Abstract
Evaluating beta diversity patterns along environmental gradients and different geographic ranges helps comprehend the roles of niche-based and spatial processes to metacommunity structure. Pond-breeding anuran composition depends on environmental predictors such as climate, vegetation type and habitat features as well as spatial processes (e.g., dispersal limitation or distances among patches). Here, we investigated the role of spatial and local (water chemistry and habitat structure) and regional (climate) environmental predictors to tadpole beta diversity (and its turnover and nestedness-resultant components) in temporary ponds distributed along a forest–grassland transition in the southern Brazilian Coastal Plain. Additionally, we investigated the role of the assessed predictors within forest and grassland sub-regions to test for congruence in their contribution across vegetation types. Turnover was the most relevant component of beta diversity. Spatial predictors alone explained beta diversity (and both components) at the broadest scale. However, the contribution of environmental and spatial predictors to beta diversity differed between sub-regions. Spatial and local environmental predictors independently explained beta diversity in the grassland, while in the forest sub-region, local predictors and spatially-structured climate were the most important. Our results revealed a complex interplay of niche-based and spatial assembly processes shaping anuran metacommunities. The different relationships of environmental and spatial predictors with beta diversity in the forest and grassland sub-regions suggest that the contribution of assembly processes to anuran metacommunity structure change with vegetation type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The composition and structure of biological communities are assembled by an interplay of processes that vary through time and space (Leibold et al. 2004). Comprehending the contribution of these processes to biodiversity patterns is one of the main goals of metacommunity ecology (Brown et al. 2017). Variation in species composition among local communities (beta diversity; sensu Whittaker 1960, 1972) is regarded as being determined by niche-based and spatial processes (Heino et al. 2015; Brown et al. 2017). The role of niche-based processes is generally inferred from the influence of abiotic factors or biotic interactions on beta diversity (Leibold et al. 2004; Cottenie 2005; Holyoak et al. 2005; Chase and Myers 2011). In turn, beta diversity can also be structured by spatial processes (i.e., variation unaccounted for by environment, normally detected through spatial predictors or geographic distances) (Soininen et al. 2007; Heino et al. 2015). Spatial processes include dispersal limitation mechanisms, in which subsets of species from the regional pool are unable to either reach all sites or colonize those too far apart (Winegardner et al. 2012; Heino et al. 2015). In fact, beta diversity can be generated by both processes (niche-based and spatial ones) under different scenarios (Logue et al. 2011; Winegardner et al. 2012; Heino et al. 2015; Brown et al. 2017).
Beta diversity metrics can be partitioned into components of turnover (i.e., species replacement among sites) and richness (i.e. variation in the number of species among sites; whenever the composition of sites with lower species richness is a subset of the richest ones, this corresponds to nestedness) (Baselga 2012; Legendre 2014). Thus, finding the environmental and spatial predictors of beta diversity allow for recognition of the processes assembling communities (Leprieur et al. 2011). In a conservationist point of view, comprehension of the processes generating beta diversity (and those promoting species replacement or loss among sites) can be a useful tool for biodiversity conservation planning (Angeler 2013; Socolar et al. 2016).
The spatial extent of the study can affect the observed patterns of beta diversity (Heino et al. 2015). Spatial extent not only is associated with the relative influence of local- (e.g., habitat heterogeneity) and regional-level (e.g., climate) predictors, but also with the relative importance of spatial processes (Heino et al. 2015). In particular, the role of regional-level environmental predictors and spatial processes are expected to become more important with increasing spatial extent, the former being more important especially for taxa with restricted dispersal ability (Declerck et al. 2011; De Bie et al. 2012; Heino 2013). Additionally, due to the synergy of processes assembling communities, different predictors may cause different patterns of metacommunity organization depending on the group and ecosystem analyzed (Soininen 2014). In temporary ponds, aside from their patchy distribution, an additional contingency for the establishment of the biota is their intermittent condition. Hence, temporary pond dwelling species must be able to effectively disperse and reach sites that vary in their environmental suitability through time (Heino et al. 2015). Such contingencies are therefore expected to enhance the role of spatial processes (i.e., dispersal limitation) over niche-based ones for the community assembly in temporary ponds (Declerck et al. 2011; De Bie et al. 2012; Heino et al. 2015).
Anurans are organisms with complex life cycles and they are important representatives of temporary pond ecosystems. Several authors have, however, recorded contrasting contributions or complex interplays between niche-based and spatial processes influencing anuran metacommunities. This is likely because the spatial distribution of anurans depends on processes that differentially affect larval (tadpoles) and adult stages. Adult occurrence is strongly influenced by pond habitat features such as vegetation structure (Rojas-Ahumada et al. 2012; Prado and Rossa-Feres 2014a; Valério et al. 2016; Couto et al. 2017). Adults also have limited dispersal ability (Sinsch 1990) and distances among water bodies can affect the distribution of amphibians (Rojas-Ahumada et al. 2012). Hence, spatial processes related to dispersal limitation assumedly have an important role in structuring anuran composition (De Bie et al. 2012; Provete et al. 2014; Silva et al. 2014; Leão-Pires et al. 2018). In turn, tadpole distribution responds to environmental predictors, such as pond hydroperiod, water chemistry, aquatic plant structure and predator occurrence (Ultsch et al. 1999; Wells 2007; Both et al. 2011; Machado et al. 2012; Moreira and Maltchik 2012; Provete et al. 2014; Couto et al. 2017; Knauth et al. 2018). Tadpoles should therefore be more influenced by niche-based processes at narrower extents, suggesting that amphibian metacommunities are governed by varying factors across time and spatial scales.
At broader scales, spatial patterns of amphibian diversity are influenced by climate (Vasconcelos et al. 2009; Melchior et al. 2017). This is because temperature and precipitation are closely associated with the metabolism of amphibians, constraining their distribution (Andrade et al. 2017; Godinho and Silva 2018; Leão-Pires et al. 2018). Landscape structure (e.g., vegetation types) can affect the importance of spatial and niche-based processes to anuran composition. Community assembly studies conducted in different forest types indicated that spatial rather than niche-based processes were better predictors of anuran beta diversity in denser vegetation types (Prado and Rossa-Feres 2014b; Provete et al. 2014), likely because these regions hold species with more specialized reproductive modes (Landeiro et al. 2014). Investigations on the relative importance of assembly processes to anuran communities in grasslands are missing, as well as comparisons with forest types.
Thus, the contributions of niche-based and spatial processes to anuran composition change among study regions and with spatial extent (Provete et al. 2014; Melchior et al. 2017; Leão-Pires et al. 2018), making results not easily comparable. In addition, assessments of the relationships of anuran beta diversity components (turnover and nestedness) with spatial and environmental predictors have yielded contrasting results. For instance, nestedness has been found to be promoted either by environmental predictors (e.g., pond area, hydroperiod, depth, predator occurrence) (Werner et al. 2007; Both et al. 2011; Moreira and Maltchik 2012) and by geographic distances (Hecnar and MCloskey 1997; Rojas-Ahumada et al. 2012; Knauth et al. 2018), suggesting that different predictors can yield similar patterns. The assessment of tadpole distribution can provide insights on the relative roles of environmental (i.e., niche-based) and spatial predictors (i.e., spatial processes) to anuran metacommunity structure (Rojas-Ahumada et al. 2012; Prado and Rossa-Feres 2014a; Knauth et al. 2018). This is because the distribution of the larval stages indicates successful establishment of anurans and site selection by adults (Júnior and Rocha 2013), and also indirectly reflects the connection of amphibians with landscape and climate.
