Abstract
Soil and water pollution is a matter of great concern in the twenty-first century, the majority of which is caused by various organic compounds, agricultural and municipal wastes, heavy metals, and microorganisms. Due to fast industrialization, mining, and other technical breakthroughs, the soil environment is continuously poisoned by heavy metals in the modern period. As a result, heavy metal contamination has become a major concern worldwide. Mycoremediation is a type of bioremediation that uses fungi to remove, degrade, or reduce the toxicity of various pollutants from various substrates. Filamentous fungi have several properties that make them suitable for heavy metal (HM) bioremediation. Fungi have a high adsorption and accumulation capability for HMs; thus, they could be helpful. Bioaccumulation, bio-adsorption, biosynthesis, biomineralization, bio-oxidoreduction, extracellular or intracellular precipitation, surface sorption, and other bio-mechanisms involved in HM tolerance and removal by fungus differ from species to species. However, the major influential parameters that affect HM bioremediation include time, pH, temperature, HM concentration, dose of fungal biomass, and shaking rate, which vary depending on the fungi and composition of the HMs. Hence, mycoremediation is thought to be a more effective strategy than traditional methods for removing hazardous chemicals, including heavy metals, from soil and water bodies in a long-term and cost-efficient manner.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Mother Nature is continuously impacted by increasing industrial and developmental activities, and as a result, foreign elements are continually being introduced to nature. These elements include heavy metals, pollutants, chemicals derived from agricultural lands, and other sources. They are the most dangerous substances and are discharged in substantial quantities that enter the ecosystem directly or indirectly. Many metals function as micronutrients when used in the wrong quantities, but when used in the right quantities, they are advantageous for the growth and development of plants and also support metabolic activity as metalloenzymes. But if used in appropriate amounts, they are not hazardous or behave like heavy metals. Heavy metals are highly soluble in water and are consumed by aquatic species that pose a significant risk to human health due to their non-biodegradability, high toxicity, and long persistence. Continuous exposure to these heavy metals is quite concerning. Thus, it is a crucial time to find a better solution. Bioremediation is an environmentally benign approach that uses fungi, bacteria, or plants to treat wastewater and other contaminated areas (Kumari et al. 2019). In intensive agricultural and horticultural systems, large amounts of fertilizers and pesticides are being routinely used on the soil and the plant to provide enough nitrogen (N), phosphorus (P), and potassium (K) as well as protection, respectively. Heavy metals are contaminants present in trace amounts in the compounds used to supply these elements, and following repeated fertilizer and pesticide applications, their presence in the soil and in the environment may dramatically increase (Jones and Jarvis 1981; Basta et al. 2005; McLaren et al. 2005; Wuana and Okieimen 2011). In addition to macronutrients, plants also need certain micronutrients in order to develop and complete their life cycle.
Some soils are deficient in the heavy metals required for healthy plant development (Lasat 1999); thus, crops can be given these by adding them to the soil or spraying them on the leaves. Occasionally, copper (Cu) and manganese (Mn) are also added to the soil to treat cereal and root crops if the soil has a deficit in the elements. When some phosphate fertilizers are applied to the soil, cadmium (Cd) and other hazardous metals such as lead (Pb), mercury (Hg), and fluoride (F) are unintentionally added to the soil (Madhavan et al. 2017). Due to the application of numerous biosolids, heavy metals like copper, arsenic, lead, cadmium, and nickel, and some other pollutants like manures from livestock, municipal sewage sludge, and composts, are unintentionally accumulated in the soil (Basta et al. 2005). Animal manures from farms, such as those from chickens, cows, and pigs, are regularly applied to pastures and crops as solids or slurries (Sumner 2000). However, copper (Cu) and zinc (Zn) are added to diets as growth promoters, and the arsenic (As) found in poultry health products in the pig and poultry sectors may have the potential to pollute the soil with metals (Sumner 2000). The manures generated by animals on such diets have high concentrations of Zn, As, and Cu. If these are often dispersed over constrained areas of land, a sizeable amount of these metals may ultimately accumulate in the soil. Most organic solid waste products that can be recycled for environmental objectives are sewage sludge or biosolids (USEPA 1994). In most of the nations that permit biosolid reuse produced by urban populations, the application of biosolid materials in the soil is a prevalent practice. The heavy metals that are most frequently discovered in biosolids include Zn, Pb, Cd, Ni, Cu, and Cr, and the concentrations of these metals are determined by the type of industrial activity, its intensity, as well as the procedure used to treat the biosolids. Under some conditions, metals added to soils during the treatment of biosolids may seep through the soil layer and possibly pollute groundwater (McLaren et al. 2005).
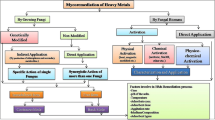
It has been a regular practice in many regions of the world for more than 400 years to apply municipal, industrial, and related effluents to land (Reed et al. 1995). As per the estimation, it was found that wastewater irrigates 20 million hectares of arable land globally. In practice, farmers are more focused on growing their yields and profits than on the benefits or threats associated with the environment. Even while wastewater effluents typically have low metal concentrations, they may eventually cause a significant metal accumulation in the soil if used to irrigate land over an extended period. The legacy of widespread distribution of metal pollutants in soil has been left to many nations by the mining and processing of metal ores in conjunction with industries. When dumped directly into natural depressions such as on-site wetlands, heavy and larger particles known as tailings that get deposited at the bottom of the flotation cell during mining can accumulate in high quantities (DeVolder et al. 2003). The extensive smelting and mining of zinc and lead ore have been contaminating the land and threatening human and ecological health. Most of the lengthy, expensive restoration techniques employed for these sites may not restore soil productivity. The environmental risk that heavy metals in soil pose to people is correlated with bioavailability. Another significant source of soil pollution is the airborne emission of lead from the combustion of gasoline containing tetraethyl lead; this dramatically raises the level of lead in urban soils and major roadways. Due to lubricating oils and tire treads, Zn and Cd may also be added to soils close to roads (USEPA 1994; Wuana and Okieimen 2011). The numerous factors which are involved in heavy metal removal are illustrated in Fig. 8.1. The purpose of this review is to provide information on heavy metals and different metalloids that are responsible for environmental pollution because of their persistency, toxicity, and accumulation in the biosphere, as well as to highlight the bioremediation methods like mycoremediation that have so far proven to be effective (Raffa et al. 2021).
8.2 Heavy Metals/Metalloids in Soil
All metals and metalloids having a density of more than 5 g cm3 have been collectively referred to as “heavy metals” (Wuana and Okieimen 2011; Pendias and Pendias 2001). Generally, common transitional metals like zinc (Zn), copper (Cu), lead (Pb), cadmium (Cd), and mercury (Hg) are referred to as “heavy metals” in this context, whereas “metalloids” refer to a class of chemical elements that exhibit properties that fall somewhere between those of metals and nonmetals and naturally occur as poly-hydroxylated species. This category often includes the chemical elements arsenic (As), boron (B), silicon (Si), antimony (Sb), germanium (Ge), and tellurium (Te), as well as less frequent elements like astatine (At) and polonium (Po). These heavy metals and metalloids in the soil or water may substantially impact human and ecological health (Chan et al. 2016). Any metallic chemical element that has a high density and has the potential to be poisonous even at low doses is referred to as a heavy (or trace) metal. Although heavy metals are easily absorbed and bioaccumulated in different plant sections, they are neither essential nor play a crucial function in cells’ metabolic pathways (Nas and Ali 2018). Cobalt (Co), nickel (Ni), copper (Cu), vanadium (V), zinc (Zn), and chromium (Cr) are heavy elements that, at low concentrations, are not poisonous (Nas and Ali 2018).
Heavy metals are an essential component of the planet and cannot be removed or degraded. Because of their propensity to collect in live cells, they are enormously hazardous. When a chemical compound gradually assembles within living things over time in contrast to its environmental concentration, this is referred to as bioaccumulation. Industrial and consumer waste, as well as acid rain, which causes weathering and the release of heavy metals into groundwater, rivers, lakes, and streams are all ways that trace metals can enter the water and harm aquatic life. Heavy metals and metalloids are present in the soil due to the parent materials. These materials come from lithogenic and human-made processes (Alloway 2013). Anthropogenic activities that are increasing the number of heavy metals in the environment include mining, smelters, foundries, burning of fossil fuels, using gasoline, waste incinerators, and other industrial operations. This affects the environment’s ability to sustain life and provide for its basic needs. The three heavy metals that are most problematic are cadmium, mercury, and lead, according to the European Monitoring and Evaluation Programme (EMEP), because of their severely detrimental impacts on human health. According to Damodaran et al. (2013), the physiology and composition of the organism’s cell wall, as well as physicochemical factors like concentration of the metal, time, temperature, pH, and ionic strength of the metal, all play a role in the intricate process by which the heavy metal removal mechanism operates. Any environmental product containing these heavy metals may be harmful to human health as well as to soil, plants, and animals. According to Singh and Kalamdhad (2011), it is highly concerning that heavy metals are absorbed by plants, subsequently accumulated, and transferred to human tissues through the food chain. Interactions between plants, fungi, bacteria, and other living and nonliving elements of the environment occur. Under stress, they often adapt metabolically to the environment by going through various mechanisms to lessen the toxicity (Abdullahi et al. 2021).
