, hindering the foragers’ ability to distinguish between the honey and sucrose treatments.
Foraging preferences could explain the elevated attraction to sucrose: it could indicate water seeking behavior, which may be particularly relevant during drought conditions (Núñez and Giurfa 1996; Pankiw et al. 2001). Sugar preferences could also play a role: while honey has a higher overall sugar concentration (~ 82%), it also has much more glucose and fructose compared to the sucrose feeder (~ 40% glucose, 35% fructose, 5% sucrose, and 20% water by weight; Doner 1977; Aljohar et al. 2018). Honey bees sometimes show a preference for sucrose over glucose or fructose when the concentrations are matched (Waller 1972), and they can have a stronger learned association with sucrose (Simcock et al. 2018). Finally, foraging on raw honey has costs: honey has a much greater viscosity and stickiness compared to sucrose, and high flow rates (a characteristic of the lower concentration sucrose solution) lead to higher recruitment and visitation (Núñez and Giurfa 1996). We also observed that many honey foragers needed to groom themselves before being able to fly back to the colony. It is possible that the bees were less motivated to return to the honey feeder due to a perception of a diminished reward value because of the extra time and effort required to collect it (Seeley 1986); these foragers may also exhibit different recruitment signaling towards naïve foragers as a result of this experience (Borofsky et al. 2020).
Robbing activity changes with food availability and the approach of winter, and correspondingly, colonies are more defensive, presumably in response to robbing attacks or other ecological cues that indicate robbing risk (Downs and Ratnieks 2000; Garbuzov et al. 2020). Such a pattern predicts increased defensiveness over the course of our experiment, and possibly that guard and forager behavior are driven by experiences unmeasured in the experiment (e.g., a history of robbing attack or an experience of failing to find floral resources). Although we found that guard defensiveness increased over the course of the season, this shift was likely driven, at least in part, by competitive interactions at the experimental food source, which also increased. Colony defensiveness shifted day to day for at least one colony as we switched the food treatment, suggesting plasticity in defensiveness even within a short seasonal time window. Thus, though broad seasonal patterns (particularly the late-season nectar dearth) may drive increased traffic at food sources including robbed victim colonies (Downs and Ratnieks 2000; Garbuzov et al. 2020), it appears unlikely that seasonal cues exclusively alter forager aggression or guard defensiveness. There is likely also important day-to-day variation in colony defensiveness that could reflect acute resource competition.
Because guard defensiveness is associated with feeder bee traffic in our experiment, at least some of that traffic originated from nearby colonies, and experimental colonies were relatively close to the feeder, an alternative explanation for our results could be that experimental colonies experienced robbing threats from foragers from other colonies, leading to increased guard defensiveness. Attempted robbing and colony invasion are characterized by increased foraging activity at the entrance of the victim colony, as a proportion of the entering foragers are robbers recruited by nestmates to the victim colony. However, we found weak evidence that foraging activity at the colony entrance predicted guard behaviors, suggesting an increased volume of entering bees, whether they were nestmates or invaders, was not sufficient to increase guard defensiveness. Traffic at the feeder, irrespective of colony foraging activity, was a much stronger predictor of guard defensiveness. In our previous study where we showed increased guard defensiveness during a robbing event, we used automated marking to show there was little, if any, interference from neighboring colonies at our experimental sites (Grume et al. 2021). Thus, it seems unlikely that interfering invaders to the experimental colony explain the patterns of guard defensiveness we observed. As discussed above, attempted invasions by nearby colonies could have occurred outside of our observation window and may explain some portion of the stable shifts in defensiveness that occurred over the season.
Our data suggesting that forager aggression increases with forager competition with conspecifics at a food resource follows a robust pattern of experienced-induced plasticity in aggression in honey bees (Alaux and Robinson 2007; Alaux et al. 2009; Rittschof and Robinson 2013; Rittschof et al. 2014; Shpigler et al. 2017; Herb et al. 2018). Honey bee aggression, typically performed in the context of nest defense, is sensitive to social and ecological information experienced throughout an individual worker bee’s life (Rittschof et al. 2015). This plasticity presumably allows honey bees to optimize their investment in costly nest defense activities (Rivera-Marchand et al. 2008), and to defend the colony collectively (Breed et al. 1990; Guzman-Novoa and Page 1994). Because nest defense involves a specialized subset of worker bees (Breed et al. 1990), these individuals are thought to be most sensitive to aggression-modulating information. However, robbing studies suggest that non-defensive specialists (foragers) also adjust their aggression in response to environmental information (Grume et al. 2021; Rittschof and Nieh 2021). Our study thus emphasizes first that there are contexts beyond nest defense that may require plasticity in aggression, and second, that individuals other than defensive specialists adjust their aggression in response to personal experience.
