Abstract
Electrogenic bacteria (EB), also known as electrigens and exoelectrogens, can transmit electrons extracellularly beyond the cell membrane to or from electron acceptors such as electrodes, oxide minerals, and other bacteria under anaerobic or microaerobic conditions. These bacteria catalyze electrochemical oxidations or reductions at an anode or cathode, respectively, to generate an electric current in microbial fuel cells. Originally, these fuel cells were inefficient and could only be used as a battery in isolated locations. Microbial fuel cell (MFC) has been used as one of the green technology sources and one of the promising approaches for green energy production. Currently, few studies highlight the electron production ability of bacteria found in MFC and the application also is underexplored. Hence, this chapter will discuss the EB ability, characteristic, and mechanism in producing electrons. How EB can be utilized in sludge degradation and what are the secondary metabolites produced by the EB that can benefit the environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In recent decades, chemical use has increased due to increased industrial, agricultural, and human activities. In addition, human social growth and a false sense of personal safety produce a large number of chemicals in both the aquatic and terrestrial environments. For example, river water is used for agriculture, bathing, and drinking in many areas worldwide. Furthermore, sewage water is used for cultivation in many impoverished nations without being treated. As a result, untreated agricultural wastewater introduced a slew of contaminants into the environment and required urgent solutions for their removal. Chemical and physical methods for removing pollutants are extensively used and successful. However, these technologies have certain drawbacks, including cost, efficiency, and the creation of secondary pollutants. As a result, biological therapy appears to be more favourable and is regarded as a green removal method. One of the methods for wastewater treatment is using microbial fuel cells that involve the utilization of Electrogenic Bacteria (EB). These bacteria are also reported to have various hydrolytic enzymes that can work well in degrading complex carbon compounds, including sludge.
2 Mechanisms of Electrogenic Bacteria
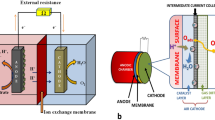
Electrogenic bacteria (EB) are a group of bacteria capable of converting chemical energy into electrical energy by carrying out the respiration process [1]. The oxidation of organic substrates would thereby generate electrons that could be received by external electron acceptors such as metal oxides, other bacteria, or even electrodes for electricity production [2, 3]. EB could be broadly classified into two categories, namely electricigens and electrotrophs [4]. Electricigens contain a subset of bacteria with the ability to completely oxidize organic compounds to carbon dioxide (CO2) and donate electrons directly to an electron acceptor whereas microorganisms that can act to reduce terminal electron acceptors are known as electrotrophs [4]. Electron transfer from the electrogenic bacteria to the electron acceptors primarily utilized two main mechanisms (Fig. 1), namely (i) Direct or non-mediated transfer as well as (ii) mediated transfer [2].
Direct electron transfer (DET) requires a direct contact between the anode surface and the microorganism’s outer membrane, whether through direct cell attachment or nanowires (pili). Pili are nanowires that connect the membrane of the microorganism to the anode surface. The benefits of pili development include the ability for many layers of biofilm microorganisms to participate in electron transfer while bulk microorganisms do not [4].
Indirect electron transfer through external or internal mediators. In this type, a redox-active material (mediator) is responsible for the electron transfer between the microorganism and the anode surface. This redox can either be exerted naturally by the microorganisms (internal) or can be added from outside (external). These mediators whether internal or external will be responsible for the electron transfer from the bulk microorganisms to the anode surface. The electron transfer in the mediated electron transfer is higher than that in the DET [4].
3 Flagella
Direct transfer of electrons would involve the establishment of physical contact between the electron acceptors and the electrogenic bacteria themselves. Micro-sized, protein-based biological extensions of electrically conductive flagella or pili known as bacterial/microbial nanowires which are found on the outer membrane of electrogenic bacteria play the vital role of transporting the electrons [2]. The nanowires are typically found in the genus of Geobacter sp. and Shewanella sp. for long-range electron transfer into the cytosol [2]. One type of nanowire known as Type IV pili are crucial in the secretion systems for effectors, microbial adherence, and locomotion in addition to making contact between the bacterial species and electron acceptors [2]. Bacteria such as Geobacter sulfurreducens were a model organism studied showing that Type IV pili were involved in the attachment of the bacteria and subsequently the translocation of the electrons during the reduction of iron (III) ions (Fe3+) [2].
4 Extracellular/Mediated Transfer
Mediated transfer, on the other hand, is a condition where the electrogenic bacteria forfeits physical contact with the electrodes and rely on endogenous redox mediators/electron shuttles instead, commonly in the forms of secondary metabolites produced by the bacteria, that shuttles the electrons produced to an external electron acceptor [2]. Electrons in mediated-transfer-utilizing bacteria are first transported to the cell surface as an aftermath of metabolic pathways. Subsequently, they are transferred into potential mediators residing in either the periplasm or outer membrane that’s capable of diffusing into the extracellular environment and finally received by external electron acceptors [2, 5]. Several compounds currently known to effectively mediate electrons would include thionine, methyl viologen, 2-hydroxy-1,4-naphtoquinone, methylene blue, humic acids, anthraquinone-2,6-disulfonic acid, and flavin [2]. Among them, flavin has been demonstrated by Marsili et al. (2008) and Velasquez-Orta et al. (2010) as a class of secondary metabolite vital in the mediation of electrons using Shewanella oneidensis as a model organism where higher concentrations and removal of flavin increased peak current by four-fold and decreased electrons transfer rate by more than 70% respectively [2].
