Abstract
Severe outbreaks of diseases are the consequences of interactions between pathogenic microorganisms and cultivated crops. Plants are recurrently exposed to various biotic stresses in their innate environment. To endure such unfavorable circumstances, they have acquired complex mechanisms to react and acclimatize to biotic stresses. Plants are host to many contagious diseases caused by both living and nonliving agencies like viruses, bacteria, fungi, nematodes, and stress conditions. Comparatively small proportions of pathogens effectively assault the host plant which results in diseases. Plant disease is a physiological disorder or structural irregularity that is destructive to the plant and its products that suppress its monetary value. Plants are sessile and responsive organisms that encounter a variety of environmental strains. Throughout their life cycle, plants respond to diverse intimidations arising from their external environment. Fungal pathogens have a meticulous impact causing a major damage and yield losses in agriculture. Due to their sessile nature plants develops a broad range of strategies that cooperatively work in defense against biotic and abiotic stresses. Plant diseases can be viewed as obstinate roadblocks to the rapid advancement in agricultural yield. Interpreting the molecular basis of plant–pathogen interaction facilitates us to select plant resistance genes. The present work is focused on the consequence of fungus Macrophomina phaseolina on the growth of chickpea plants via monitoring various biochemical parameters which play important role in defense response upon infection with the pathogen. Comparative studies on the effect of M. phaseolina toxin in chickpea as well as other host plants like Brassica oleracea, Brassica juncea, Helianthus annus, and Vigna mungo via detached leaf bioassay method are also planned.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Microorganisms and plants live in the vicinity of each other hence; they affect each other in both harmful and useful manners (Ortíz-Castro et al. 2009). Plants have developed a variety of mechanisms to counter the various stresses of both abiotic and biotic origins (Gull et al. 2019). Plants have the mechanism to identify stress, when sensed; they activated different signaling pathways to combat the stress. Different genes activated and started their expression and gene products are essentially required for defense. For successful control, plants must recognize the stress-causing organism and should trigger the best possible defense process to encounter the pathogen (Kaur et al. 2022). To develop a successful plant defense mechanism, the pathogen is the central part of the process. Plants have a high risk of diseases of biotic origin like bacterial, fungal, and viral pathogens and this results in keen loss to plant production (Hussain and Usman 2019). A number of diverse options are accessible for farmers to prevent and cure infections of crops. Some alternatives comprise development of disease-resistant varieties, biological check, rotation of crops, tillage, and use of insecticides and pesticides, etc. (Barzman et al. 2015). Substantial attempts have been achieved to devise environmentally friendly approaches for preventing and curing diseases of plants and to save mankind from health-related risks (El-Gamal Nadia et al. 2007; Food and Agriculture Organization of the United Nations Rome 2017).
Severe outbreaks of diseases are the consequences of interactions between pathogenic microorganisms and cultivated crops (Lamichhane and Venturi 2015). Plants are recurrently exposed to various biotic stresses in their innate environment (Iqbal et al. 2021). To endure in such unfavorable circumstances, they have acquired complex mechanisms to react and acclimatize to biotic stresses (Fujita et al. 2006; Isah 2019). Plants are host to many contagious diseases caused by both living and nonliving agencies like viruses, bacteria, fungi, nematodes, and stress conditions. Comparatively small proportions of pathogens effectively assault the host plant which results in diseases (Mahajan and Tuteja 2005; Guiñazú et al. 2012; Nazarov et al. 2020).
Plants are sessile and responsive organisms that encounter a variety of environmental stresses. During their life cycle, plants respond to diverse intimidations arising from their external environment. Fungal pathogens have a meticulous impact causing a major damage and yield losses in agriculture (Fujita et al. 2006; Shuping and Eloff 2017). Due to their sessile nature plants develops a broad range of strategies that cooperatively work in defense against biotic and abiotic stresses (Agrios 2005). Plant diseases can be viewed as obstinate roadblocks to the rapid advancement in agricultural yield. Interpreting the molecular basis of plant–pathogen interaction facilitates us to select plant resistance genes (Prabhukarthikeyan et al. 2020).
Fungal infection employs different pathways for colonizing host plants. Fungal pathogen either directly penetrates the cell wall or enters through the natural openings like stomata to invade the host (Zabka et al. 2008; Pawlowski and Hartman 2016). Like other plant interacting microbes, fungal pathogen secretes effectors proteins that interacts with host and invade plant defense mechanisms (Choudhary et al. 2012). Nonetheless, the plant developed diverse defense mechanisms that mark the effector proteins, which results in innate immunity that is often linked with localized cell death (Dodds et al. 2009; Selin et al. 2016). The molecular mechanisms involved in the perception, signaling, and reaction in plant–pathogen interactions are major elements in the study of accurate resistance or susceptibility (Peyraud et al. 2017). The effector proteins called elicitors are responsible for activating resistance in plants against fungal infection (Ito et al. 1997). Highlighted plant signaling pathways contribute to finding natural and man-made compounds known as elicitors that are accountable for immunity against pathogen infection (Gomez et al. 2004; AbdulMalik et al. 2020).
Plant opposes pathogen attacks by activating a broad variety of mechanisms, either constitutive or inducible, which adds to the immunity of plants against pathogens. Constitutive defenses are preexisting resistance mechanisms which control infection immediately after revelation of host to pathogen (Andersen et al. 2018). The inducible defenses are associated with quick and efficient commencement of cellular defense responses, which are induced only after contact with a pathogen (Canesi and Pruzzo 2016).
On detection of pathogen, plant activates hypersensitive response against infection. The recognition of pathogens normally occurs through the binding of avirulence (Avr) genes and R genes secreted by pathogen and plant, respectively (Balint-Kurti 2019). If the R gene of host and Avr gene of pathogen is muted or absent, no detection is possible and results in disease (Grover and Gowthaman 2003). As a consequence of putative adherence of these two host–pathogen proteins, a signaling cascade is triggered and results in a variety of plant defense mechanisms (Tyler 2002). The immune resistances are concerned with confinement of pathogens spreading out. Plants have several polymorphic R genes and their products which enable them to function as a receptor for Avr proteins secreted by several pathogens (Health 2000; Petit-Houdenot and Fudal 2017).
Plant produces many low molecular weight antibiotic phytoalexin in response to infection (Loser and Weltring 1998). Phytoalexins hold the development of bacterial and fungal pathogens in vivo and in vitro, and accumulation of these antibiotics throughout an infection can induce resistance against pathogen (Grayer and Kokubun 2001; Jeandet et al. 2014).
On the whole, structurally related phytoalexins are produced by plants of the same species and these phytoalexins enable a plant to introduce a toxic cocktail against encroaching pathogen (Ortega et al. 2005). These phytoalexins are produced in the cell adjacent to infected cell and delivered at the site of infection in the form of lipid vesicles, creating a noxious microenvironment and forbidding ecesic of pathogen. Accruement of phytoalexin is frequently linked with hypersensitive response, although phytoalexins are secreted by only living cells (Pusztahelyi et al. 2015).
Secondary metabolites like phenolic compounds having antimicrobial activity are mainly concerned with resistance against pathogens. Polyphenols are commonly found in plants and they have been reported to have numerous biological effects including antioxidant activity (Othman et al. 2019). Phenolic compounds function as phytoalexins, phytoanticipins, structural roadblocks, and regulators of pathogenicity or activators of plant defense genes against fungal bacterial and viral infection in plants (Tripathi et al. 2022). At the initial stage of defense, these phenolic compounds quickly accumulate at the site of infection, which limits or deliberate the pathogen growth and plays an essential part during resistance (Pratyusha 2022).
One of the important enzymes employed in the phenylpropanoid pathway that regulates stress by governing the synthesis of phenolic substances is Phenylalanine ammonia lyase (PAL; EC 4.3.1.5) (Wen et al. 2005; Zhang and Liu 2015). Lignin, phenolic substances, and phytoalexins add inflexibility and durability to cell walls and render barriers against pathogen (Conèeica et al. 2006; Lee et al. 2019). Involution of an antioxidant immune system in plants defends them against oxidative damage caused by ROS (reactive oxygen species) production or the cleaning of already produced reactive oxygen species (ROS) (Ali et al. 2018; Cavelcanti et al. 2007; Nita and Grzybowski 2016).
As, a result of environmental stimuli, the phenylpropanoid pathway signaling is a far-flung biochemical stress response (Chatterjee and Ghosh 2008). L-Phenylalanine is deaminated to trans-cinnamic acid by PAL (Barberan and Espin 2001), which is the initial step in the synthesis and accumulation of plants’ secondary metabolites like lignin and phytoalexins during pathogen attack (Ozyigit et al. 2007; Ramírez-Gómez et al. 2019). PAL also induces the synthesis of salicylic acid (SA) and it can regulate the defense mechanism by activating the synthesis of phenolic compounds which is responsible for inducing systemic resistance in many plants (Wen et al. 2005; Lefevere et al. 2020).
Therefore, several antioxidant catalysts such as peroxidase (POX; EC 1.11.1.7) involve in ROS production during the pathogenesis. POX is one of the key oxidoreductive enzymes that take part in cell wall synthesis like lignifications and suberization of host plant cells, oxidation of phenols during infection (Chittoor et al. 1999; Quiroga et al. 2005; Minatel et al. 2017).
Plants have developed a variety of defense mechanisms against fungi which make up the assembly of secondary metabolites, proteins, and peptides bearing antifungal activity (Keller 2019). Plants exhibit an innate immune system by developing resistance like alteration of cell wall composition and oxidative burst of cells that cure infection and biosynthesis of chemicals like phytoalexin and pathogenesis-related proteins (Nürnberger and Scheel 2001; Wan et al. 2021). Such resistance can be sparked by exposing the plant to pathogenic microbes, or synthetically by salicylic acid and jasmonic acid (Wu and Bradford 2003; Betsuyaku et al. 2018).
This type of resistance is called as Induced Systemic Resistance (IAR) or Systemic Acquired Resistance (SAR). Among all induced defense mechanisms, accumulation of “Pathogenesis Related (PR) proteins” is the most vital immunity of plants against pathogenic infection (Adrienne and Barbara 2006; Boccardo et al. 2019). For instance, degradation of chitin by chitinase is one of the key components of many pathogenic fungi cell walls (Bartnick 1968). These enzymes restrict fungal growth by inducing dissolution of hyphal tips (Mauch et al. 1988). The transfer of chitinase genes to other plants may reduce or hinder the disease symptoms (Grover and Gowthaman 2003).
