Abstract
Perioperative neurocognitive disorder (PND) is a common disorder following anesthesia and surgery, especially in the elderly. The complex cellular and molecular processes are involved in PND, but the underlying pathogenesis of which remains inconclusive due to conflicting data. A growing body of evidence has been shown that perioperative systemic inflammation plays important roles in the development of PND. We reviewed the relevant literature retrieved by a search in the PubMed database (on July 20, 2023). The search terms used were “delirium”, “post operative cognitive dysfunction”, “perioperative neurocognitive disorder”, “inflammation” and “systemic”, alone and in combination. All articles identified were English-language, full-text papers. The ones cited in the review are those that make a substantial contribution to the knowledge about systemic inflammation and PNDs. The aim of this review is to bring together the latest evidence for the understanding of how perioperative systemic inflammation mediates neuroinflammation and brain injury, how the inflammation is regulated and how we can translate these findings into prevention and/or treatment for PND.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive alteration affecting patients following anesthesia and surgery is a heterogenous set of conditions, which includes any form of acute event (postoperative delirium) and cognitive decline diagnosed up to 30 days after the procedure (delayed neurocognitive recovery) and up to 12 months (postoperative neurocognitive disorder, POCD) [1]. Previously, all forms of the impairment were called POCD, but more recently, perioperative neurocognitive disorders (PNDs) are recommended to be used as an overarching term for cognitive impairment identified in the perioperative period [1]. PND has been recognized as a consequence of anesthesia as early as 1887 [2], and a common complication of cardiac surgery in the elderly described by Bedford in 1955 [3]. Following its modern description, the agreements of definitions and diagnostic criteria of PND have been made in 2018 [1].
Since the 1950’s, advanced age has been identified to be one of the strongest associations for PND after major noncardiac surgery [4]. The incidence of PND is reported to be anywhere between 9 and 54% after surgery in adults over age 65 [5,6,7]. In fact, cognitive dysfunction is common in adult patients of all ages at hospital discharge after major non-cardiac surgery [4]. PND is associated with adverse outcomes, including significant complications including dementia, and increased mortality, thereby producing a significant burden on the healthcare system [8,9,10]. The pathogenesis of PND is multifaceted, which might be associated with anesthesia, tissue damage, neuroinflammation, surgical stress, psychological stress and so on. Growing evidence indicates that systemic inflammation following surgery and anesthesia is involved in PND, in which inflammatory signaling molecules have been identified. The purpose of this review is to summarize the literature to date concerning PND and the cellular mechanisms involved in systemic inflammation underlying the pathogenesis of PND in pre-clinical and human studies.
Systemic inflammation and cognitive dysfunction
Systemic factors (such as plasma proteins, immune cells), mediated by blood, have been shown to be as mediators of brain homeostasis and modulate brain function [11, 12]. A large number of studies have shown that systemic inflammation can induce or exacerbate neurological symptoms including anxiety, depression, and cognitive dysfunction [13, 14]. For example, systemic inflammation induced by psoriasis has been shown to be associated with an increased risk of developing dementia [15]. Another epidemiological study suggests that chronic periodontitis is closely associated with the incidence and progression of cognitive impairment [16], which is confirmed in animals [17]. Yamanaka’s study showed that systemic inflammation treated by lipopolysaccharide (LPS)-induced cognitive dysfunction in aged rats [18]. In a randomized, placebo-controlled study in healthy men, systemic inflammation induced by an intravenous injection of LPS altered functional connectivity of resting state networks in brain [19]. A single intraperitoneal injection of tumor necrosis factor-α (TNF-α) in mice increased serum level of the proinflammatory mediators in a dose- and time-dependent manner and affects the central nervous system at a neuroimmune and behavioral level [20]. In preoperational glioma patients, increased interleukin-2 (IL-2) levels were positively correlated with cognitive impairment [21]. Certain cytokines such as interferon (IFN)-α and IL-2 are often used in the treatment certain cancers and chronic diseases. Cognitive impairment was reported in those patients who received those medications [22]. In critically ill patients, the proinflammatory cytokine IL-8 was associated with delirium [23]. Clinical studies showed that higher circulating levels of IL-6 or IL-1β were associated with worse cognitive function and steeper cognitive decline [24, 25]. These results suggest that systemic inflammation may play an important role in modulating cerebral function. The periphery-to-brain communication pathways mediate inflammation-associated brain function.
Systemic inflammation following surgery/anesthesia and PND
The innate immune system is a generic component of the organism’s response to infection or tissue damage, which includes surface barriers, the complement system, and inflammatory mediators produced by immune cells [26]. Surgery triggers the innate immune system to launch a systemic inflammatory response (see Fig. 1). It is well known that tissue damage due to surgery triggers local inflammation at the surgical site, where cellular injury can release endogenous damage-associated molecular patterns (DAMPs) that activate innate immunity and promote and exacerbate the inflammatory response [27, 28]. Among the DAMPs, high mobility group box 1 (HMGB1) is the most studied. HMGB1, as a major DAMP, is a nuclear protein that is present in almost all eukaryotic cells and can be released by necrotic cells during tissue injury [29]. It can interact with multiple receptors of circulating bone marrow derived monocytes (BMDMs), including the receptor for advanced glycation end products (RAGE), toll-like receptor (TLR) 2, and TLR4 [29], and induce activation of nuclear factor-kappa B (NF-κB), and production of proinflammatory cytokines. The systemic inflammatory response is observed in rodent models during the perioperative period [30,31,32,33,34,35,36]. Similar inflammatory changes have been described in clinical samples. For example, after orthopedic surgery, statistically significant changes were present in IL-5, IL-6, IL-8, IL-10, monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein (MIP)-1α and receptor for advanced glycation end products in plasma [37]. In adult patients, the cytokine response to open cholecystectomy stimulated both the pro-inflammatory (IL-1β, IL-6 and TNF-α) and the anti-inflammatory (IL-4) components, while this response was absent in laparoscopic cholecystectomy [38]. The complement system is another key component in the inflammatory response, which can be activated by the DAMPs after surgery [39]. Clinical studies demonstrated that there is early complement component 3 (C3) activation, represented by plasma C3 depletion and upregulation of cleaved forms of C3 in patients undergoing surgery [40, 41].
The innate immune system is activated following surgical injury and releases peripheral inflammatory mediators. High mobility group box 1 protein (HMGB1) is rapidly released in response to injury, which activates nuclear factor-kappa B (NF-κB) signaling pathways in bone marrow derived monocytes (BMDMs), increasing transcription of inflammatory genes such as interleukin (IL), tumor necrosis factor alpha (TNF-α), monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein (MIP)-1α. Thus, a systemic inflammatory response is induced
The effects of anesthetic agents on the immune system have been investigated at both mechanistic and clinical levels in animals and human [42, 43]. A brief exposure to isoflurane general anesthesia, without induced surgical stress, significantly increased serum IL-1β in children undergoing MRI examination [44]. In single-drug propofol anesthesia without any surgical intervention in healthy subjects, propofol significantly decreased the levels of hepatocyte growth factor, IFN-γ-induced protein 10, and increased the levels of IL-17, IL-5, IL-7 and platelet-derived growth factor, suggesting propofol seemed to induce mixed pro- and anti-inflammatory effects on the immune system [45]. The latest literature shows that sevoflurane general anesthesia without surgery, even in older adults, did not provoke an inflammatory state in the early hours after exposure [46]. The data suggest that anesthetic agents appear to have effects on the immune system but the inflammatory responses they induce may be far outweighed by the contribution of patient and surgical factors.
