Abstract
Knowing how floral visitors forage efficiently among flowers is important to understanding plant-pollinator interactions. When bees search for rewarding flowers, they use several visual cues to detect the available floral resources. In addition to these cues, bees can recognize scent marks, which are olfactory cues left on flowers foraged by previous visitors. This behavior is well known in social bees, such as honeybees and bumblebees. Although solitary bees do not need to give information about which flowers were foraged to conspecifics, several pieces of evidence have indicated the use of scent marks. However, it is unknown whether the behavior is widely used in many different bee species. We investigated whether four different solitary bees, Colletes patellatus (Colletidae), Andrena prostomias (Andrenidae), Osmia orientalis (Megachilidae), and Tetralonia mitsukurii (Apidae), can recognize flowers that have been foraged previously by visitors within 3 min. All four bees showed rejection responses to flowers foraged by conspecifics. However, our results showed that responses to foraged flowers varied among bee species. The tendency of A. prostomias and T. mitsukurii to reject the foraged flowers was pronounced, while in C. patellatus and O. orientalis it was weak. In both A. prostomias and T. mitsukurii, the rejection rate of flowers foraged by conspecifics decreased as the time lag after the last visit increased. Both bees visited the flowers from which pollen or nectar had been artificially removed. We suggest that A. prostomias and T. mitsukurii would recognize scent marks left by previous visitors, while the other two bees would not recognize them so strongly. It is likely that the decision to use scent marks is dependent either on the richness of resources or on the complexity of floral structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many flowering plants receive visits from a wide range of pollinator species (e.g., Waser et al. 1996). The association of bees and flowers is unusually close because bees collect floral resources for adults and larvae (Minckley and Roulston 2006). Understanding the strategies bees use to efficiently exploit floral resources is beneficial to understanding complex pollination systems. If many bees share the same floral resources, depletion of flowers must occur, rendering efficient foraging more difficult. Thus, the ability to detect rewarding flowers is likely to be important for visitors. Despite difficulty in predicting the renewal and depletion of floral resources such as nectar and pollen, bees can discriminate between rewarding and non-rewarding flowers in floral patches (Marden 1984; Wetherwax 1986). They detect and use several cues to assess the depletion of floral resources, for example, visual and olfactory signals directly from pollen and nectar (Thorpe et al. 1975; Heinrich 1979; Galen and Kevan 1983; Dobson and Bergström 2000) and perceive changes in floral color (Kadmon et al. 1991; Weiss 1991; Nuttman et al. 2006). In addition to these cues, bees recognize scent marks left on flowers by previous visitors (Corbet et al. 1984; Kato 1988; Goulson et al. 1998). It is thought that the scent marks can function as both attractants and repellents. Many studies have shown that scent marks attract social bees to artificial resources (e.g., Free and Williams 1979, 1983; Williams and Poppy 1997; Cameron 1981; Schmitt and Bertsch 1990; Goulson et al. 2000; Aguilar and Sommeijer 2001; Schmidt et al. 2005). The repellent role of scent marks on natural floral resources has been verified in honeybees (Giurfa 1993; Giurfa and Núñez 1992, 1993a, b; Giurfa et al. 1994), bumblebees (e.g., Goulson et al. 1998; Stout et al. 1998; Stout and Goulson 2001, 2002), and sweat bees (Yokoi and Fujisaki 2007; Yokoi et al. 2007).
In more recent analysis, Wilms and Eltz (2007) showed that the repellent scent marks of bumblebees are footprint cues. Regarding the role of scent marks in bumblebees, the same chemical cues function as both attractive and repellent signals depending on resource volume and the context in which they are presented (Saleh and Chittka 2006; Witjes and Eltz 2007). The rate of rejection responses to scent marks decreases over time and corresponds to the rate of nectar replenishment (Stout et al. 1998; Stout and Goulson 2002). Several bee species recognize the scent marks left by visitors of different bee families or hoverflies (Stout and Goulson 2001; Gawleta et al. 2005; Reader et al. 2005; Yokoi et al. 2007; but see Williams 1998). This type of behavior improves foraging efficiency when collecting resources by reducing the time spent visiting non-rewarding flowers (Williams 1998).
