Abstract
Context
The study of habitat fragmentation is fraught with definitional and conceptual challenges. Specifically, a multi-scale perspective is needed to address apparent disagreements between landscape- and patch-based studies that have caused significant uncertainty concerning fragmentation’s effects.
Objectives
Here we test the hypothesis that habitat fragmentation alters biological communities by creating hierarchically nested selective pressures across plot-, patch-, and landscape-scales in the Thousand Island Lake, China. We assess the variation in fragmentation-diversity relationships contextualize the interplay of scale-dependent relationships.
Methods
This work utilized two datasets. At the island scale, islands were sampled to adequately capture the vast majority of species on each island using 5 × 5 m sampling cells. To capture intra-island variation, we sampled 400 m2 plots set up along an edge gradient on each island. The variation in species richness at each scale was then compared using Wilcoxon tests, Spearman rank correlations, generalized linear models, and single-large-or-several-small (SLOSS) simulations.
Results
We find that edges have little impact on plot α-diversity and between-plot β-diversity, but increase the amount of β-diversity that can be attributed to nestedness at the plot-scale. We also find that the percent habitat in the surrounding landscape has a positive effect on species richness at the patch-scale and that small islands accumulate species faster than large islands of equal total size at the landscape-scale.
Conclusions
By observing interdependent effects at each scale, we find support for the hypothesis that habitat fragmentation’s effects are hierarchically structured. Therefore, multi-scale approaches are needed to understand the patterns, processes, and consequences of fragmentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The question of why smaller, more isolated patches have fewer species than those that are larger and less isolated has intrigued biologists for generations. By the middle-to-late 1700s, European explorers had already recognized the fundamental components of what would become the field of island biogeography, including the species-area and species-isolation relationships (Lomolino et al. 2010). However, while these relationships were studied extensively over the following 200 years (Lomolino et al. 2010), a sufficiently parsimonious and dynamic explanation for their existence was not proposed until the publication of MacArthur and Wilson’s The Theory of Island Biogeography (IBT) in 1967.
MacArthur and Wilson’s accomplishment was far reaching, and only shortly after the publication of IBT, ecologists and conservation biologists began applying IBT principals to the study of habitat fragmentation (Laurance 2010) with the assumption that spatially isolated patches of habitat were in fact very similar to islands, embedded in a sea of human activity rather than the literal ocean. However, the application of IBT principals to habitat fragmentation has resulted in significant definitional and conceptual challenges, the most important of these being the scale at which habitat fragmentation is studied. The most commonly used definition of habitat fragmentation is that habitat fragmentation is the process by which large continuous tracts of habitat are broken up into smaller, more isolated remnant patches (e.g., Didham 2010; Wilcove et al. 1986; Wu 2009). This suggests that fragmentation is a landscape-scale process (Fahrig 2003, 2017), which may drive biological changes at other scales (Didham et al. 2012; Lafortezza et al. 2010; Valdés et al. 2015). However, many studies of fragmentation have occurred at the scale of the fragment, comparing the communities within patches to themselves and each other (Fahrig 2003). Such studies have consistently found that habitat fragmentation has strong negative effects on most ecologically relevant parameters (Haddad et al. 2015). In particular, the study of communities within patches has shown that edges can have marked effects on community composition (e.g., Laurance et al. 2006; Laurance et al. 2011), which suggests that fragmentation’s effects on community structure are at least partially deterministic (e.g., “species sorting” in Holyoak et al. (2005)).
However, extrapolating relationships from one scale to others is an uncertain proposition. Spatial scale-dependence is ubiquitous in ecology in different forms. Not only landscape metrics, but also some basic ecological patterns can vary across scales, such as the species-area relationship (Crawley and Harral, 2001). To take an obvious example, while it is well established that small fragments have decreased α-diversity relative to large fragments, a recent review by Fahrig (2017) found that more spatially fragmented landscapes often have more γ-diversity than less spatially fragmented landscapes if the total amount of habitat was held equal, suggesting that landscape configuration plays a more important role than patch-level effects in controlling γ-diversity at the regional scale. Further, studies which attempt to integrate spatial information from multiple scales often only predict fragmentation effects at a single scale, sometimes very local, and use the effects at this scale to assess fragmentation-mediated impacts at other scales (e.g., Bennett et al. 2006; Fardila et al. 2017; Lafortezza et al. 2010). While such studies are certainly interesting and relevant to conservation planning, they do not explore the scale-dependence of fragmentation’s effects.