The systemic inflammation following surgery contribute to persistent cognitive decline in rodent models [30,31,32,33,34,35,36, 47]. Increased levels of HMGB1 after surgery and anesthesia have been described in preclinical models of cognitive impairment [48,49,50,51]. The elevations of HMGB1 were also observed in patients with PND after major gastrointestinal surgery [52]. Another clinical study showed that surgery-induced acute systemic inflammation is followed by a rapid and transient activation of the brain immune system [53]. After coronary artery surgery, elevated postoperative concentrations of IL-6 and C-reactive protein are associated with short- and medium-term impairment of cognitive functions in patients [54]. POCD patients were associated with higher postoperative plasma levels of malondialdehyde, and higher IL-1β [55], TNF-α and IL-6 [56]. In a prospective biomarker cohort study, IL-8 exhibited a strong correlation with delirium severity in surgical patients [57]. Hyperbaric oxygen and remote ischemic preconditioning could mitigate surgery-induced cognitive impairment in animals and elderly patients, respectively, and this may be associated with the attenuation of systemic inflammation [58, 59]. Blockade of either TNF-α or IL-6 using antibodies effectively reduced POCD in rodents [60, 61]. Moreover, in a murine model of orthopedic surgery, C3a receptor blockade improved hippocampal-dependent memory function, suggesting that complement activation may play a role in the mechanisms underlying PND development [62].
These results suggest that surgery and anesthesia can induce systemic inflammation, which may play important roles in the pathophysiological process of PND, although it has been demonstrated that in young patients, there is no effect of anesthesia on postoperative cognitive functions. There is no association of inflammatory markers with respect to the patient’s cognitive status [63].
How inflammatory signals access the brain: barriers and conduits
Blood–brain barrier
The brain vasculature serves two roles: as a conduit to supply nutrients and as a barrier to block inflammatory insults [11]. The neurovascular unit (NVU), defined by the structural cellular composition of neurons, glia (e.g., astrocytes, microglia) and vascular cells (endothelium and mural cells including pericytes and smooth muscle cells), is critical to normal central nervous system (CNS) functioning [64]. The blood–brain barrier (BBB) is centrally positioned within the NVU and accomplishes these two roles [11]. BBB is formed by brain endothelial cells of the capillary wall, astrocyte end-feet and pericytes embedded in the capillary basement membrane [64], and it maintains a tightly controlled chemical composition of the neuronal milieu that is required for proper neuronal functioning [64]. Cerebral endothelial cells interact with the surrounding basal lamina, as well as astrocytic end-feet processes, pericytes and neurons [65, 66]. Astrocytes and pericytes are both involved in modulating brain endothelial permeability and in BBB maintenance [65, 66].
Growing evidence shows that BBB breakdown is associated with the pathogenesis of different neurological disorders and complex multifactorial diseases [67]. A Nation’s study showed that individuals with early cognitive dysfunction develop brain capillary damage and BBB breakdown in the hippocampus [68]. Studies in several animal models suggest changes in BBB permeability in PND [69, 70]. IL-17A in serum increases after surgical stress and contributes to PND disorders through BBB dysfunction [71]. Preclinical studies have shown that peripheral pro-inflammatory cytokines (e.g. TNF-α, IL-1,6) following anesthesia/surgery might induce BBB dysfunction (see Fig. 2). The changes in BBB permeability may be associated with influx of inflammatory mediators and contribute to cognitive impairment [51, 69]. In addition, BBB breakdown facilitates BMDMs to be recruited to the CNS [32]. The central recruitment of BMDMs is a necessary mechanism in POCD [72]. Therefore, changes in the BBB are likely partially responsible for the ways in which systemic inflammatory mediators modulate brain function following surgery and anesthesia.
The peripheral inflammatory mediators pass through the barriers and conduits to modulate brain function. There are several mechanisms existing by which peripheral inflammatory signal can be transmitted to the brain, including a transport across the blood–brain barrier (BBB) and b blood–cerebrospinal fluid (CSF) barrier (BCB); or entry via c the circumventricular regions (CVOs); e direct neural pathways (afferent vagal fibers); and d other pathways
Blood–cerebrospinal fluid barrier
The blood–cerebrospinal fluid (CSF) barrier (BCB) is another interface between the circulatory system and CNS (see Fig. 2), which shapes brain function in health and pathology by integrating signals from the brain with signals coming from the circulation [73,74,75,76]. The BCB is made of the choroid plexus (CP) present in all four ventricles of the brain. The CP is a vascular convolute, consisting of epithelial cells bound together by tight junctions and stromal compartment vascularized by highly permeable fenestrated capillaries [77]. It is involved in the production of CSF and actively regulates the synthesis, composition, and circulation of CSF [78]. There is no major diffusional barrier between the brain parenchyma and CSF. Thus, materials coming from the circulation present in CSF are free to enter the brain. Under physiological conditions, the CP is key to delivering micronutrients, growth factors, and neurotrophins to neuronal networks through the CSF from systemic blood delivered to the leaky microvasculature of the CP [79]. Understandably, the CP is crucial for the homeostatic regulation of the brain microenvironment along with the BBB [80]. In addition, the CP is an important gateway for the entry of immune cells into the brain [81]. Its dysfunction induced by inflammatory mediators or oxidative stress may cause brain damage [82,83,84,85]. A preclinical study showed that choroidal BCB dysfunction occurred 1 day after surgery and anesthesia. The alteration was associated with neuroinflammation and PND [62]. A clinical study examined BCB integrity of hospitalized patients by CSF to plasma albumin ratio. The results showed that BCB dysfunction may be relevant for delirium pathophysiology when it occurs [86]. Further study is warranted to investigate whether the systemic inflammation after surgery induces dysfunction of BCB and then mediates PND. It remains unknown whether the close contact between the CP and some brain structures makes it easier for substances derived from the CP to affect certain brain functions.
Circumventricular organs
Circumventricular organs (CVOs) are highly vascularized structures, which are located around the third and fourth ventricles and mainly include the organum vasculosum of the laminae terminalis, the subfornical organ, the area postrema and the median eminence, which are specialized brain regions characterized by the lack of a BBB [87] (see Fig. 2). Moreover, neurons and glial cells of the CVOs express cytokine receptors including IL-1 and TNF-α receptor 1 [88, 89]. Thus, circulating substances could reach the CVOs, which communicate with other brain areas and CSF through complex neural networks and tanycytes (specialized ependymal cells lining the ventricular spaces), respectively [90, 91]. It seems reasonable to suppose that inflammatory mediators in the blood following surgery and anesthesia could leak into the CVOs and regulate their functions. However, it is not clear whether the procedure significantly occurs in PND.
Vagal afferents
The vagus nerve is the tenth cranial nerve. It contains both motor (efferent) and sensory (afferent) components [92]. Its afferent nerves can carry an extensive range of signals from periphery to the brain, specifically to the nucleus tractus solitarius, and spread from there to secondary projection regions of the vagus such as the parabrachial nucleus (see Fig. 2). Vagal afferent nerves can rapidly activate central inflammatory pathways, when they are stimulated by immune factors in the periphery [93, 94]. For example, some studies found that vagal afferents induced central expression of inflammatory mediators in response to peripheral pro-inflammatory stimuli and thereby affect sleep [95,96,97]. Romanovsky’s studies conclude that chemosensitive afferent fibers traveling within the abdominal vagus constitute a necessary component of the afferent mechanism of the febrile response to low doses of blood-borne prostaglandins of the E series [98]. The data indicate that peripheral inflammation may alter central function through vagal afferents. Further studies will allow a better understanding of whether there are other neural routes by which peripheral inflammatory signals gain access to the brain except for the vagus nerve, and how these neural routes modulate brain function following surgery and anesthesia.