Heavy metal and metalloid soil pollution is a problem that affects every nation on the planet. Since heavy metal contamination cannot biodegrade and builds up in the soil, hurting people, animals, and the ecosystem for a very long time, it has drawn more attention recently. Exposure to heavy metals and metalloids is linked to various health issues, including kidney problems, developmental and neurobehavioral difficulties, bone problems, blood pressure issues, and tumor growth. These issues become pertinent when there are appreciable concentrations of heavy metals in the soil. An estimated five million locations worldwide have soil that is polluted with heavy metals and metalloids. The primary source of this pollution is frequent anthropogenic activities. Developed nations like the USA, China, Australia, and EU members tend to have more heavy metal-contaminated areas than developing nations (Brito et al. 2020; Yu et al. 2020). The average global amounts of these pollutants in soil vary depending on the kind of soil, the environment around it, and the distance from the source of contamination. Heavy metal/metalloid species continue to get attention worldwide due to the persistent nature of such damaging pollutants, which include static, durable, accumulative, and nonbiodegradable properties (Zhao et al. 2019). The soil near metal smelters has been contaminated by the heavy metal/metalloid species that metal smelting activities have discharged into the environment. The buildup of heavy metal/metalloid species poses a danger to the ecological environment, variety, functioning of soil microorganisms, food security, and human health. Soil microorganisms have a key role in defining the quality of the soil since they are the guardians of the ecosystem’s structure and functioning (Hou et al. 2019). Although soil microorganisms can influence soil properties, the physicochemical properties of the soils can also have a large influence on them. According to research, microorganisms greatly enhance soil fertility, crop health, and nutrient circulation in the soil. It is commonly acknowledged that the toxic stress brought on by heavy metals and metalloids may significantly influence the number, variety, and ecological functions of the soil’s microbial communities. For instance, heavy metal/metalloid pollutants have an impact on the ecological processes in soils that functional groups and a variety of functional genes sparked. As a result, the variety of microorganisms has diminished and the structure of soil microbial communities has changed even more. Numerous heavy metal/metalloid-tolerant bacteria may transform or eliminate heavy metals from polluted soil (Qiao et al. 2019). Bioremediation has been recognized as a promising green and sustainable method for cleaning up heavy metal pollution. Bioremediation has been recognized as a promising green and sustainable method for cleaning up heavy metal pollution. Furthermore, alterations in the chemical forms of heavy metal/metalloid species brought on by the physicochemical characteristics of soil may have a secondary effect on the makeup of microbial communities (Hu et al. 2021). Approximately 51 elements in the periodic table are considered heavy metals or metalloids. Because of their chemical properties and those of the soil, they are mobile and bioavailable in the soil. The interaction of soil components with metals and metalloids is influenced by the pH, the characteristics of the adsorbent surface, and the presence of cations and anions. Zinc (Zn), chromium (Cr), nickel (Ni), manganese (Mn), cadmium (Cd), lead (Pb), copper (Cu), and arsenic (As) are the most prevalent heavy metals and metalloids. The most hazardous substances are those with Cr, Cu, Zn, Cd, Pb, Hg, and As in them. As the underlying bedrock weathers, they are frequently discovered as ores (sulfides of Pb, Co, Fe, As, Pb, Zn, Ag, and Ni, and oxides of Se, Al, Mn, and Sb). Along with sulfides of arsenic, mercury, lead, and cadmium, chalcopyrite, CuFeS2, and pyrite, FeS2, are naturally occurring sulfides of copper and iron in the soil. In particular, ore mining and refining, using pesticides and fertilizers, and solid wastes all contribute to the environmental problem by raising the levels of heavy metals and metalloids. Heavy metals and metalloids are used in many industries, increasing market demand, and worldwide output. Many biological processes, including the nervous system, production of complex molecules, respiration systems, and control and functioning of enzymes, require trace amounts of copper, selenium, zinc, iron, vanadium, and manganese. Electronic gadgets, especially semiconductors, are made largely from metals, including iron, zinc, tin, lead, copper, and tungsten (Koller and Saleh 2018). It is clear that certain elements, particularly chromium, copper, zinc, and lead, are employed in many industries and that there is a significant annual output worldwide, wherein the United States, China, Australia, Russia, Peru, and Mexico are the major producing nations (Raffa et al. 2021). Microbial bioremediation lowers the expense of the heavy metal pollution treatment process while also being effective, economical, and ecologically benign (Mishra 2017). The primary mechanisms for microbial removal of heavy metals are biosorption, which includes ion exchange, redox reactions, adsorption of chemicals, precipitation, and formation of a complex with organic ligands; secondly, biomineralization which includes bioleaching, which involves releasing heavy metal ions from insoluble ores through dissolution or complexation; and thirdly, bio-oxidation (González Henao and Ghneim-Herrera 2021).
8.3 Mycoremediation of Soil
8.3.1 Important Fungal Species Involved in Bioremediation
Microbial bioremediation lowers the expense of the heavy metal pollution treatment process while also being effective, economical, and ecologically benign (Mishra 2017). The primary mechanisms for microbial removal of heavy metals are biosorption, biomineralization, and bio-oxidation (Jin et al. 2018; González Henao and Ghneim-Herrera 2021). Fungi are used in bioremediation because of their resistance and tolerance, which are used in some aquatic environments where they overpower heavy metals. Aspergillus niger, which functions as a multi-tolerant fungus, is one example of the growing fungi employed in the mycoremediation approach. Different fungi, including Penicillium, Aspergillus, Trichoderma, Fusarium, etc., use a variety of strategies within the cell wall to remove different kinds of heavy metals, including cell surface precipitation, detoxification, accumulation, efflux, and alterations. Contaminated water and soil keep the majority of metal-tolerant fungi separate. The nature of the fungal resistance is a result of the genetic makeup of the fungi, concentration of HMs, environmental conditions, nutritional availability, and various forms of heavy metals. These factors also affect how fungi react to metal and how resistant they are. Aspergillus flavus CR500 and Trichoderma harzianum are two examples of heavy metal-resistant fungi. According to Table. 8.1, most fungi belong to the class Ascomycetes and are resistant to heavy metals.
Aspergillus, Chaetomium, Coniochaeta, and Phoma, all Ascomycetes, have been researched for their similarities between the genomic and secrotomic to allow their presence in the breakdown of biomass and parthenogenesis in the dry environment (Hua et al. 2012; Challacombe et al. 2019). According to the investigation, it was found that all fungi can readily produce melanin because of their melanized structural makeup. Because of these qualities, they can thrive in arid environments. Some proteins have also tested positive and are found in nature and fungi. Both positive and negative interactions between heavy metals and fungi exist (Ruley et al. 2006). In a positive interaction, the presence of HMs has no effect, but in an adverse interaction, the presence of HMs can cause fungus death or growth inhibition. A new phase in removing heavy metals from a wasteland may result from the interaction of heavy metals and fungi. Numerous experts have noted that the majority of fungi can remove various metals in a viable form. Fungi have excellent qualities that can be exploited in bioremediation, and they also function as decomposers with vigorous enzymatic activity (Baker 1987). In large plants, the fungi and microorganisms in the rhizosphere play a crucial part in the synergistic mechanism, which directly increases the tolerance capacity of heavy metals. Few researchers have studied the interactions between different species of fungi and microorganisms in the remediation of heavy metals (Kumari et al. 2019). A schematic diagram of fungal remediation is depicted in Fig. 8.2.
Trichoderma fungi are well adapted to aid in eliminating lead from the environment. Hence, Trichoderma asperellum may be used in mycoremediation and may play a supporting function in soil phytoremediation (Bandurska et al. 2021). The study of the interaction between the cell surface of fungi and heavy metals is essential because the composition, structure, adsorption and absorption processes, and accumulation of heavy metals in the fungus vary from fungus to fungus (Chan et al. 2016). Myco-adsorption and mycoremediation are other terms for the adsorption of heavy metals on the surface of fungi. Heavy metals like cadmium, mercury, arsenic, chromium, and lead are used for the adsorption process using fungus-like Aspergillus sp., Thamnidium sp., etc. (Kumar and Dwivedi 2019). It is safe for biological systems to use the fungus to absorb and remove heavy metals from contaminated locations. The removal of heavy metals is seen to be a very safe and environmentally beneficial method when live creatures like fungi, or mushrooms, are used (Kumari and Kumar 2019). It is widely acknowledged that mushroom farming is an important tool for restoring, replenishing, and remediating the earth’s overburdened ecosphere and being a rapidly growing sector of the agricultural industry.
In comparison to microfungi, mushrooms are crucial in the buildup of heavy metals. Since mushrooms are thought to be more advantageous than plants due to their shorter life cycles and more adaptability, mycoremediation can be viewed as an advanced remediation method. An efficient biosorbent for hazardous metals is the mushroom. They expand rapidly in their natural habitat but lack the immobilization or deployment of specific reactor configurations necessary for other microbial sorbents. Instead, they evolve into sorbents because of their texture and other favorable properties (Damodaran et al. 2013). Elekes and Busuioc examined the levels of heavy metals in five different mushrooms taken from the Bucegi Massif in the Carpathian Mountains: Collybia butyracea, Calvatia excipuliformis, Boletus griseus, Marasmius oreades, and Hygrophorus virgineus. Compared to other species, C. excipuliformis has higher concentrations of Cu (244.864.26) and Zn (92.190.21) than other species. Additionally, compared to other mushrooms, C. butyracea and Zn C. excipuliformis have a larger bioaccumulation factor for Cu (Pihurov et al. 2019).
8.3.2 Toxic Compounds Degraded by Fungi
The intake of essential and nonessential metals is crucial for eliminating heavy metals. The internal mechanism of fungi can tolerate some metals quite easily. They have a unique level of metal tolerance (Renu and Singh 2016). Antioxidants that are both enzymatic and nonenzymatic help keep the fungus’ ability to tolerate stress in check. Within a single organism, more than one antioxidant property is present for the antioxidant mechanisms (Yang et al. 2016). In the tropical plant species Candida, the enzyme glutathione first assembles the metal glutathione complex, which causes the cellular level of oxidized glutathione to rise and aids in detoxifying metals. By generating metallothionein, which enhances fungal tolerance to cadmium, glutathione also aids in lowering the levels of toxicity (Wu et al. 1975).
Thiol is a substance utilized to signal cells and is thought crucial. Thiol synthesis has increased in the plant species Aspergillus flavus. Gamma-glutathione makes up one of the two tails of glutathione, and the other tail belongs to the thiol group. Here, the reaction between the thiol group and the glutathione results in cadmium bisglutathionate. The catalase, phenol, proline, and thiol concentration in Aspergillus flavus increases in response to the chromium stress (Salt et al. 1998). Again, in some fungus, large amounts of particular proteins are produced that can aid in the buildup and reclamation of heavy metals. Even the overproduction of these proteins causes a stressful condition known as heat shock. Organic acids can also be used to relieve heavy metal stress. Plants are protected from the stress brought on by heavy metals by the formation of organic acids. The fungus Penicillium sp. contains organic acids that help detoxify and remedy metals, including zinc, copper, cadmium, chromium, arsenic, manganese, and lead. These acids include pyruvic acid, oxalic acid, citric acid, gluconic acid, and malic acid (Kumari and Kumar 2019). These acids have metabolites that are intracellular, intercellular, and extracellular. During the phytomining of metals, these extracellular organic acids facilitate the extraction process from the low-grade mining ores. Due to the organic acid present inside the cells, all of these metals precipitate (Kumari et al. 2019). Numerous kinds of inactivated fungal biomass and live fungal cells have been used in comprehensive research on heavy metal removal by sorption utilizing fungus (Chan et al. 2016).
