Abstract
Bioelectrochemical systems (BESs) are one of the rising technologies capable of converting chemical energy into electric energy and vice versa by achieving simultaneous wastewater bioremediation. The microorganisms involved in the process are the core of BESs as they catalyse the oxidation of organic matter present in wastewater to produce electrons. While the chemical energy present in wastewater is converted into electrical energy in a microbial fuel cell (MFC), the electrical energy is being used to produce chemicals in a microbial electrolysis cell (MEC). Similarly, appropriate use of ion exchange membranes makes MFC capable of desalinating saline water and also facilitates recovery of nutrients from wastewater. The BESs have also proved its efficiency in utilising the solar energy for application in photosynthetic MFC employing microalgae as well as higher plants. Moreover, wastewater bioremediation in MFCs has extended its applicability in treating diverse waste streams starting from industrial and domestic to wastewater containing dye, organo-chloride, nitrate, ammonia, etc. Most remarkable advancement in BES research started with the recovery of value-added products including heavy metals, apart from generation of power. Even though innovative designs and low-cost efficient materials for electrodes, catalysts and proton exchange membranes (PEMs) for application in BESs have been developed, there are quite a few challenges of BESs that need to be addressed to take this technology forward. This chapter showers light on the microbial aspects of BES, with special focus on MFC, along with a thorough discussion on the recent developments in BES research emphasising its bottlenecks and challenges.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Exo-electrogens
- Desalination
- Heavy metal recovery
- Microalgae
- Recalcitrant compound removal
- Wastewater treatment
13.1 Introduction
Water is one of nature’s precious gifts to mankind that is a renewable resource, however at the same time a finite resource too. Apart from the fact that every living organism needs water for survival, water plays a crucial role in the sustainability of industrial, agricultural and production sectors. Water use has more than tripled globally since the 1950s, and one out of every six persons does not have regular access to safe drinking water. Lack of access to a safe water supply and sanitation affects the health of 1.2 billion people annually (WHO and UNICEF 2014). Water quality has been degraded by domestic and industrial pollution sources as well as non-point sources. Growing population, rising industrialisation and expanding agriculture have pushed up the water demand. The CPHEEO estimates about 70–80% of total water supplied for domestic use gets converted to sanitary wastewater and in addition to this industries too contribute a remarkable quantity of wastewater. Thus an increasing use of water indirectly increases the wastewater generation. As it is always recommended to use water wisely, same is applicable for wastewater, i.e. using wastewater wisely can make it a valuable resource. Thus, wastewater from households, industries and agriculture should not be seen as a problem but as a valuable resource, which could meet the demands for water, energy and nutrients.
A microbial fuel cell (MFC) is relatively new and emerging technology, which produces electrical energy from the chemical energy stored in the organic molecules present in wastewater or any aqueous solution (Logan et al. 2006). The working principle of an MFC is similar to that of a fuel cell with a difference that microbes act as a catalyst in anoxic anodic chamber and organic matter present in wastewater acts as fuel. Assuming a 100% conversion efficiency, theoretically 1 kg of organic matter (glucose as substrate) removed can produce 1 kWh of energy (Aelterman et al. 2006). A typical two-chambered MFC consists of an anaerobic anodic chamber containing the anode and bacterial consortia (electrogenic bacteria), a cathodic chamber containing the cathode and the terminal electron acceptor (TEA), a proton exchange membrane (PEM) separating these two chambers and the electrical circuit that allows the electron transport from anode to cathode. A dominant challenge during the commercialisation of this technology lies in the initial cost. A major part of the fabrication cost is attributed to the PEM, electrodes and energy supplied in providing oxygen. Hence, there is a need for low-cost and effective alternatives for each of these. Even though the energy consumed by providing aeration in the cathodic chamber can be eliminated by providing air cathodes, it does not provide any value addition to the technology in terms of products.
The application of microorganisms for bioremediation and biodegradation is gaining much importance in today’s world owing to the fact that these eco-friendly microorganisms do not produce any environmentally toxic by-products on biodegrading the wastewater. An MFC is a promising technology that utilises these eco-friendly microorganisms; however it offers a step forward to normal anaerobic treatment systems as it has the potential to generate direct electric power along with wastewater treatment. This chapter throws light on the basic microbial electron transfer mechanisms involved in an MFC with a detailing of techniques to confirm the same including electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), etc. Also, a short review on different applications of MFC including microbial desalination cell (MDC), microbial carbon capture cell (MCC) and sediment microbial fuel cell (SMFC) are being covered. A special focus was given to the bioremediation of wastewater, including domestic and industrial, and emphasis was laid on nitrate removal, recalcitrant removal and heavy metal recovery using MFC. The bottlenecks and future perspectives are being discussed at the end of the chapter as a guidance to take the research on MFC forward.
13.2 Electrochemically Active Biofilms
Bacteria have great adaptability and can survive in coldest to warmest condition and also eat a wide range of organic matter as well as some inorganic matters. Electrochemically active bacteria are those groups of microorganisms that can transfer electrons to external electron acceptors, which means they can give way to electricity generation. One of the major hurdles is in harnessing the power from these tiny bacterial cells. Bioelectrochemical systems (BES) can efficiently harness electrical energy out of electrochemically active bacteria. In BES, these bacteria are employed in the anaerobic anodic chamber with a purpose to harvest electrical energy from wastewater, and these are referred to as electrochemically active biofilms when developed on the electrodes. Bacteria produce electricity by extracellular electron transfer (EET), as they generate electrons while oxidation of substrate and transfer them across their cell membrane through tiny channels. The existing techniques to measure this are time-consuming and involve large sample size and complicated extraction protocol, and these techniques rupture the cell and denature the proteins.
13.2.1 Mechanisms of Electron Transfer
The electrochemically active bacterial species that exhibit the ability to directly transfer the electrons exogenously outside the cells are called electrogens (Kumar et al. 2015). Various mechanisms have been proposed for extracellular transport of electrons by electrogenic bacteria to the anodic surface. The electron transfer mechanisms can be direct electron transfer and mediated electron transfer (Fig. 13.1). Direct transfer can take place via membrane-bound cytochromes or via electrically conductive nanowires (pili), whereas, electron transfer is mediated by redox mediators or oxidation of reduced secondary metabolites in mediated electron transfer.
Direct reduction of an exogenous acceptor occurs by direct contact between the cell’s oxidoreductases and the terminal electron acceptor (electrode). Rhodoferax ferrireducens, which can quantitatively transfer electrons to graphite electrodes without the need for an electron-shuttling mediator, uses the direct transfer mechanism (Chaudhuri and Lovley 2003, b). Also, iron-reducing bacteria, Klebsiella sp. IR21, isolated from the anode biofilm of an MFC, gave a power density of 8.9 ± 3.65 mW/m2 (Lee et al. 2016). The electrons also get transferred via electrically conductive proteinaceous filaments, i.e. nanowires, produced by the bacteria. Pili of G. sulfurreducens was reported to serve as biological nanowires, transferring electrons from the cell surface to the surface of Fe(III) oxides (Reguera et al. 2005).
Some bacteria transfer electrons with the help of mediators, which are redox compounds. Mediators that transfer electrons can be secreted by bacteria as in Shewanella, which secretes flavins that mediate extracellular electron transfer (Marsili et al. 2008). Also, Citrobacter freundii Z7, isolated from the anodic biofilm of MFC inoculated with aerobic sewage sludge, gave a maximum power density of 204.5 mW/m2, and experiments indicated that the strain Z7 transferred electrons via secreted mediators (Huang et al. 2014). In some other cases, the redox compounds include artificial mediators, which are chemicals that facilitate the shuttling of electrons from inside of cell to electrodes outside the cell. For developing a novel cost-effective electrode material and power production from domestic wastewater using three different mediators, methylene blue, neutral red and 2-hydroxy-1,4-naphthoquinone were selected as electrode mediators with different concentrations, where methylene blue is reported to give a power density of 636 mW/m2 (Taskan et al. 2015). Some other artificial mediators reported so far include thionine, humic acid, potassium ferricyanide (Rahimnejad et al. 2013), anthraquinone-2-6 and others (Kumar et al. 2015).