Other conduits
Recent studies have shown that the existence of direct vascular channels connects skull bone marrow and the brain surface (meninges), capable of supplying monocytes and neutrophils to the meninges and CNS parenchyma under homeostatic and pathological conditions such as stroke, meningitis and leukaemia [99,100,101,102] (see Fig. 2). It remains unknown whether the skull bone marrow-derived neutrophils are more likely to migrate to the meninges and infiltrate the CNS parenchyma and, therefore, affect brain function through the potential skull-to-brain conduit following surgery and anesthesia.
Cellular targets of systemic inflammatory factors
Brain endothelial cells
Cerebral endothelial cells (ECs) are critical components of the BBB, which exhibit a specialized continuous rim of tight junctions and low rate of transcytosis [103]. ECs possess a number of cytokine receptors and adhesion molecules on their luminal side, so they can sense the systemic environment and respond to it [103, 104]. For example, TNF-α receptors [105] and IL-1 receptors [106] are expressed in ECs of brain venules, whose activation was found to be closely associated with local prostaglandin synthesis, microglial activation, monocyte recruitment and BBB breakdown [106,107,108,109], all of which are associated with cytokine influx and cognitive impairment [105, 109]. In addition, C3a receptor is expressed on brain endothelial cells [110]. The C3a/C3aR signaling through endothelial cells promotes vascular inflammation. The complement-mediated signaling impacts vascular health [62, 111]. Perioperative monocytes and inflammatory mediators in blood may interact with ECs, which cause loss of BBB integrity [104], upregulation of cell adhesion and cytokine receptors, and increase of cytokine production and other pro-inflammatory mediators [112, 113]. Activation and/or dysfunction of ECs induced by the peripheral mediators might play an important role in the pathophysiology of PND. But how the mediators change the ECs and impair cognitive function remains to be explored.
Pericytes
Brain pericytes are a critical component of the BBB/NVU and are located directly on the capillary wall and share a common basement membrane with endothelial cells and are in contact with astrocytes, neurons and other glial cells [76, 77]. They play important roles in many neurovascular functions such as regulation of cerebral blood flow, BBB formation and maintenance and angiogenesis [114]. Duan’s study showed that systemic inflammation induces C–C motif chemokine ligand 2 (CCL2) production by pericytes which rapidly relay the inflammatory signals from the circulatory system to neurons via chemokine CCL2 leading to neuronal hyperexcitability [115]. Pericytes secrete several proinflammatory mediators following immunological activation, including IL-1β, TNF-α, IFN-γ, IL-6 and IL-4 [104, 116]. Furthermore, pericyte degeneration has been shown to mediate changes of BBB permeability/or function and leads to cognitive impairment [68, 104, 117, 118]. The inflammatory signals may target pericytes after surgery resulting in CNS damage.
Microglia
Microglia are a type of neuroglia occurring in the CNS. They originate from the yolk sac during embryogenesis and can be defined as tissue-resident macrophages [119, 120]. It is shown that the cellular processes of microglia are extremely plastic in the healthy brain and respond rapidly to pathological conditions such as inflammation. The peripherally produced cytokines can trigger neuroinflammation by activating microglia [32, 37], resulting in direct neurotoxicity and a cognitive decline following surgery. For example, peripheral TNF-α signaling was required to stimulate microglia to produce MCP-1/CCL2 which facilitates the blood monocytes enter the CNS via transcellular and paracellular routes [32, 121, 122], and these migrated monocytes differentiate microglia-like cells, play roles complementary to those of the resident microglia. Accumulating evidence indicates that anesthesia and surgery cause different degrees of microglial activation. The activation results in an inflammatory cascade promoting the synthesis and the secretion of inflammatory mediators [123, 124]. The activated microglia are the primary source of inflammatory cytokines which regulate microglia under feedback control [125]. The amplifying neuroinflammation and microglial activation could contribute to the development of PND [31, 126,127,128,129].
Astrocytes
Astrocytes are the most common glial cell in the CNS and perform a variety of important functions, including recycling neurotransmitters, modulating metabolic homeostasis, provision of nutrients to the neurons, composing the BBB, and participating in immune responses [130]. Systemic inflammation following surgery and anesthesia induces the development of cognitive dysfunction, in which activation of astrocytes is a key component [30, 131,132,133]. The phenotype of A1 astrocytes (a subtype of reactive astrocytes) is strongly induced by IL-1α, TNF and complement component 1, subcomponent q [134], suggesting these astrocytes are hyper-reactive to systemic inflammation. Therefore, it is possible that perioperative period systemic inflammation mediators can cross the BBB and could contribute to this neurotoxic astrocyte phenotype, which release a broad range of pro-inflammatory mediators that lead to neuroinflammation and neuronal injury.
Oligodendrocytes
Oligodendrocytes are the myelinating cells of the CNS. They are the end product of a cell lineage which has to undergo a complex and precisely timed program of proliferation, migration, differentiation, and myelination to finally produce the insulating sheath of axons [135]. Activation of the immune-inflammatory response system and injuries in the neuronal cytoskeleton, oligodendrocytes, astrocytes, glial cells, and myelin sheath are involved in the pathophysiology of delirium following hip fracture surgery [136]. The oligodendrocyte lineage damage varies according to the inflammatory stimulus, i.e., systemic inflammation or cytokine IL-1β impair the oligodendrocyte lineage in the developing brain [137]. There is no information about anesthesia and surgery effect on oligodendrocytes involving in PND.
Neurons
Ultimately, all neuro-modulatory systemic inflammatory mediators must influence neurons to have an impact on cognitive function, which involves not only transduction through different types of non-neuronal cells, but also direct binding to receptors on neurons. In fact, receptors for pro-inflammatory cytokines are expressed in neurons, which can be directly bound by systemic cytokines to alter electrical properties and circuit integration. For example, IL-1 receptors were found to be expressed typically in neurons of the hippocampus [138, 139]. IL-1β induced prolonged hippocampal neurons expression of α5 γ-Aminobutyric acid sub-type A (α5GABAA) receptor whose activation appears to impair memory via activation of IL-1 receptors and p38 MAPK-dependent signaling [140]. Activation of TNF receptor expressed in neurons of the brain leads to neuronal death, which is involved in neural development and neurological diseases [141, 142]. Theoretically, neurons in the brain have ability to directly sense the inflammatory milieu of brain following surgery and anesthesia. Whether and how systemic inflammatory mediators cause neuronal and synaptic destruction and impair neurocognitive function after surgery warrants further research in the future.