It is interesting to note that our studies as well as previously published robbing studies (Free 1954) suggest that foragers may show elevated aggression even after interacting with their own nestmates at a food source. This raises an important unexplored role of context in nestmate recognition and response. Extensive studies show that guard bees use ecological context and personal/colony experience to modulate their defensiveness towards non-nestmates, being more permissive to entry when robbing threats and experiences are reduced (Downs and Ratnieks 2000; Couvillon et al. 2008). In these cases, guards presumably identify but ignore non-nestmates. However, foragers visiting heavily trafficked resources may be unable to correctly identify nestmates using typical olfactory cues, or they may respond to competitor presence using visual or tactile cues, which supersede or override any olfactory information that identifies a nestmate. Indeed, Free (1954) observed that the presence of fighting bees at a colony entrance is sufficient to induce casting, a robbing forager flight behavior, suggesting foragers respond to visual cues at a distance. Though we did not observe casting at either the feeders or our experimental colonies, Free’s observations nonetheless suggest that foragers can modify their behavior without physically contacting another bee. Perhaps in addition to transitioning to a robber-typical entry behavior, foragers, upon observing a contested resource, become more aggressive in preparation for fighting, a response that may be agnostic to nestmate identity.
Because colonies recruit nestmates to robbing targets, nestmate encounter rate is likely high during a robbing event, and thus ignoring nestmate foragers could come at some cost. How or why colonies tolerate this and other costs could be related to the value of the food resource. For example, foragers recruit nestmates to a victim colony during robbing, even after experiencing the cost of being attacked by the resident bees (Rittschof and Nieh 2021), which would typically slow recruitment, even to a feeder (Lau and Nieh 2010; Nieh 2010). Perhaps seasonal context, difficulty finding food, or food quality alters the tendency to recruit, or to perform recruitment inhibiting stop signals (Borofsky et al. 2020; Bell et al. 2021). We found that latency to return to the feeder was inversely correlated with traffic at the food source, suggesting resource scarcity may override conflict avoidance. These possibilities are the subject of on-going studies. To address these questions, it will be important to directly observe aggressive interactions between foragers at the feeder, an important piece of information we did not include in the current study.
Data availability
Raw data is publicly available: https://figshare.com/articles/dataset/Napieretal_RawData_xlsx/21953345
References
Alaux C, Robinson GE (2007) Alarm pheromone induces immediate-early gene expression and slow behavioral response in honey bees. J Chem Ecol 33:1346–1350
Alaux C, Sinha S, Hasadsri L, Hunt GJ, Guzman-Novoa E, Degrandi-Hoffman G, Uribe-Rubio JL, Southey BR, Rodriguez-Zas S, Robinson GE (2009) Honey bee aggression supports a link between gene regulation and behavioral evolution. Proceed National Acad Sci USA 106:15400–15405
Aljohar HI, Maher HM, Albaqami J, Al-Mehaizie M, Orfali R, Orfali R, Alrubia S (2018) Physical and chemical screening of honey samples available in the Saudi market: an important aspect in the authentication process and quality assessment. Saudi Pharm J 26:932–942
Balderrama NM, de Almeida LOB, Nunez JA (1992) Metabolic rate during foraging in the honeybee. J Comp Physiol B 162:440–447
Barnett CA, Bateson M, Rowe C (2007) State-dependent decision making: educated predators strategically trade off the costs and benefits of consuming aposematic prey. Behav Ecol 18:645–651
Bell HC, Hsiung K, Pasberg P, Broccard FD, Nieh JC (2021) Responsiveness to inhibitory signals changes as a function of colony size in honeybees (Apis mellifera). J R Soc Interface 18:20210570