13.2.2 Application of Electrogens in MFC
A phototrophic purple non-sulphur bacterium Rhodopseudomonas palustris DX-1 was reported to produce power density of 2720 mW/m2. This DX-1 also utilised a wide variety of substrates (volatile acids, yeast extract, and thiosulphate) for power production in different metabolic modes, proving its activity from a range of simple to complex sources of organic matter (Xing et al. 2008). A bacterial strain Ochrobactrum anthropi YZ-1 as isolated from MFC was capable of producing a power density of 89 mW/m2 using acetate as the electron donor in the U-tube MFC (Table 13.1). This strain was also capable of producing current using a wide range of substrates, including acetate, lactate, propionate, butyrate, glucose, sucrose, cellobiose, glycerol and ethanol (Zuo et al. 2008). Similarly, Acidiphilium sp. strain 3.2 Sup 5 cells that were isolated from an extreme acidic environment were able to produce high-density electrocatalytic currents, up to 3 A/m2 at a poised potential in the absence of redox mediators (Malki et al. 2008). Direct electron transfer from different anaerobically grown Shewanella putrefaciens strains without any electrochemical mediators showed electrochemical activities; however, no activities were observed in aerobically grown Shewanella putrefaciens (Kim et al. 2002).
Shewanella oneidensis DSP10 grown on graphite felt under minimal nutrient conditions gave power density of 1500 mW/m2 from the mini-MFC (Biffinger et al. 2007). Also, higher concentrations of DSP10 were sustained at pH of 7, whereas this trend was reversed at pH of 5, which is not favourable for DSP10, and this pH is not suitable for MFCs because of elevated acidity levels in anolyte (Biffinger et al. 2007). Propionibacterium freudenreichii used as biocatalyst in a glycerol-oxidising MFC gave a maximum open circuit voltage of 485 mV and a maximum power density of 14.9 mW/m2 (Reiche et al. 2016). Klebsiella pneumoniae strain L17 used as biocatalyst in MFCs achieved the maximum voltage outputs of 426.2 mV and showed the presence of an electrochemically active compound that could transfer electrons between K. pneumoniae L17 and the anode (Deng et al. 2010).
13.2.3 Biofilm Electrochemistry
An electrochemically active biofilm can interact with metal electrode, and hence it can be diagnosed through electrochemical analysis techniques including cyclic voltammetry and electrochemical impedance spectroscopy. Anodic behaviour can be diagnosed by investigating the characteristics of electron transferred to the electrode, whereas the electrons getting removed signify the cathodic behaviour. Biofilm formed by a mixed bacterial culture contains species with different metabolic activities, and the electrochemical behaviour depends on the concentration of the electrochemical bacterial species present. Even in case of a single pure culture species, concentration gradient plays a major role along with the mediator characteristics. The pH, redox potential of the anolyte as well as biofilm, availability of oxygen, etc. also play a significant role in governing the electrochemical nature of biofilm. In order to analyse the electrochemical behaviour of the bacterial species, a potential is applied with respect to a known reference potential, which may or may not cause a flow of current. The produced current is measured to relate it back to the electrode potential. The possible mechanism that controls the electrochemical reaction in the cell is assessed based on the observed current-potential relationship.
13.2.3.1 Cyclic Voltammetry: A Tool to Analyse the Biofilm Electrochemical Phenomenon
A CV analysis is absolutely necessary to analyse an electrochemical reaction occurring in any electrochemical cell. The electrogenic bacterial species forms a biofilm that can interact with the electrode material by transferring electrons produced during the bacterial metabolism to the electrode. The CV can be used to characterise the electrochemical behaviour of this biofilm by applying a potential with respect to a known reference potential. A linear polarization potential scan starting from initial potential to reach a final potential is done, and this causes flow of current, which is measured. The results of this wide range of potential give rise to the voltammogram, which is further used to investigate the possible electron transfer mechanisms. The electrogenic bacterial community, which is associated with certain redox couples, generates a steady-state current in MFC. Reduction and oxidation peaks are formed during the forward and reverse scans around the formal reduction potential of the redox couple, which can be detected by the CV. On the usage of a mixed culture inoculum, the biofilm on the electrode will contain multiple species having different metabolic activities. Hence, the electrochemical behaviour of the electrode containing biofilm will depend on electrochemical or concentration gradients formed within the biofilm. In short, the electron transfer in biofilms is associated with complete acetate oxidation to electrons, protons and CO2, where transfer of electron outside the cell is considered to be the rate limiting step. Thus, cyclic voltammetry is a promising analysis tool, which by employing of electrochemical theories explores the electron transfer mechanism in the BES.
13.2.3.2 Electrochemical Impedance Spectroscopy
The overpotentials associated with BES are normally investigated with the help of voltammetry studies. The BES are associated with several losses including ohmic loss, losses due to microbial kinetics, solution resistance losses, etc. However, voltammetric studies are not capable of identifying the contribution of each of these in the performance of BES. These individual contributions can be identified and quantified by electrochemical techniques including EIS. While the voltammetry techniques consider direct current, the EIS method involves alternating current. During EIS, voltage is applied at small sinusoidal amplitude, and the magnitude and shift in the response current is measured and analysed. The experiment is carried out at a range of frequencies as different process involved different i-v response at different frequencies. This makes it easy to understand the individual contributions, thus making it possible to quantify and identify the individual resistance as well as the overpotentials.
13.3 Introduction to Microbial Fuel Cell
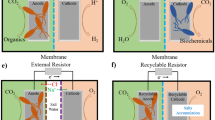
In an MFC, the chemical energy present in the wastewater is converted into electrical energy by bacterial catalysis (Logan et al. 2006). An MFC consists of basically two processes, oxidation of organic matter in an anaerobic condition and reduction of a terminal electron acceptor. However to assist the completion of the overall process in an efficient way, it is necessary to accommodate several components into an MFC (Fig. 13.2). These include a proton exchange membrane, electrodes, catalysts, circuit, external resistance, etc. Each of the components used in an MFC should satisfy a specific set of properties to get maximum output out of it, which are described in brief here.
13.3.1 Electrogenic Bacteria
The electrogenic bacteria are the core of an MFC. Earlier in 1911, when Potter first came out with the concept of producing electricity out of bacterial cells, the scope to take the technology to the field scale was very little (Potter 1911). The main challenge was in using this phenomenon in wastewater. Wastewater contains complex substrates, and hence culturing of a pure bacterial species and avoiding contamination of species in waste stream are major challenges. However, the mixed culture bacteria are not substrate specific, and hence, the efficiency on using the mixed culture in removing organic matter from wastewater is much higher as compared to the usage of pure culture for the same purpose. This challenge has led scientists to explore the possibilities to efficiently utilize the mixed bacterial culture by specifically enhancing the activity of electrogenic bacterial community (Tiwari and Ghangrekar 2015). Most of the investigations and techniques used for suppressing the methanogens were adopted based on either the techniques employed in suppressing the methanogens in the digestive tract of ruminants or those employed in enhancing the hydrogen production (Table 13.2).
13.3.2 Terminal Electron Acceptor
Oxygen upon reduction in the presence of proton yields water, which makes it the most suitable electron acceptor in an MFC. Oxygen can be either provided through aeration of catholyte or in the form of photosynthetic oxygen produced by microalgae, or even air cathode can be employed in MFC. However, the sluggish reduction kinetics of oxygen has led to the use of expensive catalysts, which forced the need of alternate terminal electron acceptor in MFC. Nitrate and heavy metals, including copper, iron, chromium, etc., have also been used as electron acceptor, with a dual purpose of treating the waste stream along with supply of electron acceptor in the cathodic chamber. Apart from this, ferricyanide, hydrogen peroxide, perchlorate, persulphate, etc. have proved to be highly efficient electron acceptors in an MFC (Table 13.3). The key point that should be kept in mind while selecting the electron acceptor is the challenges to be faced during the disposal; in other words the end product on reduction should be a valuable recovery rather than an add-on to environmental pollution. Hence, the wastewater having the potential to be an efficient electron acceptor should be targeted with an aim to treat the same without employing separate costly treatment technologies.
13.3.3 Electrode Material
High electrical conductivity, better biocompatibility, hydrophilicity, anti-corrosiveness, efficient electron transfer, cost-effectiveness, etc. are some of the factors that should be satisfied while selecting the electrode material for an MFC. The anode used in an MFC should allow efficient bacterial attachment and should have high microbe accessible surface area, such as electrode with macroscale pores that assist in internal colonisation of microbes. The electrodes used so far include carbon-based electrodes as well as non-carbonaceous electrodes like stainless steel. Carbon felt, paper, mesh, foam, etc. as well as graphite rod are some of the commonly used carbon-based electrodes, which are highly biocompatible and inert. Researchers have been exploring different forms of carbon-based electrode owing to its low cost and ease of synthesis.