Contrary to the traditional prediction that scent marking may be restricted to social Apidae (Stout et al. 1998), some evidence suggests the use of scent marks in other bee taxa: Anthidium manicatum (Gawleta et al. 2005) and Halictus aerarius (Yokoi et al. 2007; Yokoi and Fujisaki 2007). Bees are classified into seven families (Michener 2000), with evidence of scent marks both in social bees and in solitary bees found within three families, Apidae, Halictidae, and Megachilidae. Scent marking has been considered as a trait of social bees because the cue enhances the colony foraging efficiency by communicate information of floral resources to nestmates and by reducing the time searching by native foragers (Cameron 1981). Recent reports are supporting the hypothesis that scent marks are widely used in many bee species. However, the information about solitary bee species that have the ability to recognize scent marks is very scarce. To test the hypothesis that uses of scent marks are generally common in many bee species, we focused on four solitary bees from different bee families, Colletidae, Andrenidae, Megachilidae, and Apidae. The former two families have not been investigated before with evidence for foraging scent recognition. Both Colletidae and Andrenidae are among the most primitive bee families, exhibiting little or no sociality (Michener 2000).
In this study, we investigated behaviors on a flower previously visited by conspecifics in four solitary bee species by comparing (1) their ability to recognize flowers that were foraged by previous visitors, (2) the duration over which marks left by bees were functional, and (3) the responses of bees to flowers with their floral resources artificially removed. Here, we report the results for each bee species and discuss the use of scent marks by solitary bees.
Methods
Bee species and flowers
The study was conducted in a field at the Nara campus of Kinki University, Nakamachi, Nara, Japan (34°40′N, 135°43′E). For all bee species, individuals visiting flowers naturally in the field were used. Four bee–flower systems were examined. Females of each solitary bee species primarily visited its specific plant species at the field site during the study. All investigations were conducted on clear and sunny days from May to October in 2003–2005.
I. Andrena prostomias Pérez (Andrenidae)
Andrena prostomias visited Deutzia crenata Sieb. et Zucc. (Saxifragaceae) flowers that were open from May to June. All female individuals foraged for nectar and pollen while males foraged only for nectar. The D. crenata inflorescence is a panicle, and each flower has white petals and yellow anthers.
II. Colletes patellatus Pérez (Colletidae)
Colletes patellatus visited Aster ageratoides Turcz. subsp. ovbatus (Franch. et Savat.) Kitam (Compositae) that was open from August to October. Although most bees foraged for nectar and pollen, some collected only nectar. The inflorescence of A. ageratoides is a capitulum with numerous individual florets. Handling time required to forage the flower is very short.
III. Tetralonia mitsukurii Cockerell (Apidae)
Tetralonia mitsukurii visited bush clover Lespedeza bicolor (Fabaceae) flowers that were open from August to September. The observed bees foraged for nectar that was concealed in the corolla. The Lespedeza flower had papilionaceous corolla, and its petals return to their original position after a visitor leaves and appear no different from unvisited flowers to the human eye.
IV. Osmia orientalis Benoist (Megachilidae)
Osmia orientalis visited wild strawberry Rubus hirsutus (Rosaceae) flowers that were open from March to May. The flower had rosaceous corolla, and it is easy to detect pollen on the corolla. Flowers visited by bees that collected both pollen and nectar were used for the experiment.
Species systems I–IV were used to investigate bee responses to flowers that had been foraged by previous visitors. Species systems I and III were used for two experiments: the duration of scent mark efficacy and the response of bees to non-rewarding flowers. Systems II and IV were not used in these experiments because the system II plant was removed by mowing and not enough sample could be prepared of the system IV plant.
Responses to previously foraged flowers
The experiments were conducted in 2003. Our experimental design followed that of Goulson et al. (1998, 2001). The foraged single flower or inflorescences (in Compositae flowers) were removed with scissors after we confirmed the foraging behavior of bees, and were offered to either the same individual or conspecifics within 3 min using forceps. Three minutes was chosen as the maximum time between visits because it is sufficient to detect the next visitors and offer the flowers, but is much less than the time taken for floral resources to replenish in most systems. We classified the responses of subsequent visitors into three patterns following Corbet et al. (1984) and Schmitt and Bertsch (1990): hovering, the bee came within 1 cm of the flower, but did not forage on it; landing, the bee landed on the flower, but did not forage; probing, the bee landed on the flower and foraged for floral resources. Both hovering and landing were defined as rejection responses because bees did not collect floral resources. Each flower was used within 1 or 2 days after it opened. We did not know the visitation history of the flower except the last visit. However, we defined it as rewarding flowers because the last visitor collecting both nectar and pollen was confirmed. As a control (unvisited flowers), we prepared flower or inflorescences that had been covered with nylon mesh until flowering. Flowers were used only once and were discarded at the end of each test to prevent reuse.