Missing from this debate is a recognition that the variables which control biological responses to fragmentation likely vary across scales. An alternative hypothesis is that fragmentation’s effects on biological communities are hierarchical and scale-dependent (Lomolino 2000, Fig. 1), which is consistent with the Hierarchical Patch Dynamics Paradigm (Wu and Loucks 1995). Such a hypothesis would suggest that at very small spatial scales, community composition should be controlled by both local abiotic filters and the relative fitness of the species competing for resources. Larger, less isolated patches should have more diverse species assemblies due to IBT principals, strengthening inter-specific competition, and more total niche space, as they contain more types of habitat, allowing for divergent community compositions within the patch. These local and patch factors will interact with each other to alter the relative diversity of patch communities, which will in turn control regional diversity. While the proceeding sentences described the hierarchical structure from the bottom up, a top-down description would be equally valid: if there are no species in the regional pool that can survive in the niche space that fragmentation creates, niches at local and patch scales will go unoccupied. This suggests that fragmentation’s effects on biodiversity are constantly interacting within and across scales, dynamically shaping and modifying communities at all scales at all times.
Here we test the hypothesis that fragmentation modifies biological communities through hierarchically nested, scale dependent controls by simultaneously assessing fragmentations effects on woody plant communities at plot-, patch-, and landscape-scales in Thousand Island Lake, China. Specifically, we highlight the linkages between several fragmentation-species diversity relationships across multiple scales in our study system, providing further evidence that the scale of analysis is a critical component in the interpretation of studies of the biological consequences of habitat fragmentation. Finally, we close by calling for more truly multi-scale assessments of the biological consequences of habitat fragmentation.
Methods
Data sources and sampling
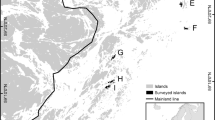
Thousand Island Lake (TIL) is a large, manmade lake located in Chun’an County of Zhejiang Province, China, with a sub-tropical monsoonal climate. Forests throughout the TIL region have long been modified by humans. These modifications culminated in the late 1950s, when trees were completely or near-completely clear-cut prior to lake formation. Lake formation resulted in the isolation of more than 1000 habitat remnants, which have since undergone secondary succession. The age of contemporary forests in the lake region are similar (approximately 60 years old, unpublished tree ring data), roughly corresponding to the end of the Great Leap Forward and the implementation of immigration policies for local people during dam construction (Wilson et al. 2016).
This work uses two datasets of woody plant richness and abundance. At the island-scale, we utilize a version of the woody plant dataset introduced in Hu et al. (2016) updated to correct misidentifications recognized in subsequent sampling. This dataset measured woody plant community structure on 29 islands in 2009–2010 by tagging and identifying each plant with diameter at breast height greater than or equal to 1 cm within 5 × 5 m sampling cells. The criteria for island selection and the detail design of the field work were described in Hu et al. (2016). Total sampled area for each island ranged from 0.02 hectare (ha) to 1.5 ha, and rarefaction analysis has shown that this dataset is sufficient for capturing the vast majority of species on the islands (Yu et al. 2012).
To capture the effects of local bioclimatic variation on communities and test fragmentation effects at the plot scale, we chose to focus on intra-patch edge effects, which are known to be a dominant sub-patch process controlling fragmentation-mediated effects (Haddad et al. 2015; Laurance et al. 2006; Laurance et al. 2011). To capture variation in community structure from patch edges to patch interiors 400 m2 sampling plots identical to those described above were set up along an edge gradient on 28 islands. Small islands, those smaller than one ha when water level is at 105 m above sea level, received one to two plots. Large islands, those greater than one ha when water level is at 105 m above sea level, received two additional plots, each more than 40 m from the island edge. To account for its much greater size, the largest island received 5 additional plots, all more than 40 m from the island edge, and one additional edge plot. The division between small and large islands was selected because a study in a similar forest identified that one ha is the minimum size for patches to maintain interior communities (Young and Mitchell 1994). Sampling for these data took place over the summers of 2014 and 2015.