In this study, we assessed the beta diversity of tadpole communities in temporary ponds distributed along a latitudinal gradient in the southern Brazilian Coastal Plain region. Our major goal was to investigate the roles of niche-based and spatial assembly processes (dispersal limitation) to tadpole metacommunity structure. For this purpose, we tested the relative contributions of local and climatic environmental predictors (proxies of niche-based processes) and spatial predictors (proxies of spatial processes) to the patterns of beta diversity (and its turnover and nestedness-resultant components). We sampled a set of locally heterogeneous ponds ranging across a gradient of climatic conditions (temperature and precipitation) and vegetation types (forest and grassland sub-regions). However, as some authors showed that the relative importance of assembly processes to amphibian metacommunities can change with the spatial extent of analysis and vegetation type (Melchior et al. 2017), we also tested the relative contributions of environmental and spatial predictors within the forest and grassland sub-regions to test for congruence in ecological processes. Based on the assumptions of low dispersal ability of adults and elevated sensitivity of tadpole stages to local habitat conditions and climate, our general hypothesis is that the relative importance of niche-based and spatial assembly processes to anuran metacommunity structure varies with the scale of analysis. In this sense, spatial processes would be the main process affecting anuran distribution at the broadest extent (Coastal Plain), while niche-based processes associated with local environmental predictors would predominate at the finest scales (within each sub-region). We thus expected: (1) spatial predictors to be the primary predictors of beta diversity at the broadest extent; and (2) pond-level variables (e.g., water chemistry, habitat structure) to be the primary predictors of beta diversity within sub-regions.
Materials and methods
Study area
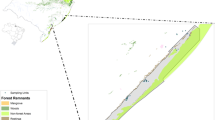
The study was conducted in the Coastal Plain region from southern Brazil (states of Rio Grande do Sul and Santa Catarina; Fig. 1). The study area is characterized by lowlands (altitudes below ~ 20 m) and sandy soils (Villwock and Tomazelli 2006) and holds a high concentration of wetlands (Maltchik 2003). The Coastal Plain presents distinct landscape and climatic characteristics between the north and south regions, including the transition between the Pampean and the Atlantic Forest biomes (IBGE 2004). Although the boundaries between the two biomes are not clearly defined in the Coastal Plain, the northern regions are more influenced by the mountains of the Serra Geral (ombrophilous forest), typical of the Atlantic Forest, while in the south open-like, shrubland landscapes (restinga) predominate (Leão et al. 2014). The southern region is considered as Pampean by some sources (IBGE 2004) (Fig. 1). The average annual temperature ranges from 12 to 22° C, and the annual rainfall, from 1000 to 1500 mm (Villwock and Tomazelli 2006).
Location of the study area and of sampling ponds along the Coastal Plain region in southernmost Brazil (adapted from: Pires et al. 2018). Ellipses indicate the sub-regions (Atlantic Forest and Pampa) within the Coastal Plain
Sampling design and tadpole collection
We sampled 24 ponds distributed along an extension of ~ 520 km in the Coastal Plain (Fig. 1). The ponds had maximum size of 1 ha and they were separated by a minimum distance of ~ 10 km. Ponds were selected according to the following criteria: reduced anthropic impact in their surroundings (e.g., mainly extensive cattle grazing areas), and artificial (man-made) ponds were not selected for sampling. The dominant aquatic vegetation in each pond varied from 1 to 2 habitat types: emergent (either with or without branched stems) and floating-leaved plants, and presence of predatory invertebrates was recorded (Pires et al. 2018). All ponds had predatory fishes (authors’ observation).
Two sampling events were carried out in the austral spring 2015 (September–November), a period that covers the breeding season of the anuran species in southern Brazil, characterized by the beginning of warmer and rainy season (Santos et al. 2007). Tadpoles were collected with a 30-cm wide frame dip-net (mesh size = 250 μm). Each sample consisted of three 1-m sweeps, taken in four different locations of each pond, totaling 12 sweeps per pond. The distance kept between each sweep was 1 m. Sweeps were conducted from the margin up to 4 m to the interior of the pond. Tadpoles were anesthetized with clove oil solution (Lucena et al. 2013) and fixed in situ with 10% formaldehyde. Samples were packed in 500-ml plastic bottles. In the laboratory, the samples were washed and the tadpoles identified to species level. Specimens are deposited in the amphibian collection of the Science and Technology Museum from the Pontifical Catholic University of Rio Grande do Sul (PUCRS).
Environmental and spatial predictors
We investigated the influence of the following environmental predictors on tadpole beta diversity:
-
1.
Local environmental predictors, consisted of two subsets: (i) water chemistry: dissolved oxygen (DO), electrical conductivity (EC), pH, total dissolved solids (TDS), water temperature (WT) and water turbidity (NTU), measured in situ with a water quality meter (HORIBA U-22). (ii) Habitat structure, represented by: pond area, measured by walking the perimeter of each pond with a GPS; pond depth, measured with a graduated stick in ten distinct points of each pond; and then classified as ‘less’ than or ‘greater’ than 30 cm; vegetation structure, classified as ‘undergrowth’ (i.e., presence of grasses and/or shrub-like vegetation less than 30-cm tall) or ‘arboreal’ (i.e., presence of shrub trees and agglomerates higher than 30-cm tall) of each pond; hydroperiod—classified as ‘long’ or ‘short’ based on the water presence at each pond between each sampling occasion. Short-hydroperiod ponds were the ones that either dried up completely or had a reduction up to 80% of their surface flooding area; connectivity (physical connection between the sampled ponds and other water bodies). Aside from pond area, all habitat structure predictors were considered as dummy variables in the data analysis. The original values of the local predictors are provided in Table S1.
-
2.
Climatic predictors, consisted of the bioclimatic data derived from temperature and precipitation measures, obtained from WorldClim (Hijmans et al. 2005). We extracted the corresponding values of 19 bioclimatic variables for the geographic location of each pond. However, in view of the elevated correlation among some of the bioclimatic variables, we conducted a principal components analysis (PCA) using the correlation matrix. The first five axes of the PCA summarized ~ 99.1% of the variation of the bioclimatic variables, and they were employed as surrogates for the climatic conditions. The PCA ordination diagram showed a clear latitudinal gradient in the distribution of the sampling sites in relation to the climactic conditions in the study area. The first axis of the PCA (PC1) explained ~ 50% of the variation in the bioclimatic dataset and segregated the ponds from the Coastal Plain into two subgroups (Fig S1A), generally corresponding to the distribution of the vegetation types occurring in the Coastal Plain (restinga and ombrophilous forest, Fig. 1). Based on this ordination, we posteriorly classified the two subsets of ponds as ‘Pampean’ and ‘Atlantic Forest’ sub-regions and separately assessed in the subsequent analyses. In general, the climate in the region covers a gradient of increasing temperatures and more seasonal precipitation to the northern areas in the region (Fig S1B).