For the development of resistant cultivars, quick methods are the need of the hour for the successful evaluation of host resistance and variability of pathogenic microorganisms (Chen et al. 2019). Alternative methods like detached leaf assay are often employed in place of whole plant evaluation and have several advantages also like precise quantification of disease development along with determination of pathogen reproduction (Aregbesola et al. 2020).
Chickpea (Cicer arietinum L.) is the second most important legume crop worldwide (Madurapperumage et al. 2021) (Fig. 14.1). Chickpea have high protein content, it is utilized as a vegetable and dry bean (Wallace et al. 2016). As, a supplement of high proteins cereals, legume crops are used as one of the most beneficial answers to protein malnutrition in developing countries (Maphosa and Jideani 2017). Fungus is major mutualistic symbionts and parasite of plants; hence, their analysis is an essential component of plant pathology and plant science (Vågsholm et al. 2020; Choudhary et al. 2012). The worldwide production of chickpea is approximately threefold of lentils and consumption is second most to dry beans among pluses (FAOSTAT 2017; ICARDA 1993). Chickpea is a self-pollinating crop and rarely cross-pollinated; only 0–1% is reported (ICRISAT 2014, 2019). The state-wise production of chickpeas is mentioned in Fig. 14.2.
Pathogenic fungi secrete phytotoxins causing damage to plant tissue having symptoms including necrosis, chlorosis, wilting, water soaking, and eventually death of plants. In various host–pathogen interactions phytotoxins have constituted as virulence factors which could be used for screening out resistant cells (Pontes et al. 2020). This soilborne fungal infection is among the most crucial biotic factors confining the yield of chickpea, leading to severe monetary loss (Tarafdar et al. 2018).
Macrophomina phaseolina (Tassi) goid a soil inhabiting fungal phytopathogen, causes charcoal rot in over 500 plant species including chickpea causing a major loss in its yield (Ghosh et al. 2016). It continues to be a disputing task in managing the soilborne disease in natural environment. It is dispersed universally and is predominant in arid, tropical, and subtropical regions, especially areas having very high temperatures and low rainfall. It is a key phytopathogenic fungus infecting a huge number of plant species and existing for up to 15 years in the soil as a saprophyte (Kaur et al. 2022). It causes severe yield losses in epidemic years (Degani et al. 2022). Climate conditions of the desert support the growth and survival of M. phaseolina hence; disease is quite common in Rajasthan and leads to heavy losses of this crop (Marquez et al. 2021).
M. phaseolina is considered as one of the soilborne fungi that infect large numbers of host plants through roots, stems, and crown regions, near the soil surface (Sinha et al. 2016). It causes several plant diseases as charcoal rot. M. phaseolina is considered as one of the soilborne fungi that infect a large number of host plants through roots, stems, and crown regions near the soil surface (Arora and Dhurwe 2019). It causes several plant diseases such as charcoal rot, leaf blight, and damping off. So, the fungus caused great economic losses. Some physiological factors that affect the growth and pathogenicity of M. phaseolina were indicated. Root infections due to soilborne pathogens are often primary restraints in the production of legume crops (Ijaz et al. 2012; Wolfgang et al. 2019). Screening of germplasm to obtain resistance or tolerant cultivar may be vital and can be used as a basis of resistance indicator in plant breeding programmes (Zhou et al. 2018).
M. phaseolina releases a numeral of phytotoxins, such as asperlin, isoasperlin, phaseolinic acid, phaseolinone, phomalactone, and phomenon (Mahato et al. 1987; Nallasivam and Fernandes 2017). Phaseolinone and phaseolinic acid seem most significant of others that cause symptoms of disease in plants comparable to those caused by the disease-causing microbes (Mathur 1968; Dhingra and Sinclair 1985; Siddiqui et al. 1979; Bhattacharya et al. 1992). The release of phaseolinone phytotoxin during the infection caused by M. phaseolina and defended by the crucial role of cell wall degrading enzymes in the disease unveiling and procession (Chan and Sackston 1973).
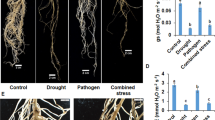
Many new emerging plant diseases are surfaced due to the increasing temperature globally at an alarming rate (Velásquez et al. 2018; Chobe et al. 2020; Palit et al. 2020) and one of them is root rot disease of chickpea and the etiological agent is Macrophomina phaseolina, a soilborne necrotrophic fungus (Marquez et al. 2021; Dell’Olmo et al. 2022). Poor apical growth, yellowish or dull green leaf, reduced vigor, and twig dieback are the symptoms shown by the plant infected with dry root rot disease (Chobe et al. 2019). Further, leaves suddenly start wilting and dried on the trees, if the extent of disease is severe (Garrett et al. 2016). Recent studies identified the southern and central states of India with more than 35% disease incidence as dry root rot hotspots (Abbas et al. 2020). There are many assumptions and one assumption suggests that climate change is the principal cause. One of the reasons identified for disease spread is rising temperature (30–35 °C) and dry soil conditions (less than 60%) which are suitable for disease progression (Ghosh et al. 2017; Adhikary et al. 2019). Scientific reports suggested that the pathogen can tolerate harsh environmental conditions such as extremities pH, temperature, and drought (Almomani et al. 2013; Burdon and Zhan 2020). Chickpea is more infection prone during flowering and podding season coupled with drought and higher temperature coincidently (Devi et al. 2022). The focus of scientists is to develop more disease-resistant varieties with better management methodology (Hatmi et al. 2015).
Chickpea Plants Get Diseases in These Conditions
They are in search of more disease favorable factors and strategies to counter the disease at the molecular level. Recently, a few genes were identified from chickpea genome which encodes for defense enzymes like chitinase and endochitinase (Iriti and Faoro 2009). Both enzymes are suitable tools for the management of dry root rot disease (Veliz et al. 2017). As stated earlier, climate change and global warming tilt the balance of hosts and pathogens toward pathogens seriously (Karthik et al. 2021).
The epidemiology of plant diseases is depended upon several environmental and climatic factors (Sharma et al. 2019), as life of pathogens are profoundly affected by the amount of greenhouse gases, humidity, rainfall, soil moisture and temperature (Sharma et al. 2015). Hence, to develop an insight of knowledge of host–pathogen interaction, studies should be taken in multivariant scenarios at cellular, physiological, and molecular levels and should also include both abiotic and biotic stress factors (Singh et al. 2021).
In many economically important plant species, the biochemical basis of disease resistance remains unexplored as yet. Such studies are not available in many common and economically important crops of Rajasthan including Chickpeas. Further, there are no reports as yet on the biochemical basis of disease resistance of the rot disease caused by M. phaseolina in Cicer arietinum plants.
The present work is focused on the consequence of fungus M. phaseolina on the growth of chickpea plants via monitoring various biochemical parameters which play important role in defense response upon infection with the pathogen (Ramegowda and Senthil-Kumar 2015). Comparative studies on the effect of M. phaseolina toxin in chickpea as well as other host plants like Brassica oleracea, Brassica juncea, Helianthus annus, and Vigna mungovia detached leaf bioassay method are also planned.
14.2 Historical Backgrounds
Physiology of disease resistance is one of the most attractive areas of research. Rather, they depend upon the natural defense response of individual cells and receive the systemic signals from the zone of infection (Amil-Ruiz et al. 2011; Nishad et al. 2020). Pathogenic microorganisms employ different life strategies that can be generally classified into obliterate the host and feed upon the substances (necrotrophs) and those that utilize a surviving host to finish their life cycle (biotrophs) (Moënne-Loccoz et al. 2015). Pathogenic necrosis is frequently caused by accumulation of poisonous substances (Abu Qamar et al. 2006). Pathogenic fungus multiply in apoplast (intercellular spaces) either inserting through stomata or hydathodes or lesions (Melotto et al. 2008; Bos et al. 2010) whereas fungi directly enter epidermal cells or enlarge hyphae on top among plant cells (Craig et al. 2009; Ojha et al. 2008; Anand et al. 2007; MikuliPetkovšek et al. 2009; Amil-Ruiz et al. 2011).
Among the antimicrobial substances produced by the plants, phenolics are the most important one. Synthesis of PAL is increased in many plant species as they detect any sort of stress such as herbicide intervention, injury, nutrient deficiency, radiation, and microbial attack (Donkor et al. 2019).
Induced disease resistance can be defined as the progression of active resistance dependent on the host plant’s chemical or physical barriers started by abiotic or biotic agents, it sensitizes the host to act in response rapidly after detecting infection in the form of accumulation of phytoalexin, phenols, lignifications, pathogenesis-related (PR) proteins and elicitation of enzymes like chitinase, peroxidase, and polyphenol oxidase (Aal et al. 2019). Induced disease resistance in plants have positive effects on plant growth and yield all of which are important characteristics for disease management strategies (Lawton et al. 1995). Several publish reports by many investigators that resistance could be systemically induced by the interaction of both biotic and abiotic stress like chemical compounds with plants. Many chemical compounds are known to induce resistance by activating defense networks in plants. Several investigators studied the effectiveness of these chemical inducers on charcoal rot disease incidence caused by M. phaseolina (Mohammad et al. 2007; El-Fiki et al. 2004). Inducer of systemic resistance sensitizes the plant to respond rapidly after infection (Glazebrook et al. 1997).
14.2.1 Host–Pathogen Interaction
There are two classes of plant resistance, i.e., host specific and nonhost specific. An interaction between a specific genotype of the host and the pathogen is categorized into host-specific resistance, also called pathogen race-specific resistance. The resistance exhibited by the whole plant species toward a specific pathogen or parasite is categorized into nonhost-specific resistance. Resistance of plant species against common pathogens is included in nonhost-specific resistance (Health 2000). However, the biochemical changes during the course of infection are almost similar in both host and nonhost-resistant plants (Somssica and Hahlbrock 1998).
