Abstract
Spatial and environmental processes influence species composition at distinct scales. Previous studies suggested that the distribution of larval anurans at the landscape-scale is influenced by environmental gradients related to adult breeding site selection, such as pond canopy cover, but not by water chemistry. However, the combined effects of spatial, pond morphology, and water chemistry variables on metacommunity structure of larval anurans have not been analyzed yet. We used a partial redundancy analysis with variation partitioning to analyze the relative influence of pond morphology (e.g., depth, area, and aquatic vegetation), water chemistry, and spatial variables on a tadpole metacommunity from southeastern Brazil. We predict that pond morphology and canopy cover will influence the metacommunity at broad spatial scales, while water chemistry would play a larger role at finer scales. We found that broad-scale spatial patterns of pond canopy cover and pond morphology strongly influenced metacommunity structure, with water chemistry being not significant. Additionally, species composition was spatially autocorrelated at short distances. We suggest that the reproductive behavior of adult anurans is driving tadpole metacommunity dynamics, since pond morphology, but not water chemistry affects breeding site selection by adults. Our results contribute to the understanding of amphibian species diversity in tropical wetlands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both environmental and spatial processes influence species diversity in ecological communities. The metacommunity concept is a multiscale approach useful to analyze the interplay between these environmental and spatial processes that regulate species composition and distribution at local and regional scales. The metacommunity concept has also advanced our understanding of how dispersal and patch heterogeneity influence species abundance and distribution in a set of local communities (Leibold et al., 2004).
Spatial patterns in metacommunities may arise from neutral- and niche-based mechanisms (Leibold et al., 2004; Diniz-Filho et al., 2012). Spatial distribution of species under niche-based mechanisms is driven by their distinct demographic characteristics as a result of differential responses to the environment (stabilizing mechanisms; Chesson, 2000). According to this view, individuals occupy sites along environmental gradients in order to maximize their fitness. Conversely, under neutral dynamics species are ecologically equivalent and have the same chance to give birth, die, migrate, and speciate (equalizing mechanisms; Chesson, 2000), and thus their abundance is dictated solely by stochastic demographic fluctuations. Consequently, species distribution would be random, but spatially autocorrelated, due to dispersal limitation of organisms (Leibold et al., 2004). Nonetheless, discerning the scale at which spatial processes influence ecological patterns remains a challenge for ecologists (Levin, 1992; Landeiro et al., 2011).
The joint influence of environmental and spatial processes on metacommunity structure has been largely studied on theoretical grounds (e.g., Leibold & McPeek, 2006). As a result, empirical studies on the effects of environmental and spatial processes on metacommunity structure of freshwater organisms are scarce (Logue et al., 2011). For instance, recent experiments with aquatic microorganisms found interacting effects of local environmental factors and dispersal on species richness and composition (Altermatt et al., 2011). Pond organisms with complex life cycles, such as anuran amphibians, are a useful model system to test metacommunity theory, since they are subjected to different spatial and environmental processes at the adult and larval stages. Most amphibians are dispersal-limited organisms that require wet environments to live and reproduce (reviewed in Smith & Green, 2005; Wells, 2007). Additionally, several anurans exhibit a marked philopatric behavior, breeding in the same pond in which they emerged as juveniles (Semlitsch, 2008). This complex habitat selection behavior coupled with specific environmental requirements may act as an endogenous ecological process (McIntire & Fajardo, 2009; Legendre & Legendre, 2012), creating spatial autocorrelation in species composition, i.e., ponds close to each other would have a similar species composition than farther ponds. Moreover, adults are the life stage that disperse and effectively connect breeding sites by oviposition (Wells, 2007; Semlitsch, 2008). As a result, both the spatial arrangement of water bodies and their surroundings play a role in the distribution of pond-breeding anurans and their larvae.