-
3.
We employed Moran’s eigenvector Maps (MEM) (Borcard et al. 2011) as spatial predictors (proxies of spatial processes). The MEM method is based on a pairwise distance matrix containing Euclidean distances between sites. This matrix is then truncated at the smallest distance connecting all sites. We used as criterion for truncation the minimum spanning tree as threshold value (default option of the dbmem function). A principal coordinate analysis (PCoA) was conducted and the positive eigenvectors retained were considered as spatial predictors.
The spatial configuration of the local environmental predictors could also potentially bias our results (if nearer ponds had similar environments, they could potentially harbor similar species composition). Therefore we conducted multivariate Mantel correlograms (Borcard and Legendre 2012; Legendre and Legendre 2012) to check for spatial autocorrelation in the dataset of local environmental predictors. We proceeded as follows: (1) we first standardized (zero-scaled mean and unit variance) the matrix of local environmental predictors (water chemistry and habitat structure). (2) we computed Mantel statistics (Pearson’s r coefficient) between the Euclidean distance matrix of the standardized local environmental predictors and a matrix containing the geographical distances between ponds. Mantel statistics were computed for distance classes d to form the Mantel correlogram. We used Sturge’s rule to compute the number of distance classes that included all ponds. Mantel statistics were tested for significance using 999 permutations using the progressive Holm’s correction method. We also ran the correlograms for the subsets of ponds from each sub-region (Pampa and Atlantic Forest). Spatial autocorrelation was not detected (p > 0.05) in any of the three datasets (full dataset of the Coastal Plain, Pampa and Atlantic Forest sub-regions). Multivariate Mantel correlograms were computed with the mantel.correlog function in the package vegan (Oksanen et al. 2019).
Data analysis
We first tested for differences in tadpole composition between sub-regions. For this purpose, we performed a non-parametric multivariate analysis of variance followed by 9999 permutations (PERMANOVA). We used as response variable a dissimilarity matrix (Bray–Curtis index) computed from the log-transformed abundance matrix of the fauna.
We calculated beta diversity for our dataset based on a presence–absence matrix. We used the Jaccard dissimilarity index (βjac) to calculate beta diversity. Afterwards, we decomposed βjac into turnover (βsim) and nestedness-resultant components (βnes), using the Podani family decomposition index (Legendre 2014) to avoid overestimation of the turnover component (Carvalho et al. 2013). βjac, βsim and βnes were used as the response variables in the analyses. Beta diversity matrices were computed with the beta.div.comp function from package adespatial (Dray et al. 2019). We checked for spatial autocorrelation in tadpole composition through multivariate Mantel correlograms (using βjac as response matrix). We also ran the correlograms for the subset of ponds from each sub-region (Pampa and Atlantic Forest). The procedures used for computation of the correlograms for βjac were similar to the ones undertaken for the local environmental predictors (see previous subsection).
We assessed the relative importance of spatial and local and climatic predictors to beta diversity (and its components) patterns using distance-based redundancy analysis (db-RDA) (Legendre and Anderson 1999). This approach allows the usage of dissimilarity matrices as response variables while retaining the basis of canonical analyses (Legendre and Legendre 2012). We used square-rooted PCoA axes derived from each dissimilarity matrix (βjac, βnes and βsim) as dependent variables, as dissimilarities were non Euclidean (Legendre 2014).
Prior to the fitting of the full models, we ran forward selection procedures on each explanatory matrix (local environmental predictors, bioclimatic PCA scores, spatial predictors) (Blanchet et al. 2008). This procedure searches for the subset of variables in the explanatory matrices that best explain variation (R2adj. values) in each response matrix (βjac, βnes and βsim). In each forward selection procedure (local environmental predictors, bioclimatic PCA scores, spatial predictors), we employed as stopping criteria for selection of variables a significance value no higher than 0.05 and R2adj. values of each explanatory variable no higher than the model containing all variables of each explanatory matrix. The matrix of local environmental predictors (water chemistry and habitat structure) was scaled to zero mean and unit variance prior to the assessment.
We ran db-RDAs for each response matrix (βjac, βnes and βsim) with the selected spatial and environmental variables retained by forward selection procedures. The significance of the full model was assessed after 999 permutations, and the explained variance was estimated with the adjusted R2 values (Peres-Neto et al. 2006). Finally, in cases where spatial predictors and environmental predictors were included in the full model, we assessed the individual and joint contribution of each fraction using variation partitioning approach (Legendre and Legendre 2012). We repeated these procedures three times: first for the full dataset of the Coastal Plain; second, for the subsets from each sub-region (Pampa and Atlantic Forest).
Forward selection procedures and the MEM matrix were respectively computed with the functions forward.sel and dbmem from package adespatial version 0.3-7 (Dray et al. 2019). PERMANOVA, db-RDAs and the variation partitioning were respectively run with the adonis, dbrda and the varpart functions from package vegan version 2.5-5 (Oksanen et al. 2019). All response matrices (Bray-Curtis distance matrix, βjac, βnes and βsim) were computed from the pooled data of the two collections. A synthetic graphical abstract describing the main components of beta diversity and the sampling design of the study (broad extent and the main vegetation type in each sub-region of the Coastal Plain) is provided in Fig. S2. A workflow summarizing all the undertaken statistical steps is provided in Fig. S3. All analyses were carried out in the statistical environment R v. 3.5.1 (R Core Team 2019).
Results
We collected 986 specimens from 28 species and five families (Table S2). The richest family was Hylidae (14), followed by Leptodactylidae (10), Bufonidae (2), Odontophrynidae (1) and Microhylidae (1). Scinax squalirostris (Lutz 1925) was the most abundant species, corresponding to 27% of all individuals collected. Multivariate Mantel correlograms did not detect spatial autocorrelation in tadpole composition (p > 0.05) in any of the assessed datasets (Coastal Plain; Pampa and Atlantic Forest sub-regions; Fig S4). Differences in tadpole composition between sub-regions were not detected by PERMANOVA (pseudo-F1,22 = 0.69; R2 = 0.03, p = 0.75).
