Abstract
Surgery can be a life-saving procedure; however, significant complications may occur after routine procedures especially in older and more frail patients. Perioperative neurocognitive disorders (PNDs), including delirium and postoperative cognitive dysfunction, are the most common complications in older adults following common procedures such as orthopedic or cardiac surgery. The consequences of PNDs can be devastating, with longer in-hospital stay, poorer prognosis, and higher mortality rates. Inflammation is gaining considerable interest as a critical driver of cognitive deficits. In this regard, resolution of inflammation, once thought to be a passive process, may provide novel approaches to treat neuroinflammation and PNDs. Herein we review the role for impaired resolution after surgery and the growing role of specialized pro-resolving mediators (SPMs) in regulating postoperative neuroinflammation and neurological complications after surgery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Astrocytes
- Blood-brain barrier
- Bone
- Brain
- Cytokines
- Delirium
- Healing
- Macrophages
- Maresins
- Memory
- Microglia
- Neuroinflammation
- Resolvins
- Surgery
Perioperative neurocognitive disorders (PNDs), previously referred to as postoperative cognitive dysfunction (POCD), include acute changes in cognitive function (i.e. delirium) and longer-lasting memory impairments [1]. PNDs have become the most common complications in older adults after common surgical procedures such as cardiac and orthopedic surgery, and negatively affect post-operative outcomes and recovery in at-risk subjects [2]. Indeed, patients who suffer from PNDs require intensive nursing care, report higher mortality rates, and become at greater risk for further complications, including permanent dementia [3, 4]. Although advanced age has been recognized as a prominent risk factor for PNDs, the pathophysiology of these complications is still poorly understood. Recent studies showed pro-inflammatory signaling molecules can be identified in the central nervous system (CNS) of both PND patients and animal models, implicating surgery-induced neuroinflammation as a key contributor in the pathophysiology of PNDs. The aim of this review is to discuss the role of dysregulated immunity after surgery and the therapeutic potential for specialized pro-resolving lipid mediators (SPMs) in the perioperative neurocognitive arena.
We and others have been interested in clarifying the pathogenesis of PNDs and developed preclinical translational models to study the impact of anesthesia and surgery on the CNS. During surgery, aside from direct effects of anesthetic agents on the CNS (reviewed in [5]), acute inflammation triggered by trauma and sterile injury can negatively affect brain function, contributing to sickness behavior and PND-like pathology [6]. If poorly controlled, dysregulated inflammation leads to more extensive organ dysfunction and tissue injury. Release of pro-inflammatory cytokines, alarmins, and leukotrienes appear to associate with distant long-term effects on the brain, contributing to neuroinflammation, neurotoxicity, and subsequent memory impairments. The mechanisms whereby systemic inflammation affects CNS function remain partly defined and are object of active investigations. Systemic cytokines have been shown to enter the brain through different immune-to-brain signaling pathways, which include humoral, neuronal, and cellular routes [7]. Using a well-established PND model of orthopedic surgery, which frequently leads to cognitive dysfunctions in humans, we first reported a key role for systemic pro-inflammatory cytokines, including interleukin (IL)-1β and tumor necrosis factor alpha (TNFα), in mediating surgery-induced neuroinflammation and cognitive decline following surgery [6, 8]. These initial results as well as work from other models of surgery-induced cognitive dysfunction, prompt for a prominent role of the systemic and humoral response in PND pathogenesis. However, peripheral cytokines in response to non-CNS surgery were also shown to disrupt the blood-brain barrier (BBB ), thus facilitating the migration of peripheral cells, including macrophages, into the brain parenchyma through activation of TNFα/nuclear factor (NF)-κB signaling pathway [9] [10]. BBB homeostasis plays fundamental role in the communication between peripheral inflammation and neuroinflammation [11]. Using fibrinogen as a classical marker of BBB disruption, we reported a significant deposition of this blood-derived molecule in the hippocampal parenchyma [12]. Similar results were reported in other surgical models, with evident BBB opening and disruption of tight junctions after anesthesia and surgery [13]. More recently we described a role for neuronal processing, including pain signaling at the level of the spinal cord, in disrupting acute neurogenesis after orthopedic surgery [14]. These factors, together with the overall activation of innate immune pathways, have been highlighted as pathogenic mechanisms underlying cognitive decline in preclinical models and in early clinical studies. Preclinical models have evaluated multiple therapeutic strategies to modulate neuroinflammation and PND-like behavior. Selective targeting of key pro-inflammatory cytokines (such as anti-TNFα and IL-1 receptor agonist) prevent neuroinflammation and improve cognitive decline in animal models [6, 15]. Macrophage-specific deletion of IKKβ, a central coordinator of TNFα activation of NF-κB, prevents BBB disruption and macrophage infiltration in the hippocampus following surgery. Moreover, harnessing the cholinergic reflex by stimulating the α7 subtype of nicotinic acetylcholine receptors (α7 nAChR) in macrophages inhibited NF-κB activity and BBB disruption, thus preventing neuroinflammation and cognitive decline following surgery [12]. Although these and several other therapies may successfully reduce postoperative neuroinflammation and limit PNDs, they often contribute to side effects by over suppressing the immune system, increasing the risk of infection and delaying wound healing, which are all crucial in the context of postoperative recovery. Thus, it is pivotal to identify safer therapeutic strategies to prevent neuroinflammation without suppressing systemic immune functions.
