Abstract
Certain anaerobic bacteria, termed electrogens, produce an electric current when electrons from oxidized organic molecules are deposited to extracellular metal oxide acceptors. In these heterotrophic “metal breathers”, the respiratory electron transport chain (R-ETC) works in concert with membrane-bound cytochrome oxidases to transfer electrons to the extracellular acceptors. The diversity of bacteria able to generate an electric current appears more widespread than previously thought, and aerobic phototrophs, including cyanobacteria, possess electrogenic activity. However, unlike heterotrophs, cyanobacteria electrogenic activity is light dependent, which suggests that a novel pathway could exist. To elucidate the electrogenic mechanism of cyanobacteria, the current studies used site-specific inhibitors to target components of the photosynthetic electron transport chain (P-ETC) and cytochrome oxidases. Here, we show that (1) P-ETC and, particularly, water photolysed by photosystem II (PSII) is the source of electrons discharged to the environment by illuminated cyanobacteria, and (2) water-derived electrons are transmitted from PSII to extracellular electron acceptors via plastoquinone and cytochrome bd quinol oxidase. Two cyanobacterial genera (Lyngbya and Nostoc) displayed very similar electrogenic responses when treated with P-ETC site-specific inhibitors, suggesting a conserved electrogenic pathway. We propose that in cyanobacteria, electrogenic activity may represent a form of overflow metabolism to protect cells under high-intensity light. This study offers insight into electron transfer between phototrophic microorganisms and the environment and expands our knowledge into biologically based mechanisms for harnessing solar energy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Electrogenic bacteria can produce an electric current by donating electrons to acceptors in the environment. In previously studied heterotrophic bacteria, electrons donated to the environment were found to be derived from oxidation of organic molecules through respiratory metabolism (Chaudhuri and Lovley 2003; Lovley 2008). In Geobacter sulfurreducens, the respiratory electron transport chain (R-ETC) transfers electrons to heme-containing c-type cytochromes on the cell surface that then catalyze dissimilatory reduction of metals in the environment (Reguera et al. 2005; Esteve-Nunez et al. 2008). In another Gram-negative electrogen, Shewanella oneidensis, electrons are transferred from an inner membrane quinone/quinol pool to the outer membrane by c-type cytochromes (Shi et al. 2007). These prior studies highlight the diverse role cytochromes play in transferring electrons from intracellular membranes to the environment in electrogenic bacteria.
Our recent studies found that diverse genera of cyanobacteria also possess electrogenic activity (Zou et al. 2009; Pisciotta et al. 2010). In contrast to heterotrophs, however, the electrogenic activity of cyanobacteria was light dependent, i.e., they donated electrons to extracellular electron acceptors under illumination (Zou et al. 2009). This raises the intriguing possibility that electrons discharged to the environment by illuminated cyanobacteria might not be derived from the biochemical oxidation of organic compounds but rather from light-driven biophotolysis of water.
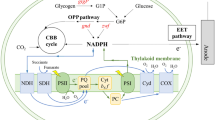
Plastoquinone (PQ) is an important photosynthetic electron transfer chain (P-ETC) energy shuttle that links photosystem II (PSII) electron flow with cytochrome b6f (Berry et al. 2002). PQ is present in substantial amounts in both the cellular (outer) membrane as well as the thylakoid membranes and is a shared component between the respiratory electron transport chain (R-ETC) and P-ETC (Paumann et al. 2005). Oxidized PQ can accept electrons from PSII, the respiratory dehydrogenases, and from photosystem I (PSI) under conditions in which the Q cycle is active. Reduced PQ normally donates electrons to cytochrome b6f, and thereby to PSI by a pathway, which is a part of the linear Z-scheme electron transfer. Importantly, the rates of electron transfer via R-ETC are very low, between 1% and 10% of maximum transfer rate via P-ETC and mostly occurs in the dark (Paumann et al. 2005; Meyer 1986). Under conditions leading to excessive reduction of the PQ pool, such as exposure to intense light, PQ can also donate electrons directly to cytochrome bd quinol oxidase (Pils and Schmetterer 2001). This provides an alternative route for electron flow, possibly involved in the electrogenic activity of cyanobacteria.
Phototrophs possess both short-term and long-term adaptation strategies to deal with the damaging effects of strong sunlight. Fluorescent dissipation of light energy as infrared radiation or heat is one short-term mechanism called non-photochemical quenching (Karapetyan 2007). Downregulation of chlorophyll expression is a longer-term adaptation strategy to high-intensity sunlight. Carotenoids are antioxidant photopigments that protect against photooxidation when upregulated. Carotenoid expression levels have been used as markers of light stress because on the order of hours to days, overall carotenoid expression is upregulated under high light stress conditions (Kilian et al. 2007).
