Abstract
Charcoal rot caused by the fungus, Macrophomina phaseolina, have emerged as serious concern for cultivation of soybean under climate change scenario worldwide. Macrophomina phaseolina causes huge annual losses to the crop and can survives in the soil mainly as microsclerotia for 2 years or longer and; germinate repeatedly during the crop-growing season. The pathogen generally attacks the young plants when their growth is retarded due to unfavourable conditions. Moreover, charcoal rot is usually most severe in older plants which have been subjected to stressful environmental conditions such as high temperature, drought, or poor fertility. The disease severity is directly related to the humidity, temperature, tillage practices and soil nutrient conditions. This review deals with the details of pathogen and its management approaches. The management of disease through stress management is the most viable solution to overcome the menace of it. Although, the fungicide is the means of disease prevention but cultural practices, irrigation management during drought and resistant cultivars are the most practical means of control as the pathogen have more than 500 plant species to inhabit. The possibilities in substantial yield reduction under present changing climate underscore the need for further research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Charcoal rot caused by the fungus, Macrophomina phaseolina, have emerged as serious concern for cultivation of soybean under climate change scenario worldwide. Macrophomina phaseolina causes huge annual losses to the crop and can survives in the soil mainly as microsclerotia for 2 years or longer and; germinate repeatedly during the crop-growing season. The pathogen generally attacks the young plants when their growth is retarded due to unfavourable conditions. Moreover, charcoal rot is usually most severe in older plants which have been subjected to stressful environmental conditions such as high temperature, drought, or poor fertility. The disease severity is directly related to the humidity, temperature, tillage practices and soil nutrient conditions. This review deals with the details of pathogen and its management approaches. The management of disease through stress management is the most viable solution to overcome the menace of it. Although, the fungicide is the means of disease prevention but cultural practices, irrigation management during drought and resistant cultivars are the most practical means of control as the pathogen have more than 500 plant species to inhabit. The possibilities in substantial yield reduction under present changing climate underscore the need for further research.
Introduction
Charcoal rot, caused by the fungus Macrophomina phaseolina (Tassi) Goidanich, is a cosmopolitan soil saprophyte and is well known as a facultative, opportunistic plant pathogen that infects plants exposed to certain stress conditions (Tesso et al. 2005). It is ranked among the five top most important soybean diseases, causing huge annual losses (Wrather et al. 1997, 2001). M. phaseolina (Tassi) Goidanich, is one of the most important soil borne pathogens, infecting over 500 plant species in more than 100 plant families around the world (Smith and Wyllie 1999). It can survive as microsclerotia (masses of fungal tissue) for 2 or more years in dry soil, but not more than 7–8 weeks in wet soils and mycelium not more than 7 days (Sinclair 1982). Being seed-borne (Kunwar et al. 1986) in nature, it is found both on the seed coat and cotyledons (Reuveni et al. 1983) and causes charcoal rot by infecting the roots due to the adherence of microsclerotia to the seed coat during germination and emergence (De Mooy and Burke 1990). Positive correlations have been reported between the inoculum level of M. phaseolina in the seedbed and disease severity (Khan 2007). Temperatures near 30 °C and dry conditions make this pathogen prevalent in regions with arid subtropical and tropical climates such as in Pakistan (Khan 2007), China (Xiaojian et al. 1988) and India (Suriandraselvan et al. 2006) where yield losses caused by this fungus can reach even 90 % of yield.