Through various enzymatic processes, fungi may change hazardous metals and metalloids into less toxic forms, changing the concentrations of heavy metals in the environment. Using mercuric reductase, fungi may detoxify organomercury compounds, and the resulting mercury, Hg(II), can then be further reduced to the more combustible elemental mercury, Hg(0). Similarly, it happens in the conversion of As(V) to As(III) by arsenate reductases, which are a few common detoxification processes that may be involved once As(V) enters the fungal cells via the phosphate transporters (Gonzalez-Chavez et al. 2011). It is shown that three contiguous genes control Saccharomyces cerevisiae’s resistance to arsenic, a transcriptional regulator (ACR1), an enzyme arsenate reductase (ACR2), and a plasma membrane arsenite efflux pump (ACR3). The methylation of inorganic arsenic to create volatile derivatives is another mechanism by which fungi are resistant to metals and metalloids. Metals and metalloids can be methylated by an enzymatic process in which the metal is transferred to the methyl group. The methylated metal compounds commonly differ from their parent compounds in terms of toxicity, solubility, and volatility. Metals that can be methylated include Pb, Hg, and Sn and metalloids like Se, As, and Te. Monomethylarsonic acid and dimethylarsenic acid can be converted to volatile trimethylarsine oxide by Candida humicola, Gliocladium roseum, and Penicillium sp. (Cullen and Reimer 1989). The heat-resistant Neosartorya fischeri was found to effectively volatilize (up to 23% of total As) (Hartmann et al. 2003). Reactive oxygen species (ROS) produced by heavy metals like copper, iron, chromium, cadmium, lead, and mercury can lead to oxidative stress, affect calcium homeostasis, and cause damage to DNA (Klaunig et al. 1998). ROS generation has the potential to make fungi poisonous and harm a variety of vital macromolecules, including lipids, proteins, and nucleic acids. Metal/metalloid tolerance in fungi has been linked to their capacity to remove ROS (Fujs et al. 2005). Due to their high thiolate sulfur content, small proteins (between 2 and 7 kDa), such as metallothioneins, can bind metal ions for storage and detoxification in both eukaryotes and prokaryotes. In addition to glutathione (GSH), glutathione disulfide or oxidized glutathione (GSSG), non-protein sulfhydryl groups (NP-SH), and protein-bound sulfhydryl groups (PB-SH), it has been shown that cysteine-rich peptides, such as phytochelatins, and other thiol substances can bind metal ions and scavenge ROS. Additionally, the response of fungi to metal/metalloid exposure or their detoxification is significantly influenced by antioxidant enzymes. Numerous antioxidant enzymes have been found in fungi, and they may neutralize ROS and its byproducts or repair the harm they cause. The ability to shield cells against metal/metalloid-induced stress has been demonstrated for superoxide dismutase (SOD), glutathione peroxidase (POD), glutathione reductase (GR), glutathione S-transferases (GSTs), and catalase (CAT) (Shen et al. 2015). Jiang et al. (2015) demonstrated that the synthesis of NP-SH, GSH, PB-SH, and GSSG, as well as the induction of antioxidant enzymes, greatly altered Oudemansiella radicata’s responses to Cu exposure or Cu detoxification.
8.3.3 Enzyme Involved in the Biodegradation of Toxic Compounds
The best tools for bioremediation are enzymes since they hasten all chemical reactions that take place on pollutants. Enzymes frequently have broad enough specificities to work on several substances with structural similarities. Additionally, it is possible to alter enzymes to enhance both their stability and function in specific situations or with particular substrates (Theerachat et al. 2012). For the bioremediation of pollutants, many distinct enzymes, such as mono- or dioxygenases, peroxidases, hydrolases, halogenases, transferases, oxidoreductases, and phosphotriesterases, are derived from a wide variety of microorganisms and plant sources as well. Every time, the soil, in addition to the air and water, is polluted by significant quantities of organic pollutants. These pollutants include pesticides and herbicides, plastics, dyes, medicines, and heavy metals. Most organic substances that need to be cleaned up on a global scale include aromatic molecules, chlorinated hydrocarbons, polymers, polycyclic aromatic hydrocarbons (PAHs), organocyanides, steroids, etc. The primary contributor to their lethality is the sturdy structure they possess. The following is an example of an enzyme that plays an essential role in bioremediation.
Hydrolases (EC3): Hydrolase enzymes such as nitrilases, aminohydrolases, and organophosphorus hydrolases are among the most helpful in the bioremediation of numerous chemicals, including pesticides and herbicides and nitrile, polymers, and organophosphorus compounds. Some other hydrolase enzymes include lipases and cutinases (Ufarté et al. 2015). Nitrilases (EC 3.5.5.1) can hydrolyze the triple bonds present between the carbon and nitrogen (known as the nitrile group) in polymers, herbicides, and plastics in a stereo-, regio-, or chemoselective manner, resulting in the production of carboxylic acid and ammonia. Many species, such as Streptomyces sp., Fusarium solani, Rhodococcus rhodochrous, Aspergillus niger, and others, can express these enzymes (Martinkova et al. 2017). Organophosphorus hydrolases (EC 3.1.8.2) are organophosphate chemicals that were produced and utilized not just as pesticides but also in warfare and the pharmaceutical industry. The enzyme known as organophosphorus hydrolase, which also goes by the name phosphotriesterase, is one of the enzymes that may be used for the bioremediation of organophosphorus chemicals. Aspergillus niger and Penicillium lilacinum are two examples of well-known fungus species that are responsible for the synthesis of this enzyme (Martinkova et al. 2017). Ligninolytic peroxidases: White-rot fungus (WRF) and other groups of fungi generate enzymes that break down lignin, and these enzymes have a wide range of uses in bioremediation. Because of the strong nonspecificity and high non-stereoselectivity of these enzymes, they are able to digest a wide variety of molecules that are resistant to degradation. These four varieties include things like laccase (LAC), lignin peroxidase (LiP), manganese peroxidase (MnP), and versatile peroxidase (VP) (Kaur et al. 2016).
Fungi can lower the toxicity of metals and metalloids through their enzymatic activity, which can affect the amounts of these compounds in the environment. The enzyme fungal organomercury lyase is responsible for the conversion of organomercury compounds to Hg(II), which may then be further reduced by mercuric dehydrogenase into the more volatile element Hg(0) (Gadd 1993). After phosphate transporters move As(V) into fungal cells, many common detoxification mechanisms may be engaged, including arsenate reductases reducing it to As(III) and AMF sequestering it (Sharples et al. 2000; Gonzalez-Chavez et al. 2011). S. cerevisiae’s resistance is caused by three contiguous genes: ACR1 (a transcriptional regulator), ACR2 (arsenate reductase), and ACR3 (plasma membrane arsenite efflux pump). Fungi may also resist metals and metalloids by methylating inorganic arsenic to form volatile derivatives. An enzymatic process transfers metals like Hg, Sn, and Pb to the methyl group, resulting in molecules with varied solubility, volatility, and toxicity (Barkay and Wagner-Döbler 2005). Trimethylarsine oxide is produced when the nonvolatile monomethylarsonic acid and dimethylarsenic acid are fermented by the microorganisms Candida humicola (Cullen and Reimer 1989), Gliocladium roseum, and Penicillium sp. (Cox and Alexander 1973). Heat-resistant Neosartorya fischeri was shown to biovolatalize (up to 23% of total As) (Cernansky et al. 2007) effectively. Scopulariopsis brevicaulis (Andrewes et al. 2000) and Cryptococcus humicola (McDougall and Blanchette 1996) have both been found to biomethylate As and Sb (Hartmann et al. 2003). Both investigations found that these fungi methylate As and Sb similarly. Iron, copper, cadmium, chromium, lead, and mercury produced reactive oxygen species (ROS), causing oxidative stress, calcium homeostasis changes, and DNA damage (Klaunig et al. 1998). ROS may destroy proteins, nucleic acids, and lipids, making fungus poisonous. Fungi with metal/metalloid tolerance can reduce ROS (Fujs et al. 2005). Metal ions may be stored and detoxified in tiny proteins like metallothioneins (2–7 kDa) in both pro- and eukaryotes because of their high thiolate sulfur content. Phytochelatins, cysteine-rich peptides, and other thiol compounds, including nonprotein sulfhydryl groups (NP-SH), protein-bound SH, GSH, and GSSG, are known to connect metal ions and scavenge reactive oxygen species (Cobbett and Goldsbrough 2002). In addition, antioxidant enzymes contribute significantly to the reactions of fungi to the presence of metals and metalloids, as well as to the detoxification of these substances (Raab et al. 2004). Antioxidant enzymes have been isolated thanks to the work done by fungi. These antioxidant enzymes have the potential to either get rid of reactive oxygen species (ROS) and their derivatives or repair the harm caused by these substances. It has been shown that the enzymes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (POD), glutathione reductase (GR), and glutathione S-transferases (GSTs) are all capable of protecting cells from the damage that is caused by metals and metalloids (Shen et al. 2015). Jiang et al. (2015) discovered in their research that the production of NP-SH, PB-SH, GSH, and GSSG, as well as the activation of antioxidant enzymes (including SOD, POD, CAT, and GR), played a significant role in Oudemansiella radicata’s reactions to copper toxicity or copper detoxification. These enzymes were found to be involved in the production of NP-SH, PB-SH, GSH, and GS.
8.3.4 Bioaugmentation and Biostimulation
8.3.4.1 Bioaugmentation
Autochthonous or allochthonous wild-type or genetically modified microorganisms are applied to contaminated hazardous waste sites through bioaugmentation to speed up the removal of unwanted substances. Oil-contaminated settings are typically the focus of bioaugmentation efforts as bioremediation (Mrozik and Piotrowska-Seget 2010).