Responses of bees to non-rewarding flowers
To test the responses of bees to flowers with artificially removed floral resources, we prepared two treatment flowers: “no nectar,” the nectar was removed with micro-capillary tubes; “no pollen,” pollen was removed using cotton paper. As a control (original), we prepared inflorescences that had been covered with nylon mesh until flowering. The treatment flowers were removed from plants with scissors, and using forceps, we offered to conspecifics within 3 min. We used the same behavior classification as described above. The treatment flowers were covered with a net until flowering and were discarded after use. This experiment was conducted in 2005.
Effective duration of scent marks
To investigate the duration of scent mark efficacy, flowers were netted after they had been foraged by a visitor. Netted flowers were then offered to conspecifics after a specific interval had passed (3, 10, 20, 40, 60, 120, or 180 min, or 24 h). In the species system I, data for 120, 180 min and 24 h were not collected. We compared the same behavior classification as described above. Flowers were discarded after use. We recorded data on rejection responses to previously foraged flowers within a specific time interval in 2004. We compared the results of effective duration with that of unvisited control flowers in 2003 because the sample size in 2004 was insufficient for comparison.
Statistical analysis
JMP (third edition; SAS Institute Inc., Cary, NC) was used for statistical analyses. A Fisher’s exact probability test was used for a comparison between unvisited flowers (control) and flowers visited by conspecifics or by the same individuals after we used the chi-square test for pre-planned comparison in each species system. All statistical tests were conducted at the 0.05 level of significance. Relationships between the time from initial to subsequent visitor foraging and the rejection rate were compared using logistic regression.
Results
Responses to previously foraged flowers
I. A. prostomias
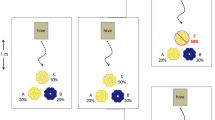
The refection rate of flowers visited by conspecific females (n = 34) and males (n = 9) was significantly higher (Fisher’s exact probability test, P < 0.001, Fig. 1a) than that of unvisited flowers (n = 20), although that of flowers visited by the same female (n = 29) was low (Fisher’s exact probability test, P = 0.064).
Proportion of rejection responses of each bee female to foraged flowers within 3 min in each plant species. All bee species visited the plant species as a specialist forager. Andrena prostomias visited Deutzia crenata (a), Colletes patellatus visited Aster ageratoides (b), Tetralonia mitsukurii visited Lespedeza bicolor (c), and Osmia arisntalis visited Rubus hirsutus (d). Different letters indicate significant difference to the unvisited control flowers. Unshaded portion (landing) and shaded portion (hovering)
II. C. patellatus
There was no detectable difference in the rejection rate between unvisited control flowers (n = 27) and flowers visited by the same female (n = 47; Fisher’s exact probability test, P = 0.2902, Fig. 1b). There was a significant difference in the rejection rate of unvisited flowers with that of flowers foraged by female conspecifics (n = 44; Fisher’s exact probability test, P = 0.0109), although the rejection rate itself was as low as 25%.
III. T. mitsukurii
There was a significant difference in the rejection rate between control flowers (n = 11) and flowers foraged by female conspecifics (n = 38; Fisher’s exact probability test, P < 0.0001, Fig. 1c) by the same female (n = 29; Fisher’s exact probability test, P = 0.0074) and by males (n = 16; Fisher’s exact probability test, P = 0.0002).
IV. O. orientalis
There was no detectable difference in the rejection rate between unvisited control flowers (n = 50) and flowers visited by the same female (n = 6; Fisher’s exact probability test, P = 1.0000, Fig. 1d). The rejection of O. orientalis to control flowers was significantly different from rejection of flowers foraged by female conspecifics (n = 51; Fisher’s exact probability test, P = 0.0058). However, the rejection rate of flowers foraged by female conspecifics was very low (15.7%).