Systemic inflammation as a therapeutic target
Neuroinflammation has become a key hallmark of neurological complications including PND [143,144,145]. As mentioned above, systemic inflammatory mediators mainly activate microglia and astrocytes, followed by enhanced neuroinflammation, resulting in neuronal and synaptic destruction and cognitive dysfunction [123, 146,147,148]. Huang’s study demonstrated that non-steroidal anti-inflammatory drugs (NSAIDs) can act rapidly to attenuate systemic inflammation and glial activation and negatively modulate neuropathological changes to improve cognition following surgery in an animal model [30]. Clinically, cyclooxygenase-2 inhibitors (a type of NSAIDs) or Dexmedetomidine (DEX, an important anesthetic adjuvant) have been associated with significantly reduced incidence of POCD and better neurocognitive function [149,150,151]. This effect may be attributed to inhibiting inflammatory mediators including IL-6, IL-8, TNF-α, and C-reactive protein [45, 152]. However, there is a study showing that DEX confer better postoperative neurocognitive function for elderly patients who received total knee arthroplasty that is unrelated to the modulation of DEX on peripheral inflammation [153]. In addition, more conventional anti-inflammatory drugs (dexamethasone) given perioperatively have yielded variable results in clinical trials [154,155,156]. Thus, the anti-inflammatory strategy as preventive and therapeutic procedures for PND remains to be further determined.
Conclusions
Systemic inflammation induced by surgery and anesthesia has been associated with brain injury and PND. The effect may be exerted via inflammatory mediators to exaggerate neuroinflammation involving in neurons, glia and other brain cells, and eventually cause neuronal and synaptic destruction (see Fig. 3). This potential mechanism suggests that regulating the perioperative inflammatory response to surgery may prevent and treat PND. However, the details of the association between surgery, peripheral and central neuroinflammation, and PND development remains unclear. Further animal and human studies are essential to elucidate the underlying cellular mechanisms. Having more insights about systemic inflammation-to-brain injury will be helpful to translate them into clinical practice.
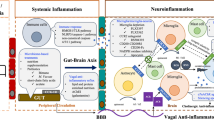
Schematic diagram showing the mechanisms involved in postoperative neuroinflammation. Inflammatory circulating mediators can induce neuroinflammation by inducing BBB breakdown involved in endothelial cell and pericyte, entering into the brain parenchyma and activating astrocytes and microglia release various additional inflammatory mediators. The inflammatory mediators can also directly act on neurons. These processes contribute to neuronal injury and perioperative neurocognitive disorder (PND)
Availability of data and materials
Not applicable.
Abbreviations
- PND:
-
Perioperative neurocognitive disorders
- POCD:
-
Postoperative neurocognitive disorder
- LPS:
-
Lipopolysaccharide
- TNF-α:
-
Tumor necrosis factor α
- IL:
-
Interleukin
- IFN:
-
Interferon
- DAMP:
-
Damage-associated molecular pattern
- HMGB1:
-
High mobility group box 1
- BMDMs:
-
Bone marrow-derived monocytes
- TLR:
-
Toll-like receptor
- NF-κB:
-
Nuclear factor-kappa B
- MCP-1:
-
Monocyte chemotactic protein-1
- MIP:
-
Macrophage inflammatory protein
- NVU:
-
Neurovascular unit
- CNS:
-
Central nervous system
- BBB:
-
Blood–brain barrier
- CSF:
-
Cerebrospinal fluid
- BCB:
-
Blood-cerebrospinal fluid barrier
- CP:
-
Choroid plexus
- CVO:
-
Circumventricular organ
- ECs:
-
Endothelial cells
- CCL2:
-
C–C motif chemokine ligand 2
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- DEX:
-
Dexmedetomidine
References
Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery 2018. Anesthesiology. 2018;129:872–9. https://doi.org/10.1097/ALN.0000000000002334.
Edwards ML, Bause GS. From dental to mental institutions: an american dentist and a british psychiatrist highlight insanity following nitrous-oxide administration. J Anesth Hist. 2018;4:133–4. https://doi.org/10.1016/j.janh.2018.02.002.
Bedford PD. Adverse cerebral effects of anaesthesia on old people. Lancet. 1955;269:259–63. https://doi.org/10.1016/s0140-6736(55)92689-1.
Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112:1179–85. https://doi.org/10.1213/ANE.0b013e318215217e.
Inouye SK, Marcantonio ER, Kosar CM, Tommet D, Schmitt EM, Travison TG, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766–75. https://doi.org/10.1016/j.jalz.2016.03.005.
Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998;351:857–61. https://doi.org/10.1016/s0140-6736(97)07382-0.
Androsova G, Krause R, Winterer G, Schneider R. Biomarkers of postoperative delirium and cognitive dysfunction. Front Aging Neurosci. 2015;7:112. https://doi.org/10.3389/fnagi.2015.00112.
Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. https://doi.org/10.1097/01.anes.0000296071.19434.1e.
Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108:8.
Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, Group I. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–55. https://doi.org/10.1097/ALN.0b013e318195b569.
Pluvinage JV, Wyss-Coray T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci. 2020;21:93–102. https://doi.org/10.1038/s41583-019-0255-9.
Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–38. https://doi.org/10.1016/j.pharmthera.2011.01.014.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. https://doi.org/10.1038/nrn2297.
Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–12. https://doi.org/10.1016/j.biopsych.2008.07.024.
Gisondi P, Sala F, Alessandrini F, Avesani V, Zoccatelli G, Beltramello A, et al. Mild cognitive impairment in patients with moderate to severe chronic plaque psoriasis. Dermatology. 2014;228:78–85. https://doi.org/10.1159/000357220.
Sung CE, Huang RY, Cheng WC, Kao TW, Chen WL. Association between periodontitis and cognitive impairment: Analysis of national health and nutrition examination survey (NHANES) III. J Clin Periodontol. 2019;46:790–8. https://doi.org/10.1111/jcpe.13155.
Xue L, Zou X, Yang XQ, Peng F, Yu DK, Du JR. Chronic periodontitis induces microbiota-gut-brain axis disorders and cognitive impairment in mice. Exp Neurol. 2020;326:113176. https://doi.org/10.1016/j.expneurol.2020.113176.
Yamanaka D, Kawano T, Nishigaki A, Aoyama B, Tateiwa H, Shigematsu-Locatelli M, et al. Preventive effects of dexmedetomidine on the development of cognitive dysfunction following systemic inflammation in aged rats. J Anesth. 2017;31:25–35. https://doi.org/10.1007/s00540-016-2264-4.
Labrenz F, Wrede K, Forsting M, Engler H, Schedlowski M, Elsenbruch S, et al. Alterations in functional connectivity of resting state networks during experimental endotoxemia - An exploratory study in healthy men. Brain Behav Immun. 2016;54:17–26. https://doi.org/10.1016/j.bbi.2015.11.010.
Biesmans S, Bouwknecht JA, Ver Donck L, Langlois X, Acton PD, De Haes P, et al. Peripheral Administration of Tumor Necrosis Factor-Alpha Induces Neuroinflammation and Sickness but Not Depressive-Like Behavior in Mice. Biomed Res Int. 2015;2015:716920. https://doi.org/10.1155/2015/716920.
Song L, Quan X, Su L, Wang K, Wang H, Wu L, et al. Inflammation and behavioral symptoms in preoperational glioma patients: Is depression, anxiety, and cognitive impairment related to markers of systemic inflammation? Brain Behav. 2020;10: e01771. https://doi.org/10.1002/brb3.1771.
Myint AM, Schwarz MJ, Steinbusch HW, Leonard BE. Neuropsychiatric disorders related to interferon and interleukins treatment. Metab Brain Dis. 2009;24:55–68. https://doi.org/10.1007/s11011-008-9114-5.
van den Boogaard M, Kox M, Quinn KL, van Achterberg T, van der Hoeven JG, Schoonhoven L, et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care. 2011;15:R297. https://doi.org/10.1186/cc10598.