As mentioned above, one of the islands is far larger (two orders of magnitude, JSE, Fig. 2) than the second largest island. To avoid overleveraging this single data point, JSE was eliminated from all analyses that used island size as a continuous variable. JSE was retained in all other cases.
Plot-scale effects
Plot-scale effects were measured as the difference in α- and β-diversity between edge and interior plots. α-diversity was measured using two complementary processes: first, as the difference in α-diversity between edge and interior plots regardless of island identity using Wilcoxon rank-sum test, and second, as the difference in mean α-diversity, paired by island. As using average pairwise comparisons of community composition can be misleading (Baselga 2013), β-diversity was measured using the Sorensen form of Baselga’s multi-site dissimilarity index (Baselga 2010 2017). Baselga’s multi-site β-diversity assesses the variation in community composition within a group and decomposes these dissimilarities into their nestedness and turnover components. Multi-site dissimilarities were assessed using the ‘betapart’ package (Baselga and Orme 2012) in R (R Core Team 2013). Because computing multi-site dissimilarities requires equal sample sizes, the number of edge plots selected had to be reduced to the maximum number of interior plots (n = 23). This was done using the function ‘beta.sample,’ which iteratively and randomly samples sites to provide dissimilarities for each sample. To assure accuracy, this random sampling was done with 100 iterations and multi-site beta-diversity was taken as the average of these 100 random samples. To assure that our results were not skewed by using presence/absence data, we also repeated this analysis using abundance-based data (see Appendix S1).
To measure the effect of island size on plots-level community composition, we first divided islands into small and large groups, using the one ha line of demarcation described above. The variation in plot mean α-diversity was assessed using a Spearman rank correlation and the difference in plot β-diversity for small and large islands was then assessed using the same methods described above for measuring edge effects. In this case sample size had to be limited to the number of plots on small islands (n = 28).
Island-scale effects
The effects of habitat fragmentation on woody plant communities at the island-scale is well studied in the TIL system (e.g., Hu et al. 2011; Hu et al. 2016; Yu et al. 2012). These works have found that area effects dominate the response of plant communities to habitat fragmentation (see Wilson et al. 2016). Beyond species richness, Liu et al. (2018) studied community dissimilarity (β-diversity) at the island scale and found that woody plant communities on small islands were less similar to one another than large islands when the number of individuals was controlled for. Rather than replicating this deep literature, we elected to simply create a semi-log species-area curve to illustrate the fragmentation effects at the island-scale.
Due to the lack of application of high-quality remote sensing imagery, few studies have explored the impact of the surrounding landscape on TIL islands beyond relatively coarse isolation metrics (e.g., distance to mainland, distance to nearest neighbor). Therefore, we elected to quantify the impact of landscape composition and configuration on island-scale species richness after first controlling for island size using high resolution SPOT-6 satellite imagery. To do so, we selected three landscape variables: (1) Percent habitat (vegetated areas) in the landscape, (2) Landscape Shape Index, (3) Coefficient of variation of habitat patch size. These indices were selected to represent landscape composition, agglomeration, and diversity, respectively, with the assumptions that patches surrounded by more habitat would have a larger pool of potential immigrants, landscapes which were more agglomerated would have more interior areas, but also potentially fewer species (Fahrig 2017), and that landscapes with a larger diversity of patch sizes would have a more diverse species pool. Each of these metrics was measured for landscapes within 500, 1000, 1500, 2000, 2500, and 3000 m buffers around each island using FRAGSTATS 4.2 (McGarigal et al. 2012). For the purposes of our study we considered all patches within the lake as potential habitat, digitalizing the vegetated edge of each island at the 1:5000 scale in ArcGIS 10.3 from high resolution (SPOT-6) satellite imagery. When buffers overlapped with the mainland, mainland habitat and matrix patches were classified from the same satellite image. Specifically, the mainland classification was implemented with ENVI 5.3 using the IsoData unsupervised classification for 10-15 initial classes and a maximum of 10 iterations. The resulting classes were then merged into two: habitat and the matrix. Post-classification processing, including interactive class editing, majority/minority analysis, clumping, and sieving, was conducted to refine and finalize the classification map. Confusion matrix analysis indicates that the final classification map has an overall accuracy of 98.10% and a Kappa coefficient of 93.21%. We assessed the impact of these variables at each buffer size on species richness after controlling for island area by calculating the residual of the semi-log species area relationship using generalized linear models of two forms, one which used three landscape variables to predict residual island species richness, and a simplified model which only used the percent of habitat in the landscape (Table 1). Model quality was assessed using the Akaike Information Criterion (Akaike 1973).