Berger D, Gotthard K (2008) Time stress, predation risk and diurnal–nocturnal foraging trade-offs in larval prey. Behav Ecol Sociobiol 62:1655–1663
Blatt J, Roces F (2001) Haemolymph sugar levels in foraging honey bees (Apis mellifera carnica): dependence on metabolic rate and in vivo measurement of maximal rates of trehalose synthesis. J Exp Biol 204:2709–2716
Bloxham L, Bateson M, Bedford T, Brilot B, Nettle D (2014) The memory of hunger: developmental plasticity of dietary selectivity in the European starling, Sturnus vulgaris. Anim Behav 91:33–40
Borofsky T, Barranca VJ, Zhou R, von Trentini D, Broadrup RL, Mayack C (2020) Hive minded: like neurons, honey bees collectively integrate negative feedback to regulate decisions. Anim Behav 168:33–44
Breed MD (1983) Nestmate recognition in honey bees. Anim Behav 31:86–91
Breed MD, Garry MF, Pearce AN, Hibbard BE, Bjostad LB, Page RE (1995) The role of wax comb in honey bee nestmate recognition. Anim Behav 50:489–496
Breed MD, Robinson GE, Page RE (1990) Division of labor during honey bee colony defense. Behav Ecol Sociobiol 27:395–401
Breed MD, Williams KR, Fewell JH (1988) Comb wax mediates the acquisition of nest-mate recognition cues in honey bees. Proc Natl Acad Sci 85:8766–8769
Carlesso D, Smargiassi S, Pasquini E, Bertelli G, Baracchi D (2021) Nectar non-protein amino acids (NPAAs) do not change nectar palatability but enhance learning and memory in honey bees. Sci Rep 11:11721
Charalabidis A, Dechaume-Moncharmont FX, Petit S, Bohan DA (2017) Risk of predation makes foragers less choosy about their food. PLoS One 12:e0187167
Couvillon MJ, Robinson EJH, Atkinson B, Child L, Dent KR, Ratnieks FLW (2008) En garde: rapid shifts in honeybee, Apis mellifera, guarding behaviour are triggered by onslaught of conspecific intruders. Anim Behav 76:1653–1658
Couvillon MJ, Schurch R, Ratnieks FL (2014a) Dancing bees communicate a foraging preference for rural lands in high-level agri-environment schemes. Curr Biol 24:1212–1215
Couvillon MJ, Schurch R, Ratnieks FL (2014b) Waggle dance distances as integrative indicators of seasonal foraging challenges. PLoS One 9:e93495
Doner LW (1977) The sugars of honey - a review. J Sci Food Agric 28:443–456
Downs SG, Ratnieks FL (2000) Adaptive shifts in honey bee (Apis mellifera L.) guarding behavior support predictions of the acceptance threshold model. Behav Ecol 11:326–333
Downs SG, Ratnieks FL, Jefferies SL, Rigby HE (2000) The role of floral oils in the nestmate recognition system of honey bees (Apis mellifera L.). Apidologie 31:357–365
Downs SG, Ratnieks FLW, Badcock NS, Mynott A (2001) Honeybee guards do not use food-derived odors to recognize non-nest mates: a test of the Odor Convergence hypothesis. Behav Ecol 12:47–50
Eiri DM, Nieh JC (2016) A nicotinic acetylcholine receptor agonist affects honey bee sucrose responsiveness and decreases waggle dancing. J Exp Biol 219:2081
Free JB (1954) The behaviour of robber honeybees. Behaviour 7:233–240
Gao J, Zhao G, Yu Y, Liu F (2010) High concentration of nectar quercetin enhances worker resistance to queen’s signals in bees. J Chem Ecol 36:1241–1243
Garbuzov M, Balfour NJ, Shackleton K, Al Toufailia H, Scandian L, Ratnieks FLW (2020) Multiple methods of assessing nectar foraging conditions indicate peak foraging difficulty in late season. Insect Conserv Divers 13(6):532–542. https://doi.org/10.1111/icad.12420
Grume GJ, Biedenbender SP, Rittschof CC (2021) Honey robbing causes coordinated changes in foraging and nest defence in the honey bee, Apis mellifera. Anim Behav 173:53–65
Guzman-Novoa E, Page RE (1994) Genetic dominance and worker interactions affect honeybee colony defense. Behav Ecol 5:91–97
Harrison JW, Palmer JH, Rittschof CC (2019) Altering social cue perception impacts honey bee aggression with minimal impacts on aggression-related brain gene expression. Sci Rep 9:14642