Mooijaart SP, Sattar N, Trompet S, Lucke J, Stott DJ, Ford I, et al. Circulating interleukin-6 concentration and cognitive decline in old age: the PROSPER study. J Intern Med. 2013;274:77–85. https://doi.org/10.1111/joim.12052.
Serantes R, Arnalich F, Figueroa M, Salinas M, Andres-Mateos E, Codoceo R, et al. Interleukin-1beta enhances GABAA receptor cell-surface expression by a phosphatidylinositol 3-kinase/Akt pathway: relevance to sepsis-associated encephalopathy. J Biol Chem. 2006;281:14632–43. https://doi.org/10.1074/jbc.M512489200.
Riera Romo M, Perez-Martinez D, Castillo Ferrer C. Innate immunity in vertebrates: an overview. Immunology. 2016;148:125–39. https://doi.org/10.1111/imm.12597.
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. https://doi.org/10.1038/nature08780.
Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62. https://doi.org/10.1146/annurev-immunol-030409-101323.
Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. https://doi.org/10.1038/nri1594.
Huang C, Irwin MG, Wong GTC, Chang RCC. Evidence of the impact of systemic inflammation on neuroinflammation from a non-bacterial endotoxin animal model. J Neuroinflammation. 2018;15:147. https://doi.org/10.1186/s12974-018-1163-z.
Hovens IB, Schoemaker RG, van der Zee EA, Heineman E, Nyakas C, van Leeuwen BL. Surgery-induced behavioral changes in aged rats. Exp Gerontol. 2013;48:1204–11. https://doi.org/10.1016/j.exger.2013.07.011.
Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–95. https://doi.org/10.1002/ana.22664.
Hovens IB, van Leeuwen BL, Nyakas C, Heineman E, van der Zee EA, Schoemaker RG. Prior infection exacerbates postoperative cognitive dysfunction in aged rats. Am J Physiol Regul Integr Comp Physiol. 2015;309:R148-59. https://doi.org/10.1152/ajpregu.00002.2015.
He Y, Li Z, Zuo YX. Nerve blockage attenuates postoperative inflammation in hippocampus of young rat model with surgical trauma. Mediators Inflamm. 2015;2015:460125. https://doi.org/10.1155/2015/460125.
Gong M, Wang G, Li G, Liu J, Sun P, Xu L, et al. Dysfunction of inflammation-resolving pathways is associated with postoperative cognitive decline in elderly mice. Behav Brain Res. 2020;386:112538. https://doi.org/10.1016/j.bbr.2020.112538.
Zhang J, Tan H, Jiang W, Zuo Z. The choice of general anesthetics may not affect neuroinflammation and impairment of learning and memory after surgery in elderly rats. J Neuroimmune Pharmacol. 2015;10:179–89. https://doi.org/10.1007/s11481-014-9580-y.
Hirsch J, Vacas S, Terrando N, Yuan M, Sands LP, Kramer J, et al. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J Neuroinflammation. 2016;13:211. https://doi.org/10.1186/s12974-016-0681-9.
Helmy SA, Wahby MA, El-Nawaway M. The effect of anaesthesia and surgery on plasma cytokine production. Anaesthesia. 1999;54:733–8. https://doi.org/10.1046/j.1365-2044.1999.00947.x.
Yang T, Velagapudi R, Terrando N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol. 2020;21:1319–26. https://doi.org/10.1038/s41590-020-00812-1.
Thordardottir S, Vikingsdottir T, Bjarnadottir H, Jonsson H Jr, Gudbjornsson B. Activation of complement following total hip replacement. Scand J Immunol. 2016;83:219–24. https://doi.org/10.1111/sji.12411.
Hoedemaekers C, van Deuren M, Sprong T, Pickkers P, Mollnes TE, Klasen I, et al. The complement system is activated in a biphasic pattern after coronary artery bypass grafting. Ann Thorac Surg. 2010;89:710–6. https://doi.org/10.1016/j.athoracsur.2009.11.049.
Yuki K, Eckenhoff RG. Mechanisms of the immunological effects of volatile anesthetics: a review. Anesth Analg. 2016;123:326–35. https://doi.org/10.1213/ANE.0000000000001403.
Stollings LM, Jia LJ, Tang P, Dou H, Lu B, Xu Y. Immune modulation by volatile anesthetics. Anesthesiology. 2016;125:399–411. https://doi.org/10.1097/ALN.0000000000001195.
Whitaker EE, Christofi FL, Quinn KM, Wiemann BZ, Xia JC, Tobias JD, et al. Selective induction of IL-1beta after a brief isoflurane anesthetic in children undergoing MRI examination. J Anesth. 2017;31:219–24. https://doi.org/10.1007/s00540-016-2294-y.
Kallioinen M, Scheinin A, Maksimow M, Langsjo J, Kaisti K, Takala R, et al. The influence of dexmedetomidine and propofol on circulating cytokine levels in healthy subjects. BMC Anesthesiol. 2019;19:222. https://doi.org/10.1186/s12871-019-0895-3.
Deiner S, Baxter MG, Mincer JS, Sano M, Hall J, Mohammed I, et al. Human plasma biomarker responses to inhalational general anaesthesia without surgery. Br J Anaesth. 2020. https://doi.org/10.1016/j.bja.2020.04.085.
Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–8. https://doi.org/10.1002/ana.22082.
Terrando N, Yang T, Wang X, Fang J, Cao M, Andersson U, et al. Systemic HMGB1 neutralization prevents postoperative neurocognitive dysfunction in aged rats. Front Immunol. 2016;7:441. https://doi.org/10.3389/fimmu.2016.00441.
Li RL, Zhang ZZ, Peng M, Wu Y, Zhang JJ, Wang CY, et al. Postoperative impairment of cognitive function in old mice: a possible role for neuroinflammation mediated by HMGB1, S100B, and RAGE. J Surg Res. 2013;185:815–24. https://doi.org/10.1016/j.jss.2013.06.043.
Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, et al. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med. 2012;18:930–7. https://doi.org/10.2119/molmed.2012.00195.
He HJ, Wang Y, Le Y, Duan KM, Yan XB, Liao Q, et al. Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neurosci Ther. 2012;18:994–1002. https://doi.org/10.1111/cns.12018.
Lin GX, Wang T, Chen MH, Hu ZH, Ouyang W. Serum high-mobility group box 1 protein correlates with cognitive decline after gastrointestinal surgery. Acta Anaesthesiol Scand. 2014;58:668–74. https://doi.org/10.1111/aas.12320.
Forsberg A, Cervenka S, Jonsson Fagerlund M, Rasmussen LS, Zetterberg H, Erlandsson Harris H, et al. The immune response of the human brain to abdominal surgery. Ann Neurol. 2017;81:572–82. https://doi.org/10.1002/ana.24909.
Hudetz JA, Gandhi SD, Iqbal Z, Patterson KM, Pagel PS. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. J Anesth. 2011;25:1–9. https://doi.org/10.1007/s00540-010-1042-y.
Ji MH, Yuan HM, Zhang GF, Li XM, Dong L, Li WY, et al. Changes in plasma and cerebrospinal fluid biomarkers in aged patients with early postoperative cognitive dysfunction following total hip-replacement surgery. J Anesth. 2013;27:236–42. https://doi.org/10.1007/s00540-012-1506-3.
Qiao Y, Feng H, Zhao T, Yan H, Zhang H, Zhao X. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: the influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 2015;15:154. https://doi.org/10.1186/s12871-015-0130-9.
Casey CP, Lindroth H, Mohanty R, Farahbakhsh Z, Ballweg T, Twadell S, et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143:47–54. https://doi.org/10.1093/brain/awz354.