Is now well appreciated that resolution of inflammation is an active process [16,17,18]. Synthesis and release of anti-inflammatory molecules is required to balance and orchestrate the overall immune response [19]. The acute inflammatory response can be divided into two stages: initiation and resolution. Resolution launches shortly after the initiation of inflammatory response [20]. Seminal work pioneered by Dr. Serhan and colleagues has demonstrated how different mediators actively participate in resolving acute inflammation in a highly regulated manner (recently reviewed in [21]). Fundamental to the resolution process are SPMs, endogenous mediators generated by polyunsaturated fatty acids (PUFA) with signaling abilities to limit inflammation [22]. Omega-3 PUFAs are important catalysts for the synthesis of potent lipid mediators that can exert pro-resolving and anti-inflammatory actions. Oxygenated metabolites derived from eicosapentaenoic acid (20:5(n-3) or EPA) and docosahexaenoic acid (22:6(n-3) or DHA), enriched in fish oils, lead to structurally distinct families of “resolution phase interaction products” [23], which include resolvins, maresins, protectins (as well as sulfide-conjugates in tissue regeneration), and lipoxins (from arachidonic acid). All SPMs display potent anti-inflammatory actions and immunoregulatory properties now tested in several pre-clinical models of acute and chronic inflammation. Indeed, SPMs inhibit the excessive swarming of neutrophils infiltration and enhance the microbial clearance function of innate immune cells [24]. SPMs also operate as “resolution agonists” to regulate the acute inflammation and limit the development of chronic inflammation; importantly they do not show immunosuppressive effects [23, 25]. The exaggerated inflammation and non-resolution of pro-inflammatory processes are characteristic of several neurological conditions and disease states like stroke, neurodegeneration, and chronic pain. Evidence from clinical studies using dietary fish oil supplementation suggest promising effects of omega 3 fatty acid in modulating inflammation both in acute and chronic conditions [26, 27]. Yet the role of SPMs in resolution of neuroinflammation and neuroprotection is just emerging. In Alzheimer’s disease (AD), circulating SPMs and receptor expression are diminished in the brain [28]. A comprehensive review on the role of SPMs in AD is provided in [29]. Here we will review the growing role of SPMs in the perioperative space, focusing on neuroinflammation in PNDs.