To test the role of the P-ETC in cyanobacterial electrogenic activity, the present study used site-specific inhibitors that target components of P-ETC (Fig. 1). Our studies revealed that P-ETC and, particularly, PSII provides the electrons that are discharged to the extracellular environment, and that PQ and cytochrome bd quinol oxidase play a key role in transferring electrons. Based on these results, we propose that the electrogenic activity may be a form of overflow metabolism that prevents cell damage under high light intensity. The discovery of light-driven electrogenic activity provides a rational foundation for developing new green technologies for converting solar energy directly into electricity using cyanobacteria.
Materials and methods
Cell cultures
Lyngbya sp. (CCMP2520) and Nostoc sp. (CCMP2511) were purchased from the Center for Culture of Marine Phytoplankton (West Boothbay Harbor, ME). Lyngbya or Nostoc were seeded into individual 150-mL transparent anodic chambers of photosynthetic microbial fuel cells (PMFCs) (Zou et al. 2009) and cultured at 24.0°C under photoautotrophic conditions using F2 media (prepared from CCMP culture media kits) under 24-h illumination cycles (12 h of light /12 h of dark) using fluorescent light source (light intensity ∼100 lx, color temperature 6,500K).
Electrogenic activity
PMFCs were constructed as previously described (Zou et al. 2009) and equipped with anodes made of carbon fibers and electrically conductive polypyrrole (Zou et al. 2010). For anode coating, 10 mg polypyrrol (20 wt.% on carbon black, Cat. N 530565 Sigma-Aldrich, St. Louis, MO) was painted over carbon fiber using a brush. PMFC's anodes served as extracellular electron acceptors. Cathodes with an effective surface area of 9.6 cm2 were constructed from wet-proofed carbon cloth (B1B, E Tek, Somerset, NJ), a carbon base layer coated with nafion-bonded platinum catalyst on the media-exposed side and four coats of PTFE gas diffusion membrane on the air side (Zou et al. 2010). In a PMFC, electrons are derived from water: 2H2O → O2 + 4H+ + 4e. Electrons arriving at the cathode via the PMFC's electrical circuit reduce oxygen in the presence of protons to reform water: O2 + 4H+ + 4e → 2 H2O.
Lyngbya or Nostoc were cultured for 2 weeks in anodic chambers of PMFCs under open circuit to allow acclimation and growth. Following acclimation/growth period, the electrogenic activity was monitored as the potential difference between the anode and the cathode of PMFCs (i.e., PMFC voltage) at 1 KΩ fixed external resistance using a digital data acquisition system (PCI-6280, National Instruments, Austin, TX) equipped with LabVIEW software (National Instruments).
Inhibitor studies
Prior to treatment with site-specific inhibitors, the electrogenic activity of each culture was monitored for at least two consecutive 24-h illumination cycles. Then, the light was kept on until the electrogenic response reached a relatively steady plateau. During the plateau, cyanobacteria were dosed with 25 μM of the inhibitors, as indicated. Following the first treatment, the light source remained on for 24 h, after which 12 h/12 h light cycling was resumed to assess any long-term effect on the positive light response. After 3 days of normal 12 h/12 h cycling, the light was kept on again to reestablish a steady plateau, during which a higher 75 μM dose was administrated. This experimental format allowed both short- and long-term inhibitor effects to be studied.
Effect of light intensity on electrogenic activity
Cultures were placed in a light box equipped with an RGB LED light source (Photon System Instruments SL-3500, Brno, Czech Republic), adapted to darkness for several hours, and then electrogenic activity of cultures was monitored by recording voltage at 1 KΩ fixed external resistance under constant illumination using blue (max = 463 nm, half-bandwidth = 22 nm) or red (lmax = 642 nm, half-bandwidth = 19 nm) light with stepwise 1,000 lx increases in light intensity, as indicated. In parallel, dissolved oxygen (DO) was measured in anode chamber in real time using Orion 4 star pH/DO meter (Thermo Orion, Beverly, MA) and DO probe (model 081010MD, Thermo Orion) using Star Navigator Plus software (Thermo Orion).
Absorbance spectroscopy
Nostoc biofilms were established as described above, and anodes of three PMFCs were electrically connected to cathodes through 1 KΩ fixed external resistances, while anodes in the other three PMFC remained electrically disconcerted. PMFCs with connected and disconnected anodes were placed into the light LED box and exposed to 4,000 lx flashing (1 min on/1 min off) red light for 72 h after which biofilms were removed and pelleted by centrifugation. Pellets were resuspended in methanol conating 50 mM ammonium acetate and sonicated 15 min in the dark prior to cell lysis using 0.1 mm glass beads shaken in a mini-bead beater (Bio-spec Products, Bartlesville, OK). Pigment extracts were centrifuged at 14K for 10 min, and the pigment containing supernatant examined using a Cary 300 Bio UV–Vis spectrophotometer (Varian Inc. Palo Alto, CA). After subtracting the background spectra of solvent, the spectra of pigments were normalized to the 665-nm chlorophyll A peak.