Landscape-scale effects
An assessment of landscape-scale fragmentation effects requires controlling for the total amount of habitat in the landscape (Fahrig 2017). To do this, we performed a single-large or several-small (SLOSS) analysis, comparing two sets of virtual landscapes, one created by aggregating both island area and species richness from the smallest island to the largest island, and another created by aggregating from the largest island to the smallest island. Such analyses are commonly used to study the landscape-scale effects of habitat fragmentation (Fahrig 2017), resulting in two dichotomous extremes, with the small to large aggregation representing the virtual landscape which has undergone the most habitat fragmentation per se, while the large to small aggregation has undergone the least habitat fragmentation per se for any given amount of habitat.
Results
Plot-scale results
When comparing edge-plots and interior-plots without controlling for island identity, plots near island edges tended to have fewer species than those in island interiors (p = 0.03, Fig. 3a). However, when comparing mean plot species richness in islands large enough to contain interior areas, this difference became negligible and statistically non-significant (p = 0.56, Fig. 3b). Further, plant communities in edge and interior plots were approximately equally dissimilar to one another (βsor = 0.83 vs. 0.81, Fig. 4a). β-diversity was primarily dependent on turnover, not nestedness, between plots, though the proportion of total β-diversity caused by the nestedness was 42% higher in edge plots than in interior plots. These results did not change when β-diversity was assessed using compositional metrics, though nestedness became slightly more important when β-diversity was measured using abundance metrics compared to presence/absence metrics (Appendix S1).
Mean plot species richness for edge and interior plots. (a) Mean plot species richness when all plots are pooled regardless of island identity. Error bars represent two standard errors of the mean. Asterisks represent statistical significance (p < 0.05). (b) Mean plot species richness of edge and interior plots paired by island. Dotted lines represent island pairs and the solid line represents the median
(a) Plot dissimilarity of edge and interior plots measured using the multi-site Sorensen family of metrics, with SIM representing the turnover component and SNE representing the nestedness component. (b) Plot dissimilarity from plots on large and small islands measured using the multi-site Sorensen family of metrics, with SIM representing the turnover component and SNE representing the nestedness component
Island size had a weak, but significant positive effect on mean plot species richness (Spearman correlation coefficient = 0.40, p = 0.04). Further, plots on large and small islands were approximately equally dissimilar from one another (βsor = 0.84 vs. 0.85 for plots on large vs. small island, respectively, Fig. 4b). Similar to edge vs. interior comparisons, plot β-diversity was primarily dependent on turnover (92–93% of total β-diversity) between plots, with the nestedness component only making up 7–8% of the observed β-diversity. Again, similar to edge vs. interior comparisons, these results did not change when β-diversity was assessed using compositional metrics, though nestedness became slightly more important when β-diversity was measured using abundance metrics compared to presence/absence metrics (Appendix S1).
Island-scale results
As expected, island species richness significantly increased with island area (R2 = 0.73, p < 0.001, Fig. 5).
We found that the inclusion of landscape configuration metrics did not improve the ability of our models to predict residual island species richness at any scale (Table 1), and therefore elected to utilize a simplified regression model that excluded configuration metrics. In doing so, we found that residual species richness was positively correlated with the amount of habitat surrounding each island (Table 1) at 500 m, 1000 m, and 1500 m buffer sizes, with the best fit (lowest AIC) occurring at the 1000 m buffer size. In no buffers was the percent habitat in the landscape correlated to island size (Spearman’s correlation coefficient range: − 0.07 to 0.16, p-value range: 0.42 to 0.96 for all buffer sizes).