Responses of bees to non-rewarding flowers
I. A. prostomias
Female rejection of the original control flower (n = 23) was not significantly different from responses to treated flowers (Fig. 2a) for either “no pollen” flowers (n = 25; Fisher’s exact probability test P = 0.1454) or “no nectar” flowers (n = 26; Fisher’s exact probability test, P = 1.0000).
III. T. mitsukurii
Female rejection of unvisited control flowers (n = 23) was not significantly different from responses to treated flowers (Fig. 2b) for either “no pollen” flowers (n = 25; Fisher’s exact probability test P = 0.6681) or “no nectar” flowers (n = 22; Fisher’s exact probability test, P = 1.0000).
Effective duration of scent marks
I. A. prostomias
The rejection rate of flowers foraged by female conspecifics decreased after 60 min (Fig. 3a). The time since the last individual visited the flower was inversely related to the proportion of flowers rejected by female conspecifics (likelihood ratio χ 21 = 14.8460, P < 0.0001). There was a significant difference between control flowers and flowers revisited within 20–60 min (Table 1).
The relationship between duration and proportion of rejection rate of visitors to the foraged flowers since last female conspecifics visited: Andrena prostomias visited Deutzia crenata (a), and Tetralonia mitsukurii visited Lespedeza bicolor (b). Bold lines show the confidence limits. Error bars represent ±SE
III. T. mitsukurii
The rejection rate of flowers foraged by female conspecifics decreased after 24 h (Fig. 3b). The time since the last individual visited the flower was inversely related to the proportion of flowers rejected by female conspecifics (likelihood ratio χ 21 = 6.4054, P = 0.0114). There was a significant difference between control flowers and flowers revisited within 180 min to 24 h (Table 1).
Discussion
Evidence suggesting the use of scent marks has been reported for only three species of solitary bees (Frankie and Vinson 1977; Gilbert et al. 2001; Gawleta et al. 2005). Our results showed the ability to recognize the foraged flowers by previous conspecifics or by same individuals in four additional solitary bee species from four different families, A. prostomias, C. patellatus, O. orientalis, and T. mitsukurii. In our results, the rejection of previously foraged flowers differed among solitary bee species. The tendency of A. prostomias and T. mitsukurii to reject the foraged flowers was pronounced, while in C. patellatus and O. orientalis it was weak. A. prostomias and T. mitsukurii showed strong rejection responses to the flowers that had been foraged by previous visitors (Fig. 1). The removal of floral resources did not influence the rejection rate (Fig. 2). Moreover, the rejection rate of previously foraged flowers decreased over time (Table 1). Although only two of the four systems were confirmed by resource removal and change of rejection rate over time, clearly they indicate that the recognition of the visited flowers might be based on olfactory cues deposited by previous visitors. The rejection rate of C. patellatus and O. orientalis against the flowers previously foraged by conspecifics was significantly higher than that against the control treatment, but it was considerably lower compared to the other two species. We suggest that these bees would not discriminate so strongly between the previously foraged and non-foraged flowers.
Why do these two types of responses to foraged flowers among bee species exist? There are two possible explanations for the low rejection rate. One explanation is that the flower species chosen in the present study always have enough resources when bees revisit. Stout et al. (1998) provide data suggesting that bees foraging for pollen rely less on scent marks. Thus, the benefits of discrimination would be lowered. It is likely that A. ageratoides foraged by C. patellatus still yield resources even after several visits, because of the numerous flower inflorescences. R. hirsutus foraged by O. orientalis may have a similar effect. We suggest that C. patellatus and O. orientalis would not need to use scent marks to discriminate between rewarding and non-rewarding flowers. Another possible explanation is that the floral morphology would influence bee’s decision of whether to use scent marks while foraging. Visits to flowers such as A. ageratoides and R. hirsutus would have very low cost because of easy access to floral resources. On the other hand, if bees visit plants with concealed floral resources, it probably takes longer to assess floral resources (Goulson et al. 2001). It is likely that the visits to flowers such as L. bicolor and D. crenata are aided by the use of scent marks. Bumblebees take more time probing morphologically complex flowers than probing simple flowers (Harder 1983; Laverty 1994). So, bumblebees rely less on scent marks when visiting simple flowers with a low handling time compared to complex flowers with a high handling time (Saleh et al. 2006). We suggest that these floral features have an effect on the decision of foraging solitary bees whether to use scent marks or not.