An ideal anode for an MFC should favour proper microbial attachment on the electrode surface, should assist in high electron transfer and should have minimum electrode resistance (Mustakeem 2015). The living biofilm on the anode acts as biocatalyst in the anodic chamber, and hence bacterial-electrode interaction is one of the critical parameters that determines the efficiency of MFC (Franks et al. 2010). The interaction is very much dependent on the nature of anode material including its surface roughness, porosity, biocompatibility, etc. (Canuto de Almeida e et al. 2019). Apart from this, the electrode material should be biocompatible, should have high electrical conductivity, should not decompose in wastewater, and should be hydrophilic and anti-corrosive (Wei et al. 2011). Hence, an investigation on low-cost long-lasting anode material, which can effectively transfer the electrons produced by electrogenic bacteria, is of high priority for effective wastewater treatment and power generation in MFC.
Graphite rod, graphite fibre, carbon felt, carbon cloth and carbon paper are some of the most extensively used carbon-based anode materials in MFC. Even though graphite rods have good conductivity and chemical stability as compared to other forms of carbon-based electrodes, Chaudhuri and Lovley observed a reduced bacterial-electrode interaction on the usage of graphite rod anode as compared to carbon felt, which was evident from the better power generation and bacterial biofilm formation in MFC with carbon felt as anode (Chaudhuri and Lovley 2003). However, the hydrophobic nature of untreated carbon felt restricts the development of biofilm and hence demands pretreatments including nitric acid pretreatment, UV/O3 pretreatment, etc. for effective biofilm growth, which otherwise adds up to the overall cost (Cornejo et al. 2015; Hidalgo et al. 2016; Neethu et al. 2018). Similarly, the short life span, cost and clogging nature reduce the scope of usage of carbon paper, cloth and fibre as electrode material (Zhou et al. 2011).
13.3.4 Proton Exchange Membrane
The protons are produced during the oxidation of organic matter in the anodic chamber by electrogenic bacteria. In addition to the completion of the electrochemical cycle and circuit, the transfer of proton to the cathodic chamber also helps in balancing the pH of the anolyte so as to provide an ambient environment for bacterial survival. Therefore, the PEM developed should be efficient enough to transfer a major portion of the protons produced in the anodic chamber. Even though proton conductivity is the primary function of a PEM, the membrane developed should also satisfy several other characteristics. As the electrogenic bacteria require an anaerobic environment for its growth and activity, the membrane separator should be able to maintain the anaerobicity of the anodic chamber by allowing minimum or no oxygen transfer from the cathodic side to the anodic side. Similarly, transfer of the substrate from the anodic chamber to cathodic chamber should not occur, which will otherwise cause substrate loss for the bacteria in the anodic chamber. One of the major challenges associated with the scaling up of MFC is the cost and stability of the electrode and separator material used. The PEM has an equal role on both these factors, and this has led to researches in developing low-cost PEMs that are stable enough and have the capacity to handle the hydraulic pressure developed (Table 13.4). The cost associated with the operation and fabrication of an MFC should be kept to minimum so as to be a low-cost alternative to the existing wastewater treatment technologies. In view of enhancing the performance of MFC, it is always recommended to use low-cost materials with high performance efficiency for fabrication.
13.3.5 Oxygen Reduction Catalyst
Oxygen is the most reliable and easily available terminal electron acceptor, which does not deliver any toxic product on reduction. However, one of the performance-hindering factors for an MFC is the sluggish oxygen reduction kinetics at the cathode, which can be resolved by the use of an oxygen reduction reaction (ORR) catalyst. High catalytic activity, high specific surface area, higher stability, non-toxicity and ease of synthesis are the major factors that are to be considered while selecting a catalyst. However, along with these properties, an ideal ORR catalyst should be cost-effective for application in an MFC, which is predominantly engineered for wastewater bioremediation. Considering the binding energy, platinum is considered to be the most efficient cathode catalyst for enhancing the ORR kinetics in an MFC. The cost associated with the same has led researchers to optimise the Pt dosing as well as to alloy it with other transition metals including Ni. Still research is progressing to explore low-cost efficient catalyst including the biochar-based catalyst as well as non-metallic catalysts.
13.3.5.1 Biomass-Derived Cathode Catalyst for Application in MFCs
A low-cost electrode as well as catalyst with high activity and durability is the need of the hour for the MFC. The reduction of oxygen in the cathodic chamber occurs either through a direct four-electron pathway or a two-step peroxide pathway. The most efficient ORR catalysts drive the reaction to a better involvement of the four-electron pathway, which leads to higher power output as well as lower production of peroxide intermediates, because the peroxide formation adversely affects the electrode and PEM. One of the major drawbacks in MFC is the sluggish ORR at the cathode, which was resolved by the usage of Pt-based catalyst. However, apart from high cost, Pt-based catalyst is associated with CO poisoning, methanol crossover and long-term instability due to particle aggregation and dissolution. Metal nanoparticle-based catalysts with a good support material have been used in MFC to achieve higher electrochemically active surface area.
A good support material should have sufficient electrical conductivity and higher surface area, which can be attained on the use of porous carbon materials. The properties of carbon-based materials including its higher electrical conductivity, stability and functionality have increased the interest of researchers in developing low-cost carbon-based electrode materials and catalysts. Hence, sustainable and ample biomass reserves can be an alternative option for the production of the same. Waste to wealth can be achieved if it is possible to convert the huge tons of agricultural as well as other biomass waste generated globally into novel catalytic or electrode materials. The selection of appropriate synthesis methods, with respect to the source, is crucial in order to obtain high surface area and reactive sites with high stability (Borghei et al. 2018). The ORR catalysts including carbon supported on Pt, N-doped carbon, heteroatom-doped carbon, Fe/Co N-doped carbon, etc. have been produced from biomass so far (Chen et al., 2011).
13.4 Applications of MFCs
13.4.1 Microbial Desalination Cell
The demand for fresh water and clean energy is driving the need for converting an MFC into an MDC, wherein desalination of saline water is attained along with wastewater treatment. Ion exchange membranes (IEMs) are most commonly used for desalination of saline water, and it is a promising tool, which when oriented properly can be applied in MDC for value-added product recovery. In an MDC, mainly the potential gradient created due to the transfer of electrons from the anodic chamber to the cathodic chamber and the concentration gradient between the desalinating chamber and its adjacent chamber are responsible for the desalination. In an investigation using a three-chambered MDC, a maximum power density of 2 W/m2 alongside removal of 90% salt from water, present in desalination chamber, was attained (Cao et al. 2009). However, three-chambered MDC is associated with certain challenges including the hindrance for passage of H+ ions from the anodic chamber to the cathodic chamber, as well as the accumulation of chloride ions in the anolyte. This decreases the anolyte pH, which poses threat to bacterial community in the anodic chamber, which can reduce the efficiency of MDC.
In order to overcome this challenge, Pradhan and Ghangrekar modified the three-chambered MDC into multi-chambered MDC, wherein the issue of pH imbalance was solved by the usage of a cation exchange membrane (CEM) adjacent to the anodic chamber, which transferred the H+ from the anolyte to the adjacent concentrate chamber (Pradhan and Ghangrekar 2014). Thus, MDC exploits wastewater as a viable substrate to yield electricity, which also has been exploited for desalination. This technology shows promising approach by offering low-cost solution for desalination of saline and brackish water (Neethu et al. 2019b). However, the use of chemical catholyte and costly cathode catalyst makes MDC unsustainable for future field-scale applications, which need to be overcome by the use of low-cost terminal electron acceptors. The dependence of the performance of MDCs on the salt concentration and MDC configuration is yet to be investigated to draw a final conclusion on it.
13.4.2 Microbial Carbon-Capture Cell
As discussed in the above section regarding the supply of low-cost electron acceptor (O2) with additional benefit of value addition, cultivation of microalgae in the catholyte is one of the alternatives (Fig. 13.3). Microalgae can provide an attractive solution for providing the photosynthetic oxygen as TEA by utilising the nutrients in wastewater along with sequestering CO2 from anodic off gas, and further it can be an excellent feedstock for biodiesel production upon harvesting. Hence, MCC is a sustainable technology that uses oxygen produced by algal biomass as electron acceptor for accomplishing concurrent electricity generation, CO2 sequestration, wastewater treatment and algal biomass production (Neethu et al. 2018). Different applications of microalgae in MFC have been investigated so far (Rajesh and Ghangrekar 2016).