5 Biofilm/Redox-Active Proteins
Membrane-bound redox-active proteins known as cytochromes are another component that helped in facilitating the short-range direct transfer of electrons generated by electrogenic bacteria to the electron acceptors [2]. Cytochromes, particularly cytochrome C (cyt-C) are among the key components in the adenosine triphosphate (ATP) synthesizing electron transport chain (ETC) process. Cyt-C acts as an intermediatory protein that passes down electrons in a serial redox reaction until they encounter a terminal electron acceptor. Cyt-C such as OmcS, OmcE, and OmcB found on the surface of Geobacter sulfurreducens are some common manifestations of the role of redox-active protein in transferring electrons which are crucial in the reduction of iron (III) oxides, an example of naturally occurring terminal electron acceptors [2]. Electron transfer occurs through biofilm formation by electrogenic bacteria where the electrons travel through the biofilm to the electron acceptors using the nanowires linked to the cytochromes.
6 Light-Dependent Direct Transfer
Photosynthetic bacteria, a group of solar energy harnessing bacteria through the use of chlorophyll in order to produce sugars [6], were also found to have electrogenic properties, with particular attention given to cyanobacteria. Cyanobacteria, a group of microorganisms having the oxygenic photosynthetic pathways of photosystems I and II (PSI and PSII) [7], were discovered to possess conserved light-dependent electrogenic activity where they own the innate ability to transfer electrons to their surroundings in response to illumination [8]. They are able to utilize the electrons from the photolysis of water by PSII which would be transmitted to extracellular electron acceptors via plastoquinone and cytochrome bd quinol oxidase during the occurrences of photosynthetic electron transport chain (P-ETC) [9]. Such an activity is driven by red and blue but not green light which shows consistencies in the predominant role of phycobilisomes, pigment-protein complexes comprising phycocyanins and allophycocyanins that absorb red light in cyanobacteria [8].
Photosynthesis in cyanobacteria begins with photolysis, a reaction where water is split into hydrogen ions, oxygen, and electrons, aided by the catalysis of proteins in PSII and driven by light energy [9]. Electrons acquired from the photolysis reaction are transported along the P-ETC from PSII to plastoquinone (P.Q.), then to the cytochrome b6f (cyt-b6f) complex, plastocyanin (P.C.), and eventually to PSI [9]. However, under a high light condition where bd quinol oxidases are maximally active to prevent the reduction of quinol pool, electrons at P.Q. would instead divert to bd quinol oxidase before being released into the surrounding medium [9]. The mechanism of the movement of electrons from the bd quinol oxidases to the environment remains unknown despite the conclusion of the study by Pisciotta et al. (2011) [9].
7 Bacteria Genera Involve as Electrogenic Bacteria (EB)
Electrogenic bacteria are organisms that can transfer electrons to extracellular electron acceptors and have the potential to be used in devices such as bioelectrochemical systems. In 2009, Logan BE wrote a review and list some of the well-known electrogenic bacteria (Table 1) [10]. Besides the reported bacterium, this subtopic highlighted some other bacteria genera that were also used extensively in microbial fuel cells (MFC).
7.1 Bacillus
Bacillus is a Gram-positive, sporulating, rod-shaped bacterium genus belonging to the Firmicutes phylum. It has around 266 named species. The plural Bacilli is the name of the bacterial class to which this genus belongs, and the term is also used to describe the form (rod/cylindrical) of specific bacteria. Bacillus species can either be obligatory aerobes, meaning they require oxygen to survive, or facultative anaerobes, meaning they can survive without it. If oxygen has been utilized or is present, cultured Bacillus species will produce the enzyme catalase [1]. Only one spore is produced by each bacterium, and it is resistant to heat, cold, radiation, desiccation, and disinfectants. Most bacilli are saprophytes meaning that they live on dead or decaying organic matter. Bacilli have a diverse array of physiologic properties that allow them to thrive in a wide range of environments, including desert sands, hot springs, and Arctic soils. Bacillus species can be thermophilic, psychrophilic, acidophilic, alkaliphilic, halotolerant, or halophilic, meaning they can thrive at pH values, temperatures, and salt concentrations that few other organisms can. Bacillus insolitus may thrive at temperatures as low as 0° Celsius. The Bacillus cell wall is a structure on the outside of the cell that serves as a second barrier between the bacteria and the environment while maintaining the rod shape and withstanding the pressure created by the cell’s turgor. Teichoic and teichuronic acids make up the cell wall. B. subtilis is the first bacterium to discover the involvement of an actin-like cytoskeleton in cell shape determination and peptidoglycan synthesis, as well as the complete collection of peptidoglycan-synthesizing enzymes. The cytoskeleton plays a crucial role in the formation and preservation of shape. Bacillus species like Bacillus cereus, Bacillus subtilis, and Bacillus megaterium have the ability to produce electricity in microbial fuel cell (MFC). Islam et al. prove that by using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS), the CV of B. cereus MFCs displayed a significant redox peak, indicating that B. cereus exhibits electrogenetic characteristics. Furthermore, the addition of B. cereus to activated sludge (AS) increased the MFC’s power generation (4.83 W/m3) and C.E. (22%) as compared to the comparable values for an MFC infected simply with AS (1.82 W/m3, 12%) [11].