14.2.1.1 Systemic Acquired Resistance
Host plants have come through from previous infection by plant pathogens then it can automatically protect against further pathogen infections. It seems that disease-causing pathogens protect the plant from further infections against homologous pathogens, although a plant may or may not carry the resistance-specific gene. The presence of these resistance-specific genes forces the plant to protect itself from subsequent microbial infection. This kind of reaction is termed Systemic Acquired Resistance (SAR). The establishment of SAR in a plant is linked with many defense reactions, like pathogenesis-related proteins and many defense-associated enzymes (Neuenschwander et al. 1996). SAR refers to a distinct signal transduction pathway that plays an important role in the ability of plants to defend themselves against the pathogen (Ryals et al. 1996; Gozzo 2003). Salicylic acid (SA) is widely distributed in plants (Raskin 1990) and plays a key role in SAR. In addition, certain natural and synthetic compounds can trigger similar plant responses (Kessmann et al. 1994; Metraux et al. 1990). The phenylalanine pathway is the main route of SA biosynthesis (Ogawa et al. 2006).
14.2.1.2 Salicylic Acid
Salicylic acid, present naturally in very low quantities in plants, is an endogenous growth regulator (Dempsey and Klessig 2017). It is the precursor of aspirin normally found in plants. It reacts with two proteins of the cell membrane. One of the proteins has catalase activity and this catalase activity is stopped when reacts with salicylic acid, resulting in the accumulation of hydrogen peroxide at the binding site locally (Ribnicky et al. 1998) (Fig. 14.3). Such form of accumulation of hydrogen peroxide brings about many reactions in cells that finally augment their resistance to pathogens (Durrant and Dong 2004). Another protein that has a high affinity to salicylic acid directly starts the expression of the gene for resistance (Zhang et al. 2007; Heidel et al. 2004).
14.2.2 Elicitors and Their Functions
Initially, the elicitor molecules only comprise phytoalexin but at present elicitors are a group of molecules generally used against different pathogen infections (Nürnberger 1999). Elicitors are divided into different classes on the basis of physical or chemical properties, types of biotic and abiotic stresses, and on the basis of their evolution and molecular organization (Table 14.1). Elicitors are classified into two main classes, “general elicitors” and “race specific elicitors” (Bektas and Eulgem 2015). General elicitors are involved in the resistant mechanism of specific and nonspecific host plants, while race-specific elicitors are able to activate pathogen resistance in particular host cultivars (Radman et al. 2003).
14.2.3 Mechanism to Defense Responses
There are multilevel complicated surveillance mechanisms found in plants by which they identify the pathogenic microorganisms and take evasive actions earlier than the pathogen would attack and cause harm to the plants. These surveillance systems are coordinated by the pre-programmed defense responses designated to the specific plant species. Innate immunity or basal resistance is the first line of defense toward the pathogen class or family. Pathogen-associated molecular patterns (PAMPs) are specific macromolecules like carbohydrates, lipids, lipopolysaccharides, proteins, and other components of microbial class or family. When PAMPs are detected by the plant cells, they trigger the basal resistance and in response plant cells prepare themselves against microbial invasions-related consequences (Rapicavoli et al. 2018). This basal resistance reacts equally for pathogenic as well as nonpathogenic microorganisms due to universal presence of these molecules (Holzmueller et al. 2007). On the other hand, pathogens responded with countermeasures to defeat the basal resistance of plants. If pathogens successfully overcome the basal resistance, then plants use another mechanism for defense: the hypersensitive response (HR) (Fig. 14.4) (Wenjuan et al. 2009).
14.2.4 Hypersensitive Responses
To activate a hypersensitive response, physical or physiological contact is necessary between pathogen and host (Health 2000). The phenomenon of HR was described in wheat plants infected with rust fungi by Stakman 1915. Hypersensitivity is a hyperimmune response shown by the host activated by the pathogen after entering the host cell (Kortekamp and zyprian 2003), and this sometimes causes death of host cell and confined the pathogen at the site. Researchers reported that apoptosis in animals is very much similar to HR in plants (Morel and Dangl 1997). Now, the above definition is made broader by the inclusion of defense gene expression (Iakimova et al. 2005). A plant is able to survive if it could identify the possible threat of different pathogens and respond appropriately through proper defense mechanisms (Nishad et al. 2020), and this event is the result of R gene of host plant and Avr gene of pathogenic microorganisms (Flor 1971). This Avr-R gene binding starts the appropriate host plant defense and results in the death of host cell also termed as HR (Levine et al. 1996). The product of R gene either works as a receptor for the Avr gene products (Yang et al. 1996) or recognizes the Avr factor indirectly through a co-receptor as supported by accumulating evidence (Dixon et al. 1994). Although resistance responses are often associated with HR, in some cases, they can occur without or with very little cell death (Bendahmane et al. 1999; Ehrenfield et al. 2005).
14.2.5 Phytoalexin
Plants produces and accumulate some antimicrobial compounds in plants when attacked by bacterial or fungal pathogen called phytoalexin (Wilkens et al. 2010). Phytoalexins are also produced in response to non-biological factors like experimental exposure to the plant with ions of heavy metals (Cu or Hg) and with UV light of a short wavelength (Grayer and Kokubun 2001). Phytoalexins are well documented in the field of plant defense. Much research has been conducted on the elicitation process and specific elicitors have been discovered (Dakora and Phillips 1996). Phytoalexins are thought to be synthesized in a cell adjacent to the infection site, in response to a signal produced either by the invading pathogen or by infected host cells (N’Guyen et al. 2021). The infected cell becomes a toxic microenvironment for the invading pathogen (Wharton and Nicholson 2000).
14.2.6 Phenylalanine Ammonia Lyase
PAL (E.C.4.3.1.5) is the enzyme that catalyze core reactions in phenylpropanoid metabolism leading to the production of plant phenolics including those that are produced under stress (Kliebenstein 2004; Jones 1984). PAL has been generally recognized as a marker of environmental stress in different plant species (MacDonald and D’Cunha 2007). It contributes to deamination of L-phenylalanine to cinnamic acid. Since PAL activity is known to affect the accumulation of polyphenols any stress such as fungal pathogen inoculation or mechanical wall injury will alter its level and consequently its products. Thus, activation of defense response pathway can be detected by measuring the PAL activity after pathogen inoculation (Lavanya et al. 2022) (Fig. 14.5).
14.2.7 Oxidative Burst
The oxidative burst is generally defined as a rapid production of high levels of ROS in response to external stimuli (Dangl and Jones 2001; Mittler et al. 2004). Hydroxyl radicals, hydrogen peroxide, and superoxide anions are the most important members of ROS (Apel and Hirt 2004). All of these species are extremely reactive and toxic which can lead to the oxidative demolition of host cells (Gechev et al. 2006). The first evidence of production of ROS came into light in 1983 when potato tuber disks were inoculated with an incompatible race of Phytophthora infestans, and superoxide radicals were generated and then transformed into hydrogen peroxide (Doke 1983). Similar findings were reported by the interaction of avirulent strain of Pseudomonas syringae strain DC300 with Arabidopsis thaliana (Alvarez et al. 1998). These investigations confirm the fact that the production of ROS in plants is thought to play an important signaling role in the activation and establishment of plant defense (Huang et al. 2019; Levine et al. 1994).
14.2.8 Peroxidase
Peroxidase is a member of the oxidoreductases (E.C.1.11.1.7) family, catalyses the oxidation of various inorganic and organic substrates in the presence of H2O2 (Koksal and Gulcin 2008). They were also reported to mediate plant defense against biological stresses (Prasannath 2017). Peroxidases worked both in favor of plants as well as against them also, some fractions of peroxidases promote growth by their indole acetic acid and oxidase activity (Takahama and Yoshitama 1998), while on the other hand, certain fractions cause cell wall rigidification and tissue lignifications resulted in retardation of growth (Passardi et al. 2005). Peroxidases studied in date palms considering their relationships with cultivar resistance to Bayoud disease (Gay and Tuzun 2000). For example, a positive correlation was established between decline in occurrence of Fusarium wilt in banana and induction of defense-related enzymes peroxidase and polyphenol oxidase (Sauerborn et al. 2002).
14.2.9 Polyphenols
Phenolic compounds are a large class of plant secondary metabolites distributed widely in the plant kingdom. Plants synthesize a variety of aromatic substances and a majority of them are either phenols or their oxygen-substituted derivatives (Othman et al. 2019; Ozyigit et al. 2007). Most of them are produced as secondary metabolites as simple phenols and phenolic acids, alkaloids, essential oils, flavones, flavonoids, flavonols, quinones, tannins, and terpenoids (Tungmunnithum et al. 2018). All of these compounds contain one or more than one benzene rings with attached hydroxyl groups that may be variously elaborated with methyl, methoxyl, amino, or glycosyl groups (Kumar and Goel 2019). Various researchers reported that a substantial increase in the production of phenolics is observed in plants in response to fungal attacks (Li and Steffens 2002).
14.2.10 Toxins of M. phaseolina
M. phaseolina was grown in vitro and it was first reported that the fungus produces phytotoxic substances and the symptoms were alike of that pathogen produces in plants (Mathur 1968). A variety of phytotoxin specifically phaseolinone, phomenon, phaseolinic acid, phomalactone, isoasperlin and asperlin are reported to be produced by M. phaseolina (Dhar et al. 1982; Mahato et al. 1987; Bhattacharya et al. 1992). Several plant pathogenic fungi produce phytotoxins related to disease progression, which interact with the cell machinery. Later one toxin was named phaseolinone (Siddiqui et al. 1979) (Fig. 14.6), later this toxin become the most studied toxin which persuades disease symptoms in host plant (Junior et al. 2021). It is a nonspecific exotoxin that is highly stable and nonbiodegradable even at high temperatures. The toxin affects germination of seeds, growth of seedlings, and tissue culture necrosis during regeneration and callusing (Abbas et al. 2018). It also inhibits the germination of seeds and causes wilting of seedlings (Vishal and Kumar 2018).
The isolation and structure of another toxin, phaseolinic acid were reported from the culture of filtrate of M. phaseolina (Mahato et al. 1987). This toxin did not affect germination of seeds or seedling growth but was able to induce nonspecific leaf necrosis in several plants (Rather et al. 2020).