8.3.4.1.1 Factors Affecting Bioaugmentation
Bioaugmentation relies on microbial consortia adapting to the location to be decontaminated. To succeed, the newly imported microbial consortia must compete with indigenous microbes, predators, and abiotic influences. According to research, it increases the biodegradation of polluted soil by enhancing remediation efficiency. Bioaugmentation is mainly done in soils with fewer pollutant-degrading microorganisms and chemicals that require multi-process treatment. Several more criteria govern soil bioaugmentation. pH, temperature, moisture, organic matter, aeration, and nutrient concentration affect bioaugmentation. Remediation is ineffective if specific soil properties are missing in nature (Yuniati 2018). According to literature, Burkholderia sp. FDS-1 degrades nitrophenolic pesticides best at 30 °C and slightly alkaline pH. Catabolic genes and enzymes are responsible for the varied catabolic actions of microbial organisms (Rivelli et al. 2013).
8.3.4.1.2 Selection of Microbes
Bioaugmenting microorganisms played different roles in polluted site augmentation in the literature. Soil augmentation uses several microbial strains or consortia. Soil contaminants influence soil quality and soil microbial populations, which perform many vital tasks. Selecting the right microbial strains or consortiums is crucial for successful soil augmentation. Fast growth, easy culturing, high pollutant concentration resistance, and tolerance of a wide variety of environmental conditions are just a few characteristics of microorganisms that must be considered when choosing a strain or microbial consortia. Soil contamination can also be remedied by harvesting beneficial microorganisms from other polluted areas that have been exposed to the same or comparable chemicals and then reintroducing them to the target area (Adams et al. 2020).
8.3.4.2 Biostimulation
Biostimulation is a low-cost and efficient green remediation strategy. Rate-limiting nutrients, including phosphorus, nitrogen, oxygen, and electron donors, are added to highly polluted areas to stimulate the local bacteria into degrading the harmful and toxic pollutants (Elektorowicz 1994).
8.3.4.2.1 Mechanism of Stimulation
Hydrocarbon bioremediation is more effective than biostimulation (Adams et al. 2020). Biostimulation is an effective hydrocarbon-degrading technique, notably for petroleum compounds and derivatives. Rate-limiting nutrients increase decontamination and boost microbial degradation capacity. In particular, biostimulation or rate-limiting nutrient input can considerably repair petroleum-contaminated locations with less efficient and metabolically deficient microbial populations. Most of the credit for this goes to the low price of carbon (C), one of the rate-limiting resources needed by native bacteria for metabolic activities involving petroleum contaminants. Thus, adding a few rate-limiting nutrients besides carbon to the soil dramatically increases petroleum breakdown. Besides rate-limiting minerals, additional nutrient-rich organic materials can accelerate restoration. Organic waste from household sewage treatment (biosolids) with nitrogen- and phosphorus-rich inorganic fertilizers accelerates petroleum hydrocarbon breakdown by 96%.
8.3.4.2.2 Factors Affecting Stimulation
Environmental factors, including pH, moisture, temperature, and others, affect biostimulation-based contaminated site bioremediation (Abdulsalam et al. 2011). The biostimulation rate is also affected by environmental physiology. In this situation, marine bioremediation might be considered. The marine ecosystem’s bioremediation rate could be better because microorganisms cannot target the polymer for destruction since wave motion dilutes or washes it out. There are examples of the harmful impacts of excessive fertilizer input to soil. An increased quantity of N and P sources can promote eutrophication, which increases algae growth and lowers water dissolved oxygen, killing aquatic life (Nikolopoulou and Kalogerakis 2009). Thus, biostimulation’s environmental dependence can restrict the method’s development or efficiency. By balancing the soil’s rate-limiting nutrient additions, biostimulation efficiently removes complex pollutants from the ecosystem (Zawierucha and Malina 2011).
Biostimulation has the potential to remediate several contaminants, including polyester polyurethanes, sulfate, and petroleum hydrocarbons. Sulfate contamination of groundwater is harmful to ecosystems and human health. They are being fixed using biostimulation. Electron-donor alteration can enhance sulfate reduction (Miao et al. 2012). Soil degradation of polyester polyurethanes is hastened by biostimulation. Foams, fibers, textiles, and synthetic leather are just a few of the many applications for polyester polyurethanes (PU), a synthetic polymer (Cosgrove et al. 2010). Like macromolecules in living organisms, these polymers form connections within themselves (such as ester and urethane linkages). The disintegration of microbes is accelerated by intramolecular interactions, which serve as attack sites for the microbes (Zheng et al. 2005). The best strategy for acclimating microbial communities in petroleum-polluted environments is called biostimulation. Consequently, the pace of cleanup was higher in adapted populations than in uncontaminated ones.
8.3.5 Agricultural Effluents and Their Mycoremediation
Modern farming techniques wholly depend on agricultural chemicals, including herbicides, pesticides, weedicides, insecticides, and fertilizers, to increase crop output; this trend has developed throughout the past century (Carvalho 2017). Agrochemicals and residues remain in the environment because agricultural effluents create pollutants. The soil ecosystem’s biota, which contains many fungal species, helps biological processes. These activities include mineralization, elemental cycling, biodegradation of organic molecules, and enhanced agricultural production due to the bioavailability of insoluble components. Agrochemicals in excessive quantities can harm the biological processes they were meant to boost. Here is a list of the fungi used in the mycoremediation process in Table 8.2.
8.4 Factors Affecting Mycoremediation
Mycoremediation is intimately linked to several crucial elements. Heavy metal remediation can be affected by pH, temperature, duration, pollutant concentration, and adsorbent dosage. Mycoremediation can be performed by either cultivating fungus or using fungal biomass, although in both circumstances, the most influential parameter is the pH of the solution. Fungal cell viability in the context of mycelial growth, metallic solubility, available active sites (functional groups) on the adsorbent, and interaction like attraction and repulsion between the adsorbent and metal ions due to the hydrogen ion (H+) isoionic effect all play a role in the removal capacity. Acidic or basic pH reduces HM sorption and fungi growth. Fungi biologically collect metal. Therefore, medium pH impacts clearance rate. Aspergillus is affected at pH 4. It was observed that biosorption was inhibited below pH 3.0 (Pundir et al. 2018). Metal cations repelled positively charged fungal biomass metal-binding active sites or ligands, limiting biosorption. Pundir et al. (2018) found that metal hydroxide formation reduces metal removal above pH 5. Fungi with more outstanding negative charges bind strongly with metal ions at higher pH. Surface functional group separation impacts it (Mohsenzadeh and Shahrokhi 2014; Rawat et al. 2020). The adsorption of fungal biomass is prevented by pH changes (Li et al. 2018). When the pH of the solution drops, the dead biomass of Auricularia polytricha, Flammulina velutipes, Pleurotus eryngii, and P. ostreatus binds Cu(II), Zn(II), and Hg(II) at quantities ranging from 5.64 to 77.39%. At pH −2 (Pourkarim et al. 2017), dead biomass from the artist’s bracket fungus is an effective source for Cr(VI) adsorption.
Temperature affects adsorption differently. The fungal (Penicillium fellutanum) endothermic process in Ni and Zn removal is from deceased cellulose hybrid with bentonite (FBC) (Rashid et al. 2016). Endothermic or exothermic adsorption reactions occur (Pourkarim et al. 2017). Active fungus clears well at 25–35 °C (Kumar and Dwivedi 2019). Elevated temperatures distort and damage biosorbent surface functional groups (active sites) and affect cell membrane integrity, microbial cell wall configuration, metal microbe complex stability, and ionization characteristics, decreasing metal bioabsorption using fungal biomass. The clearance rate drops with adsorbing species’ thermal energy (Pourkarim et al. 2017). A fungal (Penicillium fellutanum) mixture of bentonite and decomposing biomass (FBC) eliminates Ni and Zn better at 30–51 °C than at 51 °C (Rashid et al. 2016). Fungal cell wall component reorientation and chemical moiety ionization may explain the active site’s high affinity for metal ions at moderate temperatures.
Fungal biomass absorbed heavy metals faster than by mycoremediation (Salvadori et al. 2015). While fungal biomass adsorption takes minutes to hours, growing fungi can remove HM in a matter of hours to days. At 100 min, Cai et al. (2016) noticed that the combination of polyvinyl alcohol (PVA) and sodium alginate (SA) more efficiently removed Cu, Pb, and Cd from immobilized live conidia of Penicillium janthinellum strain GXCR. Dried artist’s bracket and Lepiota hystrix biomass removed Cr, Cu, and Hg(II) in 30 and 5 min, respectively (Pourkarim et al. 2017). Adsorption occurs after pollutant interaction; with the process being functional group dependent, the adsorbent surface’s active site is quickly saturated. As a result, pollutant removal will either be constant or reduce because of desorption phenomena (Hajahmadi et al. 2019). Adsorption processes have two phases: an initial quick phase that lasts a short while and an additional slower phase that lasts a long period until equilibrium.
After equilibrium, ions had trouble filling the adsorbent’s vacant active sites; hence, increasing time duration lowered the adsorption potential. Periodic intraparticle diffusion may reduce fungal biomass adsorption potential. Gupta and Balomajumder (2015) discussed dual-phase adsorption; the adsorbent’s active sites are free to bind metal ions in the first phase. The second phase removes leftover active sites and may reject pollutants and bulk phase. Because they share charges, some of the dissolved solutes may have a repulsion toward the small adsorption inorganic on the adsorbent surface, reducing adsorption and rate of interaction between the active substituent and solute particles following the second phase. Solute concentration directly impacts the adsorption rate. Growing fungus and fungal biomass remediation require it.
In developing fungus, heavy metal concentration initially increases the clearance rate, but it declines after reaching its optimal concentration. The first phenomena may be related to active locations on growing fungus, and increased interaction between HMs and developing cell fungi that encourages maximal clearance. However, time, growth, and metabolic rate regulated metal buildup and promoted metal elimination. However, greater HM concentrations are hazardous to developing fungi, slowing sorption and removal by inhibiting their metabolism and development. Lead enhances fungal biomass, predominantly filamentous, in the culture’s beginning and end phases (Samadi et al. 2017). Fungal biomass (dead and treated) affected removal rate similarly to growing fungus, initially increasing HM concentration to saturate the active site and then decreasing after equilibrium. The concentration differential between the fluid and fungal biomass allows metal ions to saturate active sites as the removal rate increases (Zang et al. 2017). Due to increased diffusion, the lower metal concentration gradient decreases transport (Chen et al. 2012). At the point of equilibrium concentration, the removal rate may decrease due to adsorbent inactivity and HM repulsion.