Landscape-scale results
When total habitat was controlled, virtual landscapes consisting of small islands had more species than virtual landscapes of large islands (Fig. 6). Further, the shapes of the species-accumulation curves were far different from one another, with the smallest to largest agglomeration presenting a roughly logarithmic curve, and the largest to smallest agglomeration presenting a roughly exponential curve (Fig. 6). Therefore, the most rapid periods of species accumulation (as a function of total habitat area) occurred when relatively small islands were added to the virtual landscape regardless of aggregation method.
Discussion
Scale specific results
Though designed to show the scale-dependence of fragmentation-mediated effects on biological communities, our study revealed several interesting results within each scale.
At the plot-scale, we found that edge effects slightly reduce species richness when island identity is ignored, but have no effect when edge and interior plots within a given large island are compared (Fig. 3). This suggests that the plot-level richness was more decreased on the small islands, which do not have interior habitat, indicating that edge effects were influenced by island-scale effects, such as the area effect. This is reinforced by our finding that plot species richness is positively correlated with island area. The reversed pattern with higher richness at edge was also detected on several large islands. We speculate that this pattern could be caused by the longer perimeter capturing more immigrants to increase plant richness at edge and stronger internal disturbance (e.g. herbivory), decreasing interior plant richness on these large islands. Further, we found that plots on large islands were not more different from one another than plots on small islands were from one another, despite the fact that large islands contained both edges and interiors. This is contrast with the results from the Biological Dynamics of Forest Fragments Project (BDFFP), which found that edge areas had significantly higher turnover rates than interiors (Laurance et al. 2006). One possible explanation for this result is that habitat heterogeneity between plots on small islands and plots on large islands was approximately equal, which is reasonable given that plots were aggregated by island size regardless of their spatial location. However, the proportion of β-diversity due to nestedness increased by 42% in edge plots relative to interior plots, which suggests that species composition was more deterministic in edge areas than interior areas. Further, nestedness was always a larger component of beta-diversity when measured using abundance based, than presence-absence based, metrics (Appendix S1). This could be because non-competitive species persist in small numbers but cannot establish populations in locations where they are at a competitive disadvantage, which suggests that some amount of species sorting is occurring in our plots. In contrast, we found that plots on large islands were no more different from one another than plots on small islands were from one another, despite the fact that large islands contain both edge and interior plots. This is also at odds with many other studies of fragmentation, which have often shown large differences between edge and interior assemblages (see Murcia (1995) for details and other idiosyncrasies in the edge-effect literature). However, this result appears to be robust to our relatively coarse grain analysis (400 m2 plots), as Liu et al. (2018) found no effect of island size on intra-island β-diversity when working at smaller spatial grains (25 m2 plots). Further, Liu et al. (2018) found that communities on small islands were more different from one another than communities on large islands when the number of individuals was controlled for. These findings could be viewed as disagreeing with our results. However, this is likely classic scale-effect, as comparing plots within islands to one another is a comparison across a gradient of proportional edge, while comparing islands to one another is a comparison across a gradient of richness.
We also found that the landscape surrounding each island had a meaningful effect on island species richness, however we did not find the direct effects of landscape configuration meaningfully increased the predictive power of our models. Therefore, we elected to simplify our model. This finding is in broad agreement with Fahrig’s (2003) classic review, which found that fragmentation per se is a weak control of fragmentation-mediated effects on biological communities relative to habitat loss at any particular time step. However, it is important to note that our model simplification was done not because we ignore the indirect effects of habitat loss on the spatial components of fragmentation (e.g., Didham et al. 2012), but because configuration metrics from our specific case did not add to the explanatory power of our models. Had the inclusion of configuration metrics significantly improved model performance, a more detailed structural equation modeling approach, such as that suggested by Didham et al. (2012), would have been appropriate.