In the catholyte, microalgae Golenkinia sp. proved to be a source of oxygen and achieved a maximum power density of 6.3 W/m3 (Hou et al. 2016). A maximum power output of 1.9 W/m2 was achieved by using microalgae as substrate in the anodic chamber of MFC (Cui et al. 2014). The marine algae Chaetoceros is reported to inhibit the growth of methanogenic archaea in anodic chamber, due to the presence of long-chain saturated fatty acid, and this MFC attained a power density of 21.43 W/m3 (Rajesh et al. 2015). Apart from improving the power generation, microalgae have the potential to remediate wastewater rich in nutrients and heavy metals (Huang et al. 2017; Logroño et al. 2017). Investigations were also done to explore the photosynthetic electrogenic activity in algae and cyanobacteria, wherein incorporating photosynthetic species in the anodic chamber of MFC gave a power density of 6.2 mW/m2 (Luimstra et al. 2014).
13.4.3 Sediment Microbial Fuel Cell
The SMFC is a modification of MFC, where oxygen is available in the overlying water, and on cathode, oxygen reduction occurs to complete the circuit by reducing it to water (Wang et al. 2014). Thus, oxygen availability is one of the major factors that govern the performance of SMFC (Fig. 13.4). Unlike most MFCs, which contain a membrane to separate the compartments containing the anode (where oxidation takes place) and the cathode (where reduction takes place), SMFC functions without membranes. A SMFC can have better application in natural water bodies, if it could power small autonomous devices; however here the low power generation has become a major challenge. A previous investigation on the effect of using different electrode materials in SMFC has reported a maximum power density of 16 mW/m2 using graphite felt electrode and 38 mW/m2 using graphite felt multiwalled carbon nanotubes (GF-MWNT) (Wang et al. 2014). On the contrary, an investigation performed using a rotating cathode for increasing oxygen availability gave a power density of 49 mW/m2 (He et al. 2007). Hence, the performance of a SMFC is dependent on several factors including the electrode material, sediment characteristics, oxygen availability in catholyte, etc. Sediment remediation, mitigation of the aquatic water pollution, algae cultivation, etc. are some of the major applications of SMFC. Incorporation of microalgae in the cathodic side of SMFC makes it a sediment microbial carbon-capture cell (SMCC) with a multiple advantage of algae cultivation and nutrient removal from the overlying pond water (Neethu and Ghangrekar 2017).
13.5 Bioremediation and Biodegradation in MFC
An MFC converts the chemical energy present in wastewater to electrical energy via the bacterial catalysis. The nature of bacterial community present in the anodic compartment is of ultimate importance in determining the efficiency of the system. Equally important is the substrate that is to be provided for the bacteria in the anodic chamber. In 1911, when Potter explored the capacity of electrogenic bacteria in producing electricity, the media used for bacterial metabolism was synthetic substrate. However, later on researchers have taken the technology to a level that even the domestic wastewater and industrial wastewater were able to replace the synthetic media for bacterial culture. This has expanded the possible applications of a BES including wastewater treatment and value-added product recovery. Apart from the normal wastewater treatment technologies practised today, a BES has an added advantage that it does not require separate units for targeting treatment of different pollutants present in wastewater. On the contrary, bioremediation can be carried out in a single bioreactor, wherein apart from removal of carbonaceous compounds, nitrification, denitrification, heavy metal removal and removal of sulphate compounds can be accomplished. The application of BES technologies in treating different wastewater streams including domestic, industrial and wastewater containing recalcitrant compounds is reviewed below.
13.5.1 Bioremediation of Domestic and Industrial Wastewater
The core of MFCs is the electrons generated in the anodic chamber, which are produced on substrate oxidation by electrogenic bacteria, and hence the type of substrate fed into the MFC is of ultimate importance. The substrate that is fed into the MFC can range from pure organic substrates including acetate, glucose, etc. to the complex substrates including cellulose, protein, fatty acids, etc. The concentration and components present in the substrate depend on the source of wastewater fed into the MFC. Apart from the domestic wastewater, which mainly contains organic compounds, industrial wastewater including agro-based industries, fertilizer industry, distilleries, dairy, etc. also have proven to be promising source of substrate for anodic bacteria in MFC. A promising organic matter removal can be achieved on usage of easily biodegradable organic compounds present in wastewater as substrate in MFC. This was much evident from an investigation carried out with different substrates, namely, glucose, fructose and sucrose, wherein the highest power density and COD removal efficiency, respectively, were achieved in MFC using glucose (136 mW/m2 and 88.5%) as substrate as compared to MFC operated with sucrose (8.8 mW/m2 and 54.2%) and fructose (3.6 mW/m2 and 67.5%).
The excellent biodegradability of organic matter by bacteria in an MFC was also experimented on the liquid fraction of pressed municipality solid waste, wherein a 94% COD removal efficiency was achieved in the anodic chamber (Koók et al. 2016). Further the better performance of MFC fed with pure substrate was evident when MFC fed with glucose gave almost three times higher power density as compared with the one having domestic wastewater as substrate to bacteria (Liu and Logan 2004). However, in addition to a better power generation, equally important is to take advantage of MFC as an environmental and energy-friendly solution to treat the wastewater generated from different sources. With this focus, research was carried out in utilising the MFC in treating different forms of wastewaters (Table 13.5).
The performance of an MFC depends on several factors including substrate characteristics, electrode, PEM, bacteria, TEA, etc. Hence, for a single substrate, the performance exhibited by MFC will vary with other parameters. For example, distillery wastewater when treated in single-chamber and double-chambered MFC gave a respective power density of 28.15 mW/m2 and 17.7 mW/m2; however, with similar COD removal efficiencies (60%), surprisingly, the same substrate (distillery wastewater) gave a power density of 1000 mW/m2 using a thermophilic MFC (Ha et al. 2012). Hence, it is difficult to judge the performance of an MFC based on the type of substrate by ignoring other dependable factors/parameters that affect the performance of MFC. A detailed performance evaluation of MFC operated with different substrates along with their operating condition is furnished below (Table.13.5).
13.5.2 Bioremediation of Nitrogen-Rich Wastewater
One of the major focuses of MFC was bioremediation of wastewater, that is, the removal of organic matter in wastewater; however equally important is the removal of nitrogen present in the wastewater, which will otherwise lead to eutrophication of receiving water body. Removal of nitrate in an MFC can be achieved either in the cathodic chamber or in the anodic chamber. In the cathodic chamber, nitrate removal can be achieved with the help of bio-cathode (algae) or by nitrate reduction to nitrogen. A total nitrogen removal of 81.6% was achieved in the cathodic side of a photosynthetic microbial fuel cell using algae in the cathodic zone (Neethu and Ghangrekar 2017). Also, a 90% total nitrogen removal was attained in a planted constructed wetland MFC, wherein root exudates of Ipomoea aquatica were utilised (Liu et al. 2013).
Nitrate can be a potential electron acceptor in the cathodic side of an MFC, on reduction of which it gets converted to nitrogen. Thus, for the first time in the literature, Clauwaert et al. (2007) reported nitrate reduction without hydrogen production in the cathodic chamber by achieving simultaneous organic matter removal in the anodic chamber. Here, the capability of Geobacter species in directly accepting electrons from the graphite felt and reducing nitrate to nitrite was best utilised for remediation of nitrate-rich wastewater, by simultaneously achieving a power density of 8 W/m3 (Clauwaert et al. 2007). Further investigation on this concept was carried out by directing the anolyte to an aerobic chamber for oxidation of ammonium to nitrate, which is again fed back to the cathodic chamber of MFC for denitrification, hence achieving a complete treatment of single wastewater stream in MFC (Virdis et al. 2008).
Apart from cathodic chamber, nitrate removal can also be achieved in the anodic chamber of an MFC. About 85% of nitrate removal was achieved in a single-chamber air cathode MFC, where the nitrate-reducing bacteria present in the anodic chamber assisted in denitrification (Sukkasem et al. 2008). Also, investigations were carried out by employing pure culture autotrophic denitrifiers, Pseudomonas sp. C27, where a power density of 40 mW/m2 was achieved (Lee et al. 2012). However, it was reported that even though the denitrifying bacterial concentration increased with an increase in the concentration of nitrate in the anodic chamber, a decrease occurred to the proportion of electrogenic bacteria in the anodic chamber (Liu and Logan 2004). This challenge was overcome by using a novel denitrifying electrogenic strain EB-1, isolated from anodic biofilm capable of giving a power density of 840 mW/m2 by achieving simultaneous denitrification (Jin et al. 2018).
Recently, Jin et al. focused on anodic denitrifying dual-chamber MFCs, which achieved a maximum simultaneous heterotrophic denitrification and electricity generation at a COD/N ratio of 5:1 in the anodic chamber; however, the electrogenic bacterial population in the anodic chamber of MFC operated with denitrifying bacteria was low as compared to the control experiment operated without denitrifying bacteria in the anodic chamber (Jin et al. 2019). Hence, the main challenge of using denitrifying bacteria in the anodic chamber is observed to be the suppression of electrogenic bacteria, which needs to be overcome by proper optimisation of operating parameters.