Results
To test the effect of site-specific inhibitors on electrogenic activity, experiments were performed using the following format. Nostoc or Lyngbya were seeded into anode chamber of PMFCs and grown under 12 h/12 h light/dark cycles for 2 weeks to establish biofilms on anode surfaces. After 2 weeks, both Nostoc and Lyngbya showed light-dependent electrogenic activity, as monitored by recording of PMFC voltage across a 1 kΩ external resistance (see “Materials and methods”). During each 12 h/12 h light/dark cycle, both cultures showed an increase in electrogenic activity upon illumination and a drop in electrogenic activity at the beginning of the 12-h dark phases (Fig. 2). The variations in the amplitude of the electrogenic activity observed within individual genera were likely due to differences in density of the biofilms formed on the anode surface. Nevertheless, all cultures showed light-dependent 24 h oscillations in electrogenic activity. Before administering inhibitors, the cyanobacterial cultures were kept under constant illumination for 30 h to establish a semi-stable plateau. Each compound was added at a low dose (25 μM) first that was followed by a higher dose of 148 h later.
Effect of the P-ETC inhibitors DCMU (a, b), CCCP (c, d), or DBMIB (e, f) on electrogenic activity of Lyngbya (left panels) or Nostoc (right panels). Both cultures were dosed first with 25 μM drug (left arrows) followed by an additional 75 μM drug (right arrows). Periods of illumination or darkness are indicated by white or black bars along x-axis, respectively
The first step of photosynthesis in cyanobacteria, the water splitting reaction, is catalyzed by PSII and powered by the energy of light. Electrons derived from water are transported along the P-ETC from PSII to PQ, then to the cyt-b6f complex, plastocyanin (PC), and eventually to PSI (Fig. 1). To assess the role of PSII in electrogenic activity, we employed two inhibitors of PSII electron transport, 3-(3,4-dichloro-phenyl)-1,1-dimethylurea (DCMU) and carbonyl cyanide m-chlorophenylhydrazone (CCCP). CCCP is thought to interfere with electron flow in PSII, whereas DCMU blocks binding of PQ to PSII preventing reduction of PQ. Addition of 25 μM DCMU was found to completely block electrogenic activity within minutes of treatment in both cultures (Fig. 2a, b). DCMU's effect on electrogenic activity was irreversible, as judged by a lack of positive light response after resumption of 12 h/12 h light/dark cycles. Treatment with 25 μM CCCP resulted in a reversible inhibition of electrogenic activity in both cultures (Fig. 2c, d). Upon resuming the light/dark cycles, the light response gradually recovered. Subsequent treatment with a higher dose of CCCP, however, fully blocked electrogenic activity (Fig. 2b). To confirm that the negative effects of DCMU or CCCP on electrogenic activity were due to site-specific inhibition of PSII and not due to side effects, we used atrazine, a compound that is structurally different from DCMU or CCCP. Like DCMU atrazine blocks the PQ-binding site on PSII and prevents electron transport from PSII to PQ (Fig. 1). Consistent with the effect of DCMU, atrazine completely blocked electrogenic activity in both cyanobacterial genera (Fig. S1a, b). While DCMU and atrazine are structurally different, both compounds have high degree of specificity against PET-C components (Gonen-Zurgil et al. 1996) and both block electron transport by competitively disrupting plastoquinone binding to the Dl protein of PSII (Matoo et al. 1981). While CCCP is a relatively non-specific PSII inhibitor; nevertheless results with this drug support the data observed with atrazine or DCMU. Together, these experiments provide evidence that electrons deposited to extracellular electron acceptors in response to illumination originate from splitting of water molecules through PSII-catalyzed biophotolysis.
To obtain further insight into the mechanism responsible for electron transport to the extracellular environment, we employed site-specific inhibitors that target electron transfer components located downstream of PSII. 2,5-dibromo-3-methyl-6-isopropyl benzoquinone (DBMIB) is known to bind specifically to cyt b6f and prevent interaction between PQ and cyt b6f, thereby, increasing the pool of reduced PQ. Crystallographic studies revealed a high-affinity DBMIB binding site on the p-side interfacial niche bounded by cytochrome f, subunit IV, and cytochrome b6 (Yan et al. 2006). Surprisingly, administration of DBMIB at a low dose was found to boost electrogenic activity in both cultures (Fig. 2e, f). Subsequent treatment with a higher dose of DBMIB had an even stronger stimulatory effect on electrogenic activity. Nostoc was more sensitive to DBMIB than Lyngbya and showed approximately threefold increase in electrogenic activity following the exposure to a high dose of DBMIB (Fig. 2f). This experiment revealed that PQ plays a key role in discharge of electrons to extracellular environment.
