Abstract
Objective and design
This study attempted to clarify the roles of endothelins and mechanisms associated with ETA/ETB receptors in mouse models of colitis.
Materials and methods
Colitis was induced by intracolonic administration of 2,4,6-trinitrobenzene sulfonic acid (TNBS, 1.5 mg/animal) or dextran sulfate sodium (DSS, 3%). After colitis establishment, mice received Atrasentan (ETA receptor antagonist, 10 mg/kg), A-192621 (ETB receptor antagonist, 20 mg/kg) or Dexamethasone (1 mg/kg) and several inflammatory parameters were assessed, as well as mRNA levels for ET-1, ET-2 and ET receptors.
Results
Atrasentan treatment ameliorates TNBS- and DSS-induced colitis. In the TNBS model was observed reduction in macroscopic and microscopic score, colon weight, neutrophil influx, IL-1β, MIP-2 and keratinocyte chemoattractant (KC) levels, inhibition of adhesion molecules expression and restoration of IL-10 levels. However, A192621 treatment did not modify any parameter. ET-1 and ET-2 mRNA was decreased 24 h, but ET-2 mRNA was markedly increased at 48 h after TNBS. ET-2 was able to potentiate LPS-induced KC production in vitro. ETA and ETB receptors mRNA were increased at 24, 48 and 72 h after colitis induction.
Conclusions
Atrasentan treatment was effective in reducing the severity of colitis in DSS- and TNBS-treated mice, suggesting that ETA receptors might be a potential target for inflammatory bowel diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis and Crohn’s disease are chronic, immunologically mediated disorders collectively referred to as inflammatory bowel diseases (IBD). Various factors, such as genetic susceptibility, persistent intestinal infection, defective microbial clearance and/or mucosal barrier function, combined with altered immune responses contribute to the pathogenesis of IBD (for review see Wallace [1] and Sartor [2]). Endothelins (ET-1, ET-2 and ET-3) act on two receptor types cloned in humans (ETA and ETB) [3] and are suggested to have a major role in colitis by their vasoconstrictor properties.
Plasma levels of endothelin-1 (ET-1) were shown to be significantly higher in patients with IBD than in healthy controls [4, 5]. Among the Crohn’s disease patients, there were more immunoreactive cells for endothelins in the submucosa than in the lamina propria whereas the converse was true for the ulcerative colitis group [6].
Local endothelin might contribute to vasculitis in IBD by inducing intestinal ischaemia through vasoconstriction, being an important step in the initiation of inflammation of the intestine [6]. In this line, a recent study have demonstrated that appendicitis/appendectomy (AA) protects against colitis in an AA murine model [7]. The same group showed that seven genes related with endothelin activity were downregulated 28 days post AA, suggesting that suppression of endothelin vasoactivity led to vascular remodeling which may contribute to the limitation of colitis in this model [8].
Data from the rat TNBS colitis model have shown that two non-selective ETA/B receptor antagonists, bosentan and Ro 48-5695, ameliorate the progression of tissue damage [9–11]. Nevertheless, there are controversies about the efficacy of the treatment regimen. Hogaboam et al. [9] have shown in a rat model of TNBS-induced colitis that bosentan was not able to ameliorate macroscopic damage when used in a protective regimen, while Anthoni and collaborators [12] demonstrated, in a mouse model of chronic DSS-induced colitis, that therapeutic administration of bosentan diminished tissue injury.
It is now widely accepted that neutrophils play a crucial role in mediating tissue damage and clinical symptoms in human and experimental colitis [13–15]. Neutrophils express two CXC chemokine receptors, CXCR1 and CXCR2 [16], which play an important role in the pathogenesis of inflammatory responses [17]. In mice, it has been shown that keratinocyte chemoattractant (KC) promotes neutrophil migration, mainly by activation of CXCR2 receptor [18]. In addition, the interaction of KC with CXCR2 may contribute to angiogenesis, which has been suggested to play an important role in the initiation and perpetuation of IBD [19].Some authors have shown that bosentan is able to reduce both the adhesion of leukocytes in colonic submucosal venules and inflammation in a dextran-induced colitis model. However, it has also been suggested that the injury-preventive role of bosentan is independent of cell infiltration [20]. Thus, in spite of the increasing evidence for a role of endothelin in IBD, the mechanisms underlying its participation, as well as the contribution of each receptor need further elucidation. In the present study, we demonstrated that TNBS-induced colitis enhanced ETA and ETB mRNA receptors; however, prepro- ET-1 and ET-2 mRNA oscillated along time. Moreover, ETA but not ETB receptor antagonists ameliorate established colitis, by decreasing cell migration and production of pro-inflammatory cytokines. Besides, we observed a reduction in E-selectin and β2-integrin, as well as, an increase in anti-inflammatory cytokines after Atrasentan treatment.
Materials and methods
Animals
Male BALB/c mice were purchased from Universidade Estadual de Campinas (Campinas, SP, Brazil) and housed in collective cages (15-20 mice per cage) at 22 ± 1 °C under a 12-h light/dark cycle (lights on at 07:00 a.m.), with free access to laboratory chow and tap water, for at least 2 weeks prior to use at 8–10 weeks of age. Experiments were performed during the light phase of the cycle. The experimental procedures were previously approved by UFSC’s Committee on the Ethical Use of Animals (CEUA, PP00062), where the study was carried out, and conducted in accordance with Brazilian regulations on animal welfare.
Induction of colitis
TNBS-induced colitis—the colitis model employed was that originally described by Morris et al. [21]. and slightly modified by Bento et al. [22]. Briefly, under anesthesia with a mixture of ketamine and xylazine (80 and 10 mg/kg, respectively, i.p.), one-day fasted mice were given TNBS (1.5 mg in 100 µL of 35% ethanol) delivered using a polyethylene PE50 catheter inserted into the colon 4 cm proximal to the anus. As the vehicle constitutes part of the colitis-inducing protocol, control mice received an equal volume of sterile 0.9% NaCl solution. Following colonic instillation, the animals were kept in a head-down position for 2 min and refrained from food and water for 4 h (in a separate cage) before transfer to their home cages.
DSS-induced colitis—this model of colitis was employed as previously described by Wirtz et al. [23] and consisted of adding 3% w/v of dextran sulfate sodium (30-50 kD, MP Biomedicals, Cleveland, OH, USA) to the animals’ drinking water for 5 days, followed by another 2 days during which they were offered plain water. Control mice received plain drinking water all along.
Treatment protocols
To evaluate the potential effects of endothelin receptor antagonists in reversing TNBS-induced colitis, mice were treated once daily for 3 days with either Atrasentan (10 mg/kg per day, i.v.; selective ETA receptor antagonist), A192621 (20 mg/kg per day, i.v.; selective ETB receptor antagonist) or vehicles. The choice of doses was made on the basis of results obtained in prior studies by our group and others [24–26]. Drug treatments started 24 h after TNBS administration, and were repeated at 48 h and 68 h. Animals were killed 72 h after TNBS administration, i.e., 4 h after receiving the last injection. Other groups of TNBS-treated mice received Dexamethasone (1 mg/kg, twice a day, s.c.), which was used as a positive control.
In the experiments involving DSS-induced colitis, mice received Atrasentan (10 mg/kg, i.v.) or vehicle once daily, starting concomitantly with DSS treatment until Day 7, and were killed 4 h after receiving the last injection. Dexamethasone and A-192621 were not tested in the DSS-treated mice.