Microglia are the primary active immune defense in the CNS responsible to maintain brain homeostasis. However, activated microglia can cause overproduction of pro-inflammatory cytokines and reactive oxidative species that can lead to persistent inflammation and exacerbate pathological changes in CNS [30, 31]. In PND microglia respond to surgical trauma following BBB opening and monocytes infiltration. Modulation of microglial activity is an attractive therapeutic targeted for multiple conditions ranging from neurodegeneration to chronic pain [32]. In vitro studies using immortalised murine microglial cell line BV-2 showed pre-incubation with resolvin D1 (RvD1 ) and E1 (RvE1) inhibit lipopolysaccharide (LPS)-induced TNF-α, IL-6 and IL-1β gene expression via regulating of miRNAs expression and NF-κB signaling pathway [33]. Similar effects of RvD1 were demonstrated in separate studies using human glioma cells or isolated primary rat microglia [34, 35]. RvD1 and maresin-1 (MaR1) also down-regulate β-amyloid (Aβ)42-induced CD11b and CD40 expression in human microglial cells. Moreover, MaR1 and aspirin triggered lipoxin A4 (ATL) both exert stimulatory effect on microglial cells to uptake Aβ42 [36, 37]. In addition to reducing the expression of M1-like markers activation of microglia, RvD1 also promotes the expression of Arg1 and Ym1 in IL-4 activated BV2 cells [38]. These in vitro findings demonstrate that SPMs are directly involved in regulating both classical and alternative microglia activation, thus may display potent therapeutic effects in inflammatory diseases of the CNS. The regulatory effects of SPMs on microglial cells have also been also verified in vivo. A study using Tg2576 mice, which overexpresses a mutant form of amyloid precursor protein (APP) and develops early AD onset, showed neuroprotective effects of ATL by shifting microglial phenotype to a more anti-inflammatory state [37]. In models of surgery-induced microglial activation, both lipoxins and resolvins have been shown to improve neuroinflammation by attenuating release of pro-inflammatory cytokines [39,40,41]. Importantly, other classes of inflammatory-stop signals can modulate neuroinflammation in PND-models. Using a rat model of cardiopulmonary bypass with deep hypothermic circulatory arrest treatment with annexin A1 (ANXA1) was able to improve cognitive outcomes and modulate microglial activation by inhibiting NF-κB p65 transcriptional activity and subsequent cytokine production [42]. Recently, we demonstrated that prophylaxis with MaR1 can rescue microglial activation after orthopedic surgery, as detected by morphological changessuch as shifting to a more ramified homeostatic morphology [43]. Thus, resolution agonists including SPMs may diminish neuroinflammation in part by regulating microglial phenotype, leading to improved cognitive outcomes. To date many of the SPMs have not been evaluated in models of PND and surgical recovery, which warrants future investigations.
Aside from microglia, astrocytes also contribute a key role in maintaining CNS homeostasis, including regulating brain blood flow [44, 45], synapse function [46], extracellular ion concentration [47], and interacting with endothelial cells to support the BBB [48]. Notably, a role for immune-regulation has emerged from these cells [49]. As an active immune regulator in CNS, astrocytes also sense stimulation and danger signals, responding by releasing glia-transmitters and communicating to neurons [50]. Together with microglia they also contribute to further activating adaptive immune defense [51] [49]. Following brain injury or in neurodegenerative diseases, astrocytes undergo pronounced transformation called “astrogliosis”. Astrogliosis changes the cellular expression and morphology of astrocytes and contribute to the repair and scarring process in CNS [52]. However, this reactive phenotype has also been suggested to become detrimental by upregulating the expression of IL-17 receptor and sphingosine 1-phosphate (S1P ) [53, 54]. This further triggers the production of pro-inflammatory cytokines and chemokines, which can lead to exacerbated neuroinflammation and neurodegeneration [55, 56] . During neuroinflammation, the status of astrocytes actions may be determined by the danger signals in the local environment and regulated in time-specific manner [56]. The controversial roles of astrocytes indicate that these cells may play key roles in cognitive function, both in health and diseases. The specific SPMs actions on astrocytes have not been thoroughly investigated. In pain models, Lipoxin A1 exerts anti-nociceptive effect by modulation of astrocytic activation [57]. Similarly, AT-RvD1 was shown to attenuate TNF-α release from spinal astrocytes and improve mechanical hypersensitivity in a rat model of carrageenan-induced peripheral inflammation [58]. In PND models we described changes in astrocytes morphology at 24 h after orthopedic surgery [41, 43]. The astrogliosis is associated with increased basal glutamatergic synaptic transmission, reduced short term plasticity and long-term potentiation in hippocampus [41]. These pathological changes in astrocytes can be eliminated by prophylaxis with aspirin triggered resolvin-D1 (AT-RvD1) as low as ~0.1 μg/kg [41] or MaR1 at ~4 μg/kg [43]. The precise mechanisms underlying the effects of AT-RvD1 and MaR1 on postoperative astrogliosis still require further exploration. Accumulated evidences suggest the pro-inflammatory activity of astrocytes may be regulated by microglia [59] via cytokines, chemokine, complement activation [60], growth factors, and other signaling molecules [61]. After surgery the CCL2/CCR2 axis has been implicated in the neuroinflammatory response; Xu et al. showed that increased CCL2 expression in astrocytes is sufficient to activate microglia and cause learning impairments [62]. Therefore, regulation of astrogliosis may indirectly regulate microglia activity and targeting cell-specific interactions may result into effective therapies for different neurological conditions, including PNDs (Figs. 4.1 and 4.2).