Finally, by comparing virtual landscapes of equal total habitat we found that groups of small islands had significantly more species than groups of large islands. Though this may be surprising to many, our finding is in agreement with Fahrig’s (2017) literature review, which found this pattern to be nearly universal. As small islands had more β-diversity than larger islands (Liu et al. 2018), this response was expected. However, it is important to note that these virtual landscapes can only capture the results of possible habitat loss given current conditions, and cannot project how the loss of small vs. large patches would impact the long-term dynamics of the system. For example, although large patches are relatively similar to one another and that groups of large patches accumulate species more slowly than groups of small patches, it is possible that these large patches may serve as important source populations for interior species. As we know that fragmentation’s effects on community composition can be time-dependent (Collins et al. 2017), it is critical that SLOSS findings are confirmed through experiments which monitor fragmentation-mediated responses through time, before they be integrated into conservation planning.
Habitat fragmentation’s hierarchical effects
Our core hypothesis was that habitat fragmentation creates hierarchically nested selective pressures across plot-, patch-, and landscape-scales. By observing meaningful variation in biological communities, across and between all three of these levels, we find support for this hypothesis.
Studying community responses at all three scales simultaneously had a significant impact on the interpretation of our results. Had our study simply focused on patch and sub-patch processes, we would have concluded that small patches were of little conservation value, though our landscape scale analysis showed quite the opposite. Similarly, had we focused only on the local landscape around each patch, we would have concluded that fragmentation per se had no measurable effect on species richness, though our SLOSS analysis suggests that habitat fragmentation per se could be a significant driver of regional diversity. Therefore, it is only by studying the impacts of fragmentation simultaneously at all of these scales, could we recognize how biological communities respond to interacting gradients of intra-patch, inter-patch, and landscape-scale controls.
Our study is substantially different from those which use independent variables generated at multiple scales to predict fragmentation effects at a single spatial scale, and use effects at this scale to assess fragmentation-mediated impacts at other scales (e.g., Lafortezza et al. 2010). Our aim was not to quantify community controls at a given scale, but rather to establish that the impacts of these controls are scale-dependent. Valdés et al. (2015) performed a somewhat similar multi-scale analysis, however, they only predicted the impact of fragmentation on the plot-, patch-, and within-patch scales. Valdés et al. (2015) found at plot-, patch-, and within-patch scales that patch-scale controls were generally more important than controls occurring at higher levels, which is precisely as predicted by the Hierarchical Patch Dynamics Paradigm (Wu and Loucks 1995) at the root of our hypothesized hierarchical control structure. In addition to this scale-dependence, the relative strength of local-, patch-, and landscape-scale controls is likely also context specific. For example, systems with strong local filters (e.g., edge effects in the BDFFP, Laurance et al. 2006; Laurance et al. 2011) will likely see more significant local and patch-scale species sorting than we observed in this study. Further, systems that are significantly isolated, such as oceanic archipelagos, could see dominant controls at the landscape and inter-patch levels. In our study, we observed little difference in the dissimilarity between plots across gradients of proportional edge (e.g., island size), though other studies have found that edge effects are detectable in the TIL system (Su et al. 2014). Our finding is reasonable, as TIL forests are secondary, re-assembling, sub-tropical forests, which are likely much more edge adapted than tropical forests, such as those studied at the BDFFP. This species-level analysis would benefit from replication at the functional-level, as unequal richness within functional groups (e.g., unequal numbers of shade-tolerant vs. shade-intolerant plant species) could create significant noise in our results. Therefore, these findings should not be taken as a statement of primacy of one scale over another, but rather as yet another contextual example that the responses of biological communities to selective pressures vary across scales, sometimes in surprising ways.