Owing to higher variability among the isolates of this pathogen, no commercial resistant soybean variety is yet available for effective management of this disease. Therefore, reducing drought stress during the reproductive stages of growth of soybean plants can help in minimizing the risk from charcoal rot. This can be done by following production systems like no-till that conserve soil moisture, maintaining proper plant populations, growing drought tolerant varieties and maintaining soil fertility. Fields with a history of severe charcoal rot should be rotated for 1–2 years with non-host crops (cereals). Fungal propagules exposed to energy stress, lose endogenous C by respiration and exudation resulting in energy (nutrient) stress, with demand for nutrients during germination, viability loss and decreased pathogenic aggressiveness (Mondal and Hyakumachi 1998). In addition, the beneficial bacterial live in rhizosphere (i.e., the region around the root) which is rich in nutrients due to the exudation of plant nutrients from the roots can influence the plant bi-directionally. One direct influence may be stimulation of plant growth and other plant health promotion (i.e. indirect influence). Hence, biological control of plant pathogens and deleterious microbes occurs through the production of antibiotics, lytic enzymes, hydrogen cyanide and siderophore or through mycoparasitism, competition for nutrients and space by bioagents that results in plant health promotion significantly. Soil application of biocontrol agents’ viz., Trichoderma viride, T. harzianum, Pseudomonas fluorescens and Bacillus subtilis effectively reduced root rot caused by soil borne pathogens in several crops (Thilgavathi et al. 2007; Loganathan et al. 2010).
Pathogen
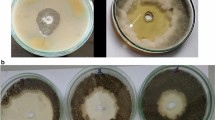
The binomial nomenclature of M. phaseolina is applied to both the microsclerotial and pycnidial anamorphs, however the microsclerotial phase is the one predominantly observed worldwide (Dhingra and Sinclair 1978). Different synonyms have been ascribed to the fungus M. phaseolina (Tassi) that includes M. phaseoli (Maubl.) Ashby, Macrophoma conchoci Swada, Sclerotium bataticola Taub. and Rhizoctonia bataticola (Taub.) (Mihail 1992). The lack of a known teleomorph has stalled its taxonomy over the years (Kulkarni and Patil 1966; Crous et al. 2006); however, a thorough phylogenetic study of 113 members of the family Botryosphaeriaceae using ribosomal DNA sequences was able to separate the genera Macrophomina and Tiarosporella (Crous et al. 2006). Although, only one species is recognized within the genus (Mihail and Taylor 1995), great variability in morphology and pathogenicity was recognized among isolates from different host species and between isolates from different parts of the same plant (Fernandez et al. 2006). Efforts were also been made to characterize the fungus population in different parts of the world based on its pathogenic variability (Karunanithi et al. 1999), morphological characteristics (Fernandez et al. 2006), as well as the molecular characteristics (Almeida et al. 2003; Jana et al. 2003; Purkayastha et al. 2006). The unstable B chromosome may be one of the mechanisms for generating variation in fungi (Miao et al. 1991). In addition, the mature hyaline and pigmented hyphal cells of Macrophomina are uninucleate, but young, growing hyphal cells and hyphal tip cells are usually multinucleate (Knox- Davies 1967). Hyphal fusion heterokaryosis after mitotic segregation and recombination may explain the occurrence of cultural types or physiological races (Punithalingam 1983). Double-stranded RNA (dsRNA) has also been reported in M. phaseolina with sizes ranging from 0.4 to 10 kbp and the number of dsRNA ranging from 1 to 10 (Pecina et al. 2000). Variations exist in pathogenicity among M. phaseolina from different geographical regions (Dhingra and Sinclair 1973). The phytotoxin produced in cultures of M. phaseolina is Botryodiplodin. Phaseolinone was not detected, which suggested that botryodiplodin may be the phytotoxin that facilitates infection (Ramezani et al. 2007). Moreover, the host species-specific conservation of a family of repeated DNA sequences in the genome of a fungal plant pathogen is a possible mechanism (Hamer et al. 1989) for isolates distinctness in this pathogen. Jana and coworkers (2005) reported the use of microsatellites markers as potential diagnostic markers for the study of the variability within closely related isolates of M. phaseolina population specific to soybean and cotton.