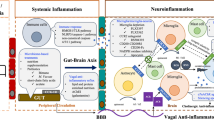
Putative mechanisms of surgery-induced neuroinflammation and cognitive dysfunction. Surgery triggers systemic pro-inflammatory cytokines that can impair the blood-brain barrier, thus allowing peripheral cells into the brain parenchyma. Macrophage infiltration activates glia, including resident microglia and astrocytes, that acutely affect processes of synaptic plasticity and hippocampal-dependent memory function. Treatment with SPMs, such as resolvins and maresins, actively promote resolution of inflammation after surgical trauma and prevent central nervous system dysfunction through the modulation of systemic and central cell types, such as monocytes, microglia, and neurons
Proposed mechanisms for neuroinflammation and surgery-induced cognitive dysfunction. Surgery has been shown to engage the innate immune system and activate a cascade of pro-inflammatory mediators, including alarmins, cytokines and eicosanoids. These molecules exert effects on the humoral and neuronal signaling overall contributing to the neuroinflammatory response. These processes are mediated not only by activation of resident microglia but also by infiltration of peripheral cells into the brain parenchyma via a disrupted blood-brain barrier. This pro-inflammatory milieu and glia dysfunction impair neuronal activity and synaptic plasticity, impinging on processes of long-term potentiation, neurotransmission, and receptor function at the synapse. In combination, these pathological hallmarks contribute to learning and memory impairments following surgical trauma
Intrinsic to the postoperative neuroinflammation is the activation of the peripheral innate immune system. Sterile injury, as during surgery, rapidly triggers the release of TNFα and alarmins into the circulation. TNFα can initiate a pro-inflammatory cytokine cascade that eventually impairs the BBB homeostasis. The BBB dysfunction in turn facilitates the migration of macrophages into the hippocampus [6, 12, 41, 43]. Inhibition of the TNFα signaling by anti-TNFα antibody or genetic abrogation of macrophage-specific IKKβ prevent postoperative BBB disruption and macrophage infiltration in the hippocampus [6, 12]. This work demonstrated the primary impact of innate immune system and systemic inflammation on the development of neuroinflammation and cognitive decline. During resolution of inflammation, SPMs stimulate the cessation of PMN influx [22, 34] and macrophage clearance of cellular and toxic debris [16, 20, 63]. The regulation of macrophage phagocytosis function by SPMs may be mediated by the switch of macrophage phenotype [16]. For example, Dalli et al. demonstrated that 10 nM of MaR1 or RvD1 lead to significant reductions in CD54 and CD80 expression and a concomitant up-regulation of CD163 and CD206 in human macrophage [64]. Moreover, M2-like macrophages are more efficient in converting DHA into MaR1 [64]. Similarly, our in vitro work indicated 10 nM MaR1 can inhibit LPS-induced TNFα release, NF-κB nuclear translocation, superoxide generation and M1-like phenotype surface markers expression in primary bone marrow derived macrophages [43]. These modulatory effects on macrophage function/phenotype may be a key mechanism for SPMs to prevent surgery induced BBB disruption, ensuing neuroinflammation, and cognitive decline [43]. Importantly, because SPMs exert both anti-inflammatory and pro-resolving effects, they are crucial to terminate inflammation but also stimulate tissue repair [65], which is fundamental in the context of perioperative recovery. In fact, RvD1 and maresins have shown to accelerate wound healing in diabetic patients [66, 67]. AT-RvD1 delivered through nanoparticles also enhance wound healing in a mice model of peritonitis [68]. We recently demonstrated that MaR1 pretreatment boosts systemic levels of IL-10 with a long-lasting trend up to 14 days after surgery [43]. Further, we found no difference in callus formation in mice treated with MaR1 compared to vehicle, suggesting MaR1 may be a safe option to be tested in future clinical trials.