13.5.3 Microbial Fuel Cell for Recalcitrant Remediation
Xenobiotic compounds are those compounds which are man-made chemicals that are present in the environment at a concentration higher than their natural concentration. Even though bacterial community is capable of degrading most of the xenobiotics, there are certain compounds that are exceptional. These synthetic compounds whose biodegradability is very slow or which are non-biodegradable and exists in environment for long are classified as recalcitrant (Faber 1979). This reluctance of microorganism in degrading the recalcitrant has been differently explained in the literature as it might be due to a large molecular size or due to difficulties in penetration or due to low solubility in water, etc. (Faber 1979). Recalcitrant compounds can range from halogenated compounds like halocarbons and polychlorinated biphenyls to complex synthetic polymers (Table 13.6). Hence, the more complex is the structure of the compound, the more difficult is its biodegradation. Genetic engineering tools have been applied to a much greater extent to modify the microorganism to make them capable of degrading the recalcitrant compounds.
Even though BESs have been mainly focused on power generation, recently the application of BES for bioremediation is gaining priority. The remediation of xenobiotic compounds using BES is a recent and upcoming promising treatment technique. Xenobiotic compounds cannot be directly degraded by microorganisms owing to its complexity and hence are not readily used for bacterial metabolism. Therefore, in most of the cases the xenobiotic compounds are treated outside the cell rather than inside the cell. Several investigations have been carried out, wherein the xenobiotic compounds act as an electron acceptor.
An anaerobic-aerobic process using single-chamber MFC has led to effective degradation of azo dye when used as substrate (Danish Khan et al. 2015). Even though the biodegradation mechanism of non-recalcitrant compounds has been widely discussed in the literature, it is important to know the mechanism of recalcitrant degradation by electrogenic bacteria. To state a few, say for azo dye degradation mechanism, the high redox potential of azo dye makes it a good electron accepting candidate, and hence higher electron transfer rates can lead to rapid reductive degradation of azo dyes in the anodic compartment of MFC (Fernando et al. 2014). Similarly, chloronitrobenzene compounds are known to have highly electron withdrawing nature and can be efficiently reduced to much lower toxic forms. However, in a different investigation, the aerobic treatment of pentachlorophenol in cathodic compartment of dual-chambered MFC also has proven to be better than its anaerobic treatment in single-chambered MFC (Khan et al. 2018). Degradation of xenobiotic compounds in the anodic chamber has been widely investigated, which includes the degradation of trichloroethane (Aulenta et al. 2011), polychlorinated biphenyls (Chun et al. 2013), refractory organic pesticide (Cao et al. 2015), hydrocarbons (Morris and Jin 2008), phenanthrene and benzene (Adelaja et al. 2017), phenanthrene (Adelaja et al. 2014), etc. Hence, the non-biodegradability nature of certain xenobiotics that poses restrictions in its biodegradability can be overcome by utilising their electron transfer mechanism and subsequent degradation in BES.
13.5.4 Value-Added Product Recovery in Microbial Fuel Cell: Heavy Metal Recovery
Even though heavy metals are inevitable part of various industrial, medical and several other applications, their presence in the wastewater causes heavy threat to the environment. The non-biodegradability of the heavy metals opens up the scope to recover heavy metal from the wastewater streams. Even though several conventional methods including precipitation, coagulation, ion exchange, etc. have been used for heavy metal removal, the MFC can come out as an emerging technology, where simultaneous removal as well as recovery of heavy metal is possible. In an MFC, the cathodic chamber, which is a destination for the electrons and protons produced in the anodic chamber, can be efficiently used for reduction of heavy metals having a higher redox potential to get reduced on accepting electrons and precipitate. For example, the introduction of Cr(VI), which is highly soluble and harmful, into the cathodic chamber of MFC gets reduced to less toxic Cr(III) (Wang et al. 2008). Similarly, Li et al. added vanadium oxide (NaVO3) to the anodic chamber, and the action of Rhodoferax ferrireducens assisted in removal of 75.8% NaVO3 in the anodic chamber while achieving a 64% electron recovery. Also, a follow-up investigation was carried out to investigate the fate of MFC on using the V in the cathodic side as electron acceptor, wherein only 26% removal of V was achieved (Zhang et al. 2012). In addition, tetrachloroaurate was used as an electron acceptor in order to recover gold efficiently. Surprisingly, a 99.98% recovery of Au was achieved for an Au(III) concentration of 200 ppm (Choi and Hu 2013). Likewise investigations have reported recovery of several other heavy metals including copper (Wang et al. 2010), silver (Yun-Hai et al. 2013), selenite (Chellamuthu et al. 2011), arsenic (Xue et al. 2013), zinc (Fradler et al. 2014), cadmium (Abourached et al. 2014), etc. Therefore, the recovery of these value-added heavy metals from the wastewater streams with simultaneous power generation takes the MFC technology a step ahead of other conventional wastewater treatment technologies.
13.6 Bottlenecks and Future Perspective
The major areas in the research on MFC that need to be focused are the reduction in fabrication cost and enhancement in performance using low-cost material for different components. The PEM, electrode and catalyst used in fabrication of MFC account for the major cost and are also the performance-determining components in MFC. Optimisation of operating conditions is equally important in enhancing the performance of MFC. Equally important is the enrichment of electrogenic bacteria on the anode, which is an inevitable element in an MFC. Rather than going for pure electrogenic species in MFC, it is always recommended to use mixed culture bacterial species considering the issue of substrate specificity and feasibility while dealing with real wastewater. Hence, there is a need to explore biological and natural techniques to suppress the methanogenic archea present in the mixed culture in order to enhance the activity of electrogenic bacteria. In MFCs, power generation is one of the major goals, and hence, microorganisms capable of generating electricity in MFCs have gained increasing research interest. Until now, experimentations have been done to understand the microbial electrogenic consortia responsible for electricity generation in MFC. Still there is a need to understand the optimum conditions for maximum bacterial activity so that it can be exploited in such a way that the electrons are diverted from natural electron acceptors to the electrode effectively.
As a key component of MFC, the PEMs are gaining extensive attention in recent years because of its selective permeability towards protons to run the MFC in highly efficient way. Protons exhibit excess mobility in aqueous system than other ions; hence in other biological systems and materials, the proton conductivity as well as water mobility increases with water uptake (Neethu et al. 2019a). However, the Nafion membrane, which is most commonly used in MFC, is associated with several limitations such as oxygen diffusion, cation accumulation, substrate crossover, durability due to fouling, high cost, etc. (Hasani-Sadrabadi et al. 2010). Biological membranes have very high water permeability and selectivity, which can improve the performance of membrane in terms of proton conductivity (Qu et al. 2013). Also, the ceramic membranes with cation exchangers proved to be a low-cost alternative to the costly Nafion membrane, however with a far low proton mass transfer coefficient than Nafion (Ghadge and Ghangrekar 2015). Hence, there is a need to explore the scope to improve the performance of the ceramic membranes as well as the use of easily available biological membranes. Also, as most of the catalysts are expensive and toxic, there is a need to explore a low-cost catalyst, which can increase the oxygen reduction reaction. Rather than synthesising or procuring catalyst, the possibility of making catalyst out of waste stream has not been experimented so far.
13.7 Summary
A microbial fuel cell is a promising low energy-consuming technology, which converts organic matter present in wastewater to electrical energy. The multiple applications of MFC make it unique as compared to other technologies used for wastewater treatment. The MFC technology still needs further development in order to harvest maximum possible electricity and attain high level of bioremediation. There are several factors, which are discussed in detailed in this chapter, that significantly affect the performance of MFCs and are required to be modified for more flexibility for its practical field-scale applications. Also, organised multidisciplinary efforts are further required for scaling up of MFC to enhance power production as well as wastewater treatability.