The dynamics of the terrestrial ecosystem surrounding ponds affect freshwater metacommunities. One aspect of the terrestrial ecosystem that strongly influences the aquatic ecosystem is pond canopy cover, which is a key gradient affecting the distribution of several freshwater organisms, from invertebrates (McCauley et al., 2008; Hoverman et al., 2011) to amphibians (Skelly et al., 1999; Werner et al., 2007). Canopy cover alters light availability and leaf litter input into ponds (Stoler & Relyea, 2010), and consequently primary productivity and decomposition (Skelly et al., 2002). As a result, larval fitness is lower in less productive, closed-canopy ponds (Skelly et al., 2002; Schiesari, 2006). Besides primary productivity, studies conducted in eastern North America show that canopy cover may affect other water chemistry variables that influence larval development, such as temperature, conductivity, and dissolved oxygen (Werner & Glennenmeier, 1999; Stoler & Relyea, 2010). As a result, the colonization of closed-canopy ponds involves a trade-off, since a few generalist species can tolerate it (Skelly et al., 1999; Werner et al., 2007), but experience lower exploitative competition and predation on the other hand (Schiesari, 2006). Nonetheless, the effects of canopy cover on anuran species seem to depend on life history strategies (e.g., larval period length; Skelly et al., 2002), on the leaf litter species (Stoler & Relyea, 2010), and on the composition of the regional species pool.
On the other hand, the influence of water chemistry on freshwater communities has been rarely investigated at the metacommunity scale (e.g., Hecnar & M’Closkey, 1996; Hájek et al., 2011). Several water chemistry variables, such as conductivity, pH, and dissolved oxygen modulate tadpole survival, development, and time to metamorphosis (reviewed in Ultsch et al., 1999). Hence, water chemistry represents the influence of within-pond environment on tadpole abundance, as expected under the species sorting perspective.
Taken together, the aforementioned environmental variables seem to differentially affect adult anurans and their larvae. On the one hand, the distribution of adult anurans seems to be more influenced by spatial processes and landscape attributes of the terrestrial ecosystem (Resetarits et al., 2005; Binckley & Resetarits, 2007; Wells, 2007). Whereas larval development, growth, and abundance might be directly influenced by pond water chemistry (Ultsch et al., 1999), although any metacommunity-scale process on adults will probably affect the distribution of their larvae. However, to the best of our knowledge, empirical studies have not tested the combined effects of spatial structure, water chemistry, and pond morphology on amphibian species composition, especially in diverse, tropical environments (see Logue et al., 2011). In particular, the characteristic complex life cycle of amphibians has the potential to simultaneously test whether different perspectives in metacommunity theory could explain community assembly.
Therefore, we hypothesized that water chemistry, and canopy cover and pond morphology variables represent two distinct mechanisms that would affect differently the metacommunity dynamics of adults and their larvae. Following McIntire & Fajardo (2009), we argued that pond morphology and canopy cover would be proxies for the effect of adult breeding site selection at the metacommunity scale, while water chemistry would represent the effects of pond environment, by influencing tadpole development and survival. Given that anurans are strongly dispersal-limited organisms and are distributed in patchy environments (ponds), it is likely that they would conform to the patch dynamics perspective. Concurrently, species sorting might also play a role, given the tendency of adult amphibians to track environmental characteristics related to breeding sites. As a result, we expect that pond morphology and canopy cover influence species composition at broad scales, while water chemistry would be more significant at finer spatial scales.
Materials and methods
Study area and sampling
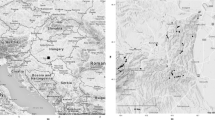
This study was carried out in the Serra da Bocaina National Park (22°40′ to 23°20′S; 44°24′ to 44°54′W), at the border between the states of São Paulo and Rio de Janeiro, southeastern Brazil (Fig. 1). Samplings were conducted on the Bocaina highlands (1,500 m a.s.l.), in São José do Barreiro, São Paulo, and extended over an area of approximately 11 km2 (ponds were 2.15 km apart in mean, maximum 6 km). The climate in this region is of the type Cwb (humid subtropical highland; Peel et al., 2007), with moderated temperatures, dry winters (between April and September) and warm summers (from October to March). The annual rainfall varies between 400 and 2,100 mm, with mean annual temperature of 22° C. The breeding season for the majority of adult anurans is during the rainy season (Garey, unpub. data). Thus, we sampled ponds in this period in order to maximize the chance of collecting most of the species.