RNA extraction and real-time PCR
Total RNA was extracted from colon samples taken from anesthetized mice treated with vehicle or TNBS using the TRizol® protocol (Invitrogen, Carlsbad, CA, USA). The Reverse Transcription assay was carried out according to the M-MLV Reverse Transcriptase protocol (Invitrogen, Carlsbad, CA, USA). Briefly, a mixture of 2 μg of total RNA, 1 μl of oligo dT 15, 1 μl of dNTP mix (10 mM) and ultra pure water (completed to 12 μl) was heated for 5 min at 65 °C and quickly chilled in ice. After further addition of 4 μl of first strand buffer [250 mM Tris–HCL (pH 8.3), 375 mM KCl and 15 mM MgCl2], 2 μl of 0.1 M Molecular Grade DTT and 1 μl of RNAseOUT®, the mixture was then incubated for 2 min at 37 °C before addition of 1 μl (200 U) of M-MLV Reverse Transcriptase. Following incubation for another 50 min at 37 °C, the reaction was interrupted by heating to 75 °C for 15 min. The c-DNAs were stored at 4 °C until performing the PCR reaction. To this effect, cDNA was amplified in triplicate samples using TaqMan® Universal PCR Master Mix Kit (Applied Biosystems, São Paulo, SP, Brazil) with specific primers, the 3’ quencher MGB and FAM-labeled probes (all from Applied Biosystems, São Paulo, SP, Brazil) for murine: endothelin ETA receptor (Mm01243722_m1), ETB receptor (Mm01224433_m1), preproendothelin-1 (Mm00438656_m1), preproendothelin-2 (Mm00432983_m1; actually prepro-vasoactive intestinal contractor) and GAPDH (NM_008084.2; used as an endogenous control for normalization purposes). The PCR reactions were performed in a 96-well Optical Reaction Plate (Applied Biosystems) and contained: 1 µL of cDNA, 5 µL master mix, 0.5 µL probe and 3.5 µL DEPC water in a total of 10 µL. Amplifications were carried out in a thermal cycler (StepOne Plus, Applied Biosystems) for 40 cycles. The fluorescence was collected at each amplification cycle and the data were analyzed using the 2−ΔΔCt method for expression of relative quantification. Expression of the target genes was calibrated against the conditions found in control animals, i.e., those which received vehicle.
Body weight change and disease activity index
Body weight was measured daily, starting on the day preceding colitis induction in the TNBS model (i.e., before fasting) and then again just prior to intracolonic TNBS or vehicle administration (after fasting) and up to 72 h after treatment. Body weight was also assessed just prior DSS administration and up to 7 days thereafter in the DSS model. In the DSS model only, the clinical disease activity index (DAI) was estimated daily using the protocol proposed by Cooper et al. [27], which ranged from 0 to 4 and was the sum of scores given for body weight loss (scored as: 0 none; 1 1–5%; 2 5–10%; 3 10–20%; 4 over 20%), stool consistency (scored as: 0 well-formed pellets; 2 loose stools; 4 diarrhea) and the presence or absence of fecal blood (scored as: 0 negative hemoccult test; 2 positive hemoccult test; 4 gross bleeding).
Macroscopic and microscopic colonic damage
Three days after TNBS administration, mice were killed and their distal colons removed, rinsed with saline and macroscopic colonic damage was evaluated using the following scoring system: 0 no damage; 1 hyperemia without ulcers; 2 hyperemia with bowel wall thickening but no ulcers; 3 one site of ulceration without bowel wall thickening; 4 two or more sites of ulceration or inflammation; 5 0.5 cm inflammation and major damage; 6, at least 1 cm major damage (for every additional 0.5 cm of damage, the score is increased by one to a maximum of 10); plus 1 for presence of diarrhea, stricture; plus 1 or 2 for presence of mild or severe adhesions, respectively [28]. To evaluate microscopic colon damage by light microscopy, samples of the distal colon were fixed immediately in 10% formaldehyde solution, embedded in paraffin, cut into 5 µm thick transversal sections, mounted on glass slides, deparaffinized and stained with H&E. Microscopic colonic damage was evaluated using the following scoring system: 0 no inflammation; 1 very low inflammation level; 2 low level of leukocyte infiltration; 3 high level of leukocyte infiltration, high vascular density and colon wall thickening; 4 transmural infiltrations, loss of goblet cells, high vascular density and colon wall thickening [29]. In the DSS model, macroscopic damage was assessed according to Kimball et al. [30]., as the sum of scores attributed to stool condition (0 normal well-formed faecal pellets; 1 loosely shaped moist pellets; 2 amorphous, moist, sticky pellets; 3 diarrhoea; plus 1 for presence of blood in stool); colon damage (0 no inflammation; 1 reddening, mild inflammation; 2 moderate or more widely distributed inflammation; 3 severe and/or extensively distributed inflammation); assessment of colon weight loss (0 for <5%; 1 for 5–14%; 2 for 15–24%; 3 for 25–35%, 4 for >35%) and colon length shortening (0 for <5%, 1 for 5–14%, 2 for 15–24%, 3 for 25–35%, 4 for >35%), up to a maximum total score of 15. The intensity of microscopic colonic damage was assessed according to the scoring system described by Rath et al. [31]. and modified by Van der Sluis et al. [32].
Myeloperoxidase assay
Neutrophil infiltration into the distal colon was assessed indirectly by measuring the myeloperoxidase (MPO) activity. Colon segments were homogenized in EDTA/NaCl buffer (pH 4.7) and centrifuged at 10,000 r.p.m. for 15 min at 4 °C. The pellet was resuspended in 0.5% hexadecyltrimethyl ammonium bromide buffer (pH 5.4) and frozen in liquid nitrogen and thawed repeatedly three times. Samples were then centrifuged again (10,000 r.p.m., 15 min, 4 °C), and 25 µL of the supernatant was used for the MPO assay. The enzymatic reaction was assessed by addition of 25 μL of 1.6 mM tetramethylbenzidine in 80 mM NaPO4, plus 100 μL of 0.3 mM H2O2. MPO activity was measured spectrophotometrically at 650 nm and the results are expressed as optical density (OD) per milligram of tissue.
Determination of cytokine levels
Colon segments were homogenized in phosphate buffer containing 0.05% Tween 20, 0.1 mM phenylmethylsulphonyl fluoride, 0.1 mM benzethonium chloride, 10 mM EDTA and 20 IU aprotinin A. The homogenates were centrifuged at 3,000×g for 10 min and the supernatants stored at −80 °C until assays for determination of levels of the cytokines IL-1β, MIP-2, KC, IL-10 and IL-13 were carried out. The amount of protein in each sample was measured using the Bradford method [33], using bovine serum albumin as a standard. The levels of each cytokine were evaluated using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s recommendations (R&D systems, Minneapolis, MN, USA), and the results are expressed in pg/mg of protein in each sample.
Adhesion molecule expression
Fresh 5 µm thick distal colon slices were incubated overnight and at 4 °C with primary anti-E-selectin (1:500), anti-P-selectin (1:500) or anti-β-integrin (1:500) antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) dissolved in Renaiscence solution (Biocare Medical, Concord, CA, USA). Prior incubation, high-temperature antigen retrieval was performed placing the slides in 10 mM trisodium citrate buffer pH 6.0, in a water bath at 95–98 °C, for 45 min. The slides were then washed twice with PBS and incubated with PicTure ™ MAX Polymer detection kit, according to the manufacturer’s protocol. The sections were counterstained lightly with Harris’s haematoxylin solution. Control and experimental tissue sections were placed on the same glass slide and processed under the same conditions. Images of colon sections stained with antibodies to E-selectin, P-selectin and β-integrin were acquired using an light microscope (Olympus, BX-41) connected to a digital camera (3.3 Mpixel QCOLOR3C, QimagingTM) and an image acquisition software (Qcapture Pro 5.1, QImagingTM). All image analyses were performed using NIH ImageJ 1.36b imaging software (National Institutes of Health, Bethesda, MD, USA). Settings for image acquisition were identical for control and experimental tissue sections, and four images were captured from each section. The total pixels intensity was determined, and data were expressed as optical density (OD).