7.2 Geobacter
Geobacter is a genus of Proteobacteria. It is rod-shaped bacteria with flagella. Geobacter is known for its remarkable electron transfer and environmental restoration abilities, which lends itself to a variety of industrial applications. The sequencing of Geobacter sulfurreducens has revealed additional information about the bacteria’s capabilities. Geobacter has been discovered to be able to transition towards metallic substances because of this sequencing. Geobacter also contains genes that allow it to operate in the presence of oxygen, according to previous studies Geobacter appears to have approximately 100 genes that code for distinct c-type cytochrome forms. Geobacter’s ability to reduce metals and generate power is due to its wide range of c-type cytochromes [2]. Geobacter was initially discovered in the Potomac River in 1987 [3]. G. sulfurreducens was later discovered in a hydrocarbon-contaminated soil sample in Oklahoma [2]. Geobacter species have also been discovered to be able to breathe through a graphite electrode. Geobacter species are anaerobic respiration bacterial species with bioremediation properties. Geobacter was discovered to be the first organism capable of oxidizing organic chemicals and metals, such as iron, radioactive metals, and petroleum compounds, into ecologically friendly carbon dioxide utilizing iron oxide or other readily accessible metals as electron acceptors.
7.3 Klebsiella
Klebsiella is a Gram-negative, oxidase-negative genus of rod-shaped bacteria with a polysaccharide-based capsule. Klebsiella species may be found all over the world. This is assumed to be owing to different sublineages generating niche adaptations and biochemical adaptations that make them more suited to a certain environment. They can be found in various environments, including water, soil, plants, insects, and people. Edwin Klebs, a German-Swiss scientist, and was given the name Klebsiella. For many years, Klebsiella bacillus was known as Friedlander bacillus named after Carl Friedlander, who characterized it. Klebsiella bacteria are found in the typical flora of humans and animals’ noses, mouths, and intestines. Klebsiella is a Gram-negative bacterium that is normally non-motile. When compared to other members of the Enterobacteriaceae family, they are shorter and thicker. The cells are rod-shaped and range in size from 0.3 to 1.5 µm width by 0.5–5.0 µm long. They come in singles, pairs, chains, and end-to-end links. Like the other members of the Enterobacteriaceae family, Klebsiella may grow on conventional lab media and has no particular growth needs. The species is aerobic but anaerobic in nature. Their optimal growing temperature is from 35° to 37° Celsius, with a pH of around 7.2 [4].
Although certain species create a visible capsule or slime layer that may be utilized for serologic identification, molecular serotyping may eventually replace this approach. On their cell surfaces, certain species of this genus often express two kinds of antigens. The first, O antigen, is a component of lipopolysaccharide (LPS), which comes in nine different types. The second is K antigen, a capsular polysaccharide with over 80 variants [5]. Both have a role in pathogenicity and serve as the foundation for serogrouping. Several vaccines have been developed based on these two key antigenic determinants [6]. Some Klebsiella species like Klebsiella pneumoniae and Klebsiella variicola are used in microbial fuel cells (MFC); however, there was not much explanation on its electrogenic mechanism can be found.
7.4 Pseudomonas
Pseudomonas is a Gram-negative Gammaproteobacteria genus that belongs to the Pseudomonadaceae family and has 191 validly recognized species [7]. Species of this genus display some characteristics: Rod-shaped, Gram-negative, flagellum one or more providing motility, aerobic, non-spore-forming, catalase-positive, and oxidase-positive. Members of the genus exhibit a wide variety of metabolic diversity, allowing them to colonize a wide range of habitats. Pseudomonas aeruginosa in its role as an opportunistic human pathogen, Pseudomonas syringae as a plant pathogen, Pseudomonas putida as a soil bacterium, and plant growth-promoting Pseudomonas fluorescens, Pseudomonas lini, Pseudomonas migulae, and P. graminis are among the best-studied species [8, 8].
The pseudomonads were discovered early in the history of microbiology due to their extensive prevalence in water and plant seeds such as dicots. Walter Migula classified Pseudomonas as a genus of Gram-negative, rod-shaped, polar-flagellated bacteria with some sporulating species in 1894 and 1900 in hazy terms [10, 11]. The latter assertion was eventually proven to be erroneous due to the reserve materials’ refractive grains. Pseudomonas pyocyanea (basonym of Pseudomonas aeruginosa) proved to be the most accurate descriptor [12].