We sampled tadpoles using a hand dipnet in 13 water bodies with different morphologies (e.g., area, depth, and aquatic vegetation) and varying degrees of canopy cover, monthly between August 2008 and January 2009. All ponds were sampled within a 1-week period each month. All ponds were fishless, and 3 out of 13 held water during only 3 months, all others were permanent ponds. Hydroperiod seems to be the main driver of the community structure of several freshwater taxa (Wellborn et al., 1996). However, since most of our ponds were permanent, we could not evaluate the role of this variable in our study system. Blind sweeps were made along the entire margins of water bodies, with effort proportional to surface area (Skelly & Richardson, 2010). Tadpoles were fixed in the field with 10% formalin. In addition, adult anurans were acoustically monitored monthly to record the species present in each water body, which helped in tadpole identification. When identification was not possible based on original descriptions, we reared tadpoles in aquaria until metamorphosis, feeding them with commercial fish food.
Prior to tadpole sampling, we recorded two sets of pond characteristics (Table S1): (i) water chemistry variables, namely: conductivity (mS/cm), dissolved oxygen (mg/l), pH, temperature (°C), and turbidity (NTU) using a Horiba U-10 multiparameter water quality checker; and (ii) pond morphology variables: surface area (m2), maximum depth (m), and aquatic vegetation (%). We estimated aquatic vegetation visually by dividing pond surface into quadrants; the percentage of aquatic vegetation was used as a proxy for habitat structural complexity, varying from 0% (low complexity) to 100% (high complexity). We also recorded pond canopy cover (%) using a spherical densitometer (Forestry Suppliers, Jackson, MS, USA); measurements were taken in four directions (N, S, E, W), and the center of the pond. Pond morphology variables were measured only once at the peak of the rainy season, whereas water chemistry was measured monthly during the sampling period (Table S1). Prior to analyses, we standardized environmental variables to zero mean and unit variance to account for their different scales of measurement. Standardizations were implemented in vegan package (Oksanen et al., 2011) in R version 2.13.2 (R Core Team, 2011).
Spatial variables
We used distance-based Moran’s Eigenvector Maps (dbMEMs; Dray et al., 2012; Legendre & Legendre, 2012) to describe spatial structures. dbMEMs (formerly called Principal Coordinates of Neighbor Matrices—PCNMs) are a class of flexible, multiscale ordination methods that produce orthogonal eigenvectors used to represent spatial relationships among sampling sites in uni- and multivariate response data (Dray et al., 2012). dbMEMs can also be divided into submodels corresponding to different spatial scales. By concentrating most of the variation, the first eigenvectors usually describe broad spatial structures, i.e., that encompass the spatial variation in the whole sampled area, while the last eigenvectors (with lower eigenvalues) describe fine spatial structures, which capture variation at the scale of sampling sites (Dray et al., 2012; Legendre & Legendre, 2012). We computed dbMEM eigenvectors from a truncated geodesic distance matrix obtained with R package fields (Furrer et al., 2011). The longest distance (2.887 km) connecting two ponds in a minimum spanning tree (Fig. S1) was used as a threshold to truncate the distance matrix. All ponds separated by distances lower than the threshold distance were connected, whereas those more distant than the threshold are disconnected. This procedure produced 7 dbMEMs. Analysis was conducted using R package PCNM (Legendre et al., 2010). Further, we implemented a forward selection procedure with double-stopping criteria (Legendre & Legendre, 2012) in R package packfor (Dray, 2009) to only select dbMEMs that significantly explained the variance in the species composition matrix. This procedure recovered 5 dbMEM (accumulated R 2adj = 0.414; Fig. S2, S3). dbMEMs 1 and 2 were arbitrarily classified as broad-scale variables, dbMEM 4 as medium scale, and dbMEMs 5 and 7 as fine-scale variables (Fig. S2). dbMEMs 1 and 2 had positive spatial autocorrelation (Moran’s I = 0.291 and 0.084, respectively), while the dbMEMs 4, 5, and 7 had negative autocorrelation (Moran’s I = −0.218, −0.068, and −0.033, respectively). All the five selected dbMEMs were used as spatial variables in the following analysis, since excluding those with negative Moran’s I seems not to change overall results (see Legendre & Legendre, 2012).