Primary cultures of peritoneal leukocytes
Naïve mice received an i.p. injection of 1 ml of 3% thioglycollate (BD, Franklin Lakes, NJ, USA). Four or 72 h later, peritoneal exudate cells were harvested by three consecutive lavages of the peritoneal cavity with 7 ml of cold phosphate-buffered saline, interspersed with gentle massages of the abdomen. Lavage samples recovered were centrifuged at 200×g for 10 min at 4 °C and subjected to hypotonic lysis to eliminate red blood cells, regardless of the presence of a red pellet. Centrifugation was repeated and after an additional wash the cells were resuspended in DMEM supplemented with 10% heat inactivated calf serum, 10 U/mL penicillin, 10 mg/mL streptomycin. Exudate cell numbers and populations were determined by Poch hemocytometry (Sysmex Corporation, São Paulo, SP, Brazil) and confirmed by total and differential cell counts using Neubauer chamber and May-Grunwald Giemsa stained cytospin preparations. Polymorphonuclear cells comprised 85–90% of the cells present in exudate samples collected at 4 h following thioglycollate injection, the remaining cells constituted mononuclear cells. Due to their high yield, relative to other cell types, these neutrophils were used without additional purification steps. Samples collected 72 h after thioglycollate injection contained mainly mononuclear cells, from which macrophages were purified by adherence to plastic, whereby 0.1 mL suspensions of 2 × 106 cells/ml in culture medium were distributed in 96-well plates, incubated for 2 h at 37 °C in 5% CO2 and washed. Cells adhered to the wells were considered macrophages. Peritoneal neutrophils and macrophages (2 × 106 cells/mL) were stimulated with lipopolysaccharide (LPS, 100 ng/mL) for 4 and 24 h, respectively, either in the presence or absence of ET-1 or ET-2 (each at 30 or 100 nM), BQ-123 (selective ETA receptor antagonist, 1 µM) or BQ-788 (selective ETB receptor antagonist, 1 µM). The choice of drugs concentrations used in in vitro experiments were based on the previous studies [34–36]. Control cells were incubated with the appropriate corresponding vehicles. After stimulation, the plate was centrifuged (200×g, 10 min) and cell-free supernatant was collected and stored at −70 °C until determination of cytokine (MIP-2, KC and IL-1β) levels as described above.
Drugs and reagents
Atrasentan and A-192621 ([2R-(2a,3b,4a)]-4-(1,3-benzodioxol-5-yl)-1-[2-(2,6-diethylphenyl)amino]-2oxoethyl]-2-(4-propoxyphenyl)-3 pyrrolidinecarboxylic acid) were kindly provided by Abbott Laboratories (Abbott Park, IL, USA). Dexamethasone, hexadecyltrimethylammonium bromide (HTAB), E. coli lipopolysaccharide (batch number 076K4085), tetramethylbenzidine (TMB), hydrogen peroxide, Tween 20, Tween 80, phenylmethylsulphonyl fluoride (PMSF), benzethonium chloride, ethylenediamine tetraacetic acid (EDTA), aprotinin, phosphate-buffered saline (PBS), eosin, haematoxylin, penicillin, streptomycin and 2,4,6-TNBS were all purchased from Sigma (St. Louis, MO, USA). BQ-123 (cyclo[D-Trp-DAsp- Pro-D-Val-Leu]) and BQ-788 (N-cis-2,6-dimethylpiperidinocarbonyl-L-g-methylleucyl-D-1-methoxycarboyl-norleucine) were from RBI (Natick, MA, USA), ET-1 and ET-2 were from American Peptides (Sunnyvale, CA, USA), ketamine was from Sespo (Sao Paulo, Brazil), xylazine was from Vertbrands (Sao Paulo, SP, Brazil), formaldehyde was from Merck (Darmstadt, Germany) and Bradford reagent was from Bio-Rad Laboratories (Richmond, CA, USA). Atrasentan was dissolved in water containing 100 µL of 0.1 N NaOH, A192621 in warm water containing 3% ethanol and 100 µL of 0.1 N NaOH, BQ-123 and BQ-788 in PBS and Dexamethasone was dissolved in saline.
Statistical analysis
All data are expressed as mean ± sem. Statistical analysis was performed using Kruskal–Wallis followed by Dunn’s tests for non-parametric data, one-way ANOVA followed by Newman-Keuls test for parametric data. All analyses were conducted using GraphPad Prism 4 software (GraphPad Software Inc., San Diego, CA). Differences with p ≤ 0.05 were considered to be statistically significant.
Results
Influence of TNBS-induced colitis on the colonic endothelinergic system
Initially, in order to investigate whether TNBS-mediated colitis might induces alterations in colonic endothelinergic system, we evaluated the mRNA expression of mRNA encoding for prepro-ET-1, prepro-ET-2 and endothelin ETA and ETB receptors, detected through quantitative RT-PCR, in colonic samples collected at 24, 48 and 72 h after intracolonic TNBS administration (Fig. 1). Samples from control mice exhibited detectable mRNA levels for all four proteins. Prepro-ET-1 and prepro-ET-2 mRNA levels were significantly diminished at 24 h after colitis induction (by 50 and 60%, respectively). While prepro-ET-1 mRNA levels returned to basal values thereafter, those of prepro-ET-2 mRNA increased 2.4-fold over baseline values at 48 h and diminished again back to control levels at 72 h after colitis induction. Notably, ETA and ETB receptor mRNA levels were both consistently up-regulated by 1.8-, 2.9- and 3.5-fold, and by 2.4-, 4.1- and 3.2-fold at 24, 48 and 72 h after TNBS administration, respectively.
Changes in colonic mRNA levels encoding for prepro-ET-1, prepro-ET-2 and endothelin ETA and ETB receptors induced by TNBS. Colonic samples from control mice exhibited detectable mRNA levels for ETA and ETB receptors and for prepro-ET-1, prepro-ET-2, ETA (a) and ETB (b) receptor mRNA levels were both consistently up-regulated at 24, 48 and 72 h after TNBS administration. Prepro-ET-1 (c) and prepro-ET-2 (d) mRNA levels were significantly diminished at 24 h after colitis induction. Prepro-ET-1 mRNA levels returned to basal values thereafter, but prepro-ET-2 mRNA increased over baseline values at 48 h and diminished again back to control levels at 72 h after colitis induction. GAPDH was used as an endogenous control. Each column represents the mean ± SEM of 4-7 mice per group. # P < 0.05, versus control group; *P < 0.05 versus TNBS-treated group
Influence of Atrasentan treatment on TNBS-induced body weight loss and macroscopic colonic damage
Unlike controls, mice given TNBS failed to regain the body weight they lost during fasting for at least 3 days after its administration (Fig. 2a). After 72 h of TNBS administration, mice developed signs of severe illness characterized by bloody diarrhea and rectal prolapse (results not shown) and their colons displayed a 2.9-fold increase in weight (Fig. 2b), as well as severe inflammation associated with hyperemia, ulceration and occasionally adhesion, leading to a high macroscopic damage score (Fig. 2c). Daily treatment with the selective ETA receptor antagonist Atrasentan (10 mg/kg/day, i.v.), starting 24 h after TNBS administration, i.e., at a time point when colitis is already established [22], and repeated at 48 and 72 h, significantly recovered body weight loss (Fig. 2a). At this same dose, Atrasentan also fully reversed the increase in colon weight and reduced the macroscopic damage score (Fig. 2b, c). In sharp contrast, identical treatment of TNBS-administered mice with the selective ETB receptor antagonist A-192621 (20 mg/kg/day, i.v.) was not able to reduce macroscopic damage, colon weight or to recover the body weight lost during fasting (Fig. 2a–c). On the other hand, Dexamethasone treatment (1 mg/kg, twice daily, s.c.), used as a positive control, markedly reduced TNBS-induced increase in colonic weight and macroscopic damage score, but failed entirely to restore body weight loss (Fig. 2a–c).