Sun L, Xie K, Zhang C, Song R, Zhang H. Hyperbaric oxygen preconditioning attenuates postoperative cognitive impairment in aged rats. Neuroreport. 2014;25:718–24. https://doi.org/10.1097/WNR.0000000000000181.
He Z, Xu N, Qi S. Remote ischemic preconditioning improves the cognitive function of elderly patients following colon surgery: A randomized clinical trial. Medicine (Baltimore). 2017;96: e6719. https://doi.org/10.1097/MD.0000000000006719.
Hu J, Feng X, Valdearcos M, Lutrin D, Uchida Y, Koliwad SK, et al. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br J Anaesth. 2018;120:537–45. https://doi.org/10.1016/j.bja.2017.11.096.
Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–22. https://doi.org/10.1073/pnas.1014557107.
Xiong C, Liu J, Lin D, Zhang J, Terrando N, Wu A. Complement activation contributes to perioperative neurocognitive disorders in mice. J Neuroinflammation. 2018;15:254. https://doi.org/10.1186/s12974-018-1292-4.
Sahoo AK, Panda N, Sabharwal P, Luthra A, Balu M, Chauhan R, et al. Effect of anesthetic agents on cognitive function and peripheral inflammatory biomarkers in young patients undergoing surgery for spine disorders. Asian J Neurosurg. 2019;14:1095–105. https://doi.org/10.4103/ajns.AJNS_173_19.
Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78. https://doi.org/10.1152/physrev.00050.2017.
Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. https://doi.org/10.1038/nrn1824.
Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–61. https://doi.org/10.1038/nature09522.
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–78. https://doi.org/10.1016/j.cell.2015.10.067.
Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–6. https://doi.org/10.1038/s41591-018-0297-y.
Yang S, Gu C, Mandeville ET, Dong Y, Esposito E, Zhang Y, et al. Anesthesia and surgery impair blood-brain barrier and cognitive function in mice. Front Immunol. 2017;8:902. https://doi.org/10.3389/fimmu.2017.00902.
Yang T, Xu G, Newton PT, Chagin AS, Mkrtchian S, Carlstrom M, et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br J Anaesth. 2019;122:350–60. https://doi.org/10.1016/j.bja.2018.10.062.
Ni P, Dong H, Wang Y, Zhou Q, Xu M, Qian Y, et al. IL-17A contributes to perioperative neurocognitive disorders through blood-brain barrier disruption in aged mice. J Neuroinflammation. 2018;15:332. https://doi.org/10.1186/s12974-018-1374-3.
Degos V, Vacas S, Han Z, van Rooijen N, Gressens P, Su H, et al. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. 2013;118:527–36. https://doi.org/10.1097/ALN.0b013e3182834d94.
Schwartz M, Baruch K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J. 2014;33:7–22. https://doi.org/10.1002/embj.201386609.
Kunis G, Baruch K, Rosenzweig N, Kertser A, Miller O, Berkutzki T, et al. IFN-gamma-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain. 2013;136:3427–40. https://doi.org/10.1093/brain/awt259.
Baruch K, Schwartz M. CNS-specific T cells shape brain function via the choroid plexus. Brain Behav Immun. 2013;34:11–6. https://doi.org/10.1016/j.bbi.2013.04.002.
Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W, et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci USA. 2013;110:2264–9. https://doi.org/10.1073/pnas.1211270110.
Redzic ZB, Segal MB. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv Drug Deliv Rev. 2004;56:1695–716. https://doi.org/10.1016/j.addr.2004.07.005.
Johanson C, Stopa E, McMillan P, Roth D, Funk J, Krinke G. The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol Pathol. 2011;39:186–212. https://doi.org/10.1177/0192623310394214.
Neman J, Chen TC. The Choroid Plexus and Cerebrospinal Fluid: Emerging Roles in CNS Development, Maintenance, and Disease Progression. Academic Press.2015;1st edition:155–65.
Kaur C, Rathnasamy G, Ling EA. The choroid plexus in healthy and diseased brain. J Neuropathol Exp Neurol. 2016;75:198–213. https://doi.org/10.1093/jnen/nlv030.
Kratzer I, Ek J, Stolp H. The molecular anatomy and functions of the choroid plexus in healthy and diseased brain. Biochim Biophys Acta Biomembr. 2020;1862:183430. https://doi.org/10.1016/j.bbamem.2020.183430.
Ott BR, Jones RN, Daiello LA, de la Monte SM, Stopa EG, Johanson CE, et al. Blood-cerebrospinal fluid barrier gradients in mild cognitive impairment and Alzheimer’s disease: relationship to inflammatory cytokines and chemokines. Front Aging Neurosci. 2018;10:245. https://doi.org/10.3389/fnagi.2018.00245.
Goldim MP, Danielski LG, Rodrigues JF, Joaquim L, Garbossa L, de Oliveira Junior AN, et al. Oxidative stress in the choroid plexus contributes to blood-cerebrospinal fluid barrier disruption during sepsis development. Microvasc Res. 2019;123:19–24. https://doi.org/10.1016/j.mvr.2018.12.001.
Mesquita SD, Ferreira AC, Gao F, Coppola G, Geschwind DH, Sousa JC, et al. The choroid plexus transcriptome reveals changes in type I and II interferon responses in a mouse model of Alzheimer’s disease. Brain Behav Immun. 2015;49:280–92. https://doi.org/10.1016/j.bbi.2015.06.008.
Zanotto C, Simao F, Gasparin MS, Biasibetti R, Tortorelli LS, Nardin P, et al. Exendin-4 reverses biochemical and functional alterations in the blood-brain and blood-CSF barriers in diabetic rats. Mol Neurobiol. 2017;54:2154–66. https://doi.org/10.1007/s12035-016-9798-1.
Hov KR, Berg JP, Frihagen F, Raeder J, Hall R, Wyller TB, et al. Blood-cerebrospinal fluid barrier integrity in delirium determined by Q-albumin. Dement Geriatr Cogn Disord. 2016;41:192–8. https://doi.org/10.1159/000443789.
Benarroch EE. Circumventricular organs: receptive and homeostatic functions and clinical implications. Neurology. 2011;77:1198–204. https://doi.org/10.1212/WNL.0b013e31822f04a0.
Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension. 2013;62:118–25. https://doi.org/10.1161/HYPERTENSIONAHA.113.01404.
Korim WS, Elsaafien K, Basser JR, Setiadi A, May CN, Yao ST. In renovascular hypertension, TNF-alpha type-1 receptors in the area postrema mediate increases in cardiac and renal sympathetic nerve activity and blood pressure. Cardiovasc Res. 2019;115:1092–101. https://doi.org/10.1093/cvr/cvy268.
Okamoto A, Fujii R, Yoshimura R, Miyata S. Transcytosis of tanycytes in the circumventricular organs of adult mouse brain. Neurosci Lett. 2022;779:136633. https://doi.org/10.1016/j.neulet.2022.136633.
Jeong JK, Dow SA, Young CN. Sensory circumventricular organs, neuroendocrine control, and metabolic regulation. Metabolites. 2021. https://doi.org/10.3390/metabo11080494.
Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. https://doi.org/10.1016/S1566-0702(00)00215-0.
Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–9. https://doi.org/10.1189/jlb.0208108.
Maier SF. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain Behav Immun. 2003;17:69–85. https://doi.org/10.1016/s0889-1591(03)00032-1.