Cytochrome oxidases mediate transfer of electrons to extracellular electron acceptors in heterotrophic bacteria (Reguera et al. 2005; Esteve-Nunez et al. 2008). To determine if cytochrome oxidases play a role in the electrogenic activity of cyanobacteria, we administered the cytochrome oxidases inhibitors KCN, azide, or pentachlorophenol (PCP) during plateau phase. Prior studies showed that KCN blocks all three cyanobacterial cytochrome oxidases (ARTO, COX, and the bd quinol oxidase) in a dose-dependent fashion, whereas azide inhibits only COX and ARTO, and PCP targets only the bd quinol oxidase (Fig. 1) (Pils et al. 1997; Pils and Schmetterer 2001; Mogi and Miyoshi 2009). KCN was found to inhibit electrogenic activity. This effect was most apparent as a steep drop in electrogenic activity in Lyngbya immediately after dosing with 75 μM KCN (Fig. 3c). Lower-dose 25-μM KCN had a little effect on electrogenic activity in Lyngbya and induced a gradual reduction in electrogenic activity in Nostoc (Fig. 3d). Azide had the least effect on electrogenic activity. Azide (25 μM) had no effect on Lyngbya and mild decrease in electrogenic activity of Nostoc (Fig. 3e, f). A mild, gradual drop was recorded in Lyngbya dosed with 75 μM azide (Fig. 3e). It is likely that dosing with KCN or azide had gradually developing toxic effects on Nostoc, as judged by the very slow and gradual drop in electrogenic activity over the course of the experiment. Accordingly, in a separate trial designed to ensure maximum, immediate inhibition of ARTO and COX, very high-dose 5 mM azide was added during plateau phase. In both genera, 5 mM azide had a negligible effect on electrogenic activity (Fig. 4).
Effect of the cytochrome oxidase inhibitors PCP (a, b), KCN (c, d), or azide (e, f) on electrogenic activity of Lyngbya (left panels) or Nostoc (right panels). Both cultures were dosed first with 25 μM drug (left arrows) followed by an additional 75 μM drug (right arrows). Periods of illumination or darkness are indicated by white or black bars along x-axis, respectively
In contrast to the mild effects of azide or KCN, dosing with 25 μM PCP resulted in a rapid and steep drop in electrogenic activity in both Nostoc and Lyngbya (Fig. 3a, b). PCP is known to be a specific inhibitor of cytochrome bd quinol oxidase (Pils et al. 1997; Schneider et al. 2001). PCP (25 μM) reduced electrogenic activity by approximately 50% in Nostoc and 25% in Lyngbya's within 1–3 h. By comparison, the same dose of KCN required over 24 h to cause a somewhat comparable 33% drop in electrogenic activity in Nostoc (Fig. 3c, d). KCN (25 μM) had virtually no effect in Lyngbya. When normal 12 h/12 h light cycling was resumed, the positive light response was observed in the PCP-treated cultures. However, following the PCP treatment, the peak amplitude of the positive light response was smaller than before treatment, and the dark phase baseline also appeared to have been reset to a lower level (Fig. 3a, b). When subsequently dosed with 75 μM PCP, electrogenic activity were reduced to baseline in both Nostoc and Lyngbya, indicating near complete inhibition by 75 μM PCP (Fig. 3a, b). The positive light response was completely abolished by 75 μM PCP (Fig. 3a, b). These experiments indicate that among three types of cytochrome oxidases present in cyanobacteria, the cytochrome bd quinol oxidase is primarily responsible for electron transfer to the environment.
While electrogenic activity was previously shown to be conserved among diverse genera of cyanobacteria (Pisciotta et al. 2010), it remains uncertain whether it has any physiological function. Here, we propose that in cyanobacteria, electrogenic activity represents one of the adaptation strategies to high light intensity. To test this hypothesis, simultaneous measurements of dissolved oxygen (DO), to monitor photosynthetic activity, and electrogenic activity were performed during stepwise increases in light intensity. Due to technical limitations, these experiments were conducted under red or blue light (the photosynthetic reactions in cyanobacteria are known to be driven by red and blue but not green light). While DO concentration was found to increase gradually at 1,000 and 2,000 lx, it reached a non-linear plateau upon continuous illumination at 3,000 lx, a sign of a decrease in photosynthesis as a result of photoinhibition (Fig. 5a, b). The electrogenic activity, however, increased at 1,000, 2,000, and 3,000 lx showing the largest increment at 3,000 lx, when the photosystem was operating under photoinhibitory light conditions. Subsequent drop in electrogenic activity and DO with continuous blue light illumination at 3,000 lx and even more at 4,000 lx could be attributed to highly damaging effects of strong blue light (Fig. 5b), as it is of much higher energy than the red light. Nevertheless, the maximum increase in electrogenic activity was observed under strong light conditions, at which cyanobacterial photosynthetic apparatus switched to photoprotection mode. This result supports the hypothesis that electrogenic activity plays a role in photoprotection.