According to Uzunoğlu et al. (2014), Sargassum acinarum can remove 100 mg L−1 of Cu(II) at its maximum rate (seaweed). While Saravanan et al. (2016) used a mixed biosorbent of custard apple seeds and A. niger to remove Cr(VI) and Ni(II), they discovered that the specific removal rate decreases with metal concentration. Zang discovered that Auricularia auricula has its maximum adsorption capacity at 200 mg L−1 Cr(VI) in effluent. Adsorption tests rely on the amount of fungal adsorbent used. Adsorbent concentrations that are higher enhance HM interaction and active site availability. When mycelial pellets were raised from 2 to 10 g L−1, rates of Cr(VI) ion elimination improved from 20.1 to 88.5%. Biosorbent also increased the number of active sites. In other investigations, the correlation between fungal adsorbent dosage and pollutant removal rate was maintained despite differences in pollutant and adsorbent (Mondal et al. 2017). Figure 8.3 shows the general process of mycoremediation of heavy metals.
8.5 Recent Advancements in Mycoremediation
Humans contaminate the environment by introducing heavy metals, radionuclides, hydrocarbons, and pollutants. The urban and industrial expansion has polluted surface and groundwater, harming persons and the ecosystem (Kour et al. 2021). Thus, the need for environmental ethics pollution-reduction methods is growing. Pesticides and herbicides protect crops from pests and weeds, enhancing productivity and yield. Agriculture has increased pesticide use. These poison ecosystems and are intransigent. Pesticides cause cancer, mutagenesis, immunosuppression, hormone imbalance, and other health problems (Gupta 2004). To use and abuse pesticides as a means of generating power, microorganisms have evolved various enzymes, activation mechanisms, and metabolic pathways (Goel et al. 2008; Kumar et al. 2021). Many different pollutants can be degraded by fungus via two different processes called mycodegradation and mycodeterioration. “Mycoremediation” describes the process of using fungus in nature to break down pollutants and garbage. Fungal species, including Phanerochaete velutina, Coriolus versicolor, and Pleurotus ostreatus, have exhibited the capacity to degrade a variety of herbicides, including atrazine (Castillo et al. 2001). Biodegradation of Granstar (tribenuron methyl) to various metabolites was shown to be most effective when performed by Aspergillus versicolor over 5 weeks (Ai-jawhari and Ai-seadi 2016). Exploring endophytic fungus aids heavy metal biosorption. Penicillium sp. and A. niger have a better biosorption capability in metal contaminant settings by binding metals present in various pollution sources. Polluted environments can absorb heavy metals through biosorption utilizing metabolically driven or physicochemical adsorption processes. Aromatic hydrocarbons are more environmentally harmful than aliphatic ones. Endophytes help their hosts degrade organic contaminants and reduce phytotoxicity by using relevant degradation pathways and metabolic capacity (Soleimani et al. 2010). Many microbes extract hydrocarbons from air, water, and soil. Biodegradation is slow. Thus, instead of depending on a specific organism, microbes from diverse genera can act together to extend degradation. Petroleum-contaminated soil, water, and surfaces include many microorganisms. Microorganisms may attach heavy metal ions to their cell walls, rendering them immobile. In addition, they may transform certain contaminants into water-soluble molecules and utilize them as food and fuel. Bioremediation is a method that can be used to speed up by the presence of microorganisms that stimulate plant growth or promote decomposition by rhizobia (Kavamura and Esposito 2010). Because of their high biomass content, fungi are helpful in the biodegradation of heavy metal-polluted areas. Because they break down so easily, convert, and cycle nutrients, fungi play a crucial role on Earth. In their groundbreaking research, Wunch et al. (1999) documented for the first time the fungi’s ability to degrade anthropogenic chemicals.
8.6 Conclusion and Future Prospectus
It is possible to increase the resistance of metal/metalloid-resistant fungus to environmental toxins using various genetic engineering techniques. Pocsi (2011), using S. cerevisiae as a model system, has suggested increasing synthesis of extracellular and intracellular metal chelators as a possible target for genetic modifications, taking off the metal scavengers to slow the metal flow, increasing the production of components of the antioxidative defense system, altering the network of regulators, and interfering with programmed cell death (apoptosis). Scientists have developed fungal strains from the endophytic fungi Mucor sp. CBRF59 by fusing protoplasts (Deng et al. 2013). Rape plants were injected with the mutant strain CBRF59T3 to increase the stress tolerance of an unidentified fungus utilized in heavy metal-polluted soil phytoremediation. Rape sprouts cultivated on Cd(II)- and Pb(II)-contaminated soils had 35–189% higher Cd(II) levels (Deng et al. 2013). Qiu et al. (2015) discovered that a modified BY-G strain was more resistant to oxidative stress, heat, furfural, hydroxymethylfurfural, and Cd than a reference strain of S. cerevisiae (II). Public opinion on bioremediation using GM microorganisms is crucial. Using genetically modified fungus to treat metals and metalloids seems promising. The intricacy of the toxic effects of metals and metalloids on cells has long escaped comprehension. Still, sequence information on their genomes has enabled postgenomic techniques to gain abundant data on the roles of their genes and the mechanisms that regulate them. Immobilization of metals by their reduction has uses in bioremediation and in creating new biomaterials and catalysts (Gadd et al. 2012). Subtracting heavy metals from wastewater and soils using a biosorption-based biosynthesis of nanoparticles can also help in the production of heavy metal nanoparticles that may be used in the technology industry (Karman et al. 2015). One innovative strategy for creating metal nanoparticles (NPs) involves using the highly organized physical and metabolic activity of microbial cells (Gericke and Pinches 2006). Compared to other microorganisms, fungi are preferable for synthesizing NPs due to their manageability, simplicity of food requirements, strong cell wall-binding capability, and high intracellular metal absorption capabilities (Sanghi and Verma 2009). Several research has examined whether fungus can detoxify polluted surroundings by producing nanomaterials and removing harmful metals. Velmurugan et al. (2010) found that Fusarium sp. from a South Korean zinc-contaminated mine could absorb up to 320 mg L−1 and produce ZnO NPs. In an aqueous solution, Hypocrea lixii dead biomass may produce CuO and NiO NPs by reducing Cu and Ni ions (Marcia Regina Salvadori et al. 2015). Rhodotorula mucilaginosa and Trichoderma koningiopsis biosynthesize Cu NPs (Salvadori et al. 2014). Nanoparticles improve fungal bioremediation. It was found that adding 1 mg L−1 Ag-NPs to Phanerochaete chrysosporium increased its Cd(II) removal by tenfold (Zuo et al. 2015). When used to remove Cd and break down 2,4-dichlorophenol, P. chrysosporium immobilized on TiO2 nanoparticles (PTNs) is a one-of-a-kind, high-value bioremediation material due to their antioxidative defense mechanism and physiological fluxes (Tan et al. 2015). Suspiciously, fungi can efficiently remove NPs from aqueous mediums. It was found that Pleurotus eryngii and Trametes versicolor can remove 86% and 61% of Al2O3 NPs, respectively. Although less effective against Co NPs, P. eryngii can eliminate 58% of Pt NPs (Jakubiak et al. 2014).
Tripathi et al. (2013) proposed many essential sustainability metrics as a means to evaluate the potential for the durable success of corrected systems. The decrease in pollutant levels and residual concentrations after the remediation are major factors illustrating the enhancement of soil physicochemical qualities, the growth of microbial biomass, the diversity of their functions in soil, and the improvement in the biodiversity component, which includes sensitive and key species. The traditional physicochemical techniques, including evaporation, electrochemical treatment, membrane technology, filtration, ion exchange, reverse osmosis, chemical precipitation, oxidation, and reduction, are desirable and potential substitutes. This is true notwithstanding the difficulties involved in applying molecular methods to increase the likelihood of remediation. Because it is capable of meeting a number of the aforementioned characteristics, fungal bioremediation is an alternative that is both successful and durable in terms of removing heavy metals and metalloids from polluted areas. The metal-resistant fungus has arisen as a possible solution to the pollution from metals and metalloids in light of recent developments in nanotechnology and our growing understanding of the life cycles of nanoparticles.