14.2.11 Pathogenic-Related Protein
Pathogenesis-related proteins were first described by Van Loon, who observed the accumulation of various novel proteins in leaves of tobacco by tobacco mosaic virus (van Loon and van Strien 1999). Plant and pathogen interaction results in infection or plant avert the infection (Abdulkhair and Alghuthaymi 2016). Like animals, plants do not have circulating phagocytes, however, to prevent microbial invasion they have a complex thick cell wall (Tyagi et al. 2014). Plants develop various defense mechanisms of innate pathogen-specific resistance such as varying composition of cell wall, synthesis of phytoalexins, oxidative burst, and pathogenesis-related proteins (Andersen et al. 2018). All of these responses are produced by plants equally whether the microorganism is virulent, nonvirulent, or nonpathogenic and can be induced artificially by low molecular weight chemical compounds and by using volatile molecules like jasmonate and salicylic acid (Wu and Bradford 2003), 2,6-Dichloro-isonicotinic acid or benzo (1,2,3) thiadiazole-7-carbothioic acid Smethyl ester (BTH) (Vallad and Goodman 2003). Such a type of response by plants is termed as Induced Systemic Resistance (IAR) or Systemic Acquired Resistance (SAR) (Oostendorp et al. 2001).
Among the responses exhibited by the host plant, production of “Pathogenesis Related (PR) proteins” is considered most important as these proteins provide systemic resistance to whole plant upon microbial invasion (Adrienne and Barbara 2006). These PR proteins are synthesized by a number of organisms throughout all kingdoms which are low molecular weight, small, cysteine rich, and basic in nature. PR proteins vary in their mechanism of action, species specificity, and primary structure (Leiter et al. 2005).
At present, 17 families have been assigned to PR proteins as per their structure, size, and properties are given in Table 14.2 (Van Loon et al. 2006).
14.2.12 Chitinase and Function in the Plant
Fungal cell wall is made by a polymer chitin (Garcia-Rubio et al. 2020) and enzyme chitinases (E.C. 3.2.1.14) catalyze the cleavage of bond between C1 and C4 of two successive N-acetyl-D-glucosamine units of chitin (Zeyen et al. 2002; Krolicka et al. 2018). Chitinases are produced by animals, plants, bacteria, and fungi. Plant produces endo-chitinases and works on fungal hyphae after invasion and hamper growth of pathogenic fungi (Sharma et al. 2016). Previous investigators investigated in vitro growth inhibition of fungi with plant chitinases (Mauch et al. 1988; Pusztahelyi 2018). The first report of chitinase induction was reported against Fusarium solani by pea plant. The protein extracted from plant was tested against 18 fungi and was found effective toward 15 fungi (Bettini et al. 1998).
One purified chitinase may inhibit only one or the same cell wall compositional fungal pathogen while a consortium of chitinases, β-1, 3-glucanase, and PR protein inhibit most of the tested fungi and establish the fact that synergistic activity is must for fungal growth inhibition (Veliz et al. 2017). A number of studies verified these results in tobacco, grapes, and chickpea (Giri et al. 1998). These investigations provide insight that a specific chitinase enzyme is produced by the host against a specified pathogen and this may not affect other pathogens. Tobacco plants produce the enzyme chitinase class I in response to F. solani (Jach et al. 1995). Several reports indicated that expression of chitinases in response to fungal pathogens is more powerful in resistant varieties when compared with susceptible varieties of tomato wheat and sugar beet (Lawrence et al. 2000; Manghwar et al. 2021).
Plants produce elicitor compounds which result in disease resistance through PR gene expression quickly and affect the cell wall synthesis of germinating fungal spores (Lavanya et al. 2022). On the basis of the primary structure of chitinase, plant chitinases have been divided into seven classes, from class I to class VII (González et al. 2015). Class III chitinases are distinctive in structure and have no similarity with other classes of plant chitinases (Gomez et al. 2004). These chitinases belong to the PR-8 family and family 18 of glycosyl hydrolases (Chen et al. 2019, 2020).
14.3 Conclusion
Chickpea (Cicer arietinum) is a self-pollinated, cool season, and rabi legume. India is the largest producer of chickpea which is the second most essential cool seasonal crop in the world. The major production areas of chickpeas are located in Maharashtra, Uttar Pradesh, Madhya Pradesh, Punjab, Rajasthan, and Haryana. Chickpea in semiarid regions suffers from charcoal root rot diseases induced by Macrophomina phaseolina (Tassi) Goid. Macrophomina phaseolina is a soil inhabiting root fungus so it is difficult to remove the pathogen from spreading the disease. This pathogen-induced disease is a global problem worldwide and is predominant in the area having scanty rainfall and high temperature in regions like arid, subtropical, and tropical areas. Hence, the present work has attempted to screen varieties of chickpeas. A positive correlation was found between peroxidase activity and resistant response of the varieties. The screening of germplasm to obtain resistant varieties is important because these can be used in plant breeding programmes, breeding lines, and mapping population for M. phaseolina.
The estimation of phenylalanine ammonia lyase, phenols, salicylic acid, and total protein activity of both control and inoculated plants were determined for its correlation with resistant response.
The observed value of polyphenols content reveals that when chickpea plant has been infected with pathogen by foliar suspension of mycelium, its phenolics concentration rises as consequence of resistance to disease. The polyphenol content has been observed to increase in inoculated plants in comparison to the control one.
PAL is the main enzyme in inducing the synthesis of salicylic acid which induces systemic acquired resistance in the host. In the present study, the resistant genotype inoculated with fungus showed increased PAL activity. The susceptible genotype showed decreased PAL activity.
Throughout pathogen infection, the secretion of salicylic acid soon increases in host with the increase in PAL catalysis, in order to combat increasing infection in plants by activating SAR.
Detached leaf assay is a rapid and economical way to do pathogenicity tests and the procedure has been used to study many host–pathogen interaction systems in dicots. The detached leaf assay is a rapid and reliable method to evaluate germplasm, breeding lines, and mapping population for M. phaseolina resistance. The method can be also used to monitor symptom development and infection parameters over time.
To expand insight into the molecular basis of resistance, defense-related genes were analyzed for their expression levels in response to pathogen attack. The gene selected for analysis was chickpea chitinase. It was observed that expression levels of chitinase genes were significantly higher in the resistant variety as compared to the susceptible one. A good correlation was observed between chitinase activity and fungal pathogen resistance.
From the results presented here, it can be inferred that the inoculation of chickpea with M. phaseolina results in activation of host defense system. Further investigations about factors that impart in induction and regulation of this gene expression may help to provide a better comprehension of plant–microbe interactions and mechanisms of plant responses to infection. Moreover, the results of these studies can be useful for constructing transgenic-resistant plants. Overall findings may suggest the mechanism of induced resistance in chickpea plants and together with the study of toxins and mechanism of gene regulation will open new and fascinating ways for plant genetic engineering for crop improvement.
Abbreviations
- Avr gene:
-
Avirulence gene
- F. solani :
-
Fusarium solani
- FAOSTAT:
-
Food and agriculture organization corporate statistical database
- H2O2:
-
Hydrogen peroxide
- HR:
-
Hypersensitive response
- IAR:
-
Induced systemic resistance
- ICARDA:
-
International Center for Agricultural Research in the Dry Areas
- ICRISAT:
-
International Crops Research Institute for Semi-Arid Tropics
- M.P :
-
Macrophomina phaseolina
- PAL:
-
Phenylalanine
- PAMPs:
-
Pathogen-associated molecular patterns
- PR proteins:
-
Pathogenesis-related proteins
- R gene:
-
Resistant gene
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- SAR:
-
Systemic acquired resistance
- UV:
-
Ultraviolet
References
Aal AEA, Abd-El-Kader DA, Khalifa MMA, Ali MAS (2019) Management of peanut cercospora leaf spot using resistant cultivars and inducer resistance chemicals. J Agric Res 46(3):665–684
Abbas MS, El-Shabrawi HM, Soliman AS, Selim AM (2018) Optimization of germination, callus induction, and cell suspension culture of African locust beans Parkia biglobosa (Jacq.). Benth J Genet Eng Biotechnol 16(1):191–201. https://doi.org/10.1016/j.jgeb.2017.10.012
Abbas HK, Bellaloui N, Butler AM, Nelson JL, Abou-Karam M, Shier WT (2020) Phytotoxic responses of soybean (Glycine max L.) to Botryodiplodin, a toxin produced by the charcoal rot disease fungus. Toxins (Basel) 12:25. https://doi.org/10.3390/toxins12010025
Abdulkhair WM, Alghuthaymi MA (2016) Plant pathogens. In: Plant growth. IntechOpen. https://doi.org/10.5772/65325
AbdulMalik NA, Kumar IS, Nadarajah K (2020) Elicitor and receptor molecules: orchestrators of plant defense and immunity. Int J Mol Sci 21(3):963. https://doi.org/10.3390/ijms21030963