Zielinski MR, Dunbrasky DL, Taishi P, Souza G, Krueger JM. Vagotomy attenuates brain cytokines and sleep induced by peripherally administered tumor necrosis factor-alpha and lipopolysaccharide in mice. Sleep. 2013;36(1227–38):38A. https://doi.org/10.5665/sleep.2892.
Luheshi GN, Bluthe RM, Rushforth D, Mulcahy N, Konsman JP, Goldbach M, et al. Vagotomy attenuates the behavioural but not the pyrogenic effects of interleukin-1 in rats. Auton Neurosci. 2000;85:127–32. https://doi.org/10.1016/S1566-0702(00)00231-9.
Kubota T, Fang J, Guan Z, Brown RA, Krueger JM. Vagotomy attenuates tumor necrosis factor-alpha-induced sleep and EEG delta-activity in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1213-20. https://doi.org/10.1152/ajpregu.2001.280.4.R1213.
Romanovsky AA, Ivanov AI, Szekely M. Neural route of pyrogen signaling to the brain. Clin Infect Dis. 2000;31(Suppl 5):S162-7. https://doi.org/10.1086/317515.
Cugurra A, Mamuladze T, Rustenhoven J, Dykstra T, Beroshvili G, Greenberg ZJ, et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. 2021. https://doi.org/10.1126/science.abf7844.
Cai R, Pan C, Ghasemigharagoz A, Todorov MI, Forstera B, Zhao S, et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat Neurosci. 2019;22:317–27. https://doi.org/10.1038/s41593-018-0301-3.
Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, Vandoorne K, et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci. 2018;21:1209–17. https://doi.org/10.1038/s41593-018-0213-2.
Yao H, Price TT, Cantelli G, Ngo B, Warner MJ, Olivere L, et al. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature. 2018;560:55–60. https://doi.org/10.1038/s41586-018-0342-5.
Galea I, Perry VH. The blood-brain interface: a culture change. Brain Behav Immun. 2018;68:11–6. https://doi.org/10.1016/j.bbi.2017.10.014.
Smyth LCD, Rustenhoven J, Park TI, Schweder P, Jansson D, Heppner PA, et al. Unique and shared inflammatory profiles of human brain endothelia and pericytes. J Neuroinflammation. 2018;15:138. https://doi.org/10.1186/s12974-018-1167-8.
D’Mello C, Riazi K, Le T, Stevens KM, Wang A, McKay DM, et al. P-selectin-mediated monocyte-cerebral endothelium adhesive interactions link peripheral organ inflammation to sickness behaviors. J Neurosci. 2013;33:14878–88. https://doi.org/10.1523/JNEUROSCI.1329-13.2013.
Liu X, Nemeth DP, McKim DB, Zhu L, DiSabato DJ, Berdysz O, et al. Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities. Immunity. 2019;50:764–6. https://doi.org/10.1016/j.immuni.2019.02.012.
Erikson K, Tuominen H, Vakkala M, Liisanantti JH, Karttunen T, Syrjala H, et al. Brain tight junction protein expression in sepsis in an autopsy series. Crit Care. 2020;24:385. https://doi.org/10.1186/s13054-020-03101-3.
Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472:113–29. https://doi.org/10.1002/cne.20052.
Engblom D, Ek M, Saha S, Ericsson-Dahlstrand A, Jakobsson PJ, Blomqvist A. Prostaglandins as inflammatory messengers across the blood-brain barrier. J Mol Med (Berl). 2002;80:5–15. https://doi.org/10.1007/s00109-001-0289-z.
Propson NE, Roy ER, Litvinchuk A, Kohl J, Zheng H. Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J Clin Invest. 2021. https://doi.org/10.1172/JCI140966.
Bhatia K, Ahmad S, Kindelin A, Ducruet AF. Complement C3a receptor-mediated vascular dysfunction: a complex interplay between aging and neurodegeneration. J Clin Invest. 2021. https://doi.org/10.1172/JCI144348.
Wu F, Liu L, Zhou H. Endothelial cell activation in central nervous system inflammation. J Leukoc Biol. 2017;101:1119–32. https://doi.org/10.1189/jlb.3RU0816-352RR.
Vizcaychipi MP, Watts HR, O’Dea KP, Lloyd DG, Penn JW, Wan Y, et al. The therapeutic potential of atorvastatin in a mouse model of postoperative cognitive decline. Ann Surg. 2014;259:1235–44. https://doi.org/10.1097/SLA.0000000000000257.
Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–405. https://doi.org/10.1038/nn.2946.
Duan L, Zhang XD, Miao WY, Sun YJ, Xiong G, Wu Q, et al. PDGFRbeta Cells Rapidly Relay Inflammatory Signal from the Circulatory System to Neurons via Chemokine CCL2. Neuron. 2018. https://doi.org/10.1016/j.neuron.2018.08.030.
Brown GC, Vilalta A. How microglia kill neurons. Brain Res. 2015;1628:288–97. https://doi.org/10.1016/j.brainres.2015.08.031.
Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med. 2018;24:326–37. https://doi.org/10.1038/nm.4482.
Jansson D, Rustenhoven J, Feng S, Hurley D, Oldfield RL, Bergin PS, et al. A role for human brain pericytes in neuroinflammation. J Neuroinflammation. 2014;11:104. https://doi.org/10.1186/1742-2094-11-104.
Greter M, Lelios I, Croxford AL. Microglia versus myeloid cell nomenclature during brain inflammation. Front Immunol. 2015;6:249. https://doi.org/10.3389/fimmu.2015.00249.
Chowen JA, Garcia-Segura LM. Microglia, neurodegeneration and loss of neuroendocrine control. Prog Neurobiol. 2020;184:101720. https://doi.org/10.1016/j.pneurobio.2019.101720.
Davoust N, Vuaillat C, Androdias G, Nataf S. From bone marrow to microglia: barriers and avenues. Trends Immunol. 2008;29:227–34. https://doi.org/10.1016/j.it.2008.01.010.
D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–102. https://doi.org/10.1523/JNEUROSCI.3567-08.2009.
Wang T, Zhu H, Hou Y, Gu W, Wu H, Luan Y, et al. Galantamine reversed early postoperative cognitive deficit via alleviating inflammation and enhancing synaptic transmission in mouse hippocampus. Eur J Pharmacol. 2019;846:63–72. https://doi.org/10.1016/j.ejphar.2018.12.034.
Buvanendran A, Kroin JS, Berger RA, Hallab NJ, Saha C, Negrescu C, et al. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104:403–10. https://doi.org/10.1097/00000542-200603000-00005.
Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–55. https://doi.org/10.1002/glia.10161.
Wang HL, Liu H, Xue ZG, Liao QW, Fang H. Minocycline attenuates post-operative cognitive impairment in aged mice by inhibiting microglia activation. J Cell Mol Med. 2016;20:1632–9. https://doi.org/10.1111/jcmm.12854.
Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight. 2017;2: e91229. https://doi.org/10.1172/jci.insight.91229.
Zhou X, Lu J, Wu T, Jiang X, Tian W, Dai W, et al. Multiple anesthesia/surgery cannot impair reference memory in adult mice. Mediators Inflamm. 2020;2020:3736912. https://doi.org/10.1155/2020/3736912.
Wang HL, Ma RH, Fang H, Xue ZG, Liao QW. Impaired spatial learning memory after isoflurane anesthesia or appendectomy in aged mice is associated with microglia activation. J Cell Death. 2015;8:9–19. https://doi.org/10.4137/JCD.S30596.