With a few exceptions, the production of pyoverdine, a luminous yellow-green siderophore under iron-limiting circumstances, is another feature associated with Pseudomonas species. Certain Pseudomonas species, such as Pseudomonas aeruginosa and Pseudomonas fluorescens, can create other forms of siderophores, such as pyocyanin and thioquinolobactin. Pseudomonas species also typically give a positive result to the oxidase test, which indicates the absence of gas formation from glucose.
One extensively use Pseudomonas species in MFC is P.aeruginosa [1]. It is exoelectrogenic bacteria that can generate secondary metabolites like pyocyanin (PYO) and 1-hydroxy-phenazine (OHPHZ) and use them as redox-active metabolites for facilitating the extracellular electron transfer between bacterial cells and electrodes in MFCs [12, 13].
7.5 Lysinibacillus
Lysinibacillus are motile, Gram-positive bacteria with rod-shaped cells that generate ellipsoidal or spherical endospores [13]. Previously, Lysinibacillus species were categorized as Bacillus species. Two previously listed species, Bacillus sphaericus and Bacillus fusiformis, were reclassified to the new genus Lysinibacillus, together with a unique species (Lysinibacillus boronitolerans). In 2007, Ahmed, Yokota A, Yamazoe, and Fujiwara suggested Lysinibacillus as a separate genus from Bacillus based on the discovery of lysine, aspartic acid, alanine, and glutamic acid in its peptidoglycan [14]. Since then, other Bacillus species have been proposed to be reclassified as Lysinibacillus. Tests for oxidase and catalase are positive, while indole and H2S generation are negative. A4 is the peptidoglycan type found on the cell wall (LyseAsp). Iso-C 15: 0 is the most abundant fatty acid in cells. MK-7 is the most abundant menaquinone, whereas diphosphatidylglycerol, phosphatidylglycerol, and ninhydrin-positive phosphoglycolipid are the most common polar lipids. The amount of G C in the sample is 35e38 mol% [14].
Lysinibacillus is well-known for its insecticidal effect against a variety of insects, including mosquitos, which are known to transmit several human illnesses. Furthermore, some Lysinibacillus species can remove heavy metals from the environment. Lysinibacillus spp. have recently grabbed researchers’ interest as plant growth promoters and disease control agents that might be employed instead of agrochemicals.
The use of Lysinibacillus spp. in MFCs is a relatively new addition to the long list of biocatalysts that have been studied for their potential in power generation. Lysinibacillus sphaericus has been shown to generate a maximum power density of 85 mW/m2 and current density of ≈270 mA/m2 using graphite felt as an electrode. The species has also been found to be efficient in utilizing proteinaceous material which is useful to treat a specific type of wastewaters like wastewater from slaughterhouses or from meat packaging industry [14]. Lysinibacillus macroides is another Lysinibacillus spp. that has been found to be electrogenic bacteria. Uma et al. (2017) investigated the adhesion of bacteria as a biofilm on pencil graphite lead using a fluorescence microscope and a scanning electron microscope (SEM) [15].
8 Sludge as Carbon-Rich Sources for EB
Global water contamination issues have stemmed from rapid industrialization and urbanization worldwide. Sludge is produced in large quantities by traditional wastewater treatment plants. In 2005, the United States produced 7.6 million tonnes of dry sludge; by 2010, this figure is expected to rise to 8.2 million tonnes [16]. In 2005, the EU generated 10 million tonnes of dry sludge [17]; from 2007 to 2013, China significantly produced 6.25 million tonnes of dry solids (DS) sludge, with output expected to rise to 3.6 million tonnes in 2010 [18]. Malaysia is no exception to the global trend of sewage sludge volume increasing annually as a developing country. Malaysia produces roughly 3 million metric tonnes of sewage sludge each year, with that number predicted to rise to 7 million metric tonnes by 2020. (Indah Water Konsortium Sdn Bhd, 2010). Due to various pollutants such as organic contaminants, pathogens, and a portion of heavy metals [19, 20], sewage sludge is considered a potential source of secondary environmental contamination. These pollutants can enter the natural ecosystem through improper disposal methods, posing a risk to human health and the environment [21]. For example, when agricultural land is treated with sewage sludge, the concentration of organic contaminants (OCs) rises above that of reference soil, and some OCs enter inhabited plant and animal tissues [22]. On the other hand, the high levels of heavy metals in sludge ash, which is utilized as a construction material, pose a significant risk of contamination. Such sludge cannot be disposed of until it has undergone the necessary treatments. Therefore, it will benefit sludge to be converted into a simpler organic compound that does not give a thread to the environment.
8.1 Composition of Sludge
During wastewater treatment, a considerable number of contaminants are removed from the liquid phase and transported to the semisolid phase, which is known as sludge. Sludge disposal standards are governed by European legislation, namely, the Urban Wastewater Treatment Directive (91/271/EEC). However, the definition varies in every country. Table 2 shows the definitions of sewage sludge according to various legal acts [23].
As a result of expanding population, industrialization, and wastewater treatment requirements, sludge generation in wastewater treatment plants (WWTP) and landfill accumulation has increased in many countries [13]. The long-term management of this hazardous waste has become a major environmental challenge due to the potential for detrimental ecological repercussions. [14]. The end product of the sludge treatment is the dewatering process to reduce the waste volume to prepare it for disposal. Before disposal, drying the sludge with a dewatering filter press reduces its weight and volume.