References
Abourached C, Catal T, Liu H (2014) Efficacy of single-chamber microbial fuel cells for removal of cadmium and zinc with simultaneous electricity production. Water Res 51:228–233. https://doi.org/10.1016/j.watres.2013.10.062
Adelaja O, Keshavarz T, Kyazze G (2014) Enhanced biodegradation of phenanthrene using different inoculum types in a microbial fuel cell. Eng Life Sci 14:218. https://doi.org/10.1002/elsc.201300089
Adelaja O, Keshavarz T, Kyazze G (2017) Treatment of phenanthrene and benzene using microbial fuel cells operated continuously for possible in situ and ex situ applications. Int Biodeterior Biodegrad 116:91. https://doi.org/10.1016/j.ibiod.2016.10.021
Aelterman P, Rabaey K, Pham HT, Boon N, Verstraete W (2006) Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ Sci Technol 40:3388. https://doi.org/10.1021/es0525511
Ali N, Anam M, Yousaf S, Maleeha S, Bangash Z (2017) Characterization of the electric current generation potential of the pseudomonas aeruginosa using glucose, fructose, and sucrose in double chamber microbial fuel cell. Iran J Biotechnol 15:216. https://doi.org/10.15171/ijb.1608
Aulenta F, Tocca L, Verdini R, Reale P, Majone M (2011) Dechlorination of trichloroethene in a continuous-flow bioelectrochemical reactor: effect of cathode potential on rate, selectivity, and electron transfer mechanisms. Environ Sci Technol 45:8444. https://doi.org/10.1021/es202262y
Behera M, Ghangrekar MM (2011) Electricity generation in low cost microbial fuel cell made up of earthenware of different thickness. Water Sci Technol J Int Assoc Water Pollut Res 64(12):2468–2473. https://doi.org/10.2166/wst.2011.822
Biffinger JC, Pietron J, Ray R, Little B, Ringeisen BR (2007) A biofilm enhanced miniature microbial fuel cell using Shewanella oneidensis DSP10 and oxygen reduction cathodes. Biosens Bioelectron 22:1672–1679. https://doi.org/10.1016/j.bios.2006.07.027
Bond DR, Lovley DR (2003) Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69:1548–1555. https://doi.org/10.1128/AEM.69.3.1548-1555.2003
Borghei M, Lehtonen J, Liu L, Rojas OJ (2018) Advanced biomass-derived electrocatalysts for the oxygen reduction reaction. Adv Mater 30. https://doi.org/10.1002/adma.201703691
Call TP, Carey T, Bombelli P, Lea-Smith DJ, Hooper P, Howe CJ, Torrisi F (2017) Platinum-free, graphene based anodes and air cathodes for single chamber microbial fuel cells. J Mater Chem A 5:23872. https://doi.org/10.1039/C7TA06895F
Canuto de Almeida e Silva T, Bhowmick GD, Ghangrekar MM, Wilhelm M, Rezwan K (2019) SiOC-based polymer derived-ceramic porous anodes for microbial fuel cells. In: Biochem Eng J, vol 148, p 29. https://doi.org/10.1016/j.bej.2019.04.004
Cao X, Huang X, Liang P, Xiao K, Zhou Y, Zhang X, Logan BE (2009) A new method for water desalination using microbial desalination cells. Environ Sci Technol 43:7148. https://doi.org/10.1021/es901950j
Cao X, Song HL, Yu CY, Li XN (2015) Simultaneous degradation of toxic refractory organic pesticide and bioelectricity generation using a soil microbial fuel cell. Bioresour Technol 189:87. https://doi.org/10.1016/j.biortech.2015.03.148
Chae PS, Rasmussen SG, Rana RR, Gotfryd K, Chandra R, Goren MA, Kruse AC, Nurva S, Loland CJ, Pierre Y, Drew D (2010) Maltose–neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat Methods 7(12):1003
Chaudhuri SK, Lovley DR (2003) Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol 21(10):1229–1232. https://doi.org/10.1038/nbt867
Chellamuthu P, Ward M, Nealson K, Kronen M (2011) Removal of selenite with microbial fuel cells utilizing shewanella oneidensis MR-1. In: Fuels and petrochemicals division—core programming topic at the 2011 AIChE annual meeting
Chen Z, Higgins D, Yu A, Zhang L, Zhang J (2011) A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ Sci 4(9):3167–3192. https://doi.org/10.1039/c0ee00558d
Choi C, Hu N (2013) The modeling of gold recovery from tetrachloroaurate wastewater using a microbial fuel cell. Bioresour Technol 133:589. https://doi.org/10.1016/j.biortech.2013.01.143
Chun CL, Payne RB, Sowers KR, May HD (2013) Electrical stimulation of microbial PCB degradation in sediment. Water Res 47:141. https://doi.org/10.1016/j.watres.2012.09.038
Clauwaert P, Rabaey K, Aelterman P, De Schamphelaire L, Pham TH, Boeckx P, Boon N, Verstraete W (2007) Biological denitrification in microbial fuel cells. Environ Sci Technol 41:3354–3360. https://doi.org/10.1021/es062580r
Cornejo JA, Lopez C, Babanova S, Santoro C, Artyushkova K, Ista L et al (2015) Surface modification for enhanced biofilm formation and electron transport in Shewanella anodes. J Electrochem Soc 162:H597. https://doi.org/10.1149/2.0271509jes
Cui Y, Rashid N, Hu N, Rehman MSU, Han JI (2014) Electricity generation and microalgae cultivation in microbial fuel cell using microalgae-enriched anode and bio-cathode. Energy Convers Manag 79:674–680. https://doi.org/10.1016/j.enconman.2013.12.032
Danish Khan M, Abdulateif H, Ismail IM, Sabir S, Zain Khan M (2015) Bioelectricity generation and bioremediation of an azo-dye in a microbial fuel cell coupled activated sludge process. PLoS One 10:e0138448. https://doi.org/10.1371/journal.pone.0138448
Deng LF, Li FB, Zhou SG, Huang DY, Ni JR (2010) A study of electron-shuttle mechanism in Klebsiella pneumoniae based-microbial fuel cells. Chin Sci Bull 55:99–104. https://doi.org/10.1007/s11434-009-0563-y
Faber MD (1979) Microbial degradation of recalcitrant compounds and synthetic aromatic polymers. Enzym Microb Technol 1:226. https://doi.org/10.1016/0141-0229(79)90041-3
Fang C, Min B, Angelidaki I (2011) Nitrate as an oxidant in the cathode chamber of a microbial fuel cell for both power generation and nutrient removal purposes. Appl Biochem Biotechnol 164:464. https://doi.org/10.1007/s12010-010-9148-0
Fernando E, Keshavarz T, Kyazze G (2014) External resistance as a potential tool for influencing azo dye reductive decolourisation kinetics in microbial fuel cells. Int Biodeterior Biodegrad 89:7. https://doi.org/10.1016/j.ibiod.2013.12.011
Fradler KR, Michie I, Dinsdale RM, Guwy AJ, Premier GC (2014) Augmenting microbial fuel cell power by coupling with supported liquid membrane permeation for zinc recovery. Water Res 55:115. https://doi.org/10.1016/j.watres.2014.02.026
Franks AE, Malvankar N, Nevin KP (2010) Bacterial biofilms: the powerhouse of a microbial fuel cell. Biofuels 1:589. https://doi.org/10.4155/bfs.10.25
Ghadge AN, Ghangrekar MM (2015) Development of low cost ceramic separator using mineral cation exchanger to enhance performance of microbial fuel cells. Electrochim Acta 166:320. https://doi.org/10.1016/j.electacta.2015.03.105
Ghasemi M, Daud WRW, Ismail AF, Jafari Y, Ismail M, Mayahi A, Othman J (2013) Simultaneous wastewater treatment and electricity generation by microbial fuel cell: Performance comparison and cost investigation of using Nafion 117 and SPEEK as separators. Desalination 325:1–6. https://doi.org/10.1016/j.desal.2013.06.013
Ha PT, Lee TK, Rittmann BE, Park J, Chang IS (2012) Treatment of alcohol distillery wastewater using a Bacteroidetes-dominant thermophilic microbial fuel cell. Environ Sci Technol 46:3022–3030
Hasan K, Grattieri M, Wang T, Milton RD, Minteer SD (2017) Enhanced bioelectrocatalysis of Shewanella oneidensis MR-1 by a naphthoquinone redox polymer. ACS Energy Lett 2:1947–1951. https://doi.org/10.1021/acsenergylett.7b00585
Hasani-Sadrabadi MM, Dashtimoghadam E, Majedi FS, Kabiri K, Solati-Hashjin M, Moaddel H (2010) Novel nanocomposite proton exchange membranes based on Nafion® and AMPS-modified montmorillonite for fuel cell applications. J Membr Sci 365:286–293. https://doi.org/10.1016/j.memsci.2010.09.014