Dynamics of dissolved oxygen and electrogenic activity monitored from Nostoc biofilms under increasing intensity of red (a) or blue (b) light. DO (thin lines) and electrogenic activity (bold lines) were monitored from Nostoc biofilms exposed to stepwise increments in light intensity increasing by 1,000 lx (as indicated) starting from dark-adapted cultures. Dotted lines represent control experiments where electrogenic activity was monitored from anodes that lacked biofilm. The experiments were repeated three times and showed consistent results. Vertical dash lines indicate the time points of stepwise increase in light intensity
To further test the photoprotection hypothesis, we investigated whether the option of discharging electrons to anode, if provided, could compensate for other adaptation mechanisms such as adjusting the photosynthetic pigment ratio. In response to high light intensity, cyanobacteria are known to upregulate the expression of carotenoids (Kilian et al. 2007). Nostoc biofilms were grown on identical anode surfaces and then exposed to high-intensity red light for 72 h, during which anodes were either connected to cathodes to provide electron flow from cyanobacteria or disconnected from cathodes. Spectroscopic analysis of extracted photopigments from connected versus disconnected Nostoc cultures revealed spectra consistent with higher light stress in the disconnected PMFCs (Fig. 6). This is seen by the shoulder around 505 nm and increased absorbance in the 400–500-nm range. The difference spectrum had a shape typical for carotenoids (Zang et al. 1997), indicating a much more substantial accumulation of carotenoids in disconnected rather than connected Nostoc cultures (Fig. 6, inset).
Absorbance spectra of photosynthetic pigments extracted from Nostoc biofilms cultivated under high-intensity red light (4,000 lx) in electrically connected (thin line) or disconnected (thick line) PMFCs. The spectra were normalized to the 665 nm chlorophyll A peak. The difference in absorbance spectrum (the disconnected PMFC minus the connected PMFC) is shown in the inset panel
Discussion
Previous studies established that phototrophic biofilms consisting of cyanobacteria display a light-dependent electrogenic activity (i.e., discharge electrons to the extracellular environment under illumination) (Zou et al. 2009; 2010; Pisciotta et al. 2010). An immediate increase in the rate of electron flow upon illumination and the rapid drop in darkness highlighted the direct relationship between light and the electrogenic activity. We proposed that light-dependent, PSII-mediated photolysis of water is the source of the electrons transmitted to the environment (Zou et al. 2009). The current studies provide evidence that (1) P-ETC and, particularly, water photolysed by PSII is the source of electrons that are discharged to extracellular environment by cyanobacteria under illumination, and (2) PQ and bd quinol oxidase serve as key carriers of electrons to the extracellular environment.
The electrogenic mechanism in cyanobacteria appears fundamentally different from those in previously described electrogens, such as Geobacter spp., where electrons originate from oxidation of organic molecules and are transported via R-ETC (Lovley 2008). Results presented here support the mechanism in which water, rather than organic compounds, is the source of electrons deposited to environment. Inhibition of PSII using DCMU, CCCP, or atrazine was found to immediately shut down electrogenic activity (Fig. 2). DCMU and atrazine inhibit electron transfer between PSII and PQ by blocking the plastoquinone binding pocket Qb on PSII (Kirilovsky et al. 1989), whereas CCCP interferes with electron flow in PSII. Long-term monitoring of electrogenic activity suggested irreversible inhibition by the specific PSII inhibitors DCMU or atrazine and reversible inhibition by the relatively non-specific PSII inhibitor CCCP (Fig. 2). Although CCCP is also known to act as a proton uncoupler in ATP synthesis (Trubitsin et al. 2005), collectively, the results with these three inhibitors illustrate that functional PSII is absolutely required for light-dependent electrogenic activity in cyanobacteria. Because the biological function of PSII is light-dependent photolysis of water, these results provide strong support that electrons donated to the environment are derived from water.
PQ appears to play a central role in the electrogenic pathway of cyanobacteria. PQ is a lipophilic carrier molecule that normally shuttles water-derived electrons from the Qa site of PSII through the thylakoid membrane to downstream cytochrome b6f complex (Roberts et al. 2004). Oxidation of reduced PQ by cyt b6f is believed to be the rate-limiting step in the chain of electron transfer between PSII and PSI (Trubitsin et al. 2005). Blocking of this transfer step with DBMIB treatment produced a large increase in electrogenic activity in both Noctoc and Lyngbya (Fig. 1). A dose-dependent relationship between amount of drug added and electrogenic activity was observed. The subsequent decrease in electrogenic activity observed over time was most likely due to drug clearance or decomposition. Mechanistically, DBMIB binds to the quinol oxidation (Qo) site on cyt b6f with high affinity, thereby increasing the pool of reduced PQ which builds up in the thylakoid membrane (Roberts et al. 2004; Yan et al. 2006). Diverting PQ-carried electrons away from cyt b6f to an alternative branch provides a plausible interpretation of DBMIB's stimulating effect. Quinol oxidase provides an alternative route for electron transfer from PQ that is apart from cyt b6f. Results with DBMIB suggest that (1) the redox state of the PQ pool dictates electrogenic output and, (2) conditions that cause over-reduction of the PQ pool lead to an increase in electrogenic activity.