The organic concentration of dewatered sewage sludge (about 84% moisture content, organic content: 33.4% protein, 6.6% lipid, and 3.3% carbohydrate on an organic basis) is high, and its volume is about one-tenth that of sewage sludge. The consistency of dewatered sewage sludge is solid. As a result, anaerobic digestion is not possible. Compared to dewatered sewage sludge, the volume of the liquidized sludge remains unchanged. The number of proteins in the liquidized sludge (27.6%) decreased through liquefaction, but the ammonium content increased. The amount of lipids in the liquidized sludge rose (13.8%). According to these findings, proteins were hydrolysed and degraded to volatile acids and ammonia via amino acids. The liquid phase (6.9% VS concentration) contains adequate anaerobic digesting substrates (15.5% lipid and 9.2% carbohydrate) [24]. These findings suggest that anaerobic liquid phase digestion could result in a higher digestion ratio and higher methane generation. The solid phase, which had a low moisture content (77.0%), may be composted and used for various purposes, including pipeline transportation and anaerobic digestion (Chapter ‘Microbial Fuel Cells (MFC) as an Alternative Energy Source: The Perceptions and Attitudes Towards Sustainable and Renewable Energy in Malaysia’, Table 3) [25].
9 Sludge Utilization by EB in MFC
Using biological decomposition of organic matter to produce energy, wastewater treatment with MFCs has been researched for the removal and recovery of contaminants such as Chemical Oxygen Demand (COD), heavy metals, and ammonia (NH3) [26]. MFCs can be used to recover high-value products such as silver (Ag) or chromium (Cr), which can improve the sustainability of the process for large-scale operations [27]. The anaerobic method is used to remediate wastewater and relies on bacteria to transport the electrons [28]. The high organic concentration shown in Table 3 of dewatered sewage sludge serve as good carbon source for the electrogenic bacteria.
9.1 Enzyme Utilization in Wastewater Treatment
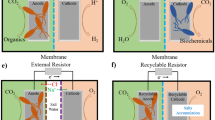
Wastewater is the source of growth of several anaerobic and facultative anaerobic bacteria, which have the ability to transfer electrons to an anode as a terminal electron acceptor and are thus classified as electrogenic bacteria [2]. At the anode of an MFC, the organic substrate is oxidized by electrochemically active microorganisms. Subsequently, the microorganisms transfer the electrons resulting from this oxidation of the anode, which then passes through an external circuit to the cathode, thus generating electricity.
Traditional activated sludge can remediate most pollutants effectively, while resistant contaminants like oil, grease, pharmaceuticals, pesticides, and plastics are difficult to be eradicated. Pharmaceuticals, insecticides, plastics, and personal care items, all of which are micropollutants, are referred to as emerging pollutants or emerging concern contaminants [29, 30]. These pollutants mainly include oil, grease, and organic micropollutants (Fig. 2). Oil and grease-containing wastewater are usually produced by dairies, oil mills, slaughterhouses, and food waste [20, 21]. The transfer rate of substrates, products, and oxygen will be harmed if oil and grease float on water surfaces [31]. The floating oil and grease may induce a filamentous microbe bloom, resulting in floating sludge, poor sedimentation, and sludge biomass loss, as well as poor performance of activated sludge [31].
Enzyme is a powerful biocatalyst that can destroy compounds in a controlled environment [32, 33]. Enzymes contain active sites that bind to specific substrates, lowering the activation energy during enzymatic operations. As a result, the reaction kinetics and specificity of these processes are quite high. There are six enzymes, and the most used ones in wastewater treatment are hydrolases and oxidoreductases [34, 35]. These two enzymes can biocatalyst most pollutants in wastewater due to their wide range of substances. Currently, commercially available enzymes include lipase, laccase, and peroxidase. Laccase and peroxidase are commonly used to remove organic micropollutants [36, 37].
Typical resistant pollutants in wastewater and their classification. Reprinted with permission from [38] (CC-BY)
9.2 Enzyme Production by Bacteria
Extracellular hydrolytic enzymes such as amylases, proteases, lipases, DNases, pullulanases, and xylanases have a wide range of potential applications in the food, feed, biomedical, and chemical industries [39, 40]. Concerns over the accumulation of micropollutants in the aquatic environment inspired a flurry of studies into biological micropollutant degradation in wastewater treatment systems. [41]. Some organic micropollutants may be hazardous and bioaccumulative, posing serious risks to human health and the environment. This bioaccumulation is usually linked to a compound with high lipid solubility and the capacity to accumulate in living creatures’ fatty tissues for an extended time. These persistent compounds migrate up the food chain, and their concentration rises as they’re absorbed and degraded in certain organs, increasing their environmental toxicity [42].