Therapeutic treatment with Atrasentan reduces the severity of TNBS-induced colitis. Mice were given TNBS (1.5 mg in 100 µL of 35% ethanol) and were treated with Atrasentan (AT, 10 mg/kg, i.v.), A192621 (20 mg/kg, i.v.), Dexamethasone (Dex, 1 mg/kg, twice a day, s.c.) or vehicle, starting 24 h after TNBS administration. Control mice received an equal volume of sterile 0.9% NaCl solution. Throughout the experiment mice were monitored for body weight loss and at 72 h after TNBS administration, colitis severity was assessed. Treatment with Atrasentan recovered mouse weight (a), decreased colon weight (b), and reduced the macroscopic score (c). Treatment with A192621 did not alter the parameters analyzed. Each column represents the mean ± S.E.M. of 5-7 mice per group. # P < 0.05, versus control group; *P < 0.05 versus TNBS-treated group
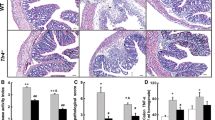
Influence of Atrasentan treatment on TNBS-induced colonic neutrophil accumulation and microscopic damage
Colonic samples taken from TNBS-treated mice 3 days after colitis induction displayed 20-fold higher MPO levels relative to controls (from 0.145 ± 0.05 to 2.9 ± 0.2 OD/mg tissue; Fig. 3a). Treatment with Atrasentan at 10 mg/kg/day or Dexamethasone (1 mg/kg/twice daily, s.c.) significantly reduced the TNBS-induced increase in colonic MPO levels by 83.0 ± 5.7 and 94.4 ± 4.1%, respectively. A-192621 (20 mg/kg/day, i.v.) also failed to modify MPO levels (data not shown) and no further experiments were carried out with this antagonist in the TNBS colitis model. Microscopic damage scores obtained by histological examination of H&E stained colon sections taken from TNBS-treated animals were 16.5-fold greater than those of control tissue samples (Fig. 3b). As illustrated in Fig. 3c, samples from TNBS-treated animals exhibited a dramatic infiltration of leucocytes into the lamina propria and colonic mucosa, substantial thickening and exudate (which suggests plasma extravasation), distortion of crypts and loss of goblet cells. Again, treatment with Atrasentan and Dexamethasone reduced microscopic damage scores by 91.4 ± 5.4 and 70.9 ± 7.3%, respectively, and largely suppressed the inflammatory changes induced by TNBS.
Therapeutic treatment with Atrasentan inhibits cell influx and improves microscopic colon damage. Mice were given TNBS (1.5 mg in 100 µL of 35% ethanol) and were treated with Atrasentan (AT, 10 mg/kg, once daily, i.v.) or Dexamethasone (Dex, 1 mg/kg, twice a day, s.c.) or vehicle, starting 24 h after TNBS administration. Control animals received an equal volume of sterile 0.9% NaCl solution. Seventy-two hours following colitis induction, the histopathological tissue damage and the MPO activity were determined. Treatment with Atrasentan decreased MPO activity (a) and the microscopic score (b). c shows colon sections from control mice, TNBS, Atrasentan (AT) or Dexamethasone-(DEX) treated mice, stained with H&E. The images are representative of at least three mice per group. Original magnifications, ×20 (c). Each column represents the mean ± SEM of five to seven mice per group. # P < 0.05 versus control group; *P < 0.05 versus TNBS-treated group
Influence of Atrasentan treatment on TNBS-induced changes in colonic adhesion molecule expression and cytokine levels
To further characterize the inflammatory changes promoted by TNBS and the anti-inflammatory properties of Atrasentan, colon samples were collected 3 days after colitis induction, sectioned and stained with antibodies against P-selectin, E-selectin or β2-integrin (Fig. 4a-c). As shown in Fig. 4, tissue sections from control mice exhibited very low levels of specific staining for P-selectin and E-selectin only in endothelial cells and for β2-integrin only in leukocytes. In comparison, P-selectin, E-selectin and β2-intregrin staining in corresponding tissues from TNBS-treated mice was increased by 5.2-, 5.7- and 3.7-fold, respectively, as revealed by densitometry. In colon samples taken from TNBS animals treated with Atrasentan (10 mg/kg/day, i.v.) or Dexamethasone (1 mg/kg/twice daily, s.c.) the intensities of immunostaining were inhibited by 76.4 ± 15.4 and 70.1 ± 18.2% for P-selectin, 85.7 ± 8.0 and 82.9 ± 12.4% for E-selectin and 73.8 ± 11.4 and 66.0 ± 13.0% for β2-integrin, respectively, relative to the values detected in tissues from TNBS mice treated with the corresponding vehicles.
Therapeutic treatment with Atrasentan decreased the expression of P-selectin, E-selectin and β2-integrin induced by TNBS. Mice were given TNBS (1.5 mg in 100 µL of 35% ethanol) and were treated with Atrasentan (AT, 10 mg/kg, once daily, i.v.), Dexamethasone (Dex, 1 mg/kg, twice a day, s.c.) or vehicle, starting 24 h after TNBS administration. Control animals received an equal volume of sterile 0.9% NaCl solution. Seventy-two hours following colitis induction, colonic tissues were collected, sectioned and stained with antibodies against P-selectin, E-selectin or β2-integrin. Treatment with Atrasentan decreased P-selectin (a), E-selectin (b) and β2-intregrin (c) expression, as observed by the immunostaining intensity. Each column represents the mean ± SEM of four mice per group. # P < 0.05, versus control group; *P < 0.05, versus TNBS-treated group
On the other hand, colonic levels of the pro-inflammatory cytokines IL-1β, MIP-2 and KC were markedly elevated at 72 h after inducing colitis with TNBS, achieving values of 6.5-, 3.4- and 3.7-fold higher than those measured in control samples, respectively. Atrasentan administration fully abrogated the TNBS-induced increases in colonic levels of IL-1β, KC and MIP-2 (96.1 ± 2.6, 95.0 ± 3.0% and >100%, respectively), and qualitatively similar results were observed following treatment with Dexamethasone (Fig. 5a–c). Conversely, TNBS-induced colitis was associated with significant decreases in the colonic levels of the anti-inflammatory cytokines IL-10 and IL-13, to values corresponding to 43.3 ± 9.4 and 58.1 ± 2.8% of those measured in colon samples from control mice. While treatment with Dexamethasone restored the levels of both anti-inflammatory cytokines to those detected in control tissues, treatment with the ETA receptor antagonist only normalized IL-10 (1.6-fold increase over corresponding value of the control TNBS-treated group; Fig. 5d, e) but not IL-13 levels.