The electrogenic activity of cyanobacteria may be a form of overflow metabolism that prevents cell damage under high light intensity. We observed a positive correlation between oxygenic photosynthetic activity (DO production) and electrogenic activity at low to moderate light levels (Fig. 5). At high light exposures, however, the rate of DO production dropped, indicating photoinhibition, whereas the electrogenic activity showed the largest increase supporting the notion that it plays a role in photoprotection. In further support of the photoprotection hypothesis, Nostoc biofilms cultivated in disconnected PMFCs under high-intensity light synthesized pigments indicative of a higher light stress response compared to Nostoc biofilms cultivated in electrically connected PMFCs (Fig. 6). The differential absorption spectrum suggests the pigments upregulated in disconnected PMFCs are carotenoids; antioxidant pigments upregulated in cyanobacteria when subjected to high light exposure (Adams et al. 2008; Kilian et al. 2007). This lends support to the hypothesis that the electrogenic activity of cyanobacteria may provide an upstream mechanism for rapidly shedding excess electrons from the P-ETC under intense light.
As noted previously, cytochromes mediate electron transfer to the environment in non-photosynthetic electrogenic bacteria such as G. sulfurreducens (Reguera et al. 2005; Esteve-Nunez et al. 2008). Cytochromes could serve a similar role in electrogenic cyanobacteria. Supportive of this, Vermaas and coauthors reported that when normal Z-scheme electron flow is disrupted by construction of a PSI knockout, electrons derived from PSII are efficiently shunted to an oxidase in the thylakoid membrane (Vermaas et al. 1994). Cyanobacteria possess at least three distinct cytochrome oxidases: ARTO, COX, and quinol oxidase (Pils and Schmetterer 2001). These three cytochromes differ in their susceptibility to drug inhibitors and so can be differentially targeted in vivo. While all three oxidases are inhibited by high-dose KCN that completely prevents electrons from leaving the PQ pool (Cooley and Vermaas 2001), at low concentrations KCN does not affect bd quinol oxidase (Pils and Schmetterer 2001). Low-dose KCN had only minimal short-term effect on electrogenic activity. Their longer-term, more gradual inhibition over the course of days suggested generalized perturbation of regular cell metabolism with only indirect effects on electrogenic output. Azide at 5 mM completely inhibits only ARTO and COX, whereas PCP selectively inhibits quinol oxidase (Pils and Schmetterer 2001). Our finding that 5 mM azide did not immediately suppress electrogenic activity (Fig. 4) indicates that COX and ARTO may not play substantial role in electrogenic activity in cyanobacteria. The process of elimination points to cytochrome bd quinol oxidase. Rapid inhibition of electrogenic activity by PCP, a drug that specifically inhibits quinol oxidase (Pils et al. 1997; Schneider et al. 2001), provides additional support for this conclusion.
The quinol oxidase gene (Cyd) is widely distributed among diverse cyanobacteria (Kufryk and Vermaas 2006). Cytochrome bd quinol oxidase's known function is to prevent over-reduction of the PQ pool and, importantly, it is maximally active under high light conditions (Berry et al. 2002; Gutthann et al. 2007). It is found in both thylakoid and cytoplasmic membranes (Berry et al. 2002; Tsunoyama et al. 2009; Howitt and Vermaas 1998). Interestingly, its distribution in thylakoid and cytoplasmic membranes appears to change depending on growth conditions (Kufryk and Vermaas 2006; Berry et al. 2002). This spatial-temporal versatility might allow bd quinol oxidase to better convey excess PSII derived electrons to the cell surface under high light conditions. Nomura and coauthors reported that a CtaDI protein is involved in acclimation to high light in a strain that lacks Cyd (bd quinol oxidase) (Nomura et al. 2006). It would be interesting to explore whether such a strain has electrogenic activity in the absence of bd quinol oxidase. Our results indicate the electrogenic path is as follow: H2O → PSII → PQ → quinol oxidase → extracellular electron acceptors. It is not yet clear if bd quinol oxidase transfers electrons directly to extracellular electron acceptors or if additional downstream components are involved. Investigation of knockout mutant strains via the microbial fuel cell-based approach holds potential to shed further light on the electrogenic pathway.