Physiochemical and biological approaches are the most common wastewater treatment technologies nowadays. Physiochemical procedures such as chemical oxidation, distillation, membrane-based separation techniques, and adsorption have been used for wastewater treatment [34]. These are treatment-oriented options, but they are exceedingly expensive and may cause more pollution and harm [35]. The three common microbial enzymes use in wastewater treatment are oxygenases, Laccases, and Cellulases. Microbial oxygenases belong to the oxidoreductase family of enzymes and are involved in the oxidation of reduced substrates by transferring oxygen from atomic oxygen (O2) to a cosubstrate such as FAD/NADH/NADPH. These enzymes play an important role in the metabolism of organic molecules by increasing their reactivity or dissolvability in water or causing the aromatic rings to be cleaved. In conjunction with multifunctional enzymes, oxygenases also intervene in the dehalogenation reactions of halogenated methanes, ethanes, and ethylenes [43].
Microbial Laccases (p-diphenol: dioxygen oxidoreductase) are multicopper oxidases produced by plants, fungi, insects, and microorganisms that catalyze the oxidation of a wide range of reduced phenolic and aromatic substrates while also reducing sub-atomic oxygen to water. Aromatic compounds, including phenols and aromatic amines, are among the most common types of poisons and are heavily regulated in many countries. Coal conversion, oil refining, resins and plastics, wood safeguarding, metal coating, colours and other synthetic chemicals, textiles, mining and dressing, and pulp and paper are just a few of the industries where they can be found in wastewater. During enzymatic hydrolysis, cellulose is debased by the cellulases to reducing sugars that yeasts or microorganisms can ferment to ethanol. In addition, Cellulases cause the removal of cellulose microfibrils, which are produced during washing and the utilization of cotton-based materials. In the paper and pulp industry, cellulase is utilized to remove ink during the recycling of paper. For as far back as a decade, there has been an interest in the enzymatic hydrolysis of cellulose. This intrigue comes from the benefits that such a procedure would offer, in particular, the conversion of lignocellulosic and cellulosic waste to a helpful energy source through the creation of sugars, ethanol, biogas, or other vigorous end products [43]. Beside the three common enzymes used in wastewater treatment, selection of electrogenic bacteria can be based on substrate specificity. For example, Nandy et al. (2013) used Lysinibacillus sphaericus in its MFCs using high protein waste due to its enzymes ability to utilize substrates mainly rich in protein components like beef extract and produce good electricity [14].
Incorporation of EB that produce hydrolytic microbial enzymes will help to treat wastewater. Industrial chemicals, pesticides, and petroleum hydrocarbons pollute water and are discovered as environmental pollutants in various aquatic and terrestrial environments due to their widespread use. The main process in the hydrolysis of organic contaminants is bacterial activity (Table 4). Only substances with a molecular mass of less than 600 Daltons can pass through cell pores. Therefore, extracellular enzyme activity is an important step in the breakdown and use of the organic compound [46].
In sludge degradation, bacteria tend to aggregate and create sludge flocs in the activated sludge process, which are made up of microbial, prokaryotic (bacteria, archaea), and eukaryotic (algae, fungal) microorganisms held together by extracellular polymeric molecules (EPS). Various studies reported that sludge flocs constitute 60%–70% of the organic fraction [45] and are further utilized by the bacteria.
Cell fractionation begins with killing microbial cells, which is done by a hydrolytic enzyme (mainly protease). An improvement in lysis efficiency can thus contribute to a reduction in overall sludge output and play a key role in lowering investment and operational costs and optimizing the current sewage treatment system. Sludge hydrolysis can be aided by hydrolase. Previous research has revealed that several microbial strains with extracellular hydrolytic enzyme secretions and other commercial hydrolytic enzymes are frequently utilized directly in reactors to accelerate sludge lysis [31]. Hydrolysis enzymes, including protease, amylase, and lipase enzymes, are the primary agents of deflocculation, hydrolysis, and oxidation of sludge-activated sludge flocs to be broken down into simpler carbohydrate molecules. The first step in protein degradation involves breaking the protein down into peptides, or into two peptides and amino acids. Next, amino acids can be transformed into organic acids with low molecular weight, ammonia, and carbon dioxide [31].
10 EB Consortium Potential for Sludge Degradation
Currently, although the key principles of MFC design and operation are well understood, the microbiological aspects remain unclear. In an MFC system, the electron transfer from the bacteria to the anode can proceed directly from their cell membrane to the anode or indirectly by means of a mediator [4]. The direct transfer method or MFC inoculated with the mixed culture exhibited significant potential for harvesting energy from organic matter and degrading organic matter [1]. Thus, the selection of a highly performing microbial consortium (either pure or mixed culture) is crucial.
Consortium or co-culture electrogenic bacteria have been shown to improve the performance in MFCs. The ecological networks between the microorganisms in a specific co-culture system containing P. aeruginosa and Klebsiella variicola were studied by Islam et al. (2018) [46]. Compared to these two bacteria alone, the co-culture showed a 3 times higher current density in MFCs. Metabolite study revealed that the fermentative metabolite (1,3-propanediol) produced by K. variicola stimulated P. aeruginosa to create more pyocyanin, resulting in improved performance of co-culture MFCs fed with palm oil mill effluent through synergistic interactions (POME). This research shows that “interspecies ecological communication” based on metabolites can improve MFC electrochemical activity [46].