Treatment with Atrasentan changes colonic cytokine production. Mice were given TNBS (1.5 mg in 100 µL of 35% ethanol) and were treated with Atrasentan (AT, 10 mg/kg, once daily, i.v.), Dexamethasone (Dex, 1 mg/kg, twice a day, s.c.) or vehicle, starting 24 h after TNBS administration. Control animals received an equal volume of sterile 0.9% NaCl solution. Seventy-two hours following colitis induction the levels of IL-1β, KC, MIP-2, IL-10 and IL-13 were measured in the colon tissue. Therapeutic treatment with Atrasentan decreased IL-1β (a), KC (b) and MIP-2 (c) production induced by TNBS. TNBS decreased IL-10 (d), and IL-13 (e) levels, and Atrasentan treatment restored IL-10, but not IL-13 levels. Each column represents the mean ± S.E.M. of 5–7 mice per group. # P < 0.05 versus control group; *P < 0.05 versus TNBS-treated group
Effects of LPS and endothelins on primary cultures of neutrophils and macrophages
The reduction in IL-1β, MIP-2 and KC levels induced by Atrasentan in TNBS-treated colon could be due to direct blockade of ETA receptors on leukocytes, which produce them, or to inhibition of cell migration into colonic tissue. We thus stimulated primary cultures of peritoneal macrophages and neutrophils with LPS, an important component of colitis-induced damage, and tested the effects of BQ-123 and BQ-788 (selective antagonists of ETA and ETB receptors, respectively; 1 μM) on IL-1β, MIP-2 and KC production. As illustrated in Fig. 6, in vitro stimulation of macrophages with LPS for 24 h increased MIP-2 and KC levels by 2.1- and 9.2-fold, respectively. Notably, MIP-2 production by LPS-stimulated macrophages was unaltered by co-incubation with endothelin-1 or endothelin-2 (30 or 100 nM), but KC production was similarly enhanced ~1.6-fold by both doses of endothelin-2 (Fig. 6b). The production of MIP-2 and KC by LPS-stimulated macrophages was significantly reduced by co-incubation with BQ-123 (80.9 ± 5.2 and 70.8 ± 8.8% inhibition, respectively), but not with BQ-788 (Fig. 6a, b). Cultured neutrophils stimulated with LPS displayed 22- and 3.2-fold increases in MIP-2 and IL-1β production over basal levels, respectively, and these responses were unaltered by co-incubation with either endothelin-1 or endothelin-2 (Fig. 6c, b). The effect of LPS on neutrophil MIP-2 production was reduced by BQ-123, but not BQ-788, whereas neither antagonist affected the increase in IL-1β production (Fig. 6c, d). Importantly, neither endothelin-1 nor endothelin-2 (30 or 100 nM) altered the basal production of any of these cytokines in control macrophages or neutrophils (i.e., incubated in the absence of LPS; data not shown).
ETA receptor blockade decreased pro-inflammatory cytokine production in pleural macrophages and neutrophils stimulated with LPS. Macrophages (2 × 106 cells/mL) or neutrophils (2 × 106 cells/mL) were stimulated in vitro with LPS (100 ng/mL), for 24 h, either in presence or absence of ET-1 or ET-2 (each at 30 or 100 nM), BQ-123 (selective ETA receptor antagonist, 1 µM) or BQ-788 (selective ETB receptor antagonist, 1 µM). BQ-123 incubation reduced MIP-2 (a) and KC (b) production by macrophages (a, b) and by neutrophils (c, d). In addition, ET-2 (30 or 100 nM) co-incubation enhanced KC production induced by LPS (b), whereas neither antagonist affected the increase in IL-1β production (d). Each point represents the mean ± SEM of four experiments performed in triplicate. # P < 0.05, versus control group; *P < 0.05, versus LPS-treated group
Effects of Atrasentan in the DSS-induced colitis model
To investigate if Atrasentan treatment would also prove to be beneficial in another preclinical model of colitis, we have tested its effects on some parameters of colitis induced by DSS addition to the drinking water of Balb/c mice for 5 days, followed by washout for another 2 days. DSS administration was associated with significant body weight loss on Days 6 and 7, increases in the clinical DAI from Day 3 onwards and colon length reduction (Fig. 7a–c, respectively). Atrasentan treatment (10 mg/kg/day, i.v.), starting concomitantly with DSS administration, fully prevented the loss of body weight and markedly attenuated the disease activity index from Day 4 onwards, particularly by ameliorating stool consistency and reducing rectal bleeding (Fig. 7a, b). In addition, as evaluated on Day 7, Atrasentan treatment partially prevented colon length reduction (Fig. 7c) and also decreased markedly colonic macroscopic and microscopic damage scores and the increase in MPO levels induced by DSS (Fig. 7d–f, respectively). As shown in Fig. 7g, histological analysis of colon sections from DSS-treated mice revealed large areas of epithelial crypt loss, prominent infiltration of leucocytes throughout the mucosa. All of these aspects were prevented by Atrasentan treatment.
Atrasentan treatment ameliorates DSS-induced colitis. Mice received DSS for 5 days followed by another 2 days during which they were offered DSS-free (i.e., plain) drinking water. Animals were treated with Atrasentan (AT, 10 mg/kg, i.v., once daily) from day 0 to day 7. DSS induced a long-lasting body weight loss (a), increased the disease activity index (b), reduced the colon length (c), and decreased the macroscopic score (d), the microscopic score (e) and the MPO levels (f). Atrasentan treatment improved all these parameters. Histological analysis showed that the large areas of epithelial crypt loss and prominent infiltration of inflammatory cells induced by DSS was minimized by Atrasentan (g). Each column represents the mean ± SEM of six mice per group. # P < 0.05, versus control group; * P < 0.05, versus DSS-treated group
Discussion
The current study provides ample evidence that consistently implicate endothelins, acting through ETA receptor-coupled mechanisms, in the manifestation of TNBS-induced colitis in mice. It shows that TNBS dynamically changes mRNA levels encoding for endothelins and their receptors, and that treatment with Atrasentan, a highly selective ETA receptor antagonist, initiated 24 h after the onset of colitis, restores body weight, reduces colonic damage, leukocyte infiltration and overproduction of pro-inflammatory cytokines and augments levels of the anti-inflammatory cytokine IL-10. Furthermore, as Atrasentan also corrected the body weight loss and reduced DAI and macroscopic and microscopic colonic damage associated with DSS-induced colitis, endothelin ETA receptor antagonists might constitute highly effective agents for treatment of IBD.
The colon contains all elements needed for a functional endothelin system, including all three endothelin isoforms, ECE-1 (endothelin-converting enzyme-1) and both ETA and ETB receptors [37–41]. ET-1 mRNA was detected in the epithelium, macrophages and stromal cells of the lamina propria in human large intestine [40, 42] and ET-1 and ET-3 peptides were found on the mucosa of the rat intestine [43].
ET-1-like immunoreactivity was also observed in the neurons of human colon, and it has been suggested that ET-1 modulates intestinal motility and secretion, acting as a neuropeptide [38, 44]. In addition, it has been reported that in rat intestine ET-2 mRNA is predominantly expressed in stromal cells of the lamina propria [39] and studies with ET-3 deficient mice have suggested that this isoform is essential for the development of enteric neurons [45]. However, no detailed information on the expression and distribution of ET-2 is available.
Regarding the endothelin receptor subtypes, the presence of both, ETA and ETB receptors, in the rats’ intestine was reported by Koseki et al. [37], which was corroborated by Yoshimura et al. [46], based on [125I]-ET-1 ligand binding assay on guinea pigs’ intestine tissue sections. These authors have showed that ET receptors were localized mainly to the mucosal layer, submucous plexus and the myenteric plexus of the muscle layer.