The overall beta diversity (βjac) in the study region was 0.396. The contribution of the turnover component was higher than the nestedness component [βsim = 0.212 (53.6%); βnes = 0.184 (46.4%)]. A similar pattern was observed for each sub-region [Pampa: βjac = 0.388 (53.6%); βsim = 0.205 (52.9%); βnes = 0.183 (47.1%); Atlantic Forest: βjac = 0.407 (52%); βsim = 0.212 (52.9%); βnes = 0.195 (48%)]. Beta diversity and its components were significantly related to environmental (local and climatic) and spatial predictors. βjac and βsim were significantly influenced (p < 0.05) only by broad-scale spatial predictors (MEM3), while a marginally significant influence of MEM1 was detected on βnes (p = 0.058; Table 1) The selected predictors accounted for a small amount of explained variance (βjac = ~ 3.5%, βsim = ~ 2.6%; βnes = ~ 1.8%) (Table 1; Figs. 2a, 3a–c).
Bar plot graphs indicating the unexplained and explained fractions of variance of tadpole beta diversity (and its turnover and nestedness-resultant component) accounted for by spatial and environmental (local and climate) predictors in intermittent ponds in the southern Brazilian Coastal Plain. Variation was partitioned for all the ponds at the broadest extent in the study region (Coastal Plain; N = 24; a) and separately for the ponds within each sub-region of the Coastal Plain (Atlantic Forest; b and Pampa; c)
Biplots of the relationships between beta diversity (and its turnover and nestedness components) and the spatial and environmental (local and climate) predictors across the scales of analysis (a–c: Coastal Plain; d–f: Atlantic Forest sub-region; g–i: Pampa sub-region. MEM Moran eigenvector map, PC principal component axis, LongH long hydroperiod
Within the Pampa and Atlantic Forest sub-regions, different relationships were detected between tadpole beta diversity and environmental and spatial predictors. In the Atlantic Forest sub-region, local (pond area and connectivity) and climatic environmental predictors (PC1), along with broad-scale (MEM1) spatial predictors explained ~ 24% of the variance of βjac. Local (pond area, connectivity and EC) and climatic environmental predictors (PC1), along with broad-scale spatial predictors (MEM1), accounted for ~ 19% of βsim. Local and climatic environmental (pH and PC4, respectively) and finer-scale spatial predictors (MEM4) explained ~ 9% of the variance of βnes (Table 1; Figs. 2b, 3d–f). In the three cases of the Atlantic Forest subset (βjac, βsim and βnes), only the fraction comprised by the local environmental predictors was statistically significant (p < 0.05) (Table 1). In the Pampa sub-region, pure contributions of broad-scale spatial (MEM1) and local environmental predictors (EC) influenced βjac, with a higher contribution of the former (~ 12% and ~ 9%, respectively; p < 0.05). βsim was influenced by EC and βnes, by hydroperiod. In both cases the R2adj. values were similar (~ 7%) (Table 1; Figs. 2c, 3g–i).
Discussion
General patterns of tadpole metacommunity structure
In this study, we detected significant contributions of local and regional environmental predictors (water chemistry and habitat structure; climate, respectively) along with pure contributions of spatial predictors to the beta diversity of tadpole communities. In general, our results indicate that anuran metacommunity structure was jointly assembled by niche-based and spatial processes in subtropical temporary ponds. Several authors demonstrated that amphibian composition is synergistically driven by environmental and spatial predictors related to niche-based and spatial assembly processes (Both et al. 2009, 2011; Rojas-Ahumada et al. 2012; Landeiro et al. 2014; Melo et al. 2014; Provete et al. 2014; Luiz et al. 2016; Leão-Pires et al. 2018). However, our observed patterns changed according to the spatial extent (i.e., environmental and spatial predictors had distinct relationships with tadpole beta diversity at the broadest extent and within each sub-region in the Coastal Plain). Spatial predictors solely influenced beta diversity at the broadest extent (Coastal Plain region). In turn, spatial and local environmental predictors influenced beta diversity in the Pampa (grassland) sub-region, while in the Atlantic Forest sub-region, local and regional environmental predictors were significant. Furthermore, spatial and environmental predictors differently influenced each component of beta diversity (turnover and nestedness) and, in this context, we thus provided evidence for a complex interplay of niche-based and spatial processes in the assembly of anuran metacommunity structure, at least at the geographical extent covered in this study.
At the broadest extent (Coastal Plain), the hypothesis that spatial processes would be the main process affecting anuran composition was loosely corroborated. The broad-scale spatial predictors (MEM1, MEM3) were the sole drivers of tadpole beta diversity and its components. The pure influence of spatial predictors (i.e., spatial processes) on metacommunity structure is usually assigned to the role of dispersal limitation (De Bie et al. 2012; Heino et al. 2015). In general, dispersal limitation is assumed to strongly influence amphibian community structure, because many species have small home ranges, while others are highly philopatric, remaining close to breeding sites (Sinsch 1990; Stebbins and Cohen 1995; Wells 2007). Despite the poor knowledge of movement ecology for South American amphibians, the few available data for Neotropical species also indicate small home ranges (Wells 2007; Table 6.5) and restricted movement patterns, including species from coastal and forest subtropical habitats, generally within 1 km (Oliveira et al. 2016; Pereira and Maneyro 2016). Additionally, we did not detect spatial autocorrelation in tadpole composition in the assessed datasets. Spatial autocorrelation was found in tadpole assemblages from subtropical Brazilian forests by Provete et al. (2014), although at much narrower extents (~ 2 km) and associated with the influence of environmental predictors. However, neither local nor climatic environmental predictors influenced beta diversity at the broadest scale of analysis in this study. In this sense, the geographic distances among ponds (~ 10 km) could have been beyond the range of dispersal of some species and a priori indicating the role of dispersal limitation in our results.
Nevertheless, the explained variance of spatial predictors for the three components of beta diversity was low (less than 4%), suggesting that the role of dispersal limitation was not straightforward across species. This is because the dispersal capacity of anurans varies among species and individuals from a single population (Semlitsch 2008), even including evidences of dispersal events reaching up to 10 km in some populations (Smith and Green 2005; Semlitsch 2008). In fact, many aspects related to amphibian dispersal and migration have not been clarified and remain poorly understood for many species (Pittman et al. 2014). For instance, dispersal can be influenced by factors associated with body size, sex and age among species (Wells 2007; Sinsch 2014).
The low explained variance and the lack of influence of the environmental predictors measured could also be an outcome of non-measured factors that influence anuran occurrence. For instance, attributes of landscape configuration such as land use and metrics of connectivity with other sites are known to affect amphibian distribution (Guerry and Hunter 2002; Prado and Rossa-Feres 2014b). Such variables were not measured and could have increased the amount of explained variation in anuran composition in this study.
Another likely explanation for our results is the occurrence of stochastic events of colonization associated with the intermittent condition of wetlands. This is the main environmental contingency in ponds (Heino et al. 2015), as a subset of species fails to disperse to all sites available for colonization (Jeffries 2005; Williams 2006). Thus, the variation in composition among sites could have arisen purely as an issue related to the impossibility by a subset of species to reach suitable sites that vary in their ‘suitability’ through time. Such contingency contributes to reduce the detected influence of niche-based assembly processes in temporary ponds (Declerck et al. 2011; Heino et al. 2015) and should be enhanced at broader scales of analysis.