References
Abdullahi UA, Khandaker MM, Shaari NEM, Alias N (2021) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. J Environ Treat Tech 9:601–608. https://doi.org/10.47277/JETT/9(3)608
Abdulsalam S, Bugaje IM, Adefila SS, Ibrahim S (2011) Comparison of biostimulation and bioaugmentation for remediation of soil contaminated with spent motor oil. Int J Environ Sci Technol 8:187–194. https://doi.org/10.1007/BF03326208
Adams GO et al (2020) Bioremediation, biostimulation and bioaugmention: a review. Int J Environ Bioremediat Biodegrad 3:28–39. https://doi.org/10.12691/ijebb-3-1-5
Ai-jawhari IFH, Ai-seadi KJY (2016) Fate of herbicide Granstar (Tribenuron methyl) in wheat field in AI-Nasiriya governorate. Int Res J Biol Sci 5:22–37
Alloway BJ (2013) Sources of heavy metals and metalloids in soils. In: Alloway BJ (ed) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Springer, Dordrecht, pp 11–50. https://doi.org/10.1007/978-94-007-4470-7_2
Andrewes P, Cullen WR, Polishchuk E (2000) Antimony biomethylation by Scopulariopsis brevicaulis: characterization of intermediates and the methyl donor. Chemosphere 41:1717–1725. https://doi.org/10.1016/s0045-6535(00)00063-1
Baker AJM (1987) Metal tolerance. New Phytol 106:93–111. https://doi.org/10.1111/j.1469-8137.1987.tb04685.x
Bandurska K, Krupa P, Berdowska A, Jatulewicz I, Zawierucha I (2021) Mycoremediation of soil contaminated with cadmium and lead by sp. Ecol Chem Eng S 28:277–286. https://doi.org/10.2478/eces-2021-0020
Barkay T, Wagner-Döbler I (2005) Microbial transformations of mercury: potentials, challenges, and achievements in controlling mercury toxicity in the environment. In: Advances in applied microbiology. Academic Press, pp 1–52. https://doi.org/10.1016/S0065-2164(05)57001-1
Basta NT, Ryan JA, Chaney RL (2005) Trace element chemistry in residual-treated soil: key concepts and metal bioavailability. J Environ Qual 34:49–63. https://doi.org/10.2134/jeq2005.0049dup
Bending GD, Friloux M, Walker A (2002) Degradation of contrasting pesticides by white rot fungi and its relationship with ligninolytic potential. FEMS Microbiol Lett 212:59–63. https://doi.org/10.1111/j.1574-6968.2002.tb11245.x
Bhandari G (2017) Mycoremediation: an eco-friendly approach for degradation of pesticides. Springer
Brito ACC, Boechat CL, de Sena AFS, de Sousa Luz Duarte L, do Nascimento CWA, da Silva YJAB et al (2020) Assessing the distribution and concentration of heavy metals in soils of an agricultural frontier in the Brazilian Cerrado. Water Air Soil Pollut 231:388. https://doi.org/10.1007/s11270-020-04760-2
Cai C-X, Xu J, Deng N-F, Dong X-W, Tang H, Liang Y et al (2016) A novel approach of utilization of the fungal conidia biomass to remove heavy metals from the aqueous solution through immobilization. Sci Rep 6:36546. https://doi.org/10.1038/srep36546
Camacho L, Sánchez J (2015) Biotechnological use of fungi for the degradation of recalcitrant agro-pesticides, vol 12. Elsevier. https://doi.org/10.1016/B978-0-12-802794-3.00012-6
Carvalho FP (2017) Pesticides, environment, and food safety. Food Energy Secur 6:48–60. https://doi.org/10.1002/fes3.108
Castillo M, Andersson A, Ander P, Stenström J, Torstensson L (2001) Establishment of the white rot fungus Phanerochaete chrysosporium on unsterile straw in solid substrate fermentation systems intended for degradation of pesticides. World J Microbiol Biotechnol 17:627–633. https://doi.org/10.1023/A:1012420422111
Cernansky S, Urík M, Ševc J, Khun M (2007) Biosorption and biovolatilization of arsenic by heat-resistant fungi (5 pp). Environ Sci Pollut Res 14:31–35. https://doi.org/10.1065/espr2006.11.361
Challacombe JF, Hesse CN, Bramer LM, McCue LA, Lipton M, Purvine S et al (2019) Genomes and secretomes of Ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. BMC Genomics 20:976. https://doi.org/10.1186/s12864-019-6358-x
Chan WK, Wildeboer D, Garelick H, Purchase D (2016) Mycoremediation of heavy metal/metalloid-contaminated soil: current understanding and future prospects. In: Purchase D (ed) Fungal applications in sustainable environmental biotechnology. Springer International Publishing, Cham, pp 249–272. https://doi.org/10.1007/978-3-319-42852-9_10
Chen S, Yue Q, Gao B, Li Q, Xu X, Fu K (2012) Adsorption of hexavalent chromium from aqueous solution by modified corn stalk: a fixed-bed column study. Bioresour Technol 113:114–120. https://doi.org/10.1016/j.biortech.2011.11.110
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182. https://doi.org/10.1146/annurev.arplant.53.100301.135154
Cosgrove L, McGeechan PL, Handley PS, Robson GD (2010) Effect of biostimulation and bioaugmentation on degradation of polyurethane buried in soil. Appl Environ Microbiol 76:810–819. https://doi.org/10.1128/AEM.00534-09
Cox DP, Alexander M (1973) Production of trimethylarsine gas from various arsenic compounds by three sewage fungi. Bull Environ Contam Toxicol 9:84–88. https://doi.org/10.1007/BF01684760
Cullen WR, Reimer KJ (1989) Arsenic speciation in the environment. Chem Rev 89:713–764. https://doi.org/10.1021/cr00094a002
Damodaran D, Balakrishnan RM, Shetty VK (2013) The uptake mechanism of Cd(II), Cr(VI), Cu(II), Pb(II), and Zn(II) by mycelia and fruiting bodies of Galerina vittiformis. Biomed Res Int 2013:149120. https://doi.org/10.1155/2013/149120
Deng Z, Zhang R, Shi Y, Hu L, Tan H, Cao L (2013) Enhancement of phytoremediation of Cd- and Pb-contaminated soils by self-fusion of protoplasts from endophytic fungus Mucor sp. CBRF59. Chemosphere 91:41–47. https://doi.org/10.1016/j.chemosphere.2012.11.065
DeVolder PS, Brown SL, Hesterberg D, Pandya K (2003) Metal bioavailability and speciation in a wetland tailings repository amended with biosolids compost, wood ash, and sulfate. J Environ Qual 32:851–864. https://doi.org/10.2134/jeq2003.8510
Dritsa V, Rigas F, Doulia D, Avramides EJ, Hatzianestis I (2009) Optimization of culture conditions for the biodegradation of Lindane by the polypore fungus Ganoderma australe. Water Air Soil Pollut 204:19. https://doi.org/10.1007/s11270-009-0022-z
Elektorowicz M (1994) Bioremediation of petroleum-contaminated clayey soil with pretreatment. Environ Technol 15:373–380. https://doi.org/10.1080/09593339409385440
Franco AR, Ferreira AC, Castro PML (2014) Co-metabolic degradation of mono-fluorophenols by the ectomycorrhizal fungi Pisolithus tinctorius. Chemosphere 111:260–265. https://doi.org/10.1016/j.chemosphere.2014.03.094
Fujs S, Gazdag Z, Poljsak B, Stibilj V, Milacic R, Pesti M et al (2005) The oxidative stress response of the yeast Candida intermedia to copper, zinc, and selenium exposure. J Basic Microbiol 45:125–135. https://doi.org/10.1002/jobm.200410480
Gadd GM (1993) Interactions of fungi with toxic metals. New Phytol 124:25–60. https://doi.org/10.1111/j.1469-8137.1993.tb03796.x
Gadd GM, Rhee YJ, Stephenson K, Wei Z (2012) Geomycology: metals, actinides and biominerals. Environ Microbiol Rep 4:270–296. https://doi.org/10.1111/j.1758-2229.2011.00283.x
Gao Y, Chen S, Hu M, Hu Q, Luo J, Li Y (2012) Purification and characterization of a novel chlorpyrifos hydrolase from Cladosporium cladosporioides Hu-01. PLoS One 7:e38137. https://doi.org/10.1371/journal.pone.0038137
Gericke M, Pinches A (2006) Biological synthesis of metal nanoparticles. Hydrometallurgy 83:132–140. https://doi.org/10.1016/j.hydromet.2006.03.019
Goel R, Zaidi MGH, Soni R, Lata K, Shouche YS (2008) Implication of Arthrobacter and Enterobacter species for polycarbonate degradation. Int Biodeterior Biodegrad 61:167–172. https://doi.org/10.1016/j.ibiod.2007.07.001
González Henao S, Ghneim-Herrera T (2021) Heavy metals in soils and the remediation potential of bacteria associated with the plant microbiome. Front Environ Sci 9:1–17
Gonzalez-Chavez MCA, del Pilar Ortega-Larrocea M, Carrillo-González R, López-Meyer M, Xoconostle-Cázares B, Gomez SK et al (2011) Arsenate induces the expression of fungal genes involved in As transport in Arbuscular mycorrhiza. Fungal Biol 115:1197–1209. https://doi.org/10.1016/j.funbio.2011.08.005
Green NA, Meharg AA, Till C, Troke J, Nicholson JK (1999) Degradation of 4-fluorobiphenyl by mycorrhizal fungi as determined by 19F nuclear magnetic resonance spectroscopy and 14C radiolabelling analysis. Appl Environ Microbiol 65:4021–4027. https://doi.org/10.1128/AEM.65.9.4021-4027.1999
Gupta PK (2004) Pesticide exposure—Indian scene. Toxicology 198:83–90. https://doi.org/10.1016/j.tox.2004.01.021
Gupta A, Balomajumder C (2015) Simultaneous adsorption of Cr(VI) and phenol onto tea waste biomass from binary mixture: multicomponent adsorption, thermodynamic and kinetic study. J Environ Chem Eng 3:785–796. https://doi.org/10.1016/j.jece.2015.03.003
Hajahmadi Z, Younesi H, Bahramifar N, Khakpour H et al (2019) Multi component isotherm for biosorption of Zn(II), Co(II) and Cd(II) from ternary mixture on top retreated dried Aspergillus niger biomass. Water Resour Ind 11:71–80
Hartmann LM, Craig PJ, Jenkins RO (2003) Influence of arsenic on antimony methylation by the aerobic yeast Cryptococcus humicola. Arch Microbiol 180:347–352. https://doi.org/10.1007/s00203-003-0600-1
Helal I, Abo-El-Seoud M (2014) Fungal biodegradation of pesticide vydate in soil and aquatic system. Springer, Berlin
Hou X, Han H, Tigabu M, Cai L, Meng F, Liu A et al (2019) Changes in soil physico-chemical properties following vegetation restoration mediate bacterial community composition and diversity in Changting, China. Ecol Eng 138:171–179. https://doi.org/10.1016/j.ecoleng.2019.07.031