Despite the evidence that release of ET-1/2 does not contribute to human IBD [47], a number of studies suggested the opposite [4, 6]. Indeed, the present study and others, using different experimental models of colitis, support the role of endothelins in the pathophysiology of this disease. In a rat model of TNBS-induced colitis, Hogaboam et al. [9]. have demonstrated that bosentan dose-dependently reduced colonic damage and MPO activity when it was given 24 h prior to colitis induction. In addition, Güllüoglu et al. [10]. reported that bosentan treatment significantly reduced MPO activity and protein oxidation level, suggesting the involvement of ETs in the pathogenesis of colonic injury in this animal model of colitis. In line with these observations, Padol et al. [11]. demonstrated that oral administration of Ro 48-5695 (10 mg/kg), a non-selective ETA/ETB receptor antagonist, ameliorated TNBS-induced damage by reducing MPO level and incidence of diarrhea and adhesions. Likewise, Lee et al. [48]. found that SM-19712, an ECE-1 inhibitor, was able to attenuate DSS-induced increases in loose stools, fecal blood, weight loss, histologic signs of inflammation, and immunostaining of PECAM-1. Furthermore, bosentan treatment was able to reduce colonic tissue damage induced by DSS mainly by reducing the adhesion of leucocytes in colonic submucosal venules [12].
Corroborating the existing data, the present study demonstrated the effectiveness of a selective ETA receptor antagonist Atrasentan in TNBS and DSS-induced colitis models in mice. In the TNBS model, Atrasentan treatment restored body weight, reduced colonic damage, leukocyte infiltration and overproduction of pro-inflammatory cytokines and augmented levels of the anti-inflammatory cytokine IL-10. Furthermore, in the DSS model, Atrasentan also corrected the body weight loss and reduced DAI and macroscopic and microscopic colonic damage, suggesting that the anti-inflammatory effect of Atrasentan was not dependent on the animal model used.
A point of controversy is the efficacy of the treatment regimen with endothelin receptor antagonists, which refers to the role of endothelins in initiating and/or maintaining the inflammatory process in the colon. Hogaboam et al. [9]. have shown in a rat model of TNBS-induced colitis, that bosentan at 30 mg/kg was able to reduce macroscopic damage, only when given in a protective regimen, but not 1 h after colitis induction. On the other hand, Anthoni and collaborators [12] demonstrated that therapeutic administration (i.e., 26–30 days after colitis induction) of bosentan at the same dose (30 mg/kg) decreased tissue injury in a mouse model of chronic DSS-induced colitis. In agreement with this latter study, our data demonstrated that the curative treatment with Atrasentan significantly attenuated colonic injury and inflammation parameters in TNBS-induced colitis in mice.
Altogether, these results may suggest differences in ETA/ETB receptors expression and/or in ET-1/ET-2 release when colitis is induced by TNBS or DSS. In this regard, it has been shown, that in the rat TNBS model of colitis, the content of prepro-ET-1 RNAm and the release of ET-1/ET-2 peptides were increased at 24 h [47]; while in the DSS-induced colitis in mice it was reported a down regulation of ET-2 peptides at this time point [41]. To further explore this hypothesis, we analyzed not only prepro-ET-1 and ET-2 RNAm but also ETA and ETB receptors expression. Our results indicated that both ET receptors were up-regulated from 24 up to 72 h after colitis induction, but prepro-ET-1 and prepro-ET-2 expression oscillated along time, suggesting important differences in the regulation of the endothelinergic system according to the experimental model employed.
A possible mechanism underlying the role of endothelins in the pathophysiology of colitis is the regulation of colon blood flow. In this regard, in the rat TNBS model of colitis it was shown a dramatic decrease in colonic blood flow, which was restored by LU-135252 (selective ETA receptor antagonist), bosentan (dual ETA/ETB receptor antagonist), and BQ-485 (ET-1 antagonist), but not by BQ-788 (selective ETB receptor antagonist), indicating that blood flow alterations were mediated by ETA receptors [20, 49]. However, Lee et al. [48]. have reported that colonic blood flow rate did not change following DSS treatment. We have also observed a participation of ETA but not ETB receptors in TNBS-induced colitis, which was associated with a decrease in cell influx, but the possibility that these changes are due to modulation of blood flow remains to be investigated.
Several studies also support a crucial role of neutrophils in mediating tissue injury and clinical symptoms in colitis [13–15]. However, increasing evidence suggests that the tissue damage may occur independently of cell migration. According to Kruschewski and colleagues [49], treatment with BQ-485 improved macroscopic injury induced by TNBS but not inhibited colonic MPO activity, while treatment with LU135252 did not ameliorate microscopic damage, but diminished leukocyte sticking. On the other hand, it was shown that treatment with bosentan was able to reduce both the adhesion of leukocytes in colonic submucosal venules and inflammation in a DSS-induced colitis model [12]. Our data reinforce this latter observation and support a role for endothelins, via ETA receptors activation, in neutrophil influx, which is, at least in part, responsible for tissue injury.
To gain further insights into the mechanisms through which ETA receptors mediate cell migration, we have assessed the content of chemotactic factors (IL-1β, MIP-2 and, KC) and the expression of adhesion molecules after colitis induction. Our results demonstrated that Atrasentan almost totally suppressed the increase of IL-1β, MIP-2 and KC, as well as, of E-selectin and β2-integrin. It is also important to point out that stimulation of endothelial cells in culture with ET-1 led to an up-regulation in the expression of ICAM-1, VCAM and E-selectin [36, 50–53].
Our present data suggest that endogenous endothelins affect recruitment of leucocytes, especially neutrophils, by regulating adhesion molecule expression in leukocytes and colonic endothelial cells. In this regard, it is important to mention that endothelins can be produced by activated PMN leucocytes infiltrating the mucosa [54], acting as important autocrine/paracrine modulators of neutrophil functions. Endothelins have been found to stimulate neutrophil migration in vitro [55–57] and in vivo [58–62]. Moreover, ET-1 induces an increase of neutrophil chemokinetics in vitro [55] and promotes neutrophil aggregation [63, 64].
Besides the regulation of neutrophil functions, endothelins are able to modulate the production of IL-8 and IL-1 in macrophages, endothelial and epithelial cells [57, 65–67]. Moreover these cytokines as well as MIP-2 and KC are important to cell migration in colon [22]. Therefore, inhibition of endothelin receptors could contribute to decrease of cell influx by diminishing chemotactic factors production. However, since the inflammatory cells produces cytokines and chemokines, the reduction on IL-1β, MIP-2 and KC levels could be just a consequence of decreased migration. To further strengthen this view, we have performed neutrophil and macrophage cultures, and have shown that BQ-123 reduced production of MIP-2 and KC in macrophages and partially decreased MIP-2 release by neutrophils. These data suggest that endothelins may modulate cell activation, mainly macrophages, decreasing cytokines production, contributing to reduction of adhesion molecules expression. In a process that depends on a cascade effect, it is difficult to pinpoint the fundamental event being affected and what represents the consequences of the primary event. It is important to mention that prepro-ET-2 levels were up regulated 48 h after TNBS administration, but prepro-ET-1 was not. In addition, ET-2 potentiates LPS-induced KC production in vitro. It might suggest that ET-2 play an important role in colonic inflammation after TNBS administration, once Atrasentan blocks ETA receptors, which are also activated by ET-2.