Lee HG, Wheeler MA, Quintana FJ. Function and therapeutic value of astrocytes in neurological diseases. Nat Rev Drug Discov. 2022;21:339–58. https://doi.org/10.1038/s41573-022-00390-x.
Zhou Y, Wu X, Ye L, Bai Y, Zhang H, Xuan Z, et al. Edaravone at high concentrations attenuates cognitive dysfunctions induced by abdominal surgery under general anesthesia in aged mice. Metab Brain Dis. 2020;35:373–83. https://doi.org/10.1007/s11011-019-00532-y.
Quiroz-Padilla MF, Guillazo-Blanch G, Sanchez MY, Dominguez-Sanchez MA, Gomez RM. Effects of excitotoxic lesion with inhaled anesthetics on nervous system cells of rodents. Curr Pharm Des. 2018;24:4–14. https://doi.org/10.2174/1381612823666170817125015.
Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–43. https://doi.org/10.1097/00000542-200703000-00007.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–7. https://doi.org/10.1038/nature21029.
Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. https://doi.org/10.1007/s00401-009-0601-5.
Maes M, Thisayakorn P, Thipakorn Y, Tantavisut S, Sirivichayakul S, Vojdani A. Reactivity to neural tissue epitopes, aquaporin 4 and heat shock protein 60 is associated with activated immune-inflammatory pathways and the onset of delirium following hip fracture surgery. Eur Geriatr Med. 2023;14:99–112. https://doi.org/10.1007/s41999-022-00729-y.
Favrais G, Bokobza C, Saliba E, Chalon S, Gressens P. Alteration of the oligodendrocyte lineage varies according to the systemic inflammatory stimulus in animal models that mimic the encephalopathy of prematurity. Front Physiol. 2022;13:881674. https://doi.org/10.3389/fphys.2022.881674.
Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987;139:459–63.
Prieto GA, Snigdha S, Baglietto-Vargas D, Smith ED, Berchtold NC, Tong L, et al. Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1beta in the aged hippocampus. Proc Natl Acad Sci USA. 2015;112:E5078-87. https://doi.org/10.1073/pnas.1514486112.
Wang DS, Zurek AA, Lecker I, Yu J, Abramian AM, Avramescu S, et al. Memory deficits induced by inflammation are regulated by alpha5-subunit-containing GABAA receptors. Cell Rep. 2012;2:488–96. https://doi.org/10.1016/j.celrep.2012.08.022.
Yang L, Lindholm K, Konishi Y, Li R, Shen Y. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J Neurosci. 2002;22:3025–32. https://doi.org/10.1523/JNEUROSCI.22-08-03025.2002.
Li R, Yang L, Lindholm K, Konishi Y, Yue X, Hampel H, et al. Tumor necrosis factor death receptor signaling cascade is required for amyloid-beta protein-induced neuron death. J Neurosci. 2004;24:1760–71. https://doi.org/10.1523/JNEUROSCI.4580-03.2004.
Subramaniyan S, Terrando N. Neuroinflammation and perioperative neurocognitive disorders. Anesth Analg. 2019;128:781–8. https://doi.org/10.1213/ANE.0000000000004053.
Kawano T, Yamanaka D, Aoyama B, Tateiwa H, Shigematsu-Locatelli M, Nishigaki A, et al. Involvement of acute neuroinflammation in postoperative delirium-like cognitive deficits in rats. J Anesth. 2018;32:506–17. https://doi.org/10.1007/s00540-018-2504-x.
Danielson M, Wiklund A, Granath F, Blennow K, Mkrtchian S, Nellgard B, et al. Neuroinflammatory markers associate with cognitive decline after major surgery: Findings of an explorative study. Ann Neurol. 2020;87:370–82. https://doi.org/10.1002/ana.25678.
Riazi K, Galic MA, Kentner AC, Reid AY, Sharkey KA, Pittman QJ. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J Neurosci. 2015;35:4942–52. https://doi.org/10.1523/JNEUROSCI.4485-14.2015.
Greenhalgh AD, David S, Bennett FC. Immune cell regulation of glia during CNS injury and disease. Nat Rev Neurosci. 2020;21:139–52. https://doi.org/10.1038/s41583-020-0263-9.
Li D, Chen M, Meng T, Fei J. Hippocampal microglial activation triggers a neurotoxic-specific astrocyte response and mediates etomidate-induced long-term synaptic inhibition. J Neuroinflammation. 2020;17:109. https://doi.org/10.1186/s12974-020-01799-0.
Yang W, Kong LS, Zhu XX, Wang RX, Liu Y, Chen LR. Effect of dexmedetomidine on postoperative cognitive dysfunction and inflammation in patients after general anaesthesia: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2019;98: e15383. https://doi.org/10.1097/MD.0000000000015383.
Lei D, Sha Y, Wen S, Xie S, Liu L, Han C. Dexmedetomidine may reduce IL-6 level and the risk of postoperative cognitive dysfunction in patients after surgery: a meta-analysis. Dose Response. 2020;18:1559325820902345. https://doi.org/10.1177/1559325820902345.
Huang JM, Lv ZT, Zhang B, Jiang WX, Nie MB. Intravenous parecoxib for early postoperative cognitive dysfunction in elderly patients: evidence from a meta-analysis. Expert Rev Clin Pharmacol. 2020;13:451–60. https://doi.org/10.1080/17512433.2020.1732815.
Li B, Li Y, Tian S, Wang H, Wu H, Zhang A, et al. Anti-inflammatory effects of perioperative dexmedetomidine administered as an adjunct to general anesthesia: a meta-analysis. Sci Rep. 2015;5:12342. https://doi.org/10.1038/srep12342.
Mei B, Xu G, Han W, Lu X, Liu R, Cheng X, et al. The benefit of dexmedetomidine on postoperative cognitive function is unrelated to the modulation on peripheral inflammation: a single-center, prospective. Randomized Study Clin J Pain. 2020;36:88–95. https://doi.org/10.1097/AJP.0000000000000779.
Glumac S, Kardum G, Sodic L, Supe-Domic D, Karanovic N. Effects of dexamethasone on early cognitive decline after cardiac surgery: a randomised controlled trial. Eur J Anaesthesiol. 2017;34:776–84. https://doi.org/10.1097/EJA.0000000000000647.
Ottens TH, Dieleman JM, Sauer AM, Peelen LM, Nierich AP, de Groot WJ, et al. Effects of dexamethasone on cognitive decline after cardiac surgery: a randomized clinical trial. Anesthesiology. 2014;121:492–500. https://doi.org/10.1097/ALN.0000000000000336.
Kluger MT, Skarin M, Collier J, Rice DA, McNair PJ, Seow MY, et al. Steroids to reduce the impact on delirium (STRIDE): a double-blind, randomised, placebo-controlled feasibility trial of pre-operative dexamethasone in people with hip fracture. Anaesthesia. 2021;76:1031–41. https://doi.org/10.1111/anae.15465.
Acknowledgements
Not applicable.
Funding
This work was supported partly by the National Natural Science Foundation of China (No. 82270997).
Author information
Authors and Affiliations
Contributions
SJ and WF conceived the idea of this review. SJ, HY and WF performed literature searching and drafted the manuscript. WF created the figures. WF and FH critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, S., Yang, H., Huang, F. et al. Systemic inflammation, neuroinflammation and perioperative neurocognitive disorders. Inflamm. Res. 72, 1895–1907 (2023). https://doi.org/10.1007/s00011-023-01792-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01792-2