Data analyses
For all subsequent analyses, we transformed the total counts of species using the Hellinger transformation (Legendre & Legendre, 2012) to homogenize variation among species abundances. None environmental variable had a variation inflation factor higher than 3 (Zuur et al., 2010). Thus, all were included in the further analysis. We employed a variation partitioning approach to disentangle species response to environmental (pond morphology, canopy cover, and water chemistry) and spatial variables (dbMEMs) driving metacommunity structure (Legendre & Legendre, 2012). Despite some recent criticism (Gilbert & Bennett, 2010; Smith & Lundholm, 2010; but see Diniz-Filho et al., 2012), this analytical technique remains useful to separate variation in species composition explained by environmental gradients, spatial autocorrelation, and spatially structured environmental gradients (i.e., variance in species composition explained by both spatial and environmental variables). We determined the exclusive and shared effects of water chemistry, pond morphology, and pond spatial network on species composition using a partial redundancy analysis (pRDA; Legendre & Legendre, 2012), with adjusted canonical R 2 values (Legendre & Legendre, 2012). Since we sampled ponds repeatedly, we used each pond and month as factors in the pRDA and then performed a randomization stratified within ponds (Lepš & Šmilauer, 2003), with 999 Monte Carlo randomizations to determine significance. We also computed a Mantel correlogram to quantify the pattern in spatial autocorrelation of community similarity, calculated as Bray–Curtis distance, and also correlograms, with the same minimum spanning tree used to calculate dbMEMs, to analyze the spatial patterns of individual environmental variables. Analyses were implemented in R packages vegan (Oksanen et al., 2011) and spdep (Bivand, 2012). To identify the environmental variables related to each spatial model, we regressed each dbMEM (explanatory variables) against the detrended pond morphology and water chemistry (predictor variables; Borcard et al., 2004). Linearity was checked visually, using Q–Q plots.
Results
We recorded tadpoles of 15 amphibian species belonging to five families. The most abundant species were Rhinella icterica (3,532 individuals), Hypsiboas sp. (aff. polytaenius) with 2,339 individuals, and Scinax sp. (aff. hayii) with 2,318 individuals. The mean species richness per pond was 2.8 (±1.45 SD; range 2–9). Chiasmocleis mantiqueira and Scinax sp. (aff. obtriangulatus) were represented by only two individuals and were not included in further analysis, since they could affect pRDA.
Both environmental variables and the spatial distribution of ponds affected metacommunity structure. Pond morphology and canopy cover accounted for 16.7% of the variation in species composition (P = 0.005), whereas water chemistry did not explain a significant proportion of it (R 2adj = 0.00; P = 0.75; Fig. 2). Pond canopy cover and aquatic vegetation were the two environmental variables that mainly influenced species composition (Table 1; Fig. 3). The spatial component alone explained about 20% (P = 0.005) of the variation in species composition. Residual variation represented 40%. Furthermore, it is noteworthy that nearly all variation (16 %) in pond morphology and canopy cover were spatially structured (Fig. 2).
Biplot of the redundancy analysis showing the major influence of A pond canopy cover and B aquatic vegetation on Hellinger-transformed data of tadpole species abundance. Isolines represent the fitted smooth surface for each environmental variable, obtained with function ordisurf in R package vegan. Only species with high scores are shown. Aplper, Aplastodiscus perviridis; Bokahe, Bokermannohyla ahenea; Denmic, Dendropsophus microps; Hyppol, Hypsiboas sp. (aff. polytaenius); Phybar, Physalaemus barrioi; Rhiict, Rhinella icterica; Scidua, Scinax sp. (aff. duartei)
Species composition showed significant, positive spatial autocorrelation in the first two distance classes (between 0.01 and 1.63 km) and negative autocorrelation in the third and fourth distance classes (between 2.43 and 3.24 km; Fig. S4). Ponds separated by 2.43 km (upper limit of the third distance class) differ in composition, probably due to contrasting values at short distances of canopy cover, area, depth, and floating vegetation (Fig. S5). Water depth was related to all spatial variables (Table 2). This suggests that the variation in water depth is not related to any particular spatial scale, which hinders its interpretation in a spatial context. Floating vegetation, canopy cover, dissolved oxygen, conductivity, and area were related to one or two broad-scale dbMEMs (cf. Table 2; Fig. S2). For example, canopy cover, conductivity, and floating vegetation were negatively related to dbMEM 2. This indicates that there is a spatial gradient in these variables, in which they increase from south to north of the sampled area, since dbMEM 2 differentiates ponds in the north from those in the south (Fig. S2). Canopy cover seems also to vary significantly at the fine scale (dbMEM 7; Table 2). Temperature, pH, and turbidity were not significantly related to any spatial variable (Table 2; Fig. S5). Thus, the strong induced spatial dependence of pond morphology and canopy cover (R 2adj = 0.163; Fig. 2) is directly influencing the spatial dynamics of amphibian species. Taken together, these results suggest that species sorting and patch dynamics related to adult breeding site selection are shaping the metacommunity structure.