Scent marks of male and female conspecifics can have repellent effects for female conspecifics in A. prostomias and T. mitsukurii. This is the first report of females able to detect flowers that were visited by males. In solitary bees, females often meet males on flowers while they are foraging. Rejection of flowers visited by males would reduce the time spent visiting less rewarding flowers. Interestingly, the rejection rate of female A. prostomias for flowers foraged by males was higher than that for flowers foraged by females, while female T. mitsukurii showed a high rejection rate for flowers foraged by either males or females. We suggest that female A. prostomias would react sensitively to the existence of males to avoid the mating approach on food sources, because of only one copulating (Maeta 2000). However, a high proportion of landing response of females to the foraged flower by males would imply the difficulty of recognition by hovering, suggesting that the scent of males was less deposited because male body size was smaller than female. So, it is likely that females have to land on the flowers foraged by males, although they want to avoid males.
Our results, combined with those of previous studies, suggest that the use of scent marks is not restricted to particular bee taxa and is certainly not restricted to the social bees. Solitary bees would not need to inform the resource assessment for conspecifics because of the absence of social cooperation (Michener 2000). In solitary bees, the chemical compounds left by previous visitors might not be deposited deliberately.
If solitary bees use cuticular hydrocarbons as a cue, very little is known about the origin of the chemical compounds and composition, while many studies demonstrate the use scent marks (Barth et al. 2008). Several studies suggest that the substance using scent marks of foraging social bees would originate from a particular gland [tarsal grand (Stout et al. 1998; Goulson et al. 2000) or claw retractor tendon grand (Jarau et al. 2004)]. In recent chemical analysis, the effects of cuticular hydrocarbons were also pointed out (Eltz 2006). Social bees recognize the scent marks left by previous solitary bees (Gawleta et al. 2005; Yokoi et al. 2007). Chemical compounds of scent marks of solitary bees may be similar to that of social bees. We suggest that solitary bees recognize the cuticular hydrocarbons, which are passively deposited on the flower, as scent marks. It is worth investigating the use of scent marks in solitary bees (Goulson et al. 2000) to elucidate the origin of using scent marks. Our study adds clear evidence for the generality of repellent scent-marking behavior in bee groups. It is necessary to investigate the discrimination ability of more solitary bee species and compare this ability among different bee groups.
References
Aguilar I, Sommeijer M (2001) The deposition of anal excretions by Melipona favosa foragers (Apidae: Meliponinae): behavioural observations concerning the location of food sources. Apidologie (Celle) 32:37–48. doi:10.1051/apido:2001109
Barth FG, Hrncir M, Jarau S (2008) Signals and cues in the recruitment behavior of stingless bees (Meliponini). J Comp Physiol [A] 194:313–327. doi:10.1007/s00359-008-0321-7
Cameron SA (1981) Chemical signals in bumblebee foraging. Behav Ecol Sociobiol 9:257–260. doi:10.1007/BF00299880
Corbet SA, Kerslake CJ, Brown C, Morland NE (1984) Can bees select nectar-rich flowers in a patch? J Apic Res 23:234–242
Dobson HEM, Bergström G (2000) The ecology and evolution of pollen odors. In: Dafni A, Hesse M, Pacini E (eds) Pollen and Pollination. Springer, Vienna, pp 63–88
Eltz T (2006) Tracing pollinator footprints on natural flowers. J Chem Ecol 32:907–915. doi:10.1007/s10886-006-9055-6
Frankie GW, Vinson SB (1977) Scent marking of passion flowers in Texas by females of Xylocopa virginica texana (Hymenoptera: Anthophoridae). J Kans Entomol Soc 50:613–625