He Z, Shao H, Angenent LT (2007) Increased power production from a sediment microbial fuel cell with a rotating cathode. Biosens Bioelectron 22(12):3252–3255
Heilmann J, Logan BE (2006) Production of electricity from proteins using a microbial fuel cell. Water Environ Res 78:531. https://doi.org/10.2175/106143005x73046
Hidalgo D, Tommasi T, Bocchini S, Chiolerio A, Chiodoni A, Mazzarino I, Ruggeri B (2016) Surface modification of commercial carbon felt used as anode for Microbial Fuel Cells. Energy 99:193. https://doi.org/10.1016/j.energy.2016.01.039
Hou Q, Nie C, Pei H, Hu W, Jiang L, Yang Z (2016) The effect of algae species on the bioelectricity and biodiesel generation through open-air cathode microbial fuel cell with kitchen waste anaerobically digested effluent as substrate. Bioresour Technol 218:902–908. https://doi.org/10.1016/j.biortech.2016.07.035
Huang J, Zhu N, Cao Y, Peng Y, Wu P, Dong W (2014) Exoelectrogenic bacterium phylogenetically related to citrobacter freundii, isolated from anodic biofilm of a microbial fuel cell. Appl Biochem Biotechnol 175:1879–1891. https://doi.org/10.1007/s12010-014-1418-9
Huang D, Song BY, He YL, Ren Q, Yao S (2017) Cations diffusion in Nafion117 membrane of microbial fuel cells. Electrochim Acta 245:654–663. https://doi.org/10.1016/j.electacta.2017.06.004
Islam MA, Ethiraj B, Cheng CK, Yousuf A, Thiruvenkadam S, Prasad R, Rahman Khan MM (2018) Enhanced current generation using mutualistic interaction of yeast-bacterial coculture in dual chamber microbial fuel cell. Ind Eng Chem Res 57:813–821. https://doi.org/10.1021/acs.iecr.7b01855
Jayashree C, Tamilarasan K, Rajkumar M, Arulazhagan P, Yogalakshmi KN, Srikanth M, Banu JR (2016) Treatment of seafood processing wastewater using upflow microbial fuel cell for power generation and identification of bacterial community in anodic biofilm. J Environ Manag 180:351. https://doi.org/10.1016/j.jenvman.2016.05.050
Jin X, Guo F, Liu Z, Liu Y, Liu H (2018) Enhancing the electricity generation and nitrate removal of microbial fuel cells with a novel denitrifying exoelectrogenic strain EB-1. Front Microbiol 9. https://doi.org/10.3389/fmicb.2018.02633
Jin X, Guo F, Ma W, Liu Y, Liu H (2019) Heterotrophic anodic denitrification improves carbon removal and electricity recovery efficiency in microbial fuel cells. Chem Eng J 370:527. https://doi.org/10.1016/j.cej.2019.03.023
Kang CS, Eaktasang N, Kwon DY, Kim HS (2014) Enhanced current production by Desulfovibrio desulfuricans biofilm in a mediator-less microbial fuel cell. Bioresour Technol 165:27–30. https://doi.org/10.1016/j.biortech.2014.03.148
Khan N, Khan MD, Nizami AS, Rehan M, Shaida A, Ahmad A, Khan MZ (2018) Energy generation through bioelectrochemical degradation of pentachlorophenol in microbial fuel cell. RSC Adv 8:20726. https://doi.org/10.1039/c8ra01643g
Kim HJ, Park HS, Hyun MS, Chang IS, Kim M, Kim BH (2002) A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzym Microb Technol 30:145–152. https://doi.org/10.1016/S0141-0229(01)00478-1
Koók L, Rózsenberszki T, Nemestóthy N, Bélafi-Bakó K, Bakonyi P (2016) Bioelectrochemical treatment of municipal waste liquor in microbial fuel cells for energy valorization. J Clean Prod 112:4406. https://doi.org/10.1016/j.jclepro.2015.06.116
Kumar R, Singh L, Wahid ZA, Din MFM (2015) Exoelectrogens in microbial fuel cells toward bioelectricity generation: a review. Int J Energy Res 39:1048. https://doi.org/10.1002/er.3305
Lee CY, Ho KL, Lee DJ, Su A, Chang JS (2012) Electricity harvest from nitrate/sulfide-containing wastewaters using microbial fuel cell with autotrophic denitrifier, Pseudomonas sp. C27. Int J Hydrog Energy 37:15827. https://doi.org/10.1016/j.ijhydene.2012.01.092
Lee YY, Kim TG, Cho K s (2016) Enhancement of electricity production in a mediatorless air–cathode microbial fuel cell using Klebsiella sp. IR21. Bioprocess Biosyst Eng 39:1005–1014. https://doi.org/10.1007/s00449-016-1579-8
Li J, Fu Q, Liao Q, Zhu X, Ye D d, Tian X (2009) Persulfate: a self-activated cathodic electron acceptor for microbial fuel cells. J Power Sources 194:269. https://doi.org/10.1016/j.jpowsour.2009.04.055
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38:4040. https://doi.org/10.1021/es0499344
Liu S, Song H, Li X, Yang F (2013) Power generation enhancement by utilizing plant photosynthate in microbial fuel cell coupled constructed wetland system. Int J Photoenergy 2013:1. https://doi.org/10.1155/2013/172010
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192. https://doi.org/10.1021/es0605016
Logroño W, Pérez M, Urquizo G, Kadier A, Echeverría M, Recalde C, Rákhely G (2017) Single chamber microbial fuel cell (SCMFC) with a cathodic microalgal biofilm: a preliminary assessment of the generation of bioelectricity and biodegradation of real dye textile wastewater. Chemosphere 176:378–388. https://doi.org/10.1016/j.chemosphere.2017.02.099
Luimstra VM, Kennedy SJ, Güttler J, Wood SA, Williams DE, Packer MA (2014) A cost-effective microbial fuel cell to detect and select for photosynthetic electrogenic activity in algae and cyanobacteria. J Appl Phycol 26:15. https://doi.org/10.1007/s10811-013-0051-2
Lusk BG, Parameswaran P, Popat SC, Rittmann BE, Torres CI (2016) The effect of pH and buffer concentration on anode biofilms of Thermincola ferriacetica. Bioelectrochemistry 112:47–52. https://doi.org/10.1016/j.bioelechem.2016.07.007
Malki M, De Lacey AL, Rodríguez N, Amils R, Fernandez VM (2008) Preferential use of an anode as an electron acceptor by an acidophilic bacterium in the presence of oxygen. Appl Environ Microbiol 74:4472–4476. https://doi.org/10.1128/AEM.00209-08
Mansoorian HJ, Mahvi AH, Jafari AJ, Amin MM, Rajabizadeh A, Khanjani N (2013) Bioelectricity generation using two chamber microbial fuel cell treating wastewater from food processing. Enzym Microb Technol 52:352. https://doi.org/10.1016/j.enzmictec.2013.03.004
Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci 105:3968–3973. https://doi.org/10.1073/pnas.0710525105
Min B, Cheng S, Logan BE (2005) Electricity generation using membrane and salt bridge microbial fuel cells. Water Res 39:1675–1686. https://doi.org/10.1016/j.watres.2005.02.002
More TT, Ghangrekar MM (2010) Improving performance of microbial fuel cell with ultrasonication pre-treatment of mixed anaerobic inoculum sludge. Bioresour Technol 101(2):562–567
Morris JM, Jin S (2008) Feasibility of using microbial fuel cell technology for bioremediation of hydrocarbons in groundwater. J Environ Sci Heal A Tox Hazard Subst Environ Eng 43:18. https://doi.org/10.1080/10934520701750389
Mustakeem (2015) Electrode materials for microbial fuel cells: nanomaterial approach. Mater Renew Sustain Energy 4. https://doi.org/10.1007/s40243-015-0063-8
Nagar H, Badhrachalam N, Rao VVB, Sridhar S (2019) A novel microbial fuel cell incorporated with polyvinylchloride/4A zeolite composite membrane for kitchen wastewater reclamation and power generation. Mater Chem Phys 224:175–185. https://doi.org/10.1016/j.matchemphys.2018.12.023
Neethu B, Ghangrekar MM (2017) Electricity generation through a photo sediment microbial fuel cell using algae at the cathode. Water Sci Technol 76:3269–3277. https://doi.org/10.2166/wst.2017.485
Neethu B, Bhowmick GD, Ghangrekar MM (2018) Enhancement of bioelectricity generation and algal productivity in microbial carbon-capture cell using low cost coconut shell as membrane separator. Biochem Eng J 133:205. https://doi.org/10.1016/j.bej.2018.02.014
Neethu B, Bhowmick GD, Ghangrekar MM (2019a) A novel proton exchange membrane developed from clay and activated carbon derived from coconut shell for application in microbial fuel cell. Biochem Eng J 148:170. https://doi.org/10.1016/j.bej.2019.05.011