Conclusion
Habitat fragmentation is a spatially and temporally dynamic process, by which the loss of habitat in the landscape alters the regional species pool, creating increasingly smaller, and more isolated patches, which sample smaller amounts of the region’s biophysical space, contain fewer species to compete with one another, and are increasingly impacted by fragmentation-mediated changes in local biophysical conditions. We hypothesized that these processes interact hierarchically, with bottom-up and top-down effects each altering biological communities in different ways across scales. We found support for this hypothesis. Plots, patches, and landscapes are likely not islands unto themselves, but rather co-dependent, interacting parts of a whole, as predicted by the Hierarchical Patch Dynamics Paradigm (Wu and Loucks 1995). Future studies of fragmentation should attempt to take these hierarchical effects into account, contextualizing the impacts of fragmentation within the constraints placed on biological communities across scales.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principal. In: Petrov BN, Caski F (eds) Proceedings of the second international symposium on information theory. Akademiai Kiado, Budapest, Hungary, pp 267–281
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Global Ecol and Biogeogr 19:134–143
Baselga A (2013) Multiple site dissimilarity quantifies compositional heterogeneity among several sites, while average pairwise dissimilarity may be misleading. Ecography 36:124–128
Baselga A (2017) Partitioning abundance-based multiple-site dissimilarity into components: balanced variation in abundance and abundance gradients. Methods Ecol and Evol 8:799–808
Baselga A, Orme CD (2012) betapart: an R package for the study of beta diversity. Methods Ecol Evol 3:808–812
Bennett AF, Radford JQ, Haslem A (2006) Properties of land mosaics: implications for nature conservation in agricultural envrionments. Biol Conserv 133:250–264
Collins CD, Banks-Leite C, Brudvig LA, Foster BG, Cook WM, Damschen EI, Andrade A, Austin M, Camargo JL, Driscoll DA, Holt RD, Laurance WF, Nicholls AO, Orrock JL (2017) Fragmentation affects plant community composition over time. Ecography 40:119–130
Crawley MJ, Harral JE (2001) Scale dependence in plant biodiversity. Science 291:864–868
Didham RK (2010) Ecological consequences of habitat fragmentation. In: Encyclopedia of life sciences. Wiley, Chichester
Didham RK, Kapos V, Ewers RM (2012) Rethinking the conceptual foundations of habitat fragmentation research. Oikos 121:161–170
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fahrig L (2017) Ecological responses to habitat fragmentation per se. Annu Rev Ecol Evol Syst 48:1–23
Fardila D, Kelly LT, Moore JL, McCarthy MA (2017) A systematic review reveals changes in where and how we have studied habitat loss and fragmentation over 20 years. Biol Conserv 212:130–138
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM, Damschen EI, Ewers RM, Foster BL, Jenkins CN, King AJ, Laurance WF, Levey DJ, Margules CR, Melbourne BA, Nicholls AI, Orrock JL, Song DX, Townshend JR (2015) Habitat fragmentation and it’s lasting impact on Earth’s ecosystems. Sci Adv 1:e1500052
Holyoak M, Leibold MA, Mouquet N, Holt RD, Hoopes MF (2005) Metacommunities: a framework for large-scale community ecology. In: Holyoak M, Leibold MA, Holt RD (eds) Metacommunities: spatial dynamics and ecological communities. University of Chicago Press, Chicago
Hu G, Feely KJ, Wu J, Xu GF, Yu MJ (2011) Determinants of plant species richness and patterns of nestedness in fragmented landscapes: evidence from land-bridge islands. Landscape Ecol 26:1405–1417
Hu G, Feely KJ, Yu MJ (2016) Habitat fragmentation drives plant community assembly processes across life stages. PLoS ONE 11:e0159572
Lafortezza R, Coomes DA, Kapos V, Ewers RM (2010) Assessing the impacts of fragmentation on plant communities in New Zealand: scaling from survey plots to landscapes. Global Ecol Biogeogr 19:741–754
Laurance WF (2010) Beyond island biogeography theory: understanding habitat fragmentation in the real world. In: Losos JB, Ricklefs RE (eds) The theory of island biogeography revisited. Princeton University Press, Princeton, NJ