Discussion
We found that both the pure spatial and environmental components related to pond morphology and canopy cover accounted for a large and significant fraction of the variation in species composition. More importantly, the gradients of canopy cover and pond morphology were spatially structured. These results point to a combination of different metacommunity processes, in which both patch dynamics and species sorting play key roles. Additionally, the induced spatial dependence of variables from the surrounding terrestrial ecosystem (e.g., canopy cover) and pond morphology, which represent the effect of adult breeding site selection, suggest that the landscape-scale distribution of anuran larvae is essentially influenced by behavioral decisions of adults in response to spatially structured environmental gradients.
The broad-scale spatial pattern, which represents the scale of the sampled area, of pond morphology variables and canopy cover influenced tadpole metacommunity dynamics. This is supported by our results showing that canopy cover and pond morphology are related to broad-scale dbMEMs, while most of water chemistry variables did not show a spatial pattern. Those variables are known to influence breeding site selection by adults (Resetarits et al., 2005; Binckley & Resetarits, 2007). Canopy cover was lower in the northernmost ponds of our study area. As a result, open-canopy specialist species were more abundant in ponds of that region (except D. minutus and B. ahenea, which occur more widely, but are less abundant elsewhere). Canopy cover is a key gradient determining amphibian distribution in wetlands (Skelly et al., 1999; Werner et al., 2007), by influencing larval growth and development (Skelly et al., 2002; Schiesari, 2006; Stoler & Relyea, 2010). This preference for open- versus closed-canopy ponds allows species to coexist locally (Chesson, 2000; Chase et al., 2005), by maximizing the fitness of individuals from species with distinct life history strategies in each of those contrasting environments. Additionally, pond depth and area had a strong spatial dependence and also influenced species composition. Deeper and larger ponds have more physical space and resource diversity, allowing more species to breed. Furthermore, a taller water column allows the colonization of larvae that occupy different microhabitats (e.g., nektonic, benthic). These results support the idea that stabilizing niche differences (Chesson, 2000) related to environmental requirements for adult reproduction, which varied at the metacommunity scale, is a key mechanism structuring this metacommunity.
We also found that the pure spatial component (~20%) seems to play a larger role in shaping species composition, compared to pond morphology variables alone (16%). The high variation accounted for by the pure spatial component can be attributed either to unmeasured environmental variables or pure spatial processes, such as dispersal limitation (Landeiro et al., 2011; Legendre and Legendre, 2012). Dispersal ability varies among species in a metacommunity, and populations of some species may be more isolated than others. Dispersal in adult anurans is usually restricted to the surroundings of one breeding site or a cluster of nearby ponds (Semlitsch, 2008). As a consequence, it is difficult to assign a single mechanism (e.g., dispersal limitation, philopatry) to the pure spatial component in our study. Future studies should use a recent analysis (Diniz-Filho et al., 2012) to tease apart these two processes. Despite that limitation, previous studies, using microsatellite markers as an indirect way to estimate dispersal in anurans (Newman & Squire, 2001 and references therein), did not find population differentiation at the scale of 2 km. These data agree with our results about spatial autocorrelation in species composition, since we also did not find a differentiation in species composition in ponds separated by 2.43 km. The spatial autocorrelation in species composition at short distances seems to be driven by the induced spatial dependence of canopy cover and pond morphology variables, such as aquatic vegetation, depth, and area. Indeed, several niche-based processes predict dispersal limitation, especially when associated with spatially heterogeneous environments, demographic stochasticity, or strong, asymmetric competition (Chesson, 2000; Chase et al., 2005). Thus, despite the lack of data for dispersal ability, these results suggest that both dispersal limitation and species response to spatially structured environmental gradients (Shurin et al., 2009) could be involved in determining the dynamics of this larval metacommunity.