Hu X, Wang J, Lv Y, Liu X, Zhong J, Cui X et al (2021) Effects of heavy metals/metalloids and soil properties on microbial communities in farmland in the vicinity of a metals smelter. Front Microbiol 12:707786. https://doi.org/10.3389/fmicb.2021.707786
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211:317–331. https://doi.org/10.1016/j.jhazmat.2011.10.016
Jakubiak M, Giska I, Asztemborska M, Bystrzejewska-Piotrowska G (2014) Bioaccumulation and biosorption of inorganic nanoparticles: factors affecting the efficiency of nanoparticle mycoextraction by liquid-grown mycelia of Pleurotus eryngii and Trametes versicolor. Mycol Prog 13:525–532. https://doi.org/10.1007/s11557-013-0933-3
Jiang J, Qin C, Shu X, Chen R, Song H, Li Q et al (2015) Effects of copper on induction of thiol-compounds and antioxidant enzymes by the fruiting body of Oudemansiella radicata. Ecotoxicol Environ Saf 111:60–65. https://doi.org/10.1016/j.ecoenv.2014.09.014
Jin Y, Luan Y, Ning Y, Wang L (2018) Effects and mechanisms of microbial remediation of heavy metals in soil: a critical review. Appl Sci 8:1336. https://doi.org/10.3390/app8081336
Jones LHP, Jarvis SC (1981) The fate of heavy metals. In: Green DJ, Hayes MHB (eds) The chemistry of soil processes. Wiley, New York, p 593
Karman SB, Diah SZM, Gebeshuber IC (2015) Raw materials synthesis from heavy metal industry effluents with bioremediation and phytomining: a biomimetic resource management approach. Adv Mater Sci Eng 2015:185071. https://doi.org/10.1155/2015/185071
Kaur H, Kapoor S, Kaur G (2016) Application of ligninolytic potentials of a white-rot fungus Ganoderma lucidum for degradation of lindane. Environ Monit Assess 188:588. https://doi.org/10.1007/s10661-016-5606-7
Kavamura VN, Esposito E (2010) Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol Adv 28:61–69. https://doi.org/10.1016/j.biotechadv.2009.09.002
Klaunig JE, Xu Y, Isenberg JS, Bachowski S, Kolaja KL, Jiang J et al (1998) The role of oxidative stress in chemical carcinogenesis. Environ Health Perspect 106(Suppl):289–295. https://doi.org/10.1289/ehp.98106s1289
Koller M, Saleh HM (2018) Introductory chapter: introducing heavy metals. In: Saleh HE-DM, Aglan RF (eds) Heavy metals. IntechOpen, Rijeka, p 74783. https://doi.org/10.5772/intechopen.74783
Kour D, Kaur T, Devi R, Yadav A, Singh M, Joshi D et al (2021) Beneficial microbiomes for bioremediation of diverse contaminated environments for environmental sustainability: present status and future challenges. Environ Sci Pollut Res Int 28:24917–24939. https://doi.org/10.1007/s11356-021-13252-7
Kumar V, Dwivedi SK (2019) Hexavalent chromium reduction ability and bioremediation potential of Aspergillus flavus CR500 isolated from electroplating wastewater. Chemosphere 237:124567. https://doi.org/10.1016/j.chemosphere.2019.124567
Kumar P, Dash B, Suyal DC, Gupta SB, Singh AK, Chowdhury T et al (2021) Characterization of arsenic-resistant Klebsiella pneumoniae RnASA11 from contaminated soil and water samples and its bioremediation potential. Curr Microbiol 78:3258–3267. https://doi.org/10.1007/s00284-021-02602-w
Kumari P, Kumar P (2019) An overview of phytomining: a metal extraction process from plant species. JETIR 6:2349–5162
Kumari P, Aley P, Mehta C, Kumar P (2019) Mycoremediation of heavy metals. JETIR 6:325–342
Lasat MM (1999) Phytoextraction of metals from contaminated soil: a review of plant/soil/metal interaction and assessment of pertinent agronomic issues. J Hazard Subst Res 2:1–25. https://doi.org/10.4148/1090-7025.1015
Li J, Sun Y, Jiang X, Chen B, Zhang X (2018) Arbuscular mycorrhizal fungi alleviate arsenic toxicity to Medicago sativa by influencing arsenic speciation and partitioning. Ecotoxicol Environ Saf 157:235–243. https://doi.org/10.1016/j.ecoenv.2018.03.073
Madhavan A, Sindhu R, Parameswaran B, Sukumaran RK, Pandey A (2017) Metagenome analysis: a powerful tool for enzyme bioprospecting. Appl Biochem Biotechnol 183:636–651. https://doi.org/10.1007/s12010-017-2568-3
Mao J, Guan W (2016) Fungal degradation of polycyclic aromatic hydrocarbons (PAHs) by Scopulariopsis brevicaulis and its application in bioremediation of PAH-contaminated soil. Acta Agric Scand Sect B Soil Plant Sci 66:399–405. https://doi.org/10.1080/09064710.2015.1137629
Martinkova L, Rucka L, Nesvera J, Patek M (2017) Recent advances and challenges in the heterologous production of microbial nitrilases for biocatalytic applications. World J Microbiol Biotechnol 33:8. https://doi.org/10.1007/s11274-016-2173-6
McDougall DN, Blanchette RA (1996) Metal ion adsorption by pseudosclerotial plates of Phellinus weirii. Mycologia 88:98–103. https://doi.org/10.1080/00275514.1996.12026628
McLaren RG, Clucas LM, Taylor MD, Hendry T (2005) Leaching of macronutrients and metals from undisturbed soils treated with metal-spiked sewage sludge. 3. Distribution of residual metals. Aust J Soil Res 43:159–170
Miao Z, Brusseau ML, Carroll KC, Carreón-Diazconti C, Johnson B (2012) Sulfate reduction in groundwater: characterization and applications for remediation. Environ Geochem Health 34:539–550. https://doi.org/10.1007/s10653-011-9423-1
Mir-Tutusaus JA, Masís-Mora M, Corcellas C, Eljarrat E, Barceló D, Sarrà M et al (2014) Degradation of selected agrochemicals by the white rot fungus Trametes versicolor. Sci Total Environ 500:235–242. https://doi.org/10.1016/j.scitotenv.2014.08.116
Mishra GK (2017) Microbes in heavy metal remediation: a review on current trends and patents. Recent Pat Biotechnol 11:188–196. https://doi.org/10.2174/1872208311666170120121025
Mohsenzadeh F, Shahrokhi F (2014) Biological removing of cadmium from contaminated media by fungal biomass of Trichoderma species. J Environ Health Sci Eng 12:102. https://doi.org/10.1186/2052-336X-12-102
Mondal NK, Samanta A, Dutta S, Chattoraj S (2017) Optimization of Cr(VI) biosorption onto Aspergillus niger using 3-level Box-Behnken design: equilibrium, kinetic, thermodynamic and regeneration studies. J Genet Eng Biotechnol 15:151–160. https://doi.org/10.1016/j.jgeb.2017.01.006
Mrozik A, Piotrowska-Seget Z (2010) Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiol Res 165:363–375. https://doi.org/10.1016/j.micres.2009.08.001
Nas FS, Ali M (2018) The effect of lead on plants in terms of growing and biochemical parameters: a review. MOJ Ecol Environ Sci 3:265–268. https://doi.org/10.15406/mojes.2018.03.00098
Nikolopoulou M, Kalogerakis N (2009) Biostimulation strategies for fresh and chronically polluted marine environments with petroleum hydrocarbons. J Chem Technol Biotechnol 84:802–807. https://doi.org/10.1002/jctb.2182
Oliveira BR, Penetra A, Cardoso VV, Benoliel MJ, Barreto Crespo MT, Samson RA et al (2015) Biodegradation of pesticides using fungi species found in the aquatic environment. Environ Sci Pollut Res Int 22:11781–11791. https://doi.org/10.1007/s11356-015-4472-0
Pendias AK, Pendias H (2001) Trace metals in soils and plants, 2nd edn. CRC Press, Boca Raton
Pihurov M, Vlăduț V, Bordean DM, Ferdes M (2019) Mycoremediation of heavy metal—contaminated soil. Springer, Berlin, pp 423–428
Pinto AP, Rodrigues SC, Caldeira AT, Teixeira DM (2016) Exploring the potential of novel biomixtures and Lentinula edodes fungus for the degradation of selected pesticides. Evaluation for use in biobed systems. Sci Total Environ 541:1372–1381. https://doi.org/10.1016/j.scitotenv.2015.10.046
Pocsi I (2011) Toxic metal/metalloid tolerance in fungi—a biotechnology-oriented approach. In: Cellular effects of heavy metals. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0428-2_2
Pointing SB (2001) Feasibility of bioremediation by white-rot fungi. Appl Microbiol Biotechnol 57:20–33. https://doi.org/10.1007/s002530100745
Pourkarim S, Ostovar F, Mahdavianpour M, Moslemzadeh M (2017) Adsorption of chromium(VI) from aqueous solution by artist’s bracket fungi. Sep Sci Technol 52:1733–1741. https://doi.org/10.1080/01496395.2017.1299179
Pundir R, Chary GHVC, Dastidar MG (2018) Application of Taguchi method for optimizing the process parameters for the removal of copper and nickel by growing Aspergillus sp. Water Resour Ind 20:83–92. https://doi.org/10.1016/j.wri.2016.05.001
Qiao Z, Cao M, Wang D, Liao S, Wang G (2019) Sphingosinicella humi sp. nov., isolated from arsenic-contaminated farmland soil and emended description of the genus Sphingosinicella. Int J Syst Evol Microbiol 69:498–503. https://doi.org/10.1099/ijsem.0.003186
Qiu Z, Deng Z, Tan H, Zhou S, Cao L (2015) Engineering the robustness of Saccharomyces cerevisiae by introducing bifunctional glutathione synthase gene. J Ind Microbiol Biotechnol 42:537–542. https://doi.org/10.1007/s10295-014-1573-6
Raab A, Feldmann J, Meharg AA (2004) The nature of arsenic-phytochelatin complexes in Holcus lanatus and Pteris cretica. Plant Physiol 134:1113–1122. https://doi.org/10.1104/pp.103.033506
Raffa CM, Chiampo F, Shanthakumar S (2021) Remediation of metal/metalloid-polluted soils: a short review. Appl Sci 11:1–23. https://doi.org/10.3390/app11094134
Ramadevi C, Mahendra N, Prasad M (2012) Mycodegradation of Malathion by a soil fungal isolate, Aspergillus niger. Int J Basic Appl Chem Sci 2:108–115
Rashid A, Bhatti HN, Iqbal M, Noreen S (2016) Fungal biomass composite with bentonite efficiency for nickel and zinc adsorption: a mechanistic study. Ecol Eng 91:459–471. https://doi.org/10.1016/j.ecoleng.2016.03.014
Rawat AP, Kumar V, Singh DP (2020) A combined effect of adsorption and reduction potential of biochar derived from Mentha plant waste on removal of methylene blue dye from aqueous solution. Sep Sci Technol 55:907–921. https://doi.org/10.1080/01496395.2019.1580732