Herb BR, Shook MS, Fields CJ, Robinson GE (2018) Defense against territorial intrusion is associated with DNA methylation changes in the honey bee brain. BMC Genomics 19:216
Higginson AD, Barnard CJ (2004) Accumulating wing damage affects foraging decisions in honeybees (Apis mellifera). Ecological Entomology 29:52–59
Lau CW, Nieh JC (2010) Honey bee stop-signaling production: temporal distribution and effect of feeder crowding. Apidologie 41:87–95
Li-Byarlay H, Rittschof CC, Massey JH, Pittendrigh BR, Robinson GE (2014) Socially responsive effects of brain oxidative metabolism on aggression. Proc Natl Acad Sci U S A 111:12533–12537
Mao W, Schuler MA, Berenbaum MR (2013) Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc Natl Acad Sci U S A 110:8842–8846
Moore AJ, Breed MD, Moor MJ (1987) The guard honey bee: ontogeny and behavioural variability of workers performing a specialized task. Anim Behav 35:1159–1167
Mujagic S, Erber J (2009) Sucrose acceptance, discrimination and proboscis responses of honey bees (Apis mellifera L.) in the field and the laboratory. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195:325–339
Nieh JC (2010) A negative feedback signal that is triggered by peril curbs honey bee recruitment. Curr Biol 20:310–315
Njoroge TM, Calla B, Berenbaum MR, Stone CM (2021) Specific phytochemicals in floral nectar up-regulate genes involved in longevity regulation and xenobiotic metabolism, extending mosquito life span. Ecol Evol 11:8363–8380
Núñez JA, Giurfa M (1996) Motivation and regulation of honey bee foraging. Bee World 77:182–196
Pamminger T, Becker R, Himmelreich S, Schneider CW, Bergtold M (2019) The nectar report: quantitative review of nectar sugar concentrations offered by bee visited flowers in agricultural and non-agricultural landscapes. PeerJ 7:e6329
Pankiw T, Waddington KD, Page RE Jr (2001) Modulation of sucrose response thresholds in honey bees (Apis mellifera L.): influence of genotype, feeding, and foraging experience. J Comp Physiol A 187:293–301
Peck DT, Seeley TD (2019) Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honey bee colonies to their neighbors. PLoS One 14:e0218392
Prado A, Requier F, Crauser D, Le Conte Y, Bretagnolle V, Alaux C (2020) Honeybee lifespan: the critical role of pre-foraging stage. Royal Soc Open Sci 7(11). https://doi.org/10.1098/rsos.200998
Preston SR, Palmer JH, Harrison JW, Carr HM, Rittschof CC (2019) The impacts of maternal stress on worker phenotypes in the honey bee. Apidologie 50:704–719
Reader T, MacLeod I, Elliott PT, Robinson OJ, Manica A (2005) Inter-order interactions between flower-visiting insects: foraging bees avoid flowers previously visited by hoverflies. J Insect Behavior 18:51–57
Ribbands CR, Kalmus H, Nixon HL (1952) New evidence of communication in the honeybee colony. Nature 170:438–440
Richard FJ, Holt HL, Grozinger CM (2012) Effects of immunostimulation on social behavior, chemical communication and genome-wide gene expression in honey bee workers (Apis mellifera). BMC Genomics 13:1–17
Rittschof CC (2017) Sequential social experiences interact to modulate aggression but not brain gene expression in the honey bee (Apis mellifera). Front Zool 14:1–10
Rittschof CC, Bukhari SA, Sloofman LG, Troy JM, Caetano-Anolles D, Cash-Ahmed A, Kent M, Lu X, Sanogo YO, Weisner PA, Zhang H, Bell AM, Ma J, Sinha S, Robinson GE, Stubbs L (2014) Neuromolecular responses to social challenge: common mechanisms across mouse, stickleback fish, and honey bee. Proc Natl Acad Sci U S A 111:17929–17934
Rittschof CC, Coombs CB, Frazier M, Grozinger CM, Robinson GE (2015) Early-life experience affects honey bee aggression and resilience to immune challenge. Sci Rep 5:15572
Rittschof CC, Nieh JC (2021) Honey robbing: could human alterations to the environment change a rare foraging tactic into a maladaptive behavior? Curr Opin Insect Sci 45:54–90
Rittschof CC, Robinson GE (2013) Manipulation of colony environment modulates honey bee aggression and brain gene expression. Genes Brain Behav 12:802–811