Also of interest were the data showing significant recovery of the anti-inflammatory cytokine IL-10 after Atrasentan treatment. This effect might be explained by the decrease of neutrophil influx [68]. This cytokine is known to deactivate respiratory burst and to inhibit proinflammatory mediator production, playing a negative modulatory role, with an effect on PMN accumulation and chemokine generation [69]. On the other hand, Atrasentan failed to restore IL-13 levels, a cytokine that has been reported to present immunoregulatory properties in patients with inflammatory bowel disease [70]. The fact that IL-10 and IL-13 can be released by different cell types could explain the differential effect of Atrasentan.
In conclusion, in the present study we have demonstrated a major role for endothelins, possibly ET-2, in maintaining colitis inflammatory parameters in two different models. Our findings indicated that ETA receptor inhibition decreased infiltration of neutrophils into the inflamed tissues by reducing levels of chemotactic factors and expression of adhesion molecules, contributing greatly to the reduction of tissue damage and signs of inflammation. Of high interest, Atrasentan has a curative property being able to attenuate TNBS-induced colitis after the establishment of inflammation. Our data indicates that the ETA receptor may represent an interesting and attractive target for the management of IBD, for which current therapies remain inadequate.
References
Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6–21.
Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407.
Masaki T. Historical review: endothelin. Trends Pharmacol Sci. 2004;25:219–24.
Letizia C, Boirivant M, De Toma G, Cerci S, Subioli S, Scuro L, et al. Plasma levels of endothelin-1 in patients with Crohn’s disease and ulcerative colitis. Ital J Gastroenterol Hepatol. 1998;30:266–9.
Kanazawa S, Tsunoda T, Onuma E, Majima T, Kagiyama M, Kikuchi K. VEGF, basic-FGF, and TGF-beta in Crohn’s disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am J Gastroenterol. 2001;96:822–8.
Murch SH, Braegger CP, Sessa WC, MacDonald TT. High endothelin-1 immunoreactivity in Crohn’s disease and ulcerative colitis. Lancet. 1992;339:381–5.
Cheluvappa R. A novel model of appendicitis and appendectomy to investigate inflammatory bowel disease pathogenesis and remediation. Biol Proced Online. 2014;16:10.
Cheluvappa R, Eri R, Luo AS, Grimm MC. Endothelin and vascular remodelling in colitis pathogenesis–appendicitis and appendectomy limit colitis by suppressing endothelin pathways. Int J Colorectal Dis. 2014;29:1321–8.
Hogaboam CM, Muller MJ, Collins SM, Hunt RH. An orally active non-selective endothelin receptor antagonist, bosentan, markedly reduces injury in a rat model of colitis. Eur J Pharmacol. 1996;309:261–9.
Gulluoglu BM, Kurtel H, Gulluoglu MG, Yegen C, Aktan AO, Dizdaroglu F, et al. Role of endothelins in trinitrobenzene sulfonic acid-induced colitis in rats. Digestion. 1999;60:484–92.
Padol I, Huang JQ, Hogaboam CM, Hunt RH. Therapeutic effects of the endothelin receptor antagonist Ro 48-5695 in the TNBS/DNBS rat model of colitis. Eur J Gastroenterol Hepatol. 2000;12:257–65.
Anthoni C, Mennigen RB, Rijcken EJ, Laukotter MG, Spiegel HU, Senninger N, et al. Bosentan, an endothelin receptor antagonist, reduces leucocyte adhesion and inflammation in a murine model of inflammatory bowel disease. Int J Colorectal Dis. 2006;21:409–18.
Buanne P, Di Carlo E, Caputi L, Brandolini L, Mosca M, Cattani F, et al. Crucial pathophysiological role of CXCR2 in experimental ulcerative colitis in mice. J Leukoc Biol. 2007;82:1239–46.
Wallace JL, McKnight W, Asfaha S, Liu DY. Reduction of acute and reactivated colitis in rats by an inhibitor of neutrophil activation. Am J Physiol. 1998;274:G802–8.
Peterson CG, Sangfelt P, Wagner M, Hansson T, Lettesjo H, Carlson M. Fecal levels of leukocyte markers reflect disease activity in patients with ulcerative colitis. Scand J Clin Lab Invest. 2007;67:810–20.
Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–15.
Hipkin RW, Deno G, Fine J, Sun Y, Wilburn B, Fan X, et al. Cloning and pharmacological characterization of CXCR1 and CXCR2 from Macaca fascicularis. J Pharmacol Exp Ther. 2004;310:291–300.
Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, et al. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J Biol Chem. 2007;282:11658–66.
Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–9.
Deniz M, Cetinel S, Kurtel H. Blood flow alterations in TNBS-induced colitis: role of endothelin receptors. Inflamm Res. 2004;53:329–36.
Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803.
Bento AF, Leite DF, Claudino RF, Hara DB, Leal PC, Calixto JB. The selective nonpeptide CXCR2 antagonist SB225002 ameliorates acute experimental colitis in mice. J Leukoc Biol. 2008;84:1213–21.
Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–6.
Wessale JL, Adler AL, Novosad EI, Calzadilla SV, Dayton BD, Marsh KC, et al. Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: ex vivo and in vivo studies. Clin Sci (Lond). 2002;103(Suppl 48):112S–7S.
Claudino RF, Marcon R, Bento AF, Chichorro JG, Rae GA. Endothelins implicated in referred mechanical hyperalgesia associated with colitis induced by TNBS in mice. Can J Physiol Pharmacol. 2010;88:661–7.
Rosano L, Spinella F, Di Castro V, Nicotra MR, Albini A, Natali PG, et al. Endothelin receptor blockade inhibits molecular effectors of Kaposi’s sarcoma cell invasion and tumor growth in vivo. Am J Pathol. 2003;163:753–62.
Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49.
Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29–36.
Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–90.
Kimball ES, Wallace NH, Schneider CR, D’Andrea MR, Hornby PJ. Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol Motil. 2004;16:811–8.
Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE Jr, Balish E, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945–53.
Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–29.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Talati M, West J, Blackwell TR, Loyd JE, Meyrick B. BMPR2 mutation alters the lung macrophage endothelin-1 cascade in a mouse model and patients with heritable pulmonary artery hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299:L363–73.
Ruetten H, Thiemermann C. Endothelin-1 stimulates the biosynthesis of tumour necrosis factor in macrophages: ET-receptors, signal transduction and inhibition by dexamethasone. J Physiol Pharmacol. 1997;48:675–88.
Zouki C, Baron C, Fournier A, Filep JG. Endothelin-1 enhances neutrophil adhesion to human coronary artery endothelial cells: role of ET(A) receptors and platelet-activating factor. Br J Pharmacol. 1999;127:969–79.
Koseki C, Imai M, Hirata Y, Yanagisawa M, Masaki T. Autoradiographic distribution in rat tissues of binding sites for endothelin: a neuropeptide? Am J Physiol. 1989;256:R858–66.
Inagaki H, Bishop AE, Escrig C, Wharton J, Allen-Mersh TG, Polak JM. Localization of endothelinlike immunoreactivity and endothelin binding sites in human colon. Gastroenterology. 1991;101:47–54.
de la Monte SM, Quertermous T, Hong CC, Bloch KD. Regional and maturation-associated expression of endothelin 2 in rat gastrointestinal tract. J Histochem Cytochem. 1995;43:203–9.
Egidy G, Juillerat-Jeanneret L, Korth P, Bosman FT, Pinet F. The endothelin system in normal human colon. Am J Physiol Gastrointest Liver Physiol. 2000;279:G211–22.