Symptoms
Charcoal rot can infect soybeans at any growth stage; however, the worst infection is typically seen during the reproductive phase. M. phaseolina overwinters as sclerotia in the soil and infected plant debris and can remain viable for several years (at least 2 years). Under favorable conditions (e.g., higher soil temperatures and low water potential), the sclerotia germinate and colonize the plants (Olaya et al. 1996). M. phaseolina can grow rapidly in infected plants and produce large amount of sclerotia that clog the vascular tissue, resulting in disease symptoms ranging from leaf yellowing, wilting to plant death (Wyllie 1989). Charcoal rot symptoms usually appear under high temperature conditions (28–35 °C) and low soil moisture, or when unfavourable environmental circumstances stress the plant (Wyllie 1988; Sinclair and Backman 1989). Although initial infection occurs at the seedling stage, it usually remains latent until the soybean plant approaches maturity (growth stages R5–R7) (Short et al. 1978). Diseased plants may wilt and prematurely die with senesced leaves remaining attached to petioles. Seed yield is frequently reduced under these conditions. The diagnostic symptoms of charcoal rot on prematurely dying or dead plants are the sloughing of cortical tissues from the lower stem and tap root and the speckled grey appearance of these infected tissues due to abundant formation of microsclerotia in vascular, cortical, and pith tissues (Smith and Carvil 1997). Other soybean diseases such as Sudden Death Syndrome (SDS), Brown Stem Rot (BSR) or stem canker may cause these symptoms as well. The distinguishing characteristic of charcoal rot is black speckling within the lower stem from microsclerotia. These black specks look like charcoal briquettes, thus the name charcoal rot. Additionally, reddish-brown to black streaks form in the vascular tissue as well. Charcoal rot is a root and stem disease that commonly occurs in hot, dry weather conditions. Therefore, symptoms of charcoal rot are also referred as dry-weather wilt or summer wilt, because it often occurs when plants are under heat and drought stresses (Smith and Wyllie 1999). These stresses can also occur in irrigated soybeans causing losses from 6 % to 33 % in experimental plots (Mengistu et al. 2011) and the combination of stress and the presence of M. phaseolina cause higher yield loss on soybeans than drought alone. This disease is most severe when plants are stressed from lack of moisture or nutrients, at excessive plant populations or where soil compaction, other diseases or nematodes or improperly applied pesticides impair root development. Charcoal rot symptoms typically appear when soybeans approach maturity. The earliest symptoms are smaller than normal sized leaves, which become chlorotic, then turn brown, but remain attached to the petiole giving the entire plant a dull greenish-yellow appearance. In many cases, these plants wilt and die. The pathogen attacks the plant throughout the season, often causing progressive debilitation of the host. After flowering, a light gray or silvery discoloration of the epidermal and sub-epidermal tissues develops in the taproot and the lower part of the stem. The best diagnostic symptom is found when the epidermis is peeled away from the stem exposing numerous small, black bodies of microsclerotia that are frequently produced in the xylem and pith of the stem and may block water flow.
Infection Process
The first reports of Mp (M. phaseolina) infection process in soybean were made by Ammon et al. (1974, 1975), which were based on scanning electron microscopy analyses. They suggested that penetration through soybean cell walls occurred as a result of mechanical pressure and/or chemical softening. Ilyas and Sinclair (1974) described the formation of intra- xylem sclerotia in wound-inoculated soybean plants, lacking the characterization of the initial penetration stages. Inside host tissues, Mp develops thin hyaline walls, which are presumed to be more permeable with increased potential for resource exchange with the host (Barrow and Aaltonen 2001; Barrow 2003). The development of Mp structures with swelled and pigmented walls, produced either inter- and intracellularly. These structures, similar to appressoria, were previously described as hyphopodia by Howard (1997). He described appressoria as structures that develop from swellings at the tips of conidial germ tubes and hyphopodia as structures that arise from mature vegetative hyphae. Hyphopodia have been defined as structures that allow the spreading of the fungus after infection of the plant, and they might enhance penetration or survival (Howard 1997; Solomon et al. 2006). Hyphopodia generally are melanized and deposition of melanin, for instance, in the fungal cell wall of appressoria is associated with the generation of intracellular turgor pressure that provides the necessary force for plant penetration (Money et al. 1998). The fungus has been shown to infect cotyledons, roots or stems either pre-emergence or post-emergence stages and microsclerotia form appressoria over host epidermal cells. The developing hyphae enter and grow between the epidermal cells inter- and intracellularly, and attack cells by mechanical or enzymatic action. However, the intracellular colonization occurs after lamella and cell wall disintegration (Ammon et al. 1974). Following epidermal and cortex invasion, M. phaseolina colonizes the vascular system developing microsclerotia on xylem vessels which may lead to their blocking causes wilt symptoms in soybean and other hosts (Ilyas and Sinclair 1974).