Other studies also reported some outstanding performance of co-culture bacteria in MFC. For instance, Venkataraman et al. (2011) [47] reported that the fermentation product (2,3-butanediol) produced by Enterobacter aerogens stimulated the production of mediator by P. aeruginosa that boosted up the current density by 14-fold in co-culture MFC compared to their monocultures. In another study, Wang et al. (2015) [48] reported that the metabolite-enabled mutualistic interaction between Shawanella oneidensis and E. coli helped to achieve higher power generation in co-culture MFC compared to the monocultures in MFC. Thus, there is huge potential that the utilization of a perfect EB consortium could help the MFC performance in degrading the sludge and at the same time produce good current generation.
11 Conclusion
The chapter represents how electrogenic bacteria (EB) play essential parts in MFC, the potential of this microbe in converting the organic sources of any pollutants could benefit the world in handling the continuous waste production. The optimization of EB as a single or consortium bacteria should be explored especially in managing sludge problems. This is due to the versatility of the MFCs in converting any organic source to the current generation which is very suitable for sludge valorization. More detailed analyses should also be done in exploring the potential of the EBs as well, there were still undiscovered mechanisms that need to be understood despite the significant advancements. Up until now, there are still many EB that has not yet been identified and characterized. More discoveries are likely to be made due to an accurate analysis of the microbial diversity within EB that emerged in various habitats and advancements in culture conditions and analytical equipment. Additionally, employing inocula from harsh settings will help scientists find new electrogenic bacteria and possibly new ways to transmit electrons to electrodes. Future tools such as genomic and transcriptomic analyses could help to understand the amount and type of genetic changes that accumulate in evolving populations on electrodes over several generations. Over evolution, mutation rates can fluctuate, favourably accumulating genetic variations that are better suited to the MFC environment. Genetic differences between derived and ancestor organisms can be detected on a whole-genome level. This will absolutely help the researcher to set up the MFC more efficiently.
References
Agrawal K et al (2019) Microbial fuel cell: a boon in bioremediation of wastes. Microbial wastewater treatment. Elsevier, pp 175–194
Slate AJ et al (2019) Microbial fuel cells: an overview of current technology. Renew Sustain Energy Rev 101:60–81
Tahernia M et al (2020) Characterization of electrogenic gut bacteria. ACS Omega 5(45):29439–29446
Drendel G et al (2018) Microbial fuel cells, related technologies, and their applications. Appl Sci 8(12)
Uria N, Ferrera I, Mas J (2017) Electrochemical performance and microbial community profiles in microbial fuel cells in relation to electron transfer mechanisms. BMC Microbiol 17(1):1–12
Pollard TD et al (ed) (2017) Chapter 19—Mitochondria, chloroplasts, peroxisomes, in cell biology, 3rd edn. Elsevier, pp 317–329
Sánchez-Baracaldo P, Cardona T (2020) On the origin of oxygenic photosynthesis and Cyanobacteria. New Phytol 225(4):1440–1446
Pisciotta JM, Zou Y, Baskakov IV (2010) Light-dependent electrogenic activity of Cyanobacteria. PLoS One 5(5):e10821
Pisciotta JM, Zou Y, Baskakov IV (2011) Role of the photosynthetic electron transfer chain in electrogenic activity of cyanobacteria. Appl Microbiol Biotechnol 91(2):377–385
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7(5):375–381
Islam MA et al (2017) Electrogenic and antimethanogenic properties of Bacillus cereus for enhanced power generation in anaerobic sludge-driven microbial fuel cells. Energy Fuels 31(6):6132–6139
Bellin DL et al (2014) Integrated circuit-based electrochemical sensor for spatially resolved detection of redox-active metabolites in biofilms. Nat Commun 5(1):1–10
Chen W et al (2015) An U.V.—vis spectroelectrochemical approach for rapid detection of phenazines and exploration of their redox characteristics. Biosens Bioelectron 64:25–29
Nandy A, Kumar V, Kundu PP (2013) Utilization of proteinaceous materials for power generation in a mediatorless microbial fuel cell by a new electrogenic bacteria Lysinibacillus sphaericus VA5. Enzyme Microb Technol 53(5):339–344
Uma Vanitha M et al (2017) Microbial fuel cell characterization and evaluation of Lysinibacillus macroides MFC02 electrigenic capability. World J Microbiol Biotechnol 33(5):91
Rorat A et al (2019) Sanitary and environmental aspects of sewage sludge management. Ind Munic Sludge pp 155–180
Kelessidis A, Stasinakis AS (2012) Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manage 32(6):1186–1195
Yang G, Zhang G, Wang H (2015) Current state of sludge production, management, treatment and disposal in China. Water Res 78:60–73
Raheem A et al (2018) Opportunities and challenges in sustainable treatment and resource reuse of sewage sludge: a review. Chem Eng J 337:616–641