The relative contribution of spatial and environmental predictors to beta diversity varied between the sub-regions assessed in the Coastal Plain. This result agrees with previous studies that demonstrated that the relative importance of such predictors changed according to the study region and spatial extent (Melchior et al. 2017). In the same study, the authors indicated that the relative importance of spatial and environmental predictors also changed among forest types. Most references are restricted to tropical forests, and studies comparing the relative importance of assembly processes between subtropical forest and grassland habitats have not been conducted. Although previous studies in the southern Brazilian Coastal Plain found that anuran composition was mainly correlated with habitat-level predictors (Machado et al. 2012; Moreira and Maltchik 2012; Moreira et al. 2016), we also expected to find higher influence of spatial predictors in grasslands. This is because landscape characteristics such as forest distribution can play a key role in structuring anuran communities, as several anuran species have specialized reproductive habits, being closely associated with riparian vegetation (Hecnar and MCloskey 1997) and thus less prone to disperse across open environments (Guerry and Hunter 2002). In this study, spatial and local environmental predictors explained tadpole beta diversity in the Pampa sub-region (grasslands), while local and climatic predictors did in the Atlantic Forest sub-region. In this context, grasslands landscapes present a greater challenge to distribution and can thus explain the predominance of spatial processes in the Pampa (grassland portion) of the Coastal Plain. In turn, the predominance of spatial processes in forest ecosystems is often related to the occurrence of species with more specialized reproductive modes, with species that show terrestrial reproduction presenting stronger spatial patterns (Landeiro et al. 2014). Though, species composition did not change between biomes (as shown by PERMANOVA), suggesting that the majority of the recorded species in this study has widespread distribution in the Coastal Plain (including the other grassland and forest ecoregions in southern Brazil), and might be thus considered generalist with regards to their reproductive modes. This might be the major explanation for the lack of spatial processes within the Atlantic Forest portion of the study region.
Tadpole beta diversity and relationships with environmental predictors
Tadpole beta diversity was mainly structured by turnover in the study region. This result is in agreement with previous studies that partitioned beta diversity of tadpole communities at local and regional extents (Silva et al. 2014; Melchior et al. 2017; Knauth et al. 2018). Landscape characteristics can play a key role in promoting turnover in anuran communities. In this context, higher turnover is expected to occur in ecotonal regions (i.e., ecological transition zones) due to the wide variety of habitats that these regions provide (Ward et al. 1999; Lawler et al. 2010). In fact, broad-scale studies that covered different landscapes also showed predominance of turnover in anuran communities (Silva et al. 2014; Melchior et al. 2017). As the study area comprises a transition between different landscape types (mixed ombrophilous forest in the northern areas of the Coastal Plain region, and grasslands in the south), this contingency likely accounted for the predominance of turnover.
Additionally, distinct environmental predictors explained beta diversity (and components) within each sub-region. These results suggest that different drivers play distinct roles on anuran metacommunity structure. In the Pampa sub-region, pond hydroperiod promoted nestedness, while electrical conductivity influenced turnover. The influence of hydroperiod on the distribution of tadpole species is well known. Although more exact measures of hydroperiod length are missing due to the spatial extent of this study, recent studies showed that even small changes in hydroperiod in South American grassland ponds have been related to species loss and nested patterns of anuran composition (Both et al. 2011; Moreira and Maltchik 2012; Knauth et al. 2018). The role of the length of water availability in driving tadpole species composition can occur either directly by constraining larval development (Babbitt et al. 2003; Amburgey et al. 2012; Pintar and Resetarits 2018) or through co-variation with other important regulators of tadpole distribution such as predator (fishes, invertebrates) occurrence (Both et al. 2011; Knauth et al. 2018), factors that can prevent certain species from establishing.
Electrical conductivity was found to influence tadpole composition (Both et al. 2009; Leão-Pires et al. 2018). This variable is in some cases highly correlated with dissolved solids (Babini et al. 2017). Most authors relate the role of both variables in affecting tadpoles through changes in the environment productivity or as surrogates of pollution (Wilbur 1987; Ultsch et al. 1999; Pellet et al. 2004). In addition, Leão-Pires et al. (2018) detected changes in composition of tadpoles in ponds with different levels of conductivity. In fact, alterations in such drivers can potentially affect tadpole activities such as feeding and development (Babini et al. 2017; Leão-Pires et al. 2018), ultimately impacting their overall composition.
Local predictors (pond area and connectivity) were correlated with the turnover component in the Atlantic Forest sub-region. In our context, connectivity was considered as the physical connection with other water bodies. Previous studies showed that the occurrence of some anuran species can be affected by the unconnected condition of waterbodies (Rodrigues et al. 2010). Furthermore, connectivity is associated with increasing distances to other waterbodies, which can affect anuran community, especially through dispersal limitation (Menin et al. 2011; Landeiro et al. 2014; Prado and Rossa-Feres 2014a). Physical connectivity is also related to the frequency of hydrological disturbances, which can lead to changes in abiotic conditions and promote replacement of non-adapted species, thus acting as a strong driver of anuran beta diversity (Knauth et al. 2018; Ramalho et al. 2018).
Spatially-structured climate also influenced beta diversity in the Atlantic Forest, although with weaker explanatory power. Though many studies found that climatic gradients are important drivers of the spatial distribution of neotropical anurans (Silva et al. 2014; Vasconcelos et al. 2014; Luiz et al. 2016), this result is probably associated with the spatial extent. Melchior et al. (2017) demonstrated that the relative importance of climate to tadpole beta diversity changed according to the scale of analysis considered. Specifically, the ‘pure’ effect of climate on anuran beta diversity was restricted and detected only at biogeographic scale of analysis, and when different forest types were compared. In the same study, analyses within dense forest types (ombrophilous forest) have also showed that spatially structured variables had lower importance to anuran beta diversity than pure environmental conditions. This result is similar to Silva et al. (2014), which also demonstrated that the relative importance of long-term climate for amphibian distribution was lower in relation to current environmental condition.
Conclusions
Beta diversity was influenced by spatial and environmental (local and climate) predictors in subtropical temporary ponds. However, the relative contribution of spatial and environmental predictors to beta diversity changed with spatial extent within the study area (forest and grassland sub-regions). At the broadest extent, spatial predictors with limited explanatory power predominated in explaining beta diversity, likely due to dispersal limitation and stochastic processes associated with the intermittent condition of temporary ponds. In turn, spatial and pond-level environmental predictors explained beta diversity in the grassland sub-region, while pond-level and regional environmental predictors (spatially-structured climate), in the forested sub-region.