Neethu B, Pradhan H, Sarkar P, Ghangrekar MM (2019b) Application of ion exchange membranes in enhancing algal production alongside desalination of saline water in microbial fuel cell. MRS Adv 4:1077. https://doi.org/10.1557/adv.2019.170
Ogugbue CJ, Ebode EE, Leera S (2015) Electricity generation from swine wastewater using microbial fuel cell. J Ecol Eng 16:26. https://doi.org/10.12911/22998993/60450
Pandit S, Khilari S, Roy S, Pradhan D, Das D (2014) Improvement of power generation using Shewanella putrefaciens mediated bioanode in a single chambered microbial fuel cell: effect of different anodic operating conditions. Bioresour Technol 166:451–457. https://doi.org/10.1016/j.biortech.2014.05.075
Parkash A (2016) Utilization of distillery wastewater for electricity generation using microbial fuel cell. J Appl Emerg Sci 6(2):79–86
Penteado ED, Fernandez-Marchante CM, Zaiat M, Cañizares P, Gonzalez ER, Rodrigo MAR (2016) Energy recovery from winery wastewater using a dual chamber microbial fuel cell. J Chem Technol Biotechnol 91:1802. https://doi.org/10.1002/jctb.4771
Potter MC (1911) Electrical effects accompanying the decomposition of organic compounds. Proc R Soc B Biol Sci 84:260–276. https://doi.org/10.1098/rspb.1911.0073
Pradhan H, Ghangrekar MM (2014) Multi-chamber microbial desalination cell for improved organic matter and dissolved solids removal from wastewater. Water Sci Technol 70:1948. https://doi.org/10.2166/wst.2014.438
Qu X, Alvarez PJJ, Li Q (2013) Applications of nanotechnology in water and wastewater treatment. Water Res 47:3931–3946. https://doi.org/10.1016/j.watres.2012.09.058
Rahimnejad M, Najafpour GD, Ghoreyshi AA, Bakeri G, Talebnia F, Oh SE (2013) Investigation of different mediators in microbial fuel cell with cyclic voltammeter. Pakistan. J Biotechnol 10:37–51
Rajesh PP, Ghangrekar MM (2016) Bioelectricity generation from marine algae chaetoceros using microbial fuel cell. https://doi.org/10.1007/978-81-322-2773-1_22
Rajesh PP, Noori MT, Ghangrekar MM (2014) Controlling methanogenesis and improving power production of microbial fuel cell by lauric acid dosing. Water Sci Technol 70(8):1363–1369. https://doi.org/10.2166/wst.2014.386
Rajesh PP, Jadhav DA, Ghangrekar MM (2015) Improving performance of microbial fuel cell while controlling methanogenesis by Chaetoceros pretreatment of anodic inoculum. Bioresour Technol 180:66–71. https://doi.org/10.1016/j.biortech.2014.12.095
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101. https://doi.org/10.1038/nature03661
Reiche A, Sivell J l, Kirkwood KM (2016) Electricity generation by Propionibacterium freudenreichii in a mediatorless microbial fuel cell. Biotechnol Lett 38:51–55. https://doi.org/10.1007/s10529-015-1944-8
Sekar AD, Jayabalan T, Muthukumar H, Chandrasekaran NI, Mohamed SN, Matheswaran M (2019) Enhancing power generation and treatment of dairy waste water in microbial fuel cell using cu-doped iron oxide nanoparticles decorated anode. Energy 172:173. https://doi.org/10.1016/j.energy.2019.01.102
Sukkasem C, Xu S, Park S, Boonsawang P, Liu H (2008) Effect of nitrate on the performance of single chamber air cathode microbial fuel cells. Water Res 42:4743. https://doi.org/10.1016/j.watres.2008.08.029
Tao HC, Li W, Liang M, Xu N, Ni JR, Wu WM (2011) A membrane-free baffled microbial fuel cell for cathodic reduction of cu(II) with electricity generation. Bioresour Technol 102:4774. https://doi.org/10.1016/j.biortech.2011.01.057
Tartakovsky B, Guiot SR (2006) A comparison of air and hydrogen peroxide oxygenated microbial fuel cell reactors. Biotechnol Prog 22:241–246. https://doi.org/10.1021/bp050225j
Taskan E, Ozkaya B, Hasar H (2015) Combination of a novel electrode material and artificial mediators to enhance power generation in an MFC. Water Sci Technol 71:320–328. https://doi.org/10.2166/wst.2014.487
Tiwari BR, Ghangrekar MM (2015) Enhancing electrogenesis by pretreatment of mixed anaerobic sludge to be used as inoculum in microbial fuel cells. Energy Fuels 29(5):3518–3524. https://doi.org/10.1021/ef5028197
Virdis B, Rabaey K, Yuan Z, Keller J (2008) Microbial fuel cells for simultaneous carbon and nitrogen removal. Water Res 42:3013. https://doi.org/10.1016/j.watres.2008.03.017
Wang G, Huang L, Zhang Y (2008) Cathodic reduction of hexavalent chromium [Cr(VI)] coupled with electricity generation in microbial fuel cells. Biotechnol Lett 30:1959–1966. https://doi.org/10.1007/s10529-008-9792-4
Wang Z, Lim B, Lu H, Fan J, Choi C (2010) Cathodic reduction of Cu2+ and electric power generation using a microbial fuel cell. Bull Kor Chem Soc 31:2025. https://doi.org/10.5012/bkcs.2010.31.7.2025
Wang D-B, Song T-S, Guo T, Zeng Q, Xie J (2014) Electricity generation from sediment microbial fuel cells with algae-assisted cathodes. Int J Hydrog Energy 39(25):13224–13230. https://doi.org/10.1016/j.ijhydene.2014.06.141
Wei J, Liang P, Huang X (2011) Recent progress in electrodes for microbial fuel cells. Bioresour Technol 102:9335. https://doi.org/10.1016/j.biortech.2011.07.019
WHO & UNICEF (2014) Progress on sanitation and drinking-water—2014 update. The Joint Monitoring Programme for Water Supply and Sanitation … http://apps.who.int/iris/bitstream/10665/81245/1/9789241505390_eng.pdf?ua=1
Xing D, Zuo Y, Cheng S, Regan JM, Logan BE (2008) Electricity generation by Rhodopseudomonas palustris DX-1. Environ Sci Technol 42:4146–4151. https://doi.org/10.1021/es800312v
Xue A, Shen ZZ, Zhao B, Zhao HZ (2013) Arsenite removal from aqueous solution by a microbial fuel cell-zerovalent iron hybrid process. J Hazard Mater 261:621. https://doi.org/10.1016/j.jhazmat.2013.07.072
You S, Zhao Q, Zhang J, Jiang J, Zhao S (2006) A microbial fuel cell using permanganate as the cathodic electron acceptor. J Power Sources 162:1409. https://doi.org/10.1016/j.jpowsour.2006.07.063
Yun-Hai W, Bai-Shi W, Bin P, Qing-Yun C, Wei Y (2013) Electricity production from a bio-electrochemical cell for silver recovery in alkaline media. Appl Energy 112:1337. https://doi.org/10.1016/j.apenergy.2013.01.012
Zhang BG, Zhou SG, Zhao HZ, Shi CH, Kong LC, Sun JJ, Yang Y, Ni JR (2010) Factors affecting the performance of microbial fuel cells for sulfide and vanadium (V) treatment. Bioprocess Biosyst Eng 33:187. https://doi.org/10.1007/s00449-009-0312-2
Zhang B, Feng C, Ni J, Zhang J, Huang W (2012) Simultaneous reduction of vanadium (V) and chromium (VI) with enhanced energy recovery based on microbial fuel cell technology. J Power Sources 204:34. https://doi.org/10.1016/j.jpowsour.2012.01.013
Zhou M, Chi M, Luo J, He H, Jin T (2011) An overview of electrode materials in microbial fuel cells. J Power Sources 196:4427. https://doi.org/10.1016/j.jpowsour.2011.01.012
Zuo Y, Xing D, Regan JM, Logan BE (2008) Isolation of the exoelectrogenic bacterium Ochrobactrum anthropi YZ-1 by using a U-tube microbial fuel cell. Appl Environ Microbiol 74:3130–3137. https://doi.org/10.1128/AEM.02732-07
Acknowledgements
The research project was supported by the Department of Biotechnology, Ministry of Science and Technology, Government of India (BT/EB/PAN IIT/2012), providing the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ghangrekar, M.M., Neethu, B. (2020). Bioelectrochemical System for Bioremediation and Energy Generation. In: Shah, M. (eds) Microbial Bioremediation & Biodegradation. Springer, Singapore. https://doi.org/10.1007/978-981-15-1812-6_13
Download citation
DOI: https://doi.org/10.1007/978-981-15-1812-6_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1811-9
Online ISBN: 978-981-15-1812-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)