Takizawa S, Uchide T, Adur J, Kozakai T, Kotake-Nara E, Quan J, et al. Differential expression of endothelin-2 along the mouse intestinal tract. J Mol Endocrinol. 2005;35:201–9.
Massai L, Carbotti P, Cambiaggi C, Mencarelli M, Migliaccio P, Muscettola M, et al. Prepro-endothelin-1 mRNA and its mature peptide in human appendix. Am J Physiol Gastrointest Liver Physiol. 2003;284:G340–8.
Takahashi K, Jones PM, Kanse SM, Lam HC, Spokes RA, Ghatei MA, et al. Endothelin in the gastrointestinal tract. Presence of endothelinlike immunoreactivity, endothelin-1 messenger RNA, endothelin receptors, and pharmacological effect. Gastroenterology. 1990;99:1660–7.
Escrig C, Bishop AE, Inagaki H, Moscoso G, Takahashi K, Varndell IM, et al. Localisation of endothelin like immunoreactivity in adult and developing human gut. Gut. 1992;33:212–7.
Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, et al. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–85.
Yoshimura M, Yamashita Y, Kan S, Niwa M, Taniyama K. Localization of endothelin ETB receptors on the myenteric plexus of guinea-pig ileum and the receptor-mediated release of acetylcholine. Br J Pharmacol. 1996;118:1171–6.
McCartney SA, Ballinger AB, Vojnovic I, Farthing MJ, Warner TD. Endothelin in human inflammatory bowel disease: comparison to rat trinitrobenzenesulphonic acid-induced colitis. Life Sci. 2002;71:1893–904.
Lee S, Carter PR, Watts MN, Bao JR, Harris NR. Effects of the endothelin-converting enzyme inhibitor SM-19712 in a mouse model of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2009;15:1007–13.
Kruschewski M, Anderson T, Loddenkemper C, Buhr HJ. Endothelin-1 receptor antagonist (LU-135252) improves the microcirculation and course of TNBS colitis in rats. Dig Dis Sci. 2006;51:1461–70.
McCarron RM, Wang L, Stanimirovic DB, Spatz M. Endothelin induction of adhesion molecule expression on human brain microvascular endothelial cells. Neurosci Lett. 1993;156:31–4.
Ishizuka T, Takamizawa-Matsumoto M, Suzuki K, Kurita A. Endothelin-1 enhances vascular cell adhesion molecule-1 expression in tumor necrosis factor alpha-stimulated vascular endothelial cells. Eur J Pharmacol. 1999;369:237–45.
Lin CC, Lin WN, Hou WC, Hsiao LD, Yang CM. Endothelin-1 induces VCAM-1 expression-mediated inflammation via receptor tyrosine kinases and Elk/p300 in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2015;309:L211–25.
Ohanian J, Forman SP, Katzenberg G, Ohanian V. Endothelin-1 stimulates small artery VCAM-1 expression through p38MAPK-dependent neutral sphingomyelinase. J Vasc Res. 2012;49:353–62.
Sessa WC, Kaw S, Hecker M, Vane JR. The biosynthesis of endothelin-1 by human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1991;174:613–8.
Elferink JG, de Koster BM. Endothelin-induced activation of neutrophil migration. Biochem Pharmacol. 1994;48:865–71.
Elferink JG, de Koster BM. Modulation of human neutrophil chemotaxis by the endothelin-B receptor agonist sarafotoxin S6c. Chem Biol Interact. 1996;101:165–74.
Hofman FM, Chen P, Jeyaseelan R, Incardona F, Fisher M, Zidovetzki R. Endothelin-1 induces production of the neutrophil chemotactic factor interleukin-8 by human brain-derived endothelial cells. Blood. 1998;92:3064–72.
Sampaio AL, Rae GA, Henriques MG. Participation of endogenous endothelins in delayed eosinophil and neutrophil recruitment in mouse pleurisy. Inflamm Res. 2000;49:170–6.
Sampaio AL, Rae GA, Henriques MG. Effects of endothelin ETA receptor antagonism on granulocyte and lymphocyte accumulation in LPS-induced inflammation. J Leukoc Biol. 2004;76:210–6.
Conte Fde P, Barja-Fidalgo C, Verri WA Jr, Cunha FQ, Rae GA, Penido C, et al. Endothelins modulate inflammatory reaction in zymosan-induced arthritis: participation of LTB4, TNF-alpha, and CXCL-1. J Leukoc Biol. 2008;84:652–60.
Verri WA Jr, Cunha TM, Magro DA, Guerrero AT, Vieira SM, Carregaro V, et al. Targeting endothelin ETA and ETB receptors inhibits antigen-induced neutrophil migration and mechanical hypernociception in mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:271–9.
Zarpelon AC, Pinto LG, Cunha TM, Vieira SM, Carregaro V, Souza GR, et al. Endothelin-1 induces neutrophil recruitment in adaptive inflammation via TNFalpha and CXCL1/CXCR2 in mice. Can J Physiol Pharmacol. 2012;90:187–99.
Gomez-Garre D, Guerra M, Gonzalez E, Lopez-Farre A, Riesco A, Caramelo C, et al. Aggregation of human polymorphonuclear leukocytes by endothelin: role of platelet-activating factor. Eur J Pharmacol. 1992;224:167–72.
Lopez-Farre A, Caramelo C, Esteban A, Alberola ML, Millas I, Monton M, et al. Effects of aspirin on platelet-neutrophil interactions. Role of nitric oxide and endothelin-1. Circulation. 1995;91:2080–8.
Cunningham ME, Huribal M, Bala RJ, McMillen MA. Endothelin-1 and endothelin-4 stimulate monocyte production of cytokines. Crit Care Med. 1997;25:958–64.
Peng H, Chen P, Cai Y, Chen Y, Wu QH, Li Y, et al. Endothelin-1 increases expression of cyclooxygenase-2 and production of interlukin-8 in hunan pulmonary epithelial cells. Peptides. 2008;29:419–24.
Speciale L, Roda K, Saresella M, Taramelli D, Ferrante P. Different endothelins stimulate cytokine production by peritoneal macrophages and microglial cell line. Immunology. 1998;93:109–14.
Lomas-Neira JL, Chung CS, Grutkoski PS, Miller EJ, Ayala A. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol. 2004;76:58–64.
Saleem S, Dai Z, Coelho SN, Konieczny BT, Assmann KJ, Baddoura FK, et al. IL-4 is an endogenous inhibitor of neutrophil influx and subsequent pathology in acute antibody-mediated inflammation. J Immunol. 1998;160:979–84.
Kucharzik T, Lugering N, Weigelt H, Adolf M, Domschke W, Stoll R. Immunoregulatory properties of IL-13 in patients with inflammatory bowel disease; comparison with IL-4 and IL-10. Clin Exp Immunol. 1996;104:483–90.
Acknowledgements
The kind donations of Atrasentan and A-192621 by Dr. Terry Opgenorth (Abbott Laboratories, Abbott Park, USA) is gratefully acknowledged. The study was supported by the Brazilian National Research Council (CNPq), Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (Capes) and Fundacao de Amparo a Ciencia e Tecnologia do Estado de Santa Catarina (Funcitec).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interests.
Additional information
Responsible Editor: Mauro Teixeira.
Rights and permissions
About this article
Cite this article
Claudino, R.F., Leite, D.F., Bento, A.F. et al. Potential role for ET-2 acting through ETA receptors in experimental colitis in mice. Inflamm. Res. 66, 141–155 (2017). https://doi.org/10.1007/s00011-016-1001-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-1001-7