Abu Qamar S, Chen X, Dhawan R, Bluhm B, Sameron LS, Dietrich RA, Mengiste T (2006) Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to botrytis infection. Plant J 48:28–44
Adhikary NK, Chowdhury MR, Begum T, Mallick R (2019) Integrated management of stem and root rot of sesame (Sesamum indicum L.) caused by (Tassi) Goid. Int J Curr Microbiol Appl Sci 8:804–808. https://doi.org/10.20546/ijcmas.2019.804.089
Adrienne CS, Barbara JH (2006) Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot Cell 5:1941–1949
Agrios GN (2005) (2005) Plant disease caused by nematodes. In: Agrios GN (ed) Plant pathology, 5th edn. Elsevier Academic, London, p 608
Ali M, Cheng Z, Ahmad H, Hayat S (2018) Reactive oxygen species (ROS) as defenses against a broad range of plant fungal infections and case study on ROS employed by crops against Verticillium dahliae wilts. J Plant Interact 13(1):353–363. https://doi.org/10.1080/17429145.2018.1484188
Almomani F, Alhawatema M, Hameed K (2013) Detection, identification and morphological characteristic of: the charcoal rot disease pathogens isolated from infected plants in Northern Jordan. Arch Phytopathol Plant Prot 46:1005–1014. https://doi.org/10.1080/03235408.2012.756174
Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in establishment of plant immunity. Cell 92:773–784
Amil-Ruiz F, Blanco-Portales R, Muñoz-Blanco J, Caballero JL (2011) The strawberry plant defense mechanism: a molecular review. Plant Cell Physiol 52:1873–1903
Anand T, Thiruvergadam R, Gandhi K, Velapan P, Ramasamy S (2007) Chemically and biologically mediated systemic resistance in cucumber (Cucumis sativus L.) against Pseudoperonospora cubensis and Erysiphe cichoracearum. Phytopathol Meditrr 46:259–271
Andersen EJ, Ali S, Byamukama E, Yen Y, Nepal MP (2018) Disease resistance mechanisms in plants. Genes 9(7):339. https://doi.org/10.3390/genes9070339
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Aregbesola E, Ortega-Beltran A, Falade T, Jonathen G, Hearne S, Bandopadhyay R (2020) A detached leaf assay to rapidly screen for resistance of maize to Bipolaris maydis, the causal agent of southern corn leaf blight. Eur J Plant Pathol 156:133–145. https://doi.org/10.1007/s10658-019-01870-4
Arora MK, Dhurwe U (2019) causal organism of charcoal rot: review article. East Afr Scholars J Agric Life Sci 2(2). https://doi.org/10.36349/easjals.2019.v02i02.006
Balint-Kurti P (2019) The plant hypersensitive response: concepts, control and consequences. Mol Plant Pathol 20(8):1163–1178. https://doi.org/10.1111/mpp.12821
Barberan TF, Espin JC (2001) Phenolics compounds and related enzymes as determinants of quality of fruits and vegetables. J Sci Food Agric 81:853–876
Bartnick GS (1968) Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol 22:87–108
Barzman M, Bàrberi P, Birch ANE, Boonekamp P, Graf B, Hommel B, Jensen JE, Kiss J, Kudsk P, Lamichhane JR, Messéan A, Moonen A, Ratnadass A, Ricci P, Sarah J, Sattin M (2015) Eight principles of integrated pest management. Agron Sustain Dev 35:1199–1215. https://doi.org/10.1007/s13593-015-0327-9
Bektas Y, Eulgem T (2015) Synthetic plant defense elicitors. Front Plant Sci 5:804. https://doi.org/10.3389/fpls.2014.00804
Bendahmane A, Kanyuka K, Baulcombe DC (1999) The Rx gene from potato controls separate virus resistance and cell death response. Plant Cell 11:781–792
Betsuyaku S, Katou S, Takebayashi Y, Sakakibara H, Nomura N, Fukuda H (2018) Salicylic acid and jasmonic acid pathways are activated in spatially different domains around the infection site during effector-triggered immunity in Arabidopsis thaliana. Plant Cell Physiol 59(1):8–16. https://doi.org/10.1093/pcp/pcx181
Bettini P, Cosi E, Pellegrini MG, Turbanti L, Vendramin G, Buiatti M (1998) Modification of competence for in vitro response to Fusarium oxysporum in tomato cells. III. PR-protein gene expression and ethylene evolution in tomato cell lines transgenic for phytohormone-related bacterial genes. Theor Appl Genet 97:575–583
Bhattacharya D, Dhar TK, Ali E (1992) An enzyme immunoassay of phaseolinone and its application in estimation of the amount of toxin in -infected seeds. Appl Environ Microbiol 58:1970–1974
Boccardo NA, Segretin ME, Hernandez I, Mirkin FG, Chacon O, Lopez Y, Borrás-Hidalgo O, Bravo-Almonacid FF (2019) Expression of pathogenesis-related proteins in transplastomic tobacco plants confers resistance to filamentous pathogens under field trials. Sci Rep 9:279. https://doi.org/10.1038/s41598-019-39568-6
Bos JIB, Prince D, Pitino M, Maffei ME, Win J, Hogenhout SA (2010) A functional genomics approach identifies candidate effectors from the aphid species Myzuspersicae (Green Peach Aphid). PLoS Gen 6:e1001216
Burdon JJ, Zhan J (2020) Climate change and disease in plant communities. PLoS Biol 18(11):e3000949. https://doi.org/10.1371/journal.pbio.3000949
Canesi L, Pruzzo C (2016) Specificity of innate immunity in bivalves: a lesson from bacteria. Environ Sci. https://doi.org/10.1016/B978-0-12-803252-7.00006-0
Cavelcanti FR, Resende ML, Lima SP, Silveira JA, Oliveira JT (2007) Activities of antioxidant enzymes and photosynthetic responses in tomato pre-treated by plant activators and inoculated by Xanthomonas vesicatoria. Physiol Mol Plant Pathol 68:198–208
Chan YH, Sackston WE (1973) Non-specificity of the necrosis inducing toxin of Sclerotium bataticola. Can J Bot 51:690–692
Chatterjee A, Ghosh SK (2008) Alteration in biochemical components in mesta plants infected with yellow vein mosaic disease. Brazilian J Plant Physiol 20:267–275
Chen A, Sun J, Matthews A, Armas-Egas L, Chen N, Hamill S, Mintoff S, Tran-Nguyen LTT, Batley J, Aitken EAB (2019) Assessing variations in host resistance to Fusarium oxysporum f sp. cubense Race 4 in Musa species, with a focus on the subtropical race 4. Front Microbiol 10:1062. https://doi.org/10.3389/fmicb.2019.01062
Chen W, Jiang X, Yang Q (2020) Glycoside hydrolase family 18 chitinases: the known and the unknown. Biotechnol Adv 43:107553. https://doi.org/10.1016/j.biotechadv.2020.107553
Chickpea: An ancient crop for the modern world. http://exploreit.icrisat.org/page/chickpea/685/60, ICRISAT, Downloaded 26 January 2014
Chittoor JM, Leach JE, White FF (1999) Induction of peroxidase during defense against pathogens. In: Datta SK, Muthukrishnan S (eds) Pathogenesis related proteins in plants. CRC Press, Boca Raton, pp 171–193
Chobe DR, Singh R, Tarafdar A, Chandran US, Ghosh R, Sharma M (2019) Deciphering the response of putative mutants against Rhizoctonia bataticola [(Taub.) Butler] causing dry root rot of chickpea. J Mycol Plant Pathol 49(4):358–367
Chobe DR, Tarafdar A, Chandran USS, Sudharani SM (2020) First report of Ectophoma multirostrata causing root rot in chickpea. Plant Dis 104:1866–1866. https://doi.org/10.1094/PDIS-01-19-0121-PDN
Choudhary MI, Zafar S, Khan NT, Ahmad S, Noreen S, Marasini BP, Al-Khedhairy AA, Rahman AU (2012) Biotransformation of dehydroepiandrosterone with and β-glucuronidase inhibitory activity of transformed products. J Enzyme Inhib Med Chem 27:348–355
Conèeica LF, Ferreres F, Tavores RM, Dios AC (2006) Induction of phenolic compounds in Hypericum pertoralum L. cells by Colletotricohum gloeosprioides elicitation. Phytochemistry 67:149–155
Craig A, Ewan R, Mesmar J, Gudipati V, Sadanandom A (2009) E3 ubiquitin ligases and plant innate immunity. J Exp Bot 60:1123–1132
Dakora FD, Phillips DA (1996) Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol Mol Plant Pathol 49:1–20
Dangl JL, Jones JD (2001) Plant pathogens and integrated defense responses to infection. Nature 411:826–833
Degani O, Becher P, Gordani A (2022) Pathogenic interactions between Macrophomina phaseolina and Magnaporthiopsis maydis in mutually infected cotton sprouts. Agriculture 12:255. https://doi.org/10.3390/agriculture12020255
Dell’Olmo E, Tripodi P, Zaccardelli M, Sigillo L (2022) Occurrence of on chickpea in Italy: pathogen identification and characterization. Pathogens. 11(8):842. https://doi.org/10.3390/pathogens11080842
Dempsey DA, Klessig DF (2017) How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans? BMC Biol 15:23. https://doi.org/10.1186/s12915-017-0364-8
Devi P, Jha UC, Prakash V, Kumar S, Parida SK, Paul PJ, Prasad PVV, Sharma KD, Siddique KHM, Nayyar H (2022) Response of physiological, reproductive function and yield traits in cultivated chickpea (Cicer arietinum L.) under heat stress. Front Plant Sci 13:880519. https://doi.org/10.3389/fpls.2022.880519
Dhar TK, Siddiqui KAI, Ali E (1982) Structure of phaseolinone, a novel phytotoxin from Macrophomina phaseolina. Tetrahedron Lett 23:5459–5462
Dhingra OD, Sinclair JB (1985) Basic plant pathology methods. CBS Publishers and Distributors Delhi, 435 p
Dixon RA, Harrison MJ, Lamb CJ (1994) Early events in the activation of plant defense responses. Annu Rev Phytopathol 32:479–501
Dodds PN, Rafiqi M, Gan PH, Hardham AR, Jones DA, Ellis JG (2009) Effectors obiotrophic fungi and oomycetes: pathogenicity factors and triggers of host resistance. New Phytol 183:993–1000
Doke N (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber to infection with an incompatible race of Phytophthora infestans and to the hypal wall components. Physiol Plant Pathol 23:345–357
Donkor D, Mirzahosseini Z, Bede J, Bauce E, Despland E (2019) Detoxification of host plant phenolic aglycones by the spruce budworm. PLoS One 14(5):e0208288. https://doi.org/10.1371/journal.pone.0208288
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
Ehrenfield N, Canon P, Stance C, Medina C, Arce-Johnson P (2005) Tobamovirus coat protein CPCg induces an HR-like response in sensitive tobacco plants. Mol Cells 19:418–427