Epidemiology
The mycelium in the soil is not considered to be a primary source of inoculum (Meyer et al. 1974), however, the sclerotia serve as the prime sources of inocula (Papavizas and Klag 1975) for disease initiation. The occurrence of sclerotia in plant debris allows the fungus to live in soil, even in the absence of a host for 2 or more years, depending on soil conditions (Wantanabe et al. 1970). Seed, soil and plant remains are the sources of primary inoculum (Reuveni et al. 1983) and the severity of the disease is directly related to the number of live sclerotia in the soil. Under dry soil conditions, the fungus can remain viable as sclerotia for more than 10 months. Pathogenicity is optimal between 28 and 35 °C (Dhingra and Sinclair 1978) and host water stress is another principal factor favouring development of the disease (Pearson et al. 1984; Mayek-Perez et al. 2002). In addition, charcoal rot incidence is much higher when plants are exposed to prolonged drought and high temperature stress during grain development (Tesso et al. 2005). Mechanical injury, high plant density and insect attacks are considered to be predisposing factors for transmission of the disease (Ahmad et al. 1991). The severity of infection depends on relative humidity, temperature, the nature of the isolate, climatic region and host cultivar. In some agricultural systems in which soil is generally low in easily available nutrients and consequently poor in microbial biomass, activity and diversity, such as those systems under conventional tillage, the suppression of soil borne plant pathogens is more difficult to attain (Vargas Gil et al. 2008). Almeida and co-workers (2003) reported higher densities of microesclerotia in soybean roots, in plots under conventional tillage, and stated that tillage has also been considered an important factor in the spread of fungal propagules in soil. Moreover, according to those authors, high temperature has also been mentioned as a factor that predisposes plants to infection by M. phaseolina, low soil moisture being the most important factor for infection. It is well known that no-till systems are cooler than conventional ones, mainly due to the crop residue layer on the soil surface. Direct seeded systems do not provide suitable soil conditions for the spread of the pathogen and reduce the stress conditions of the plants. Accordingly, a significant negative correlation between charcoal rot and water holding capacity but a positive correlation between the disease and sand content, which is reasonably considered that sandy soils usually retains less water than silty or clayey ones (Perez-Brandán et al. 2012). Charcoal rot undergoes rapid development under strong water content depletion (Pedgaonkar and Mayee 1990), therefore, cultivars that show reduced water depletion rates and a stable cellular turgor are resistant to charcoal rot (Mayek-Perez et al. 2002). Besides, the pathogen specialization to the host also seems to be related to stem nitrogen composition, and is promoted at low water availability (Pearson et al. 1987). Infection by nematodes can provide a favourable substrate for the development of the fungus by disrupting and damaging the vascular tissues and bringing physiological changes, and therefore increasing the severity of charcoal rot. Ross (1965) documented the interaction of Heterodera glycines Inchinohe (soybean cyst nematode) and M. phaseolina separately in disease complexes and explained that disruption of vascular tissues resulting from infection by H. glycines increased the susceptibility of the host to water stress. Stress-related nitrogenous compounds such as asparagines and prolines are utilized efficiently by M. phaseolina and this could explain the positive correlation between H. glycines and population of fungus (Pearson et al. 1987) and as a result of interaction with fungi, the populations of sedentary nematodes are suppressed (Powell 1971). Two season soybeans crop or late planting may add greater severity to charcoal rot.
Management
Plant diseases are considered as an important biotic constraint, where an interaction between host, pathogen and the environment occur and leads to significant crop losses worldwide. Most plants are immune or completely resistant to almost all pathogens. However, owing to co-evolution of host and pathogen, pathogens overcome the natural resistance of particular hosts through mechanism of specialization under favourable environmental conditions. Therefore, the success of any disease management strategy should