Free JB, Williams IH (1979) Communication by pheromones and other means in Apis florae colonies. J Apic Res 18:16–25
Free JB, Williams IH (1983) Scent-marking of flowers by honeybees. J Apic Res 22:86–90
Galen C, Kevan PG (1983) Bumblebee foraging and floral scent dimorphism: Bombus kirbyellus Curtis (Hymenoptera: Apidae) and Polemonium viscosum Nutt. (Polemoniaceae). Can J Zool 61:1207–1391
Gawleta N, Zimmermann Y, Eltz T (2005) Repellent foraging recognition across bee families. Apidologie (Celle) 36:325–330. doi:10.1051/apido:2005018
Gilbert F, Azmeh S, Barnard C, Behnke J, Collins SA, Hurst J et al (2001) Individually recognizable scent marks on flowers made by a solitary bee. Anim Behav 61:217–229. doi:10.1006/anbe.2000.1542
Giurfa M (1993) The repellent scent-mark of the honeybee Apis mellifera ligustica and its role as a communication cue during foraging. Insectes Soc 40:59–67. doi:10.1007/BF01338832
Giurfa M, Núñez JA (1992) Honeybees mark with scent and reject recently visited flowers. Oecologia 89:113–117. doi:10.1007/BF00319022
Giurfa M, Núñez JA (1993a) Efficient floret inspection by honeybees in capitula of Carduud acanthoides. Ecol Entomol 18:116–122. doi:10.1111/j.1365-2311.1993.tb01192.x
Giurfa M, Núñez JA (1993b) Visual modulation of a scent-marking activity in the honeybee, Apis mellifera L. Naturwissenschaften 80:376–379. doi:10.1007/BF01138797
Giurfa NM, Núñez JA, Backhaus W (1994) Odour and colour information in the honeybee, Apis mellifera L. J Comp Physiol [A] 175:773–779. doi:10.1007/BF00191849
Goulson D, Hawson SA, Stout JC (1998) Foraging bumblebees avoid flowers already visited by conspecifics or by other bumblebee species. Anim Behav 55:199–206. doi:10.1006/anbe.1997.0570
Goulson D, Stout JC, Langley J, Hughes WOH (2000) Identity and function of scent marks deposited by foraging bumblebees. J Chem Ecol 26:2897–2911. doi:10.1023/A:1026406330348
Goulson D, Chapman JW, Hughes WOH (2001) Discrimination of unrewarding flowers by bees: direct detection of rewards and use of repellent scent marks. J Insect Behav 14:669–677. doi:10.1023/A:1012231419067
Harder LD (1983) Flower handling efficiency of bumble bees: morphological aspects of probing time. Oecologia 57:274–280. doi:10.1007/BF00379591
Heinrich B (1979) Resource heterogeneity and patterns of movement in foraging bumblebees. Oecologia 40:235–245. doi:10.1007/BF00345321
Jarau S, Hrncir M, Ayasse M, Schulz C, Francke W, Zucchi R, Barth FG (2004) A stingless bee (Melipona seminigra) marks food sources with a pheromone from its claw retractor tendons. J Chem Ecol 30:793–804
Kadmon R, Shmida A, Selten R (1991) Within-plant foraging behavior of bees and its relationship to nectar distribution in Anchusa strigosa. Isr J Bot 40:283–294
Kato M (1988) Bumblebee visits to Impatiens spp.: pattern and efficiency. Oecologia 76:364–370
Laverty TM (1994) Bumble bee learning and flower morphology. Anim Behav 47:531–545. doi:10.1006/anbe.1994.1077
Maeta Y (2000) Tajima Gakuonji-no Utsugi-himehanabachi. Kaiyusya, Tokyo
Marden JM (1984) Remote perception of floral nectar by bumblebees. Oecologia 64:232–240. doi:10.1007/BF00376876
Michener CD (2000) The bees of the world. Johns Hopkins, Baltimore
Minckley RL, Roulston TH (2006) Incidental mutualisms and pollen specialization among bees. In: Waser NM, Ollerton J (eds) Plant-pollinator interactions: from specialization to generalization. University of Chicago, Chicago, pp 69–98
Nuttman CV, Semida FM, Zalat S, Willmer PG (2006) Visual cues and foraging choices: bee visits to floral colour phases in Alkanna orientalis (Boraginaceae). Biol J Linn Soc Lond 87:427–435. doi:10.1111/j.1095-8312.2006.00582.x
Reader T, MacLeod I, Elliott PT, Robinson OJ, Manica A (2005) Inter-order interactions between flower-visiting insects: Foraging bees avoid flowers previously visited by hoverflies. J Insect Behav 18:51–57. doi:10.1007/s10905-005-9346-8