El-Fiki AII, Mohamed FG, El-Deeb AA, Khalifa MMA (2004) Some applicable methods for controlling sesame charcoal rot disease () under greenhouse conditions. Egypt J Phytopathol 32:87–101
El-Gamal Nadia G, Abd-El-Kareem F, Fotouh YO, El-Mougy NS (2007) Induction of systemic resistance in potato plants against late and early blight diseases using chemical inducers under greenhouse and field conditions. Res J Agric Biol Sci 3:73–81
FAOSTAT (2017) Available online at: www.fao.org/faostat/en/#home
Flor HH (1971) Current status of the gene–for-gene concept. Annu Rev Phytopathol 9:275–296
Fujita K, Komatsu K, Tanaka K (2006) An in vitro model for studying vascular injury after laser microdissection. Histochem Cell Biol 125:509–514
Garcia-Rubio R, de Oliveira HC, Rivera J, Trevijano-Contador N (2020) The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front Microbiol 10:2993. https://doi.org/10.3389/fmicb.2019.02993
Garrett KA, Nita M, De Wolf ED, Esker PD, Gomez-Montano L, Sparks AH (2016) Plant pathogens as indicators of climate change. In: Letcher TM (ed) Climate change. Elsevier, Amsterdam, pp 325–338. https://doi.org/10.1016/B978-0-444-63524-2.00021-X
Gay PA, Tuzun S (2000) Involvement of a novel peroxidase isozyme and lignifications in hydathodes in resistance to black rot disease in cabbage. Can J Bot 78:1144–1149
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioassays 28:1091–1101
Ghosh R, Tarafdar A, Sharma M (2016) Rapid detection of Fusarium oxysporum f. sp. ciceris from disease infested chickpea fields by loop-mediated isothermal amplification. Indian Phytopathol 69:47–50
Ghosh R, Tarafdar A, Sharma M (2017) Rapid and sensitive diagnoses of dry root rot pathogen of chickpea (Rhizoctonia bataticola (Taub.) Butler) using loop-mediated isothermal amplification assay. Sci Rep 7:42737. https://doi.org/10.1038/srep42737
Giri AP, Harsulkar AH, Deshpande VV, Sainan MN, Gupta VS, Ranjekar PK (1998) Chickpea defensive proteinase inhibitors can be inactivated by Podborer gut proteinases. Plant Physiol 116:393–401
Glazebrook J, Rogers EE, Ausubel FM (1997) Use of Arabidopsis for genetic dissection of plant defense responses. Annu Rev Genet 31:547–569
Gomez LD, Noctor G, Knight MR, Foyer CH (2004) Regulation of calcium signalling and gene expression by glutathione. J Exp Bot 55:1851–1856
González LMG, El Kayal W, Morris JS, Cooke JEK (2015) Diverse chitinases are invoked during the activity-dormancy transition in spruce. Tree Genetics Genomes 11:41. https://doi.org/10.1007/s11295-015-0871-0
Gozzo F (2003) Systemic acquired resistance in crop protection: from nature to a chemical approach. J Agric Food Chem 51:4487–4503
Grayer RJ, Kokubun T (2001) Plant-fungal interactions: the search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 56:253–263
Grover A, Gowthaman R (2003) Strategies for development of fungus resistant transgenic plants. Curr Sci 84:330–340
Guiñazú LB, Andrés JA, Rovera M, Rosas SB (2012) Isolation and characterization of rhizobacteria antagonistic to (Tassi) Goid, causal agent of Alfalfa damping-off. In: Malik A, Grohmann E (eds) Environmental protection strategies for sustainable development strategies for sustainability, pp 329–339
Gull A, Lone AA, Wani NUI (2019) Biotic and abiotic stresses in plants. In: Abiotic and biotic stress in plants. IntechOpen. https://doi.org/10.5772/intechopen.85832
Hatmi S, Gruau C, Trotel-Aziz P, Villaume S, Rabenoelina F, Baillieul F, Eullaffroy P, Clement C, Ferchichi A, Aziz A (2015) Drought stress tolerance in grapevine involves activation of polyamine oxidation contributing to improved immune response and low susceptibility to Botrytis cinerea. J Exp Bot 66:775–787. https://doi.org/10.1093/jxb/eru436
Health M (2000) Non host resistance and nonspecific plant defense. Curr Opin Plant Biol 3:315–319
Heidel AJ, Clarke JD, Antonovics J, Dong X (2004) Fitness costs of mutations affecting the systemic acquired resistance pathway in Arabidopsis thaliana. Genetics 168:2197–2206
Holzmueller EJ, Jose S, Jenkins MA (2007) Influence of calcium, potassium and magnesium on Cornus florida L. density and resistance to dogwood anthracnose. Plant Soil 290:189–199
Huang H, Ullah F, Zhou D-X, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800. https://doi.org/10.3389/fpls.2019.00800
Hussain F, Usman F (2019) Fungal biotic stresses in plants and its control strategy. In: Abiotic and biotic stress in plants. IntechOpen. https://doi.org/10.5772/intechopen.83406
Iakimova E, Atanassov A, Woltering E (2005) Chemical and pathogen-induced programmed cell death in plants. Biotechnol Eq 19:124–138
ICARDA (1993) Descriptors for Chickpea (Cicer arietinum L.), International Board for Plant Genetic Resources, Rome, Italy; International Crops Research Institute for the Semi-Arid Tropics, Patancheru, India and International Center for Agricultural Research in the Dry Areas, Aleppo, Syria. ISBN: 92-9043-137-7
ICRISAT Happenings (2019) Real-time surveillance decrypts dry root rot spread in Central India. https://www.icrisat.org/real-time-surveillance-decrypts-dry-root-rot-spread-in-central-india/. Accessed 26 Apr 2019
Ijaz S, Sadaqat HA, Khan MN (2012) A review of the impact of charcoal rot (). J Agric Sci:1–6
Iqbal Z, Iqbal MS, Hashem A, Abd Allah EF, Ansari MI (2021) Plant defense responses to biotic stress and its interplay with fluctuating dark/light conditions. Front Plant Sci 12:631810. https://doi.org/10.3389/fpls.2021.631810
Iriti M, Faoro F (2009) Chemical diversity and defense metabolism: how plants cope with pathogens and ozone pollution. Int J Mol Sci 10:3371–3399
Isah T (2019) Stress and defense responses in plant secondary metabolites production. Biol Res 52:39. https://doi.org/10.1186/s40659-019-0246-3
Ito Y, Kaku H, Shibuya N (1997) Identification of a high-affinity binding protein for N-acetylchitooligosaccharide elicitor in the plasma membrane of suspension-cultured rice cells by affinity labelling. Plant J 12:347–356. https://doi.org/10.1046/j.1365-313X.1997.12020347.x
Jach G, Görnhardt B, Mundy J, Logemann J, Pinsdorf E, Leah R, Schell J, Maas C (1995) Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J 8:97–109
Jeandet P, Hébrard C, Deville M-A, Cordelier S, Dorey S, Aziz A, Crouzet J (2014) Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 19(11):18033–18056. https://doi.org/10.3390/molecules191118033
Jones DH (1984) Phenylalanine ammonia lyase: regulation of its induction and role in plant development. Phytochemistry 23:1349–1359
Junior MCP, Pandini JA, Hein DPR, da Silveira JA, Stangarlin JR, Portz RL, Bonett LP (2021) Phaseolin induction on common-bean cultivars and biological control of Colletotrichum lindemuthianum 89 race by Baccharis trimera (Less.) Dc. Braz Arch Biol Technol e21200816. https://doi.org/10.1590/1678-4324-75years-2021200816
Karthik S, Reddy MS, Yashaswini G (2021) Climate change and its potential impacts on insect-plant interactions. In: The nature, causes, effects and mitigation of climate change on the environment. IntechOpen. https://doi.org/10.5772/intechopen.98203
Kaur S, Samota MK, Choudhary M, Choudhary M, Pandey AK, Sharma A, Thakur J (2022) How do plants defend themselves against pathogens-biochemical mechanisms and genetic interventions. Physiol Mol Biol Plants 28:485–504. https://doi.org/10.1007/s12298-022-01146-y
Keller NP (2019) Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol 17:167–180. https://doi.org/10.1038/s41579-018-0121-1
Kessmann H, Staub T, Hofmann C, Maetzke T, Herzog J, Ward E, Uknes S, Ryals J (1994) Induction of systemic acquired disease resistant in plants by chemicals. Annu Rev Phytopathol 32:439–459
Kliebenstein DJ (2004) Secondary metabolites and plant/environment interactions: a view through Arabidopsis thaliana tinged glasses. Plant Cell Environ 27:675–684
Koksal E, Gulcin I (2008) Purification and characterization of peroxidase from cauliflower (Brassica oleracea L. var. botrytis) buds. Protein Pept Lett 15:320–326
Kortekamp A, Zyprian E (2003) Characterization of plasmopara-resistance in grapevine using in vitro plants. J Plant Physiol 160:1393–1400
Krolicka M, Hinz SWA, Koetsier MJ, Joosten R, Eggink G, van den Broek LAM, Boeriu CG (2018) Chitinase Chi1 from Myceliophthora thermophila C1, a thermostable enzyme for chitin and chitosan depolymerization. J Agric Food Chem 66(7):1658–1669. https://doi.org/10.1021/acs.jafc.7b04032
Kumar N, Goel N (2019) Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol Rep 24:e00370
Lamichhane JR, Venturi V (2015) Synergisms between microbial pathogens in plant disease complexes: a growing trend. Front Plant Sci 6:385. https://doi.org/10.3389/fpls.2015.00385
Lavanya SN, Niranjan-Raj S, Jadimurthy R, Sudarsan S, Srivastava R, Tarasatyavati C, Rajashekara H, Gupta VK, Nayaka SC (2022) Immunity elicitors for induced resistance against the downy mildew pathogen in pearl millet. Sci Rep 12:4078. https://doi.org/10.1038/s41598-022-07839-4
Lawrence CB, Singh NP, Qui J, Gardner RG, Tuzun S (2000) Constitutive hydrolytic enzymes are associated with polygenic resistance of tomato to Alternaria solani and may function as an elicitor release mechanism. Physiol Mol Plant Pathol 57:211–220
Lawton KA, Weymann K, Friedrich L, Vernooij B, Uknes S, Ryals J (1995) Systemic acquired resistance in Arabidopsis requires salicylic acid but not ethylene. Mol Plant-Microbe Interact 8:863–870
Lee M, Jeon HS, Kim SH, Chung JH, Roppolo D, Lee H, Cho HJ, Tobimatsu Y, Ralph J, Park OK (2019) Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. EMBO J 38:e101948. https://doi.org/10.15252/embj.2019101948