Contrary to our initial predictions, water chemistry variables did not influence tadpole species composition. This result is in agreement with previous studies (Hecnar & M’Closkey, 1996; Brodman et al., 2003) that analyzed the effects of water chemistry on broad-scale amphibian distribution. The development and survival of tadpoles is strongly influenced by water chemistry (reviewed in Ultsch et al., 1999) and pond drying, which affect water chemistry (Wellborn et al., 1996). Adult anurans seem to select pond to oviposit according to the presence of conspecifics and fish (Resetarits et al., 2005). Therefore, tadpole survival and development are affected by water chemistry, but its effects on the distribution of species between ponds could be weakened by variables that influence adult habitat selection behavior. Additionally, water chemistry variables seemed not to vary in a wide range at the landscape-scale in our study area. Furthermore, phenotypic plasticity (Miner et al., 2005) could play a role in mediating species coexistence and their response to water chemistry in our system. The high adaptive plasticity of tadpoles in response not only to predators (Relyea, 2004), but also to local environmental variables, food availability, and pond drying (Newman, 1992) may be a possible mechanism responsible for the lack of influence of water chemistry on species composition.
In summary, we have shown that the induced spatial dependence of pond morphology and canopy cover strongly influenced the metacommunity structure of tadpoles. These findings posit a strong role for adult behavior in determining larval ecology. Our results should be useful to explain the distribution of other organisms with similar complex life cycles, whose biomass export play a key role in linking aquatic and terrestrial ecosystems (Earl et al., 2011). The effect of the surrounding terrestrial landscape in determining community assembly of freshwater organisms deserves further investigation. For example, understanding how the spatial dynamics of terrestrial landscape and species composition modulate aquatic communities may contribute to studies modeling the flux of species and subsides, trophic interactions, and metaecosystem dynamics (Massol et al., 2011). Future studies should also evaluate how different dispersal abilities and philopatry generate spatial patterns in anuran metacommunities at multiple scales. In addition, metacommunity simulations in an environmentally homogeneous domain could evaluate how philopatric behavior and neutral dynamics create spatial patterns.
References
Altermatt, F., S. Schreiber & M. Holyoak, 2011. Interactive effects of disturbance and dispersal directionality on species richness and composition in metacommunities. Ecology 92: 859–870.
Binckley, C. A. & W. J. Resetarits, 2007. Effects of forest canopy on habitat selection in treefrogs and aquatic insects: implications for communities and metacommunities. Oecologia 153: 951–958.
Bivand, R., 2012. spdep: Spatial Dependence: Weighting Schemes, Statistics and Models. R package version 0.5-53. http://CRAN.R-project.org/package=spdep. Accessed 6 April 2014.
Borcard, D., P. Legendre, C. Avois-Jacquet & H. Tuomisto, 2004. Dissecting the spatial structure of ecological data at multiple scales. Ecology 85: 1826–1832.
Brodman, R., J. Ogger, T. Bogard, A. J. Long, R. A. Pulver, K. Mancuso & D. Falk, 2003. Multivariate analyses of the influences of water chemistry and habitat parameters on the abundances of pond-breeding amphibians. Journal of Freshwater Ecology 18: 425–436.
Chase, J. M., P. Amarasekare, K. Cottenie, A. Gonzalez, R. D. Holt, M. Holyoak, M. F. Hoopes, M. A. Leibold, M. Loreau, N. Mouquet, J. B. Shurin & D. Tilman, 2005. Competing theories for competitive metacommunities. In Holyoak, M., M. A. Leibold & R. D. Holt (eds), Metacommunities: Spatial Dynamics and Ecological Communities. Chicago University Press, Chicago: 335–354.
Chesson, P., 2000. Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics 31: 343–366.
Diniz-Filho, J. A. F., T. Siqueira, A. A. Padial, T. F. Rangel, V. L. Landeiro & L. M. Bini, 2012. Spatial autocorrelation analysis allows disentangling the balance between neutral and niche processes in metacommunities. Oikos 121: 201–210.
Dray, S., 2009. packfor: Forward Selection with Permutation (Canoco p. 46). R package version 0.0-7/r58. http://R-Forge.R-project.org/projects/sedar/. Accessed 6 April 2014.
Dray, S., R. Pélissier, P. Couteron, M. J. Fortin, P. Legendre, P. R. Peres-Neto, E. Bellier, R. Bivand, F. G. Blanchet, M. De Cáceres, A. B. Dufour, E. Heegaard, T. Jombart, F. Munoz, J. Oksanen, J. Thioulouse & H. H. Wagner, 2012. Community ecology in the age of multivariate multiscale spatial analysis. Ecological Monographs 82: 257–275.