Overall, we are starting to uncover the potential for resolution agonists in the context of perioperative disorders and surgical recovery. Further research is needed to evaluate the exact mechanisms of action of different SPMs on different CNS cell types, and whether specific mediators may better target the immune response triggered by surgical trauma. SPMs have been detected in human cerebrospinal fluid, establishing proof-of-principle that may serve as biomarkers for disease progression in neurological conditions like multiple sclerosis [69] and clinical PND [43]. These mediators can also be modified by dietary interventions, for example omega-3 PUFA [70], thus providing attractive strategies to intervene before an elective surgical procedure. SPMs biomarkers may take us a step closer to personalized approaches and targeted interventions to treat common complications, like postoperative pain and PNDs. As coined by Serhan and Savill ‘alpha’ programs ‘omega’ (i.e. the beginning programs the end) [20] could not better define the importance of the temporal events that orchestrate the acute inflammatory response in the perioperative space. Further characterizing these complex molecular events may provide novel and urgently needed approaches to safely treat PND and overall improve brain health for the millions of patients that undergo surgery every year.
References
Evered L et al (2018) Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth 121:1005–1012. https://doi.org/10.1016/j.bja.2017.11.087
American geriatrics society expert panel on postoperative delirium in older, A (2015) Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg 220(136):148–e131. https://doi.org/10.1016/j.jamcollsurg.2014.10.019
Chen CW et al (2014) Increased risk of dementia in people with previous exposure to general anesthesia: a nationwide population-based case-control study. Alzheimers Dement 10:196–204. https://doi.org/10.1016/j.jalz.2013.05.1766
Steinmetz J et al (2009) Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 110:548–555. https://doi.org/10.1097/ALN.0b013e318195b569
Vutskits L, Xie Z (2016) Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci 17:705–717. https://doi.org/10.1038/nrn.2016.128
Terrando N et al (2010) Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A 107:20518–20522. https://doi.org/10.1073/pnas.1014557107
Capuron L, Miller AH (2011) Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 130:226–238. https://doi.org/10.1016/j.pharmthera.2011.01.014
Cibelli M et al (2010) Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol 68:360–368. https://doi.org/10.1002/ana.22082
Terrando N et al (2011) Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol 70:986–995. https://doi.org/10.1002/ana.22664
Degos V et al (2013) Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology 118:527–536. https://doi.org/10.1097/ALN.0b013e3182834d94
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14:133–150. https://doi.org/10.1038/nrneurol.2017.188
Terrando N et al (2011) Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol 70:986–995. https://doi.org/10.1002/ana.22664
Yang S et al (2017) Anesthesia and surgery impair blood-brain barrier and cognitive function in mice. Front Immunol 8:902. https://doi.org/10.3389/fimmu.2017.00902
Zhang MD et al (2016) Orthopedic surgery modulates neuropeptides and BDNF expression at the spinal and hippocampal levels. Proc Natl Acad Sci USA 113:E6686–E6695. https://doi.org/10.1073/pnas.1614017113
Cibelli M et al (2010) Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol 68:360–368. https://doi.org/10.1002/ana.22082
Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510:92–101. https://doi.org/10.1038/nature13479
Serhan CN, Chiang N (2013) Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol 13:632–640. https://doi.org/10.1016/j.coph.2013.05.012
Serhan CN, Chiang N, Dalli J (2017) New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol Asp Med 64:1–17. https://doi.org/10.1016/j.mam.2017.08.002
Serhan CN, Savill J (2005) Resolution of inflammation: the beginning programs the end. Nat Immunol 6:1191–1197. https://doi.org/10.1038/ni1276