Saleh N, Chittka L (2006) The importance of experience in the interpretation of conspecific chemical signals. Behav Ecol Sociobiol 61:215–220. doi:10.1007/s00265-006-0252-7
Saleh N, Ohashi K, Thomson JT, Chittka L (2006) Facultative use of repellent scent mark in foraging bumblebees: complex vs. simple flowers. Anim Behav 71:847–854. doi:10.1016/j.anbehav.2005.06.014
Schmitt U, Bertsch A (1990) Do foraging bumblebees scent-mark food sources and does it matter? Oecologia 82:137–144. doi:10.1007/BF00318545
Schmidt VM, Zucchi R, Barth FG (2005) Scent marks left by Nannotrigona testaceicornis at the feeding site: cues rather than signals. Apidologie 36:285–291
Stout JC, Goulson D (2001) The use of conspecific and interspecific scent marks by foraging bumblebees and honeybees. Anim Behav 62:183–189. doi:10.1006/anbe.2001.1729
Stout JC, Goulson D (2002) The influence of nectar secretion rates on the responses of bumblebees (Bombus spp.) to previously visited flowers. Behav Ecol Sociobiol 52:239–246. doi:10.1007/s00265-002-0510-2
Stout JC, Goulson D, Allen JA (1998) Repellent scent-marking of flowers by a guild of foraging bumblebees (Bombus spp.). Behav Ecol Sociobiol 43:317–326. doi:10.1007/s002650050497
Thorpe RW, Briggs DL, Estes JR, Ericksin EH (1975) Nectar fluorescence under ultraviolet irradiation. Science 189:476–478. doi:10.1126/science.189.4201.476
Waser NM, Chittka L, Price MV, Williams NM, Ollerton J (1996) Generalization in pollination systems, and why it matters. Ecology 77:1043–1060. doi:10.2307/2265575
Weiss MR (1991) Floral colour changes as cues for pollinators. Nature 354:227–229. doi:10.1038/354227a0
Wetherwax PB (1986) Why do honeybees reject certain flowers? Oecologia 69:567–570. doi:10.1007/BF00410364
Williams CS (1998) The identity of the previous visitor influences flower rejection by nectar-collecting bees. Anim Behav 56:673–681. doi:10.1006/anbe.1998.0794
Williams CS, Poppy GM (1997) Responses of individual honey bees to artificial feeders visited by themselves and to feeders visited by hivemates. J Apic Res 36:105–108
Wilms J, Eltz T (2007) Foraging scent marks of bumblebees: footprint cues rather than pheromone signals. Naturwissenschaften. doi:10.1007/s00114-007-0298-z
Witjes S, Eltz T (2007) Influence of scent deposits on flower choice: experiments in an artificial flower array with bumblebees. Apidologie (Celle) 38:12–18. doi:10.1051/apido:2006048
Yokoi T, Fujisaki K (2007) Repellent scent-marking behaviour of the sweat bee Halictus (Seladonia) aerarius during flower foraging. Apidologie (Celle) 38:482–504. doi:10.1051/apido:2007034
Yokoi T, Goulson D, Fujisaki K (2007) The use of heterospecific scent marks by the sweat bee Halictus aerarius. Naturwissenschaften 94:1021–1024. doi:10.1007/s00114-007-0285-4
Acknowledgments
We are grateful to Dave Goulson, School of Biological and Environmental Science, University of Stirling, and Pablo J. Perez-Goodwyn, Laboratory of Insect Ecology, Kyoto University, for careful proofreading of the manuscript. We thank Takayoshi Nishida and members of the Laboratory of Insect Ecology, Kyoto University, for helpful comments on an early draft of the manuscript. We are also grateful to Tsuyoshi Sugimoto, Yasuyuki Sakuratani, and Ikuo Kandori, Laboratory of Entomology, Kinki University, for valuable advice and permission to use the university campus. This work was supported in part by the Twenty-First Century COE Program for Innovative Food and Environmental Studies Pioneered by Entomomimetic Sciences from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yokoi, T., Fujisaki, K. Recognition of scent marks in solitary bees to avoid previously visited flowers. Ecol Res 24, 803–809 (2009). https://doi.org/10.1007/s11284-008-0551-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-008-0551-8