In the current study, representatives of two cyanobacterial genera, Nostoc and Lyngbya were used to test the extent to which the electrogenic pathways is generic. Nostoc is a heterocyst-forming, filamentous cyanobacterium surrounded by a relatively thin sheath. The thin sheath could explain why Nostoc appeared to be more rapidly affected when dosed with inhibitors such as PCP or DBMIB than Lyngbya, which has a thick sheath (Figs. 2 and 3). The consistency of the inhibitor-induced changes in the two genera, however, suggests a conserved electrogenic pathway. While the patterns of drug-induced changes in electrogenic activity were similar for both genera, each genera showed some variations. This probably relates to genera-dependent factors such as differential drug permeability through thick versus thin sheaths, different detoxification rates or differences in susceptibility of electron transfer components to drugs.
To combat climate change and address growing 21st century energy demand, society needs to develop innovative solutions. On a global scale, cyanobacteria could play substantial role in the new energy landscape (Dismukes et al. 2008; Greenwell et al. 2010). These microbial phototrophs use sunlight energy to consume atmospheric CO2 and fix it into energy-dense organic material. They can perform additional useful functions like wastewater treatment and phytoremediation of nutrient pollution (Roeselers et al. 2008). The present study sheds light on the recently discovered but yet poorly understood electrogenic activity of cyanobacteria. Alternative strategies for building PMFCs that do not rely on cyanobacterial electrogenic activity but exploit phototrophs in other ways, such as for converting solar energy into biomass or hydrogen have been recently reviewed (Rosenbaum et al. 2010) and, therefore, are not discussed here.
In cyanobacteria, the P-ETC and, particularly, PQ and quinol oxidase play a major role in transferring electrons derived from water to extracellular acceptors. In this regard, the electrogenic pathway of cyanobacteria is dissimilar to heterotrophic electrogenic bacteria where the electrons are derived from organic material and are transferred principally through the R-ETC (Lovley 2008). By identifying PQ and cytochrome bd quinol oxidase as crucial links in the light-driven electrogenic pathway of cyanobacteria, the present study provides a rational foundation by which this natural process might be manipulated for renewable energy production. By increasing electron traffic through PQ and cytochrome bd quinol oxidase, it may be possible to enhance the rate for converting solar energy into electricity using cyanobacteria.
References
Adams WW, Zarter CR, Mueh KE, Amiard V, Demmig-Adams B (2008) Energy dissipation and photoinhibition: a continuum of photoprotection. In: Demmig-Adams B, Adams WW, Mattoo AK (eds) Photoprotection, photoinhibition, gene regulation, and environment. Springer, Dordrecht, pp 49–64
Berry S, Schneider D, Vermaas WFJ, Rogner M (2002) Electron transport routs in whole cells of Synechocystis sp. strain PCC 6803: the role of the cytochrome bd-type oxidase. Biochemistry 41:3422–3429
Chaudhuri SK, Lovley DR (2003) Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol 21:1229–1232
Cooley JW, Vermaas WF (2001) Succinate dehydrogenase and other respiratory pathways in thylakoid membranes of Synechocystis sp. strain PCC 6803: capacity comparisons and physiological function. J Bacteriol 183:4251–4258
Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC (2008) Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr Opin Biotechnol 19:235–240
Esteve-Nunez A, Sosnik J, Visconti P, Lovley DR (2008) Fluorescent properties of c-type cytochromes reveal their potential role as an extracytoplasmic electron sink in Geobacter sulfurreducens. Environ Microbiol 10:497–505
Gonen-Zurgil Y, Carmeli-Schwartz Y, Sukenik A (1996) Selective effect of the herbicide DCMU on unicellular algae—a potential tool to maintain monoalgal mass culture of Nannochloropsis. J Appl Phycol 8:415–419
Greenwell HC, Laurens LM, Shields RJ, Lovitt RW, Flynn KJ (2010) Placing microalgae on the biofuels priority list: a review of the technological challenges. J Royal Soc Interface (in press)
Gutthann F, Egert M, Marques A, Appel J (2007) Inhibition of respiration and nitrate assimilation enhances photohydrogen evolution under low oxygen concentrations in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1767:161–169
Howitt CA, Vermaas WF (1998) Quinol and cytochrome oxidases in the cyanobacterium Synechocystis sp. PCC 6803. Biochemistry 37:17944–17951
Karapetyan NV (2007) Non-photochemical quenching of fluorescence in cyanobacteria. Biochemistry 72:1127–1135
Kilian O, Steunou AS, Fazeli F, Bailey S, Bhaya D, Grossman AR (2007) Responses of a thermophilic Synechococcus isolate from the microbial mat of Octopus Spring to light. Appl Environ Microbiol 73:4268–4278