Serhan CN, Savill J (2005) Resolution of inflammation: the beginning programs the end. Nat Immunol 6:1191–1197. https://doi.org/10.1038/ni1276
Serhan CN, Levy BD (2018) Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 128:2657–2669. https://doi.org/10.1172/JCI97943
Serhan CN et al (2000) Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med 192:1197–1204
Serhan CN et al (2007) Resolution of inflammation: state of the art, definitions and terms. FASEB J 21:325–332. https://doi.org/10.1096/fj.06-7227rev
Malawista SE, de Boisfleury Chevance A, van Damme J, Serhan CN (2008) Tonic inhibition of chemotaxis in human plasma. Proc Natl Acad Sci USA 105:17949–17954. https://doi.org/10.1073/pnas.0802572105
Recchiuti A, Serhan CN (2012) Pro-resolving lipid mediators (SPMs) and their actions in regulating miRNA in novel resolution circuits in inflammation. Front Immunol 3:298. https://doi.org/10.3389/fimmu.2012.00298
Langerhuus SN et al (2012) The effect of dietary fatty acids on post-operative inflammatory response in a porcine model. APMIS 120:236–248. https://doi.org/10.1111/j.1600-0463.2011.02834.x
Rosell M, Wesley AM, Rydin K, Klareskog L, Alfredsson L (2009) Dietary fish and fish oil and the risk of rheumatoid arthritis. Epidemiology 20:896–901. https://doi.org/10.1097/EDE.0b013e3181b5f0ce
Wang X et al (2015) Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement 11(40):50 e41–50 e42. https://doi.org/10.1016/j.jalz.2013.12.024
Whittington RA, Planel E, Terrando N (2017) Impaired resolution of inflammation in Alzheimer’s disease: a review. Front Immunol 8:1464. https://doi.org/10.3389/fimmu.2017.01464
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69. https://doi.org/10.1038/nrn2038
Polazzi E, Contestabile A (2002) Reciprocal interactions between microglia and neurons: from survival to neuropathology. Rev Neurosci 13:221–242
Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR (2018) Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron 100:1292–1311. https://doi.org/10.1016/j.neuron.2018.11.009
Rey C et al (2016) Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav Immun 55:249–259. https://doi.org/10.1016/j.bbi.2015.12.013
Serhan CN et al (2002) Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 196:1025–1037
Xu MX et al (2013) Resolvin D1, an endogenous lipid mediator for inactivation of inflammation-related signaling pathways in microglial cells, prevents lipopolysaccharide-induced inflammatory responses. CNS Neurosci Ther 19:235–243. https://doi.org/10.1111/cns.12069
Zhu M et al (2016) Pro-resolving lipid mediators improve neuronal survival and increase Abeta 42 phagocytosis. Mol Neurobiol 53:2733–2749. https://doi.org/10.1007/s12035-015-9544-0
Medeiros R et al (2013) Aspirin-triggered lipoxin A4 stimulates alternative activation of microglia and reduces Alzheimer disease-like pathology in mice. Am J Pathol 182:1780–1789. https://doi.org/10.1016/j.ajpath.2013.01.051
Li L et al (2014) Resolvin D1 promotes the interleukin-4-induced alternative activation in BV-2 microglial cells. J Neuroinflammation 11:72. https://doi.org/10.1186/1742-2094-11-72
Feng X et al (2017) Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight 2:e91229. https://doi.org/10.1172/jci.insight.91229
Su X et al (2012) Dysfunction of inflammation-resolving pathways is associated with exaggerated postoperative cognitive decline in a rat model of the metabolic syndrome. Mol Med 18:1481–1490. https://doi.org/10.2119/molmed.2012.00351
Terrando N et al (2013) Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J 27:3564–3571. https://doi.org/10.1096/fj.13-230276
Zhang Z et al (2017) Neuroprotective effects of annexin A1 tripeptide after deep hypothermic circulatory arrest in rats. Front Immunol 8:1050. https://doi.org/10.3389/fimmu.2017.01050
Yang T et al (2019) Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br J Anaesth 122:350–360. https://doi.org/10.1016/j.bja.2018.10.062
Parri R, Crunelli V (2003) An astrocyte bridge from synapse to blood flow. Nat Neurosci 6:5–6. https://doi.org/10.1038/nn0103-5
Gordon GR, Mulligan SJ, Mac Vicar BA (2007) Astro control cerebrovasculature Glia. Astrocyte Control Cerebrovascu 55:1214–1221. https://doi.org/10.1002/glia.20543
Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431. https://doi.org/10.1016/j.tins.2009.05.001
Walz W (2000) Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int 36:291–300
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53. https://doi.org/10.1038/nrn1824
Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145. https://doi.org/10.1016/j.it.2007.01.005
Fiacco TA, Agulhon C, McCarthy KD (2009) Sorting out astrocyte physiology from pharmacology. Annu Rev Pharmacol Toxicol 49:151–174. https://doi.org/10.1146/annurev.pharmtox.011008.145602
Sorg O, Magistretti PJ (1992) Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: blockade by protein synthesis inhibition. J Neurosci 12:4923–4931
Anderson MA et al (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200. https://doi.org/10.1038/nature17623
Colombo E et al (2014) Fingolimod may support neuroprotection via blockade of astrocyte nitric oxide. Ann Neurol 76:325–337. https://doi.org/10.1002/ana.24217
Choi JW et al (2011) FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A 108:751–756. https://doi.org/10.1073/pnas.1014154108
Qian Y et al (2007) The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 8:247–256. https://doi.org/10.1038/ni1439
Colombo E, Farina C (2016) Astrocytes: Key regulators of neuroinflammation. Trends Immunol 37:608–620. https://doi.org/10.1016/j.it.2016.06.006
Svensson CI, Zattoni M, Serhan CN (2007) Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J Exp Med 204:245–252. https://doi.org/10.1084/jem.20061826
Abdelmoaty S et al (2013) Spinal actions of lipoxin A4 and 17(R)-resolvin D1 attenuate inflammation-induced mechanical hypersensitivity and spinal TNF release. PLoS One 8:e75543. https://doi.org/10.1371/journal.pone.0075543
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46:957–967. https://doi.org/10.1016/j.immuni.2017.06.006
Liddelow SA et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. https://doi.org/10.1038/nature21029
Rothhammer V et al (2018) Microglial control of astrocytes in response to microbial metabolites. Nature 557:724–728. https://doi.org/10.1038/s41586-018-0119-x
Xu J et al (2017) Astrocyte-derived CCL2 participates in surgery-induced cognitive dysfunction and neuroinflammation via evoking microglia activation. Behav Brain Res 332:145–153. https://doi.org/10.1016/j.bbr.2017.05.066
Schwab JM, Chiang N, Arita M, Serhan CN (2007) Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447:869–874. https://doi.org/10.1038/nature05877
Dalli J et al (2013) The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J 27:2573–2583. https://doi.org/10.1096/fj.13-227728
Serhan CN, Chiang N, Van Dyke TE (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8:349–361. https://doi.org/10.1038/nri2294
Tang Y et al (2013) Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes 62:618–627. https://doi.org/10.2337/db12-0684
Hong S et al (2014) Maresin-like lipid mediators are produced by leukocytes and platelets and rescue reparative function of diabetes-impaired macrophages. Chem Biol 21:1318–1329. https://doi.org/10.1016/j.chembiol.2014.06.010
Norling LV et al (2011) Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol 186:5543–5547. https://doi.org/10.4049/jimmunol.1003865
Pruss H et al (2013) Proresolution lipid mediators in multiple sclerosis - differential, disease severity-dependent synthesis – a clinical pilot trial. PLoS One 8:e55859. https://doi.org/10.1371/journal.pone.0055859
Mas E, Croft KD, Zahra P, Barden A, Mori TA (2012) Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem 58:1476–1484. https://doi.org/10.1373/clinchem.2012.190199
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yang, T., Terrando, N. (2019). The Evolving Role of Specialized Pro-resolving Mediators in Modulating Neuroinflammation in Perioperative Neurocognitive Disorders. In: Honn, K., Zeldin, D. (eds) The Role of Bioactive Lipids in Cancer, Inflammation and Related Diseases. Advances in Experimental Medicine and Biology, vol 1161. Springer, Cham. https://doi.org/10.1007/978-3-030-21735-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-21735-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21636-8
Online ISBN: 978-3-030-21735-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)