Earl, J. E., T. M. Luhring, B. K. Williams & R. D. Semlitsch, 2011. Biomass export of salamanders and anurans from ponds is affected differentially by changes in canopy cover. Freshwater Biology 56: 2473–2482.
Furrer, R., D. Nychka & S. Sain, 2011. fields: Tools for Spatial Data. R package version 6.6. http://CRAN.R-project.org/package=fields. Accessed 6 April 2014.
Gilbert, B. & J. R. Bennett, 2010. Partitioning variation in ecological communities: do the numbers add up? Journal of Applied Ecology 47: 1071–1082.
Hájek, M., J. Roleček, K. Cottenie, K. Kintrová, M. Horsák, A. Poulíčková, P. Hájková, M. Fránková & D. Dítě, 2011. Environmental and spatial controls of biotic assemblages in a discrete semi-terrestrial habitat: comparison of organisms with different dispersal abilities sampled in the same plots. Journal of Biogeography 38: 1683–1693.
Hecnar, S. & R. M’Closkey, 1996. Amphibian species richness and distribution in relation to pond water chemistry in south-western Ontario, Canada. Freshwater Biology 36: 7–15.
Hoverman, J. T., C. J. Davis, E. E. Werner, D. K. Skelly, R. A. Relyea & K. L. Yurewicz, 2011. Environmental gradients and the structure of freshwater snail communities. Ecography 34: 1049–1058.
Landeiro, V. L., W. E. Magnusson, A. S. Melo, H. M. V. Espírito-Santo & L. M. Bini, 2011. Spatial eigenfunction analyses in stream networks: do watercourse and overland distances produce different results? Freshwater Biology 56: 1184–1192.
Legendre, P. & L. Legendre, 2012. Numerical Ecology, 3rd ed. Elsevier, Oxford.
Legendre, P., D. Borcard, G. Blanchet & S. Dray, 2010. PCNM: PCNM Spatial Eigenfunction and Principal Coordinate Analyses. R package version 2.1/r82. http://R-Forge.R-project.org/projects/sedar/. Accessed 6 April 2014.
Leibold, M. A. & M. A. Mcpeek, 2006. Coexistence of the niche and neutral perspectives in community ecology. Ecology 87: 1399–1410.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau & A. Gonzalez, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Lepš, J. & P. Šmilauer, 2003. Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge.
Levin, S. A., 1992. The problem of pattern and scale in ecology. Ecology 73: 1943–1967.
Logue, J. B., N. Mouquet, H. Peter & H. Hillebrand, 2011. Empirical approaches to metacommunities: a review and comparison with theory. Trends in Ecology & Evolution 26: 482–491.
Massol, F., D. Gravel, N. Mouquet, M. W. Cadotte, T. Fukami & M. A. Leibold, 2011. Linking community and ecosystem dynamics through spatial ecology. Ecology Letters 14: 313–323.
McCauley, S. J., C. J. Davis, R. A. Relyea, K. L. Yurewicz, D. K. Skelly & E. E. Werner, 2008. Metacommunity patterns in larval odonates. Oecologia 158: 329–342.
McIntire, E. J. B. & A. Fajardo, 2009. Beyond description: the active and effective way to infer processes from spatial patterns. Ecology 90: 46–56.
Miner, B. G., S. E. Sultan, S. G. Morgan, D. K. Padilla & R. A. Relyea, 2005. Ecological consequences of phenotypic plasticity. Trends in Ecology and Evolution 20: 685–692.
Newman, R. A., 1992. Adaptive plasticity in amphibian metamorphosis. BioScience 42: 671–678.
Newman, R. A. & T. Squire, 2001. Microsatellite variation and fine-scale population structure in the wood frog (Rana sylvatica). Molecular Ecology 10: 1087–1100.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens & H. Wagner, 2011. vegan: Community Ecology Package. R package version 1.17-12. http://CRAN.R-project.org/package=vegan. Accessed 6 April 2014.
Peel, M. C., B. L. Finlayson & T. A. McMahon, 2007. Updated world map of the Köppen–Geiger climate classification. Hydrology and Earth System Sciences 11: 1633–1644.
R Core Team, 2011. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org.
Relyea, R. A., 2004. Fine-tuned phenotypes: tadpole plasticity under 16 combinations of predators and competitors. Ecology 85: 172–179.