Lefevere H, Bauters L, Gheysen G (2020) Salicylic acid biosynthesis in plants. Front Plant Sci 11:338. https://doi.org/10.3389/fpls.2020.00338
Leiter E, Szappanos H, Oberparleiter C, Kaiserer L, Csernoch L, Pusztahelyi T, Emri T, Pócsi I, Salvenmoser W, Marx F (2005) Antifungal protein PAF severely affects the integrity of the plasma membrane of Aspergillus nidulans and induces an apoptosis-like phenotype. Antimicrob Agents Chemother 49:2445–2453
Levine A, Tenhaken R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C (1996) Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Boil 6:427–437
Li L, Steffens JC (2002) Over expression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Plants 215:239–247
Loser K, Weltring KM (1998) Induction of a polyubiquitin gene (ubi1) by potato phytoalexins and heat shock in Gibberella pulicaris. Curr Genet 34:404–409
MacDonald MJ, D’Cunha GB (2007) A modern view of phenylalanine ammonia-lyase. Biochem Cell Biol 85:273–282
Madurapperumage A, Tang L, Thavarajah P, Bridges W, Shipe E, Vandemark G, Thavarajah D (2021) Chickpea (Cicer arietinum L.) as a source of essential fatty acids – a biofortification approach. Front Plant Sci 12:734980. https://doi.org/10.3389/fpls.2021.734980
Mahajan S, Tuteja N (2005) Mini review cold, salinity and drought stresses: an overview. Archiv Biochem Biophys 444:139–158
Mahato SB, Siddiqui KAI, Bhattacharya G, Ghosal T, Miyahara K, Sholichin M, Kawasaki T (1987) Structure and stereochemistry of phaseolinic acid: a new acid from. J Nat Prod 50:245–247
Manghwar H, Hussain A, Ali Q, Saleem MH, Abualreesh MH, Alatawi A, Ali S, Munis MFH (2021) Disease severity, resistance analysis, and expression profiling of pathogenesis-related protein genes after the inoculation of Fusarium equiseti in wheat. Agronomy 11(11):2124. https://doi.org/10.3390/agronomy11112124
Maphosa Y, Jideani VA (2017) The role of legumes in human nutrition. In: Functional food-improve health through adequate food. IntechOpen. https://doi.org/10.5772/intechopen.69127
Marquez N, Giachero ML, Declerck S, Ducasse DA (2021) General characteristics of pathogenicity and methods of control. Front Plant Sci 12:634397. https://doi.org/10.3389/fpls.2021.634397
Mathur SB (1968) Production of toxins and pecteolytic enzymes by two isolates of Sclerotium bataticola Taub. and their role in pathogenesis. Phytopathology 62:327–333
Mauch F, Mauch-maini B, Boller T (1988) Antifungal hydrolases in pea tissue ii. Inhibition of fungal growth by combination of chitinase and β- 1,3 glucanases. Plant Physiol 88:936–942
Melotto M, Mecey C, Niu Y, Chung HS, Katsir L, Yao J, Zeng W, Thines B, Staswick P, Browse J, Howe GA, He SY (2008) A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J 55:979–988
Metraux JP, Signer H, Ryals J, Ward E, Bem MW, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverardi B (1990) Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 250:1004–1006
MikuliPetkovšek M, Stampar F, Veberic R (2009) Accumulation of phenolic compounds in apple in response to infection by the scab pathogen, Venturia inaequalis. Physiol Mol Plant Pathol 74:60–67
Minatel IO, Borges CV, Ferreira MI, Gomez HAG, Chen CO, Lima GPP (2017) Phenolic compounds: functional properties, impact of processing and bioavailability. In: Soto-Hernandez M, Palma-Tenango M, Garcia-Mateos MR (eds) Phenolic compounds - biological activity. IntechOpen. https://doi.org/10.5772/66368
Mittler R, Vnderauwera S, Gollery M, Breusegen FV (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Moënne-Loccoz Y, Mavingui P, Combes C, Normand P, Steinberg C (2015) Microorganisms and biotic interactions. In: Bertrand JC, Caumette P, Lebaron P, Matheron R, Normand P, Sime-Ngando T (eds) Environmental microbiology: fundamentals and applications. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9118-2_11
Mohammad R, Thomas W, Hamed K, Abbas JL, Richard ET, Gabriel LB, Sciumbato (2007) Soybean charcoal rot disease fungus Macrophomina phaseolina in Mississippi produces the phytotoxin (-) botryodiplodin but no detectable phaseolinone. J Nat Prod 70:128–129
Morel JB, Dangl JL (1997) The hypersensitive response and the induction of cell death in plants. Cell Death Differ 4:671–683
N’Guyen GQ, Raulo R, Porquier A, Iacomi B, Pelletier S, Renou J-P, Bataillé-Simoneau N, Campion C, Hamon B, Kwasiborski A, Colou J, Benamar A, Hudhomme P, Macherel D, Simoneau P, Guillemette T (2021) Responses of the necrotrophic fungus Alternaria brassisicola to the Indolic Phytoalexin Brassinin. Front Plant Sci 11:611643. https://doi.org/10.3389/fpls.2020.611643
Nallasivam JL, Fernandes RA (2017) A protecting-group-free synthesis of (+)-nephrosteranic, (+)- protolichesterinic, (+)- nephrosterinic, (+)- phaseolinic, (+)-rocellaric acids and (+)- methylenolactocin. Org Biomol Chem 15:708–716. https://doi.org/10.1039/C6OB02398C
Nazarov PA, Baleev DN, Ivanova MI, Sokolova LM, Karakozava MV (2020) Infectious plant diseases: etiology, current status, problems and prospects in plant protection. Acta Naturae 12(3):46–59. https://doi.org/10.32607/actanaturae.11026
Neuenschwander U, Lawton K, Ryals J (1996) Systemic acquired resistance. In: Stacey G, Keen NT (eds) Plant microbe interaction. Chapman and Hall, New York, pp 81–106
Nishad R, Ahmed T, Rahman VJ, Kareem A (2020) Modulation of plant defense system in response to microbial interactions. Front Microbiol 11:1298. https://doi.org/10.3389/fmicb.2020.01298
Nita M, Grzybowski A (2016) The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev 3164734. https://doi.org/10.1155/2016/3164734
Nürnberger T (1999) Signal perception in plant pathogen defense. Cell Mol Life Sci 55:167–182
Nürnberger T, Scheel D (2001) Signal transmission in plant immune response. Trends Plant Sci 6:372–374
Ogawa D, Nakajima N, Seo S, Mitsuhara S, Kamad H, Ohashi Y (2006) The phenylalanine pathway is the main route of salicylic acid biosynthesis in Tobacco mosaic virus-infected tobacco leaves. Plant Biotechnol 23:392–398
Ojha S, Chakraborty MR, Dutta S, Chatterjee NC (2008) Influence of VAM on nutrient uptake and growth of custard-apple. Asian J Exp Sci 22:221–224
Oostendorp M, Kuz W, Dietrich B, Staub T (2001) Induced resistance in plants by chemicals. Eur J Plant Pathol 107:19–28
Ortega X, Velasquez JC, Pérez LM (2005) IP3 production in the hypersensitive response of lemon seedlings against Alternaría alternate involves active protein tyrosine kinases but not a G-protein. Biol Res 38:89–99
Ortíz-Castro R, Contreras-Cornejo HA, Macías-Rodríguez L, López-Bucio J (2009) The role of microbial signals in plant growth and development. Plant Signal Behav 4(8):701–712. https://doi.org/10.4161/psb.4.8.9047
Othman L, Sleiman A, Abdel-Massih RM (2019) Antimicrobial activity of polyphenols and alkaloids in Middle Eastern plants. Front Microbiol 10:911. https://doi.org/10.3389/fmicb.2019.00911
Ozyigit II, Kahraman MV, Ercan O (2007) Relation between explant age, total phenols and regeneration response in tissue cultured cotton (Gossypium hirsutum L.). Afr J Biotechnol 6:3–8
Palit P, Kudapa H, Zougmore R, Kholova J, Whitbread A, Sharma M, Varshney RK (2020) An integrated research framework combining genomics, systems biology, physiology, modelling and breeding for legume improvement in response to elevated CO2 under climate change scenario. Curr Plant Biol 22:100149. https://doi.org/10.1016/j.cpb.2020.100149
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24:255–265
Pawlowski ML, Hartman GL (2016) Infection mechanisms and colonization patterns of fungi associated with soybean. In: Fungal pathogenicity. IntechOpen. https://doi.org/10.5772/62305
Petit-Houdenot Y, Fudal I (2017) Complex interactions between fungal avirulence genes and their corresponding plant resistance genes and consequences for disease resistance management. Front Plant Sci 8:1072. https://doi.org/10.3389/fpls.2017.01072
Peyraud R, Dubiella U, Barbacci A, Genin S, Raffaele S, Roby D (2017) Advances on plant-pathogen interactions from molecular toward systems biology perspectives. Plant J 90(4):720–737. https://doi.org/10.1111/tpj.13429
Pontes JGM, Fernandes LS, dos Santos RV, Tasic L, Fill TP (2020) Virulence factors in the phytopathogen–host interactions: an overview. J Agric Food Chem 68(29):7555–7570. https://doi.org/10.1021/acs.jafc.0c02389
Prabhukarthikeyan SR, Parameswaran C, Keerthana U, Teli B, Jag PTK, Cayalvizhi B, Panneerselvam P, Senapati A, Nagendran K, Kumari S, Yadav MK, Aravindan S, Sanghamitra S (2020) Understanding the plant-microbe interactions in CRISPR/CAS9 era: indeed a sprinting start in marathon. Curr Genomics 21(6):429–443. https://doi.org/10.2174/1389202921999200716110853
Prasannath K (2017) Plant defense-related enzymes against pathogens: a review. AGRIEAST. J Agric Sci 11(1):38–48. https://doi.org/10.4038/agrieast.v11i1.33
Pratyusha S (2022) Phenolic compounds in the plant development and defense: an overview. In: Hasanuzzaman M, Nahar K (eds) Plant stress physiology- perspectives in agriculture. IntechOpen. https://doi.org/10.5772/intechopen.102873
Pusztahelyi T (2018) Chitin and chitin-related compounds in plant–fungal interactions. Mycology 9(3):189–201. https://doi.org/10.1080/21501203.2018.1473299
Pusztahelyi T, Holb IJ, Pócsi I (2015) Secondary metabolites in fungus-plant interactions. Front Plant Sci 6:573. https://doi.org/10.3389/fpls.2015.00573
Quiroga M, Guerrero C, Botella MA, Barcelo A, Amaya I, Medina MI, Alonso FJ, de Forchetti SM, Tigier H, Castro VV, Fontes MSW (2005) Plant defense and antimicrobial peptides. Prot Pept Lett 12(1):11–16. https://doi.org/10.2174/0929866053405832