Feng L, Luo J, Chen Y (2015) Dilemma of sewage sludge treatment and disposal in China. Environ Sci Technol 49(8):4781–4782
Ruiz-Gómez N et al (2017) Co-pyrolysis of sewage sludge and manure. Waste Manage 59:211–221
Meng X-Z et al (2016) Organic contaminants in Chinese sewage sludge: a meta-analysis of the literature of the past 30 years. Environ Sci Technol 50(11):5454–5466
Wiśniowska E et al (2019) 10—Sludge legislation-comparison between different countries. In: Prasad MNV et al (eds) Industrial and municipal sludge. Butterworth-Heinemann, pp 201–224
Inoue S et al (1996) Organic composition of liquidized sewage sludge. Biomass Bioenerg 10(1):37–40
Xu Y, Gong H, Dai X (2020) High-solid anaerobic digestion of sewage sludge: achievements and perspectives. Front Environ Sci Eng 15(4):71
Jadhav DA, Ghosh Ray S, Ghangrekar MM (2017) Third generation in bio-electrochemical system research—a systematic review on mechanisms for recovery of valuable by-products from wastewater. Renew Sust Energy Rev 76:1022–1031
Munoz-Cupa C et al (2021) An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci Total Environ 754:142429
Logan BE et al (2019) Electroactive microorganisms in bioelectrochemical systems. Nat Rev Microbiol 17(5):307–319
Sauvé S, Desrosiers M (2014) A review of what is an emerging contaminant. Chem Cent J 8(1):1–7
Teodosiu C et al (2018) Emerging pollutants removal through advanced drinking water treatment: a review on processes and environmental performances assessment. J Clean Prod 197:1210–1221
Cammarota M, Freire D (2006) A review on hydrolytic enzymes in the treatment of wastewater with high oil and grease content. Biores Technol 97(17):2195–2210
Brandelli A, Sala L, Kalil SJ (2015) Microbial enzymes for bioconversion of poultry waste into added-value products. Food Res Int 73:3–12
Yao Y et al (2020) Insights into the improvement of the enzymatic hydrolysis of bovine bone protein using lipase pretreatment. Food Chem 302:125199
Mishra B et al (2020) Engineering biocatalytic material for the remediation of pollutants: a comprehensive review. Environ Technol Innov, pp 101063
Varga B et al (2019) Enzymatic treatment and subsequent toxicity of organic micropollutants using oxidoreductases—A review. J Clean Prod 221:306–322
Ji C et al (2016) Biocatalytic degradation of carbamazepine with immobilized laccase-mediator membrane hybrid reactor. J Membr Sci 502:11–20
Stadlmair LF et al (2017) Mass spectrometry based in vitro assay investigations on the transformation of pharmaceutical compounds by oxidative enzymes. Chemosphere 174:466–477
Feng S et al (2021) Roles and applications of enzymes for resistant pollutants removal in wastewater treatment. Biores Technol 335:125278
Sánchez-Porro C et al (2003) Diversity of moderately halophilic bacteria producing extracellular hydrolytic enzymes. J Appl Microbiol 94(2):295–300
Sonune N, Garode A (2018) Isolation, characterization and identification of extracellular enzyme producer Bacillus licheniformis from municipal wastewater and evaluation of their biodegradability. Biotechnol Res Innov 2(1):37–44
Grandclément C et al (2017) From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: a review. Water Res 111:297–317
Burkhardt-Holm P (2011) Linking water quality to human health and environment: the fate of micropollutants. Institute of Water Policy Working Paper, vol 3, p 2011
Pandey K et al (2017) Application of microbial enzymes in industrial waste water treatment. Int. J. Curr. Microbiol. Appl. Sci 6(8):1243–1254
Karigar CS, Rao SS (2011) Role of microbial enzymes in the bioremediation of pollutants: a review. Enzyme Res 2011:805187
Xu Q (2003) New equipment, techniques and technology of sludge treatment
Islam MA et al (2018) An insight of synergy between Pseudomonas aeruginosa and Klebsiella variicola in a microbial fuel cell. ACS Sust Chem Eng 6(3):4130–4137
Venkataraman A et al (2011) Metabolite-based mutualism between Pseudomonas aeruginosa PA14 and Enterobacter aerogenes enhances current generation in bioelectrochemical systems. Energy Environ Sci 4(11):4550–4559
Wang VB et al (2015) Metabolite-enabled mutualistic interaction between Shewanella oneidensis and Escherichia coli in a co-culture using an electrode as electron acceptor. Sci Rep 5(1):1–11
Acknowledgements
Universiti Sains Malaysia (Malaysia) financially supported this research article under the APEX ERA Grant: 1001/PBIOLOGI/881005. The author (Amira Suriaty Yaakop) would like to thank Universiti Sains Malaysia for funding this project.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yaakop, A.S., Hong, O.K., Salman, S.M. (2023). Utilization of Electrogenic Bacteria Consortium for Sewage Sludge Treatment via Organic Compound Degradation. In: Mohd Zaini Makhtar, M., Shukor, H., Yaser, A.Z. (eds) Microbial Fuel Cell (MFC) Applications for Sludge Valorization. Green Energy and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-99-1083-0_7
Download citation
DOI: https://doi.org/10.1007/978-981-99-1083-0_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-1082-3
Online ISBN: 978-981-99-1083-0
eBook Packages: EnergyEnergy (R0)