We provided evidence that anuran metacommunity structure was jointly assembled by niche-based and spatial processes along a forest–grassland transition, although the relative contribution of assembly processes to tadpole metacommunity structure varies between landscapes with different vegetation types. Our results thus suggest a complex dynamics of niche-based and spatial assembly processes to anuran metacommunity structure. Such intricate relationships can be explained by the multiple processes operating along the life history of amphibians. On account of the complex life cycle and the vastly different ecological requirements of adult and larval stages of amphibians, the relative influence of each assembly process structuring adult and tadpole assemblages likely changes among life stages, yet they are somehow linked to one another. For instance, the predominance of spatial predictors and spatially-structured climate can be the outcome of processes influencing adults. This is because the adult stage is responsible for the dispersal among patches and because adults are highly constrained by physiological tolerances related to climate (Silva et al. 2014; Leão-Pires et al. 2018). The relationships between local environmental predictors and beta diversity, in turn, more likely reflect effects on the tadpole stages, which are more prone to respond to pond-level features such as habitat structure and water chemistry (Provete et al. 2014).
Our results can be useful for policies aiming at anuran conservation in temporary ponds. For instance, the higher importance of climate in the forest landscape suggests that communities from forested habitats could be more sensitive to the forecasted climatic changes for the next decades rather than communities inhabiting open landscapes. In addition, the higher importance of spatial predictors in the grasslands suggests that reduction of forested habitat could increase the role of dispersal limitation in the assembly of the local composition of anuran communities.
References
Amburgey S, Funk WC, Murphy M, Muths E (2012) Effects of hydroperiod duration on survival, developmental rate, and size at metamorphosis in boreal chorus frog tadpoles (Pseudacris maculata). Herpetologica 68:456–467. https://doi.org/10.1655/HERPETOLOGICA-D-11-00093
Andrade DV, Bevier CR, Carvalho JE (2017) Amphibian and reptile adaptations to the environment: interplay between physiology and behavior. CRC Press, Boca Raton
Angeler DG (2013) Revealing a conservation challenge through partitioned long-term beta diversity: increasing turnover and decreasing nestedness of boreal lake metacommunities. Divers Distrib 19:772–781. https://doi.org/10.1111/ddi.12029
Babbitt KJ, Baber MJ, Tarr TL (2003) Patterns of larval amphibian distribution along a wetland hydroperiod gradient. Can J Zool 81:1539–1552. https://doi.org/10.1139/z03-131
Babini S, Martina LC, Luque E et al (2017) Anuran larvae diet from agroecosystem’s ponds: environmental quality and implications for their populations. J Limnol 76:137–147. https://doi.org/10.4081/jlimnol.2016.1455
Baselga A (2012) The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob Ecol Biogeogr 21:1223–1232. https://doi.org/10.1111/j.1466-8238.2011.00756.x
Blanchet G, Legendre P, Borcard D (2008) Forward selection of spatial explanatory variables. Ecology 89:2623–2632. https://doi.org/10.1890/07-0986.1
Borcard D, Legendre P (2012) Is the Mantel correlogram powerful enough to be useful in ecological analysis? A simulation study. Ecology 93:1473–1481. https://doi.org/10.1890/11-1737.1
Borcard D, Legendre P, Avois-Jacquet C, Tuomisto H (2011) Dissecting the spatial structure of ecological data at multiple scales. Ecology 85:1826–1832. https://doi.org/10.1890/03-3111
Both C, Solé M, Dos Santos TG, Cechin SZ (2009) The role of spatial and temporal descriptors for neotropical tadpole communities in southern Brazil. Hydrobiologia 624:125–138. https://doi.org/10.1007/s10750-008-9685-5
Both C, Cechin SZ, Melo AS, Hartz SM (2011) What controls tadpole richness and guild composition in ponds in subtropical grasslands? Austral Ecol 36:530–536. https://doi.org/10.1111/j.1442-9993.2010.02183.x
Brown BL, Sokol ER, Skelton J, Tornwall B (2017) Making sense of metacommunities: dispelling the mythology of a metacommunity typology. Oecologia 183:643–652. https://doi.org/10.1007/s00442-016-3792-1
Carvalho JC, Cardoso P, Borges PAV et al (2013) Measuring fractions of beta diversity and their relationships to nestedness: a theoretical and empirical comparison of novel approaches. Oikos 122:825–834
Chase JM, Myers JA (2011) Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc B Biol Sci 366:2351–2363. https://doi.org/10.1098/rstb.2011.0063
Cottenie K (2005) Integrating environmental and spatial processes in ecological community dynamics. Ecol Lett 8:1175–1182. https://doi.org/10.1111/j.1461-0248.2005.00820.x
Couto AP, Ferreira E, Torres RT, Fonseca C (2017) Local and landscape drivers of pond-breeding amphibian diversity at the northern edge of the Mediterranean. Herpetologica 73:10–17. https://doi.org/10.1655/HERPETOLOGICA-D-16-00020.1
De Bie T, De Meester L, Brendonck L et al (2012) Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecol Lett 15:740–747. https://doi.org/10.1111/j.1461-0248.2012.01794.x
Declerck SAJ, Coronel JS, Legendre P, Brendonck L (2011) Scale dependency of processes structuring metacommunities of cladocerans in temporary pools of High-Andes wetlands. Ecography (Cop) 34:296–305. https://doi.org/10.1111/j.1600-0587.2010.06462.x
Dray S, Bauman D, Blanchet G, Borcard D, Clappe S, Guenard G, Jombart T, Larocque G, Legendre P, Madi N, Wagner HH (2019) Package ‘adespatial: multivariate multiscale spatial analysis.’ R package version 0.3-7
Godinho MBC, Silva FR (2018) The influence of riverine barriers, climate, and topography on the biogeographic regionalization of Amazonian anurans. Sci Rep 8:3427. https://doi.org/10.1038/s41598-018-21879-9
Guerry AD, Hunter ML (2002) Amphibian distributions in a landscape of forests and agriculture: an examination of landscape composition and configuration. Conserv Biol 16:745–754. https://doi.org/10.1046/j.1523-1739.2002.00557.x
Hecnar SJ, MCloskey RT (1997) Patterns of nestedness and species association in a pond-dwelling amphibian fauna patterns of nestedness and species association in a pond-dwelling amphibian fauna. Oikos 80:371–381. https://doi.org/10.2307/3546605
Heino J (2013) Does dispersal ability affect the relative importance of environmental control and spatial structuring of littoral macroinvertebrate communities? Oecologia 171:971–980. https://doi.org/10.1007/s00442-012-2451-4
Heino J, Melo AS, Siqueira T et al (2015) Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshw Biol 60:845–869. https://doi.org/10.1111/fwb.12533