Laurance WF, Nascimento HEM, Laurance SG, Andrade A, Ribeiro JELS, Giraldo JP, Lovejoy TE, Condit R, Chave J, Harms KE, D’Angelo S (2006) Rapid decay of tree-community composition in Amazonian forest fragments. PNatl Acad Sci 103:19010–19014
Laurance WF, Camargo JLC, Luizāo RCC, Laurance SG, Pimm SL, Bruna EM, Stouffer PC, Williamson GB, Benitez-Malvido J, Vasconcelos HL, Van Houtan KS, Zartman CE, Boyle SA, Didham RK, Andrade A, Lovejoy TE (2011) The fate of Amazonian forest fragments: a 32-year investigation. Biol Conserv 144:56–67
Liu JL, Velland M, Wang ZH, Yu MJ (2018) High beta diversity among small islands is due to environmental heterogeneity rather than ecological drift. J Biogeogr 45:2252–2261
Lomolino MV (2000) A call for a new paradigm of island biogeography. Global Ecol Biogeogr 9:1–6
Lomolino MV, Brown JH, Sax DF (2010) Island biogeography theory: reticulations and reintegration of “a biogeography of the species”. In: Losos JB, Ricklefs RE (eds) The theory of island biogeography revisited. Princeton University Press, Princeton, NJ
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton, NJ
McGarigal K, Cushman SA, Ene E (2012) FRAGSTATS v4: spatial pattern analysis program for categorical and continuous maps: computer software program produced by the authors at the University of Massachusetts, Amherst. Available at the following web site: http://www.umass.edu/landeco/research/fragstats/fragstats.html
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62
R Core Team (2013). R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. http://www.R-project.org/
Su X, Yuan J, Hu G, Xu G, Yu MJ (2014) Edge effect of the plant community structure on land-bridge islands in the Thousand Island Lake. Chin J Appl Ecol 24:77–84 (in Chinese with English abstract)
Valdés A, Lenoir J, Gallet-Moron E, Andrieu E, Brunet J, Chabrerie O, Closset-Kopp D, Cousins SAO, Deconchat M, De Frenne P, De Smedt P, Diekmann M, Hansen K, Hermy M, Kolb A, Liira J, Lindgren J, Naaf T, Paal T, Prokofieva I, Scherer-Lorenzen M, Wulf M, Verheyen K, Decocq G (2015) The contribution of patch-scale conditions is greater than that of macroclimate in explaining local plant diversity in fragmented forests across Europe. Global Ecol Biogeogr 24:1094–1105
Wilcove DS, McLellan CH, Dobson AP (1986) Habitat fragmentation in the temperate zone. In: Soulé ME (ed) Conservation biology: the science of diversity. Sinauer Assocociates, MA
Wilson MC, Chen XY, Corlett RT, Didham RK, Ding P, Holt RD, Holyoak M, Hu G, Hughes AC, Jiang L, Laurance WF, Liu JJ, Pimm SL, Robinson SK, Russo SE, Si XF, Wilcove DS, Wu JG, Yu MJ (2016) Habitat fragmentation and biodiversity conservation: key findings and future challenges. Landscape Ecol 31:219–227
Wu JG (2009) Ecological dynamics in fragmented landscapes. In: Levin S (ed) Princeton Guide to Ecology. Princeton University Press, Princeton, NJ
Wu JG, Loucks OL (1995) From balance of nature to hierarchical patch dynamics: a paradigm shift in ecology. Q Rev Biol 70:439–486
Young A, Mitchell N (1994) Microclimate and vegetation edge effects in a fragmented podocarp-broadleaved forest in New Zealand. Biol Conserv 67:63–72
Yu MJ, Hu G, Feely KJ, Wu JG, Ding P (2012) Richness and composition of plants and birds on land-bridge islands: effects of island attributes and differential responses of species groups. J Biogeogr 39:1124–1133
Acknowledgements
We thank Raphael Didham, Ignacio Fernandez, and Bingbing Zhou for their insightful comments during the conception and execution of this study. We also thank the Jinfeng Yuan and countless other students, post-docs, professors and local people who worked to collect the woody-plant data utilized here. This work was supported by the National Science Foundation of the United States (NSF DEB-1342754 and DEB-1342757), the National Natural Science Foundation of China (NSFC 31361123001 and 31570524), and the First Class of Disciplines-B of Zhejiang Province (Civil Engineering).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wilson, M.C., Hu, G., Jiang, L. et al. Assessing habitat fragmentation’s hierarchical effects on species diversity at multiple scales: the case of Thousand Island Lake, China. Landscape Ecol 35, 501–512 (2020). https://doi.org/10.1007/s10980-019-00961-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-019-00961-1