Reed SC, Crites RW, Middlebrooks JE (1995) Natural systems for waste management and treatment. McGraw-Hill, New York, p 433
Renu AM, Singh K (2016) Heavy metal removal from wastewater using various adsorbents: a review. J Water Reuse Desalin 7:387–419. https://doi.org/10.2166/wrd.2016.104
Rivelli V, Franzetti A, Gandolfi I, Cordoni S, Bestetti G (2013) Persistence and degrading activity of free and immobilised allochthonous bacteria during bioremediation of hydrocarbon-contaminated soils. Biodegradation 24:1–11. https://doi.org/10.1007/s10532-012-9553-x
Ruley AT, Sharma NC, Sahi SV, Singh SR, Sajwan KS (2006) Effects of lead and chelators on growth, photosynthetic activity and Pb uptake in Sesbania drummondii grown in soil. Environ Pollut 144:11–18. https://doi.org/10.1016/j.envpol.2006.01.016
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Biol 49:643–668
Salvadori MR, Ando RA, do Nascimento CAO, Corrêa B (2014) Bioremediation from wastewater and extracellular synthesis of copper nanoparticles by the fungus Trichoderma koningiopsis. J Environ Sci Health A 49:1286–1295. https://doi.org/10.1080/10934529.2014.910067
Salvadori MR, Ando RA, Nascimento CAO, Corrêa B (2015) Extra and intracellular synthesis of nickel oxide nanoparticles mediated by dead fungal biomass. PLoS One 10:e0129799. https://doi.org/10.1371/journal.pone.0129799
Samadi S, Karimi K, Behnam S (2017) Simultaneous biosorption and bioethanol production from lead-contaminated media by Mucor indicus. Biofuel Res J 4:545–550. https://doi.org/10.18331/BRJ2017.4.1.4
Sanghi R, Verma P (2009) Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour Technol 100:501–504. https://doi.org/10.1016/j.biortech.2008.05.048
Saravanan A, Kumar PS, Preetha B (2016) Optimization of process parameters for the removal of chromium(VI) and nickel(II) from aqueous solutions by mixed biosorbents (custard apple seeds and Aspergillus niger) using response surface methodology. Desalin Water Treat 57:14530–14543. https://doi.org/10.1080/19443994.2015.1064034
Sene L, Converti A, Secchi GAR, de Simão RCG (2010) New aspects on atrazine biodegradation. Braz Arch Biol Technol 53:487–496. https://doi.org/10.1590/S1516-89132010000200030
Seo J, Jeon J, Kim S-D, Kang S, Han J, Hur H-G (2007) Fungal biodegradation of carbofuran and carbofuran phenol by the fungus Mucor ramannianus: identification of metabolites. Water Sci Technol 55:163–167. https://doi.org/10.2166/wst.2007.051
Sharples JM, Meharg AA, Chambers SM, Cairney JW (2000) Mechanism of arsenate resistance in the ericoid mycorrhizal fungus Hymenoscyphus ericae. Plant Physiol 124:1327–1334. https://doi.org/10.1104/pp.124.3.1327
Shen M, Zhao D-K, Qiao Q, Liu L, Wang J-L, Cao G-H et al (2015) Identification of glutathione S-transferase (GST) genes from a dark septate endophytic fungus (Exophiala pisciphila) and their expression patterns under varied metals stress. PLoS One 10:e0123418. https://doi.org/10.1371/journal.pone.0123418
Silambarasan S, Abraham J (2012) Ecofriendly method for bioremediation of chlorpyrifos from agricultural soil by novel fungus Aspergillus terreus JAS1. Water Air Soil Pollut 224:1369. https://doi.org/10.1007/s11270-012-1369-0
Singh S (2017) Microbe-induced degradation of pesticides. Springer, Cham. https://doi.org/10.1007/978-3-319-45156-5
Singh J, Kalamdhad AS (2011) Effects of heavy metals on soil, plants, human health and aquatic life. Int J Res Chem Environ 1:15–21
Singh BK, Kuhad R (1999) Biodegradation of lindane (gamma-hexachlorocyclohexane) by the white-rot fungus Trametes hirsutus. Lett Appl Microbiol 28:238–241
Soleimani M, Afyuni M, Hajabbasi MA, Nourbakhsh F, Sabzalian MR, Christensen JH (2010) Phytoremediation of an aged petroleum contaminated soil using endophyte infected and non-infected grasses. Chemosphere 81:1084–1090. https://doi.org/10.1016/j.chemosphere.2010.09.034
Spagnoletti FN, Chiocchio VM (2020) Tolerance of dark septate endophytic fungi (DSE) to agrochemicals in vitro. Rev Argent Microbiol 52:43–49. https://doi.org/10.1016/j.ram.2019.02.003
Spina F, Cecchi G, Landinez-Torres A, Pecoraro L, Russo F, Wu B et al (2018) Fungi as a toolbox for sustainable bioremediation of pesticides in soil and water. Plant Biosyst Int J Deal Aspects Plant Biol 152:474–488. https://doi.org/10.1080/11263504.2018.1445130
Sumner ME (2000) Beneficial use of effluents, wastes, and biosolids. Commun Soil Sci Plant Anal 31:1701–1715. https://doi.org/10.1080/00103620009370532
Tan Q, Chen G, Zeng G, Chen A, Guan S, Li Z et al (2015) Physiological fluxes and antioxidative enzymes activities of immobilized Phanerochaete chrysosporium loaded with TiO2 nanoparticles after exposure to toxic pollutants in solution. Chemosphere 128:21–27. https://doi.org/10.1016/j.chemosphere.2014.12.088
Theerachat M, Emond S, Cambon E, Bordes F, Marty A, Nicaud J-M et al (2012) Engineering and production of laccase from Trametes versicolor in the yeast Yarrowia lipolytica. Bioresour Technol 125:267–274. https://doi.org/10.1016/j.biortech.2012.07.117
Tripathi P, Singh PC, Mishra A, Chauhan PS, Dwivedi S, Bais RT et al (2013) Trichoderma: a potential bioremediator for environmental clean up. Clean Technol Environ Policy 15:541–550. https://doi.org/10.1007/s10098-012-0553-7
Ufarté L, Laville É, Duquesne S, Potocki-Veronese G (2015) Metagenomics for the discovery of pollutant degrading enzymes. Biotechnol Adv 33:1845–1854. https://doi.org/10.1016/j.biotechadv.2015.10.009
USEPA (1994) A plain English guide to the EPA part 503 biosolids rule
Uzunoğlu D, Gürel N, Özkaya N, Özer A (2014) The single batch biosorption of copper(II) ions on Sargassum acinarium. Desalin Water Treat 52:789403. https://doi.org/10.1080/19443994.2013.789403
Velmurugan P, Shim J, You Y, Choi S, Kamala-Kannan S, Lee K-J et al (2010) Removal of zinc by live, dead, and dried biomass of Fusarium spp. isolated from the abandoned-metal mine in South Korea and its perspective of producing nanocrystals. J Hazard Mater 182:317–324. https://doi.org/10.1016/j.jhazmat.2010.06.032
Wang J, Hirai H, Kawagishi H (2012) Biotransformation of acetamiprid by the white-rot fungus Phanerochaete sordida YK-624. Appl Microbiol Biotechnol 93:831–835. https://doi.org/10.1007/s00253-011-3435-8
Wu L, Bradshaw AD, Thurman DA (1975) The potential for evolution of heavy metal tolerance in plants. III. The rapid evolution of copper tolerance in Agrostis stolonifera. Heredity 34:165–187
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol 2011:402647. https://doi.org/10.5402/2011/402647
Wunch KG, Alworth WL, Bennett JW (1999) Mineralization of benzo[a]pyrene by Marasmiellus troyanus, a mushroom isolated from a toxic waste site. Microbiol Res 154:75–79. https://doi.org/10.1016/S0944-5013(99)80038-X
Xiao P, Mori T, Kamei I, Kiyota H, Takagi K, Kondo R (2011) Novel metabolic pathways of organochlorine pesticides dieldrin and aldrin by the white rot fungi of the genus Phlebia. Chemosphere 85:218–224. https://doi.org/10.1016/j.chemosphere.2011.06.028
Yang H, Li J, Du G, Liu L (2016) Biotechnology of microbial enzymes: production. In: Biocatalysis and industrial applications. Elsevier, Amsterdam
Yu F, Lin J, Xie D, Yao Y, Wang X, Huang Y et al (2020) Soil properties and heavy metal concentrations affect the composition and diversity of the diazotrophs communities associated with different land use types in a mining area. Appl Soil Ecol 155:103669. https://doi.org/10.1016/j.apsoil.2020.103669
Yuniati MD (2018) Bioremediation of petroleum-contaminated soil: a review bioremediation of petroleum-contaminated soil: a review. IOP Conf Ser Earth Environ Sci 118:1–7
Zang T, Cheng Z, Lu L, Jin Y, Xu X, Ding W et al (2017) Removal of Cr(VI) by modified and immobilized Auricularia auricula spent substrate in a fixed-bed column. Ecol Eng 99:358–365. https://doi.org/10.1016/j.ecoleng.2016.11.070
Zawierucha I, Malina G (2011) Bioremediation of contaminated soils: effects of bioaugmentation and biostimulation on enhancing biodegradation of oil hydrocarbons. In: Singh A, Parmar N, Kuhad RC (eds) Bioaugmentation, biostimulation and biocontrol. Springer, Berlin, pp 187–201. https://doi.org/10.1007/978-3-642-19769-7_8
Zhao X, Huang J, Lu J, Sun Y (2019) Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol Environ Saf 170:218–226. https://doi.org/10.1016/j.ecoenv.2018.11.136
Zheng Y, Yanful EK, Bassi AS (2005) A review of plastic waste biodegradation. Crit Rev Biotechnol 25:243–250. https://doi.org/10.1080/07388550500346359
Zuo Y, Chen G, Zeng G, Li Z, Yan M, Chen A et al (2015) Transport, fate, and stimulating impact of silver nanoparticles on the removal of Cd(II) by Phanerochaete chrysosporium in aqueous solutions. J Hazard Mater 285:236–244. https://doi.org/10.1016/j.jhazmat.2014.12.003
Acknowledgments
Authors are thankful to their respective institutions for providing facilities to develop this book chapter.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Ethics declarations
There are no conflicts of interest to be declared.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ray, M.K., Panda, J., Panda, B.P., Mohanta, T.K., Mohanta, Y.K. (2023). Mycoremediation of Heavy Metals and/or Metalloids in Soil. In: Sarma, H., Joshi, S. (eds) Land Remediation and Management: Bioengineering Strategies. Springer, Singapore. https://doi.org/10.1007/978-981-99-4221-3_8
Download citation
DOI: https://doi.org/10.1007/978-981-99-4221-3_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-4220-6
Online ISBN: 978-981-99-4221-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)