Kirilovsky DL, Ajlani G, Picaud M, Etienne AL (1989) Mutations responsible for high light sensitivity in an atrazine-resistant mutant of Synechocystis 6714. Plant Mol Biol 13:355–363
Kufryk GI, Vermaas WF (2006) Sll1717 affects the redox state of the plastoquinone pool by modulating quinol oxidase activity in thylakoids. J Bacteriol 188:1286–1294
Lovley DR (2008) The microbe electric: conversion of organic matter to electricity. Curr Opin Biotechnol 19:1–8
Matoo AK, Pick U, Hoffman-Falk H, Edelman M (1981) The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the “proteinaceous shield” regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Acad Natl Sci U S A 78:1572–1576
Meyer J (1986) Photosynthetic and respiratory electron transport in a cyanobacterium. Photosynth Res 9:1573–1579
Mogi T, Miyoshi H (2009) Properties of cytochrome bd plastoquinol oxidase from the cyanobacterium Synechocystis sp. PCC 6803. J Biochem 145:395–401
Nomura CT, Persson S, Shen G, Inoue-Sakamoto K, Bryant DA (2006) Characterization of two cytochrome oxidase operons in the marine cyanobacterium Synechococcus sp. PCC 7002: inactivation of ctaDI affects the PS I:PS II ratio. Photosynth Res 87:215–228
Paumann M, Regelsberger G, Obinger C, Peschek GA (2005) The bioenergetic role of dioxygen and the terminal oxidase(s) in cyanobacteria. Biochim Biophys Acta 1707:231–253
Pils D, Schmetterer G (2001) Characterization of three bioenergetically active respiratory terminal oxidases in the cyanobacterium Synechocystis sp. strain PCC 6803. FEMS Microbiol Lett 203:217–222
Pils D, Gregor W, Schmetterer G (1997) Evidence for in vivo activity of three distinct respiratory terminal oxidases in the cyanobacterium Synechocystis sp. strain PCC6803. FEMS Microbiol Lett 152:83–88
Pisciotta J, Zou Y, Baskakov IV (2010) Light-dependent electrogenic activity of cyanobacteria. PLoS ONE 5:e10821
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101
Roberts AG, Bowman MK, Kramer DM (2004) The inhibitor DBMIB provides insight into the functional architecture of the Qo site in the cytochrome b6f complex. Biochemistry 43:7707–7716
Roeselers G, Loosdrecht MC, Muyzer G (2008) Phototrophic biofilms and their potential applications. J Appl Phycol 20:227–235
Rosenbaum M, He Z, Angenent LT (2010) Light energy to bioelectricity: photosynthetic microbial fuel cells. Curr Opin Biotechnol 21:1–6
Schneider D, Berry S, Rich P, Seidler A, Rogner M (2001) A regulatory role of the PetM subunit in a cyanobacterial cytochrome b6f complex. J Biol Chem 276:16780–16785
Shi L, Squier TC, Zachara JM, Fredrickson JK (2007) Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol Microbiol 65:12–20
Trubitsin BV, Ptushenko VV, Koksharova OA, Mamedov MD, Vitukhnovskaya LA, Grigor'ev IA, Semenov AY, Tikhonov AN (2005) EPR study of electron transport in the cyanobacterium Synechocystis sp. PCC 6803: oxygen-dependent interrelations between photosynthetic and respiratory electron transport chains. Biochim Biophys Acta 1708:238–249
Tsunoyama Y, Bernát G, Dyczmons NG, Schneider D, Rögner M (2009) Multiple Rieske proteins enable short- and long-term light adaptation of Synechocystis sp. PCC 6803. J Biol Chem 284:27875–27883
Vermaas WF, Shen G, Styring S (1994) Electrons generated by photosystem II are utilized by an oxidase in the absence of photosystem I in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett 337:103–108
Yan J, Kurisu G, Cramer WA (2006) Intraprotein transfer of the quinone analogue inhibitor 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone in the cytochrome b6f complex. Proc Acad Natl Sci U S A 103:69–74
Zang LY, Sommerburg O, van Kuijk FJGM (1997) Absorbance changes of carotenoids in different solvents. Free Radic Biol Med 23:1086–1089
Zou Y, Pisciotta J, Billmyre RB, Baskakov IV (2009) Photosynthetic microbial fuel cells with positive light response. Biotechnol Bioeng 104:939–946
Zou Y, Pisciotta J, Baskakov IV (2010) Nanostructured polypyrrole-coated anode for sun-powered microbial fuel cells. Bioelectrochem 79:50–56
Acknowledgments
This research was supported by Elkins Professorship Award to IVB.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Suppl. Fig. 1
Effect of atrazine on electrogenic activity of Lyngbya (a) or Nostoc (b). Both cultures were dosed first with 25 μM of atrazine (left arrows) followed by an additional 75 μM (right arrows). Periods of illumination or darkness are indicated by white or black bars along x-axis, respectively (JPEG 42 kb)
Rights and permissions
About this article
Cite this article
Pisciotta, J.M., Zou, Y. & Baskakov, I.V. Role of the photosynthetic electron transfer chain in electrogenic activity of cyanobacteria. Appl Microbiol Biotechnol 91, 377–385 (2011). https://doi.org/10.1007/s00253-011-3239-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3239-x