Rivera-Marchand B, Giray T, Guzmán-Novoa E (2008) The cost of defense in social insects: insights from the honey bee. Entomol Exp Appl 129:1–10
Roitberg BD, Keiser S, Hoffmeister T (2010) State-dependent attacks in a mosquito. Physiol Entomol 35:46–51
Sasaki T, Pratt SC (2018) The psychology of superorganisms: collective decision making by insect societies. Annu Rev Entomol 63:259–275
Schmid-Hempel P, Kacelnik A, Houston AI (1985) Honeybees maximize efficiency by not filling their crop. Behav Ecol Sociobiol 17:61–66
Seeley TD (1986) Social foraging by honeybees: how colonies allocate foragers among patches of flowers. Behav Ecol Sociobiol 19:343–354
Seeley TD (1989) Social foraging in honey bees: how nectar foragers assess their colony’s nutritional status. Behav Ecol Sociobiol 24:181–199
Seeley TD (1994) Honey bee foragers as sensory units of their colonies. Behav Ecol Sociobiol 34:51–62
Seeley TD (1997) Honey bee colonies are group-level adaptive units. Am Nat 150:s22–s41
Seeley TD, Tovey CA (1994) Why search time to find a food-storer bee accruately indicates the relative rates of nectar collecting and nectar processing in honey bee colonies. Anim Behav 47:311–316
Shafir S, Waite T, Smith B (2002) Context-dependent violations of rational choice in honeybees ( Apis mellifera ) and gray jays ( Perisoreus canadensis ). Behav Ecol Sociobiol 51:180–187
Shpigler HY, Saul MC, Murdoch EE, Cash-Ahmed AC, Seward CH, Sloofman L, Chandrasekaran S, Sinha S, Stubbs LJ, Robinson GE (2017) Behavioral, transcriptomic and epigenetic responses to social challenge in honey bees. Genes Brain Behav 16:579–591
Simcock NK, Gray H, Bouchebti S, Wright GA (2018) Appetitive olfactory learning and memory in the honeybee depend on sugar reward identity. J Insect Physiol 106:71–77
Skelhorn J, Rowe C (2007) Predators’ toxin burdens influence their strategic decisions to eat toxic prey. Curr Biol 17:1479–1483
van Alphen JJM, Bernstein C, Driessen G (2003) Information acquisition and time allocation in insect parasitoids. Trends Ecol Evol 18:81–87
Van Nest BN, Moore D (2018) How to train a honey bee. J Undergrad Neurosci Educ 17:T1–T11
Verdolin JL (2006) Meta-analysis of foraging and predation risk trade-offs in terrestrial systems. Behav Ecol Sociobiol 60:457–464
Waller GD (1972) Evaluating responses of honey bees to sugar solutions using an artificial flower feeder. Ann Entomol Soc Am 65:857–862
Wilson EO (1971) The insect societies. Belknap Press of Harvard University Press, Cambridge, Massachusetts
Acknowledgements
We thank Jimmy Harrison and Anna Foose for help with experimental set-up and honey bee colony management.
Funding
This work was funded by a University of Kentucky Office of Undergraduate Research Summer Research Fellowship (to T.C.N.), the National Science Foundation (to C.C.R., IOS-2045901), the National Institute of Food and Agriculture Hatch Program (to C.C.R., 1012993), the Kentucky Agriculture Development Fund (to C.C.R., A2019-0266), and the Foundation for Food and Agriculture Research (to C.C.R., 549049).
Author information
Authors and Affiliations
Contributions
T.C.N, R.R.W., and C.C.R conceptualized and designed the study. T.C.N., R.R.W., and C.W.K. collected data. T.C.N. drafted an original version of the manuscript. C.C.R. performed the data analysis and drafted the final version of the manuscript. R.R.W. and C.W.K. contributed to the writing of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was designed so that there was no known mortality impacts on honey bee individuals or colonies.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Communicated by D. Naug
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Napier, T.C., Westwick, R.R., Kane, C.W. et al. Evaluating the cues that coordinate a shift towards the robbing foraging tactic in the honey bee (Apis mellifera). Behav Ecol Sociobiol 77, 46 (2023). https://doi.org/10.1007/s00265-023-03321-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03321-x