Radman R, Saez T, Bucke C, Keshavarz T (2003) Elicitation of plants and microbial cell system. Biotechnol App Biochem 37:91–102
Ramegowda V, Senthil-Kumar M (2015) The interactive effects of simultaneous biotic and abiotic stresses on plants: mechanistic understanding from drought and pathogen combination. J Plant Physiol 176:47–54. https://doi.org/10.1016/j.jplph.2014.11.008
Ramírez-Gómez XS, Jiménez-García SN, Campos VB, Campos MLG (2019) Plant metabolites in plant defense against pathogens. In: Plant diseases - current threats and management trends. IntechOpen. https://doi.org/10.5772/intechopen.87958
Rapicavoli JN, Blanco-Ulate B, Muszyński A, Figueroa-Balderas R, Morales-Cruz AM, Azadi P, Dobruchowska JM, Castro C, Cantu D, Roper MC (2018) Lipopolysaccharide O-antigen delays plant innate immune recognition of Xylella fastidiosa. Nat Commun 9:390. https://doi.org/10.1038/s41467-018-02861-5
Raskin I (1990) Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 43:439–463
Rather BA, Mir IR, Masood A, Anjum NA, Khan NA (2020) Nitric oxide pre-treatment advances seed germination and alleviates copper-induced photosynthetic inhibition in Indian Mustard. Plants 9(6):776. https://doi.org/10.3390/plants9060776
Ribnicky DM, Shulaev V, Raskin I (1998) Intermediates of salicylic acid biosynthesis in tobacco. Plant Physiol 118:565–572
Ryals J, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt M (1996) Systemic acquired resistance. Plant Cell 8:1809–1819
Sauerborn J, Buschmann H, Ghiasi GK, Kogel KH (2002) Benzothiadiazole activates resistance in sunflower (Helianthus annuus) to the root parasitic weed Orobanche Cumana. Phytopathology 92:59–64
Selin C, de Kievit TR, Belmonte MF, Fernando WGD (2016) Elucidating the role of effectors in plant-fungal interactions: progress and challenges. Front Microbiol 7:600. https://doi.org/10.3389/fmicb.2016.00600
Sharma M, Ghosh R, Pande S (2015) Dry root rot (Rhizoctonia bataticola (Taub.) Butler): an emerging disease of chickpea- where do we stand? Arch Phytopathol Plant Prot 48:13–16, 797–812. https://doi.org/10.1080/03235408.2016.1140564
Sharma M, Sengupta A, Ghosh R, Agarwal G, Tarafdar A, Nagavardhini A, Pande S, Varshney RK (2016) Genome wide transcriptome profiling of Fusarium oxysporum f sp. ciceris conidial germination reveals new insights into infection-related genes. Sci Rep 6:37353. https://doi.org/10.1038/srep37353
Sharma M, Ghosh R, Tarafdar A, Rathore A, Chobe DR, Kumar AV, Gaur PM, Samineni S, Gupta O, Singh NP, Saxena DR, Saifulla M, Pithia MS, Ghante PH, Mahalinga DM, Upadhyay JB, Harer PN (2019) Exploring the genetic cipher of chickpea (Cicer arietinum L.) through identification and multi-environment validation of resistant sources against Fusarium wilt (Fusarium oxysporum f. sp. ciceris). Front Sustain Food Syst 3:78. https://doi.org/10.3389/fsufs.2019.00078
Shuping DSS, Eloff JN (2017) Use of plants to protect plants and food against fungal pathogens: a review. Afr J Tradit Complement Altern Med 14(4):120–127. https://doi.org/10.21010/ajtcam.v14i4.14
Siddiqui KAI, Gupta AK, Paul AK, Banerjee AK (1979) Purification and properties of a heat-resistant exotoxin produced by Macrophomina phaseolina (Tassi) Goid in culture. Experientia 35:1222–1223
Singh M, Singh J, Maurya S, Kumar S, Meena AK, Sharma P, Lakhran L (2021) Root rot disease incited by in arid legumes and their management: a review. Legume Res LR-4714:1–7. https://doi.org/10.18805/LR-4714
Sinha R, Gupta A, Senthil-Kumar M (2016) Understanding the impact of drought on foliar and xylem invading bacterial pathogen stress in chickpea. Front Plant Sci 7:902. https://doi.org/10.3389/fpls.2016.00902
Somssica IE, Hahlbrock K (1998) Pathogen defense in plants-a paradigm of biological complexity. Trends Plant Sci 3:86–90
Stakman EC (1915) Relation between Puccina graminis and plants highly resistant to its attack. J Agric Res 4:193–199
Takahama U, Yoshitama K (1998) Hydoxycinnamic acid esters enhance peroxidase-dependent oxidation of differences in the enhancement among the esters. J Plant Res 111:97–100
Tarafdar A, Rani TS, Chandran USS, Ghosh R, Chobe DR, Sharma M (2018) Exploring combined effect of abiotic (soil moisture) and biotic (Sclerotium rolfsii Sacc.) stress on collar rot development in chickpea. Front Plant Sci 9:1154. https://doi.org/10.3389/fpls.2018.01154
Tripathi R, Tewari R, Singh KP, Keswani C, Minkina T, Srivastava AK, De Corato U, Sansinenea E (2022) Plant mineral nutrition and disease resistance: a significant linkage for sustainable crop protection. Front Plant Sci 13:883970. https://doi.org/10.3389/fpls.2022.883970
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines 5(3):93. https://doi.org/10.3390/medicines5030093
Tyagi H, Jha S, Sharma M, Giri J, Tyagi AK (2014) Rice SAPs are responsive to multiple biotic stresses and overexpression of OsSAP1, an A20/AN1 zinc-finger protein, enhances the basal resistance against pathogen infection in tobacco. Plant Sci 225:68–76
Tyler BM (2002) Molecular basis of recognition between Phytophthora pathogens and their hosts. Annu Rev Phytopathol 40:137–167
Vågsholm I, Arzoomand NS, Boqvist S (2020) Food security, safety, and sustainability- getting the trade-offs right. Front Sustain Food Syst 4:16. https://doi.org/10.3389/fsufs.2020.00016
Vallad GE, Goodman RM (2003) Systemic acquired resistance and induced systemic resistance in conventional agriculture. Alliance Crop Soil Environ Sci Soc 44:1920–1934
van Loon LC, van Strien EA (1999) Families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55:85–97
van Loon LC, Rep M, Pieterse CMJ (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44:135–162
Velásquez AC, Castroverde CDM, He SY (2018) Plant and pathogen warfare under changing climate conditions. Curr Biol 28(10):PR619-R634. https://doi.org/10.1016/j.cub.2018.03.054
Veliz EA, Martínez-Hidalgo P, Hirsch AM (2017) Chitinase-producing bacteria and their role in biocontrol. AIMS Microbiol 4(3):689–705. https://doi.org/10.3934/microbiol.2017.3.689
Vishal B, Kumar PP (2018) Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front Plant Sci 9:838. https://doi.org/10.3389/fpls.2018.00838
Wallace TC, Murray R, Zelman KM (2016) The nutritional value and health benefits of chickpeas and hummus. Nutrients 8(12):766. https://doi.org/10.3390/nu8120766
Wan J, He M, Hou Q, Zou L, Yang Y, Wei Y, Chen X (2021) Cell wall associated immunity in plants. Stress Biol 1:3. https://doi.org/10.1007/s44154-021-00003-4
Wen Z, Liao W, Chen S (2005) Production of cellulase/β-glucosidase by the mixed fungi culture of Trichoderma reesei and Aspergillus phoenicis on dairy manure. Appl Biochem Biotechnol:93–104
Wenjuan L, Ping H, Jiyun J (2009) Potassium influenced phenylalanine ammonia lyase, peroxidases and polyphenol oxidases in Fusarium graminearum infected maize (Zea mays L.). In: Proceedings of international plant nutrition colloquium XVI
Wharton PS, Nicholson RL (2000) Temporal synthesis and radiolabelling of the sorghum 3-deoxyanthocyannidin phytoalexins and the antocyanin, cyaniding 3-dimaloynl glucoside. New Phytol 145:457–469
Wilkens A, Paulsen J, Wray V, Winterhalter P (2010) Structures of two novel trimeric stilbenes obtained by horseradish peroxidase catalyzed biotransformation of trans-resveratrol and viniferin. J Agric Food Chem 58:6754–6761
Wolfgang A, Taffner J, Guimarães RA, Coyne D, Berg G (2019) Novel strategies for soil-borne diseases: exploiting the microbiome and volatile-based mechanisms toward controlling Meloidogyne-based disease complexes. Front Microbiol 10:1296. https://doi.org/10.3389/fmicb.2019.01296
Wu C, Bradford KJ (2003) Class I chitinase and â-1,3-glucanase are differentially regulated by wounding, methyl jasmonate, ethylene and gibberellin in tomato seeds and leaves. Plant Physiol 133:263–273
Yang Y, Yuan Q, Gabriel DW (1996) Water soaking Function(s) of xcmh1005 are redundantly encoded by members of the Xanthomonas avr/pth gene family. Mol Plant Microbe Interact 9:105–113
Zabka M, Lesniak W, Prus W, Kuznicki J, Filipek A (2008) Sgt1 has co-chaperone properties and is up-regulated by heat shock. Biochem Biophys Res Commun 370:179–183
Zeyen RJ, Kruger WM, Lyngkjaer MF, Carver TLW (2002) Differential effects of D-mannose and 2-deoxy-D-glucose on attempted powdery mildew fungal infection of inappropriate and appropriate Gramineae. Physiol Mol Plant Pathol 61:315–323
Zhang X, Liu C (2015) Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol Plant 8(1):17–27. https://doi.org/10.1016/j.molp.2014.11.001
Zhang X, Dai Y, Xiong Y, Defraia C, Li J, Dong X, Mou Z (2007) Overexpression of arabidopsis map kinase 7 leads to activation of plant basal and systemic acquired resistance. Plant J 52:1066–1079
Zhou M, Tang Y, Deng X, Ruan C, Ding M, Shao J, Tang Y, Wu Y, (2018) Description of cultivated common buckwheat. In: Zhou M, Suvorova G, Woo SH (eds) Buckwheat germplasm in the world, pp 53–60. https://doi.org/10.1016/B978-0-12-811006-5.00006-9
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Preeti, Panwar, D., Saini, P., Vats, J.K. (2023). Effect of Temperature and Defense Response on the Severity of Dry Root Rot Disease in Chickpea Caused by Macrophomina phaseolina. In: Mathur, P., Kapoor, R., Roy, S. (eds) Microbial Symbionts and Plant Health: Trends and Applications for Changing Climate. Rhizosphere Biology. Springer, Singapore. https://doi.org/10.1007/978-981-99-0030-5_14
Download citation
DOI: https://doi.org/10.1007/978-981-99-0030-5_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-0029-9
Online ISBN: 978-981-99-0030-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)