Hijmans RJ, Cameron SE, Parra JL et al (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. https://doi.org/10.1002/joc.1276
Holyoak M, Leibold MA, Holt RD (2005) Metacommunities: spatial dynamics and ecological communities. University of Chicago Press, Chicago
IBGE (2004) Mapa de Biomas do Brasil. Primeira Aproximação. Escala 1:5.000.000. http://www.terrabrasilis.org.br/ecotecadigital/index.php/estantes/mapas/563-mapa-de-biomas-do-brasil
Jeffries M (2005) Small ponds and big landscapes: the challenge of invertebrate spatial and temporal dynamics for European pond conservation. Aquat Conserv Mar Freshw Ecosyst 15:541–547
Júnior VNTB, Rocha CFD (2013) Tropical tadpole assemblages: which factors affect their structure and distribution? Oecol Aust 17:217–228. https://doi.org/10.4257/oeco.2013.1702.04
Knauth DS, Felipe L, Moreira B, Maltchik L (2018) Partitioning tadpole beta diversity in highland ponds with different hydroperiods. Freshw Sci 37:380–388. https://doi.org/10.1086/697926
Landeiro VL, Waldez F, Menin M (2014) Spatial and environmental patterns of Amazonian anurans: differences between assemblages with aquatic and terrestrial reproduction, and implications for conservation management. Nat Conserv 12:42–46. https://doi.org/10.4322/natcon.2014.008
Lawler JJ, Shafer SL, Bancroft BA, Blaustein AR (2010) Projected climate impacts for the amphibians of the Western Hemisphere. Conserv Biol 24:38–50
Leão TCC, Fonseca CR, Peres CA, Tabarelli M (2014) Predicting extinction risk of Brazilian Atlantic Forest angiosperms. Conserv Biol 28:1349–1359. https://doi.org/10.1111/cobi.12286
Leão-Pires TA, Luiz AM, Sawaya RJ (2018) The complex roles of space and environment in structuring functional, taxonomic and phylogenetic beta diversity of frogs in the Atlantic Forest. PLoS One. https://doi.org/10.1371/journal.pone.0196066
Legendre P (2014) Interpreting the replacement and richness difference components of beta diversity. Glob Ecol Biogeogr 23:1324–1334. https://doi.org/10.1111/geb.12207
Legendre P, Anderson MJ (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol Monogr 69:1–24
Legendre P, Legendre LFJ (2012) Numerical ecology, 3rd edn. Elsevier, Amsterdam
Leibold MA, Holyoak M, Mouquet N et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x
Leprieur F, Tedesco PA, Hugueny B et al (2011) Partitioning global patterns of freshwater fish beta diversity reveals contrasting signatures of past climate changes. Ecol Lett 14:325–334. https://doi.org/10.1111/j.1461-0248.2011.01589.x
Logue JB, Mouquet N, Peter H, Hillebrand H (2011) Empirical approaches to metacommunities: a review and comparison with theory. Trends Ecol Evol 26:482–491. https://doi.org/10.1016/j.tree.2011.04.009
Lucena CAS, Calegari BB, Pereira EHL, Dallegrave E (2013) O uso de óleo de cravo na eutanásia de peixes March 2013. Bol Soc Bras Ictiol 105:20–24
Luiz AM, Leão-Pires TA, Sawaya RJ (2016) Geomorphology drives amphibian beta diversity in Atlantic Forest Lowlands of southeastern Brazil. PLoS One 11:1–16. https://doi.org/10.1371/journal.pone.0153977
Machado IF, Moreira LFB, Maltchik L (2012) Effects of pine invasion on anurans assemblage in southern Brazil coastal ponds. Amphib Reptil 33:227–237. https://doi.org/10.1163/156853812X638518
Maltchik L (2003) Three new wetlands inventories in Brazil. Interciencia 28:421–423
Melchior LG, Rossa-Feres DC, Silva FR (2017) Evaluating multiple spatial scales to understand the distribution of anuran beta diversity in the Brazilian Atlantic Forest. Ecol Evol 7:2403–2413. https://doi.org/10.1002/ece3.2852
Melo M, Fava F, Pinto HB, Nomura F (2014) Are assemblages of aquatic-breeding anurans (Amphibia) niches structured or neutral? Biotropica 46:608–614. https://doi.org/10.1111/btp.12130
Menin M, Waldez F, Lima AP (2011) Effects of environmental and spatial factors on the distribution of anuran species with aquatic reproduction in central Amazonia. Herpetol J 21:255–261
Moreira LFB, Maltchik L (2012) Assessing patterns of nestedness and co-occurrence in coastal pond anuran assemblages. Amphibia-Reptilia 33:261–271. https://doi.org/10.1163/156853812X641721
Moreira LFB, Moura RG, Maltchik L (2016) Stop and ask for directions: factors affecting anuran detection and occupancy in Pampa farmland ponds. Ecol Res 31:65–74. https://doi.org/10.1007/s11284-015-1316-9
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) Package ‘vegan’. Community ecology package, version 2.5-5
Oliveira M, Aver GF, Moreira LFB, Colombo P, Tozetti AM (2016) Daily movement and microhabitat use by the Blacksmith Treefrog Hypsiboas faber (Anura: Hylidae) during the breeding season in a subtemperate forest of Southern Brazil. South Am J Herpetol 11:89–97. https://doi.org/10.2994/sajh-d-16-00017.1
Pellet J, Hoehn S, Perrin N (2004) Multiscale determinants of tree frog (Hyla arborea L.) calling ponds in western Switzerland. Biodivers Conserv 13:2227–2235
Pereira G, Maneyro R (2016) Movement patterns in a Uruguayan population of Melanophryniscus montevidensis (Philippi, 1902) (Anura: bufonidae) using photo-identification for individual recognition. South Am J Herpetol 11:119–126. https://doi.org/10.2994/sajh-d-15-00020.1
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625. https://doi.org/10.1890/0012-9658(2006)87%5b2614:vposdm%5d2.0.co;2
Pintar MR, Resetarits WJ (2018) Variation in pond hydroperiod affects larval growth in Southern Leopard Frogs, Lithobates sphenocephalus. Copeia 106:70–76. https://doi.org/10.1643/CE-17-696
Pires MM, Stenert C, Maltchik L (2018) Drivers of beta diversity of Odonata along a forest–grassland transition in southern Brazilian coastal ponds. Freshw Sci 37:357–366. https://doi.org/10.1086/697925
Pittman SE, Osbourn MS, Semlitsch RD (2014) Movement ecology of amphibians: a missing component for understanding population declines. Biol Conserv 169:44–53
Prado VHM, Rossa-Feres DC (2014a) The influence of niche and neutral processes on a neotropical anuran metacommunity. Austral Ecol 39:540–547. https://doi.org/10.1111/aec.12114
Prado VHM, Rossa-Feres DDC (2014b) Multiple determinants of anuran richness and occurrence in an agricultural region in south-eastern Brazil. Environ Manage 53:823–837. https://doi.org/10.1007/s00267-014-0241-y
Provete DB, Gonçalves-Souza T, Garey MV et al (2014) Broad-scale spatial patterns of canopy cover and pond morphology affect the structure of a Neotropical amphibian metacommunity. Hydrobiologia 734:69–79
R Core Team (2019) R: a language and environment for statistical computing
Ramalho WP, Machado IF, Vieira LJS (2018) Do flood pulses structure amphibian communities in floodplain environments? Biotropica 50:338–345. https://doi.org/10.1111/btp.12523
Rodrigues DJ, Lima AP, Magnusson WE, Costa FRC (2010) Temporary pond availability and tadpole species composition in central Amazonia. Herpetologica 66:124–130. https://doi.org/10.1655/09-020R2.1