Resetarits, W., C. A. Binckley & D. R. Chalcraft, 2005. Habitat selection, species interactions, and processes of community assembly in complex landscapes. In Holyoak, M., M. A. Leibold & R. D. Holt (eds), Metacommunities: Spatial Dynamics and Ecological Communities University of Chicago Press, Chicago. Chicago University Press, Chicago: 374–398.
Schiesari, L., 2006. Pond canopy cover: a resource gradient for anuran larvae. Freshwater Biology 51: 412–423.
Semlitsch, R. D., 2008. Differentiating migration and dispersal processes for pond-breeding amphibians. Journal of Wildlife Management 72: 260–267.
Shurin, J. B., K. Cottenie & H. Hillebrand, 2009. Spatial autocorrelation and dispersal limitation in freshwater organisms. Oecologia 159: 151–159.
Skelly, D. K. & J. L. Richardson, 2010. Larval sampling. In Dodd, C. K. (ed.), Amphibian ecology and conservation: a handbook of techniques. Oxford University Press, Oxford: 55–70.
Skelly, D. K., E. E. Werner & S. A. Cortwright, 1999. Long-term distributional dynamics of a michigan amphibian assemblage. Ecology 80: 2326–2337.
Skelly, D. K., L. K. Freidenburg & J. M. Kiesecker, 2002. Forest canopy and the performance of larval amphibians. Ecology 83(4): 983–992.
Smith, M. A. & D. M. Green, 2005. Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography 28: 110–128.
Smith, T. W. & J. T. Lundholm, 2010. Variation partitioning as a tool to distinguish between niche and neutral processes. Ecography 33: 648–655.
Stoler, A. B. & R. A. Relyea, 2010. Living in the litter: the influence of tree leaf litter on wetland communities. Oikos 120: 862–872.
Ultsch, G. R., D. F. Bradford & J. Freda, 1999. Physiology: coping with the environment. In McDiarmid, R. W. & R. Altig (eds), Tadpoles: The Biology of Anuran Larvae. Chicago University Press, Chicago: 189–214.
Wellborn, G. A., D. K. Skelly & E. E. Werner, 1996. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics 27: 337–363.
Wells, K. D., 2007. The Ecology and Behavior of Amphibians. University of Chicago Press, Chicago.
Werner, E. E. & K. S. Glennemeier, 1999. Influence of forest canopy cover on the breeding pond distributions of several amphibian species. Copeia 1999: 1–12.
Werner, E. E., D. K. Skelly, R. A. Relyea & K. L. Yurewicz, 2007. Amphibian species richness across environmental gradients. Oikos 116: 1697–1712.
Zuur, A. F., E. N. Ieno & C. S. Elphick, 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1: 3–14.
Acknowledgments
We are indebted to M. Almeida Neto, F. Altermatt, L. M. Bini, J. W. Fox, V. L. Landeiro, G. F. Livingston, T. F. Rangel, and T. Siqueira for providing useful insights to this manuscript. A. Bispo and B. Vilela helped with the map. IBAMA provided collecting permits (#14474) and the staff of the Serra da Bocaina National Park kindly provided logistical support and housing. This paper is part of DBP master’s-thesis at the graduate program in Biologia Animal, supported by fellowships from FAPESP (#2008/55744-6) and CAPES-DS. We greatly acknowledge the input received from L.C. Schiesari and L. Casatti as members of DBP’s examining committee. MVG and TG-S were supported by FAPESP doctoral fellowships (#2008/50575-1, 2008/58979-4, respectively). DCRF thanks FAPESP (2010/52321-7) and CNPq (563075/2010-4). IAM was supported by a Jovem Pesquisador Grant from FAPESP (01/13341-3 and 06/56007-0). During the final preparation of the manuscript, DBP was supported by a CAPES-DS and CAPES-PDSE doctoral fellowships. We also thank all those who helped in the field and lab work. Voucher specimens are housed at the tadpole collection of DZSJRP-UNESP.
Note added in proof
Hypsiboas sp. (aff. polytaenius) was formally described as Hypsiboas bandeirantes, Caramaschi and Cruz, 2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Lee B. Kats
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Provete, D.B., Gonçalves-Souza, T., Garey, M.V. et al. Broad-scale spatial patterns of canopy cover and pond morphology affect the structure of a Neotropical amphibian metacommunity. Hydrobiologia 734, 69–79 (2014). https://doi.org/10.1007/s10750-014-1870-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-1870-0