Rojas-Ahumada DP, Landeiro VL, Menin M (2012) Role of environmental and spatial processes in structuring anuran communities across a tropical rain forest. Austral Ecol 37:865–873
Santos TG, Rossa-Feres DC, Casatti L (2007) Diversidade e distribuição espaço-temporal de anuros em região com pronunciada estação seca no sudeste do Brasil. Iheringia Série Zool 1:37–49. https://doi.org/10.1590/S0073-47212007000100007
Semlitsch RD (2008) Differentiating migration and dispersal processes for pond-breeding amphibians. J Wildl Manage 72:260–267
Silva FR, Almeida-Neto M, Arena MVN (2014) Amphibian beta diversity in the Brazilian atlantic forest: contrasting the roles of historical events and contemporary conditions at different spatial scales. PLoS One. https://doi.org/10.1371/journal.pone.0109642
Sinsch U (1990) Migration and orientation in anuran amphibians. Ethol Ecol Evol 2:65–79
Sinsch U (2014) Movement ecology of amphibians: from individual migratory behaviour to spatially structured populations in heterogeneous landscapes. Can J Zool 92:491–502. https://doi.org/10.1139/cjz-2013-0028
Smith MA, Green D (2005) Dispersal and the metapopulation paradigm in amphibian ecology and conservation: Are all amphibian populationsmetapopulations? Ecography 28:110–128. https://doi.org/10.1111/j.0906-7590.2005.04042.x
Socolar JB, Gilroy JJ, Kunin WE, Edwards DP (2016) How should beta-diversity inform biodiversity conservation? Conservation targets at multiple spatial scales. Trends Ecol Evol 31:67–80. https://doi.org/10.1016/j.tree.2015.11.005
Soininen J (2014) A quantitative analysis of species sorting across organisms and ecosystems. Ecology 95:3284–3292
Soininen J, McDonald R, Hillebrand H (2007) The distance decay of similarity in ecological communities. Ecography (Cop) 30:3–12. https://doi.org/10.1111/j.2006.0906-7590.04817.x
Stebbins RC, Cohen NW (1995) Declining amphibians. A natural history of amphibians. Princeton University Press, Princeton, pp 210–251
Ultsch GR, Bradford DF, Freda J (1999) Physiology: coping with the environment. In: McDiarmid R, Altig R (eds) The biology of anuran larvae. The University of Chicago Press, Tadpoles, pp 189–214
Valério LM, Dorado-Rodrigues TF, Chupel TF et al (2016) Vegetation structure and hydroperiod affect anuran composition in a large Neotropical wetland. Herpetologica 72:181–188. https://doi.org/10.1655/Herpetologica-D-14-00069.1
Vasconcelos TS, Santos TG, Rossa-Feres DC, Haddad CF (2009) Influence of the environmental heterogeneity of breeding ponds on anuran assemblages from southeastern Brazil. Can J Zool 87:699–707. https://doi.org/10.1139/Z09-058
Vasconcelos TS, Prado VHM, Silva FR, Haddad CFB (2014) Biogeographic distribution patterns and their correlates in the diverse frog fauna of the atlantic forest hotspot. PLoS One 9:1–9. https://doi.org/10.1371/journal.pone.0104130
Villwock JA, Tomazelli LJ (2006) Planície Costeira do Rio Grande do Sul: gênese e paisagem atual. In: Becker FG, Ramos RA, Moura LA (eds) Biodiversidade. Regiões da Lagoa do Casamento e dos Butiazais de Tapes, planície costeira do Rio Grande do Sul. Ministério do Meio Ambiente, Brasília, pp 20–33
Ward JV, Tockner K, Schiemer F (1999) Biodiversity of floodplain river ecosystems: ecotones and connectivity. River Res Appl 15:125–139
Wells KD (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Werner EE, Skelly DK, Relyea RA, Yurewicz KL (2007) Amphibian species richness across environmental gradients. Oikos 116:1697–1712. https://doi.org/10.1111/j.0030-1299.2007.15935.x
Whittaker RH (1960) Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr 30:279–338
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21:213–251
Wilbur HM (1987) Regulation of structure in complex systems: experimental temporary pond communities. Ecology 68:1437–1452
Williams DD (2006) The biology of temporary waters. Oxford University Press, New York
Winegardner AK, Jones BK, Ng ISY et al (2012) The terminology of metacommunity ecology. Trends Ecol Evol 27:253–254. https://doi.org/10.1016/j.tree.2012.01.007
Acknowledgements
This study was part of DSK Master course dissertation at the Biology Graduate Program from UNISINOS University. This research was funded by CNPq (Brazilian National Council for Scientific and Technological Development), as part of the project entitled ‘Effects of climate change on wetland biodiversity in southern Brazil: A space-time approach’ (Grant number 474892/2013-1). CAPES (Coordination for the Improvement of Higher Education Personnel) granted a Master scholarship to DSK. CNPq granted a postdoctoral research fellowship to MMP at the Biology Graduate Program from UNISINOS University (Grant number 151152/2018-7). LM and CS have been continuously funded by CNPq via Research Productivity grants.
Author information
Authors and Affiliations
Contributions
DSK collected and conducted the specimen identification and participated in the drafting of the manuscript. MMP analyzed the data and drafted the manuscript. LM and CS conceived the project and coordinated several aspects of the study, including revision of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
We declare that the data collection complied with current Brazilian environmental laws (SISBIO-n. 36365-2).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
27_2019_658_MOESM1_ESM.tiff
Fig. S1. Graphical outputs of the first two principal components (PC1 and PC2) of the principal component analysis (PCA) of the WorldClim bioclimatic variables for the 24 ponds studied. (A) Plot of the ponds; (B) plot of the correlation (loadings) of the variables. (TIFF 2741 kb)
27_2019_658_MOESM2_ESM.tif
Fig. S2. Conceptual figure summarizing the objectives of the study. (A) The two components of beta diversity (turnover and nestedness/richness; adapted from: Baselga 2012). (B) Sampling design of the study emphasizing the main vegetation types (Pampa and Atlantic Forest) in the study region (Coastal Plain) and the scales of analysis (broad extent and sub-regions). (TIFF 1306 kb)
27_2019_658_MOESM3_ESM.tif
Fig. S3. Step by step representation of the undertaken statistical procedures for the analyses of the relationships between tadpole beta diversity and spatial predictors and environmental drivers. (TIFF 1290 kb)
27_2019_658_MOESM4_ESM.tiff
Fig. S4. Graphic outputs of the multivariate Mantel correlograms used for the testing of spatial autocorrelation in each dataset (Coastal Plain, Atlantic Forest and Pampa subsets). (TIFF 1129 kb)
Rights and permissions
About this article
Cite this article
Knauth, D.S., Pires, M.M., Stenert, C. et al. Disentangling the role of niche-based and spatial processes on anuran beta diversity in temporary ponds along a forest–grassland transition. Aquat Sci 81, 63 (2019). https://doi.org/10.1007/s00027-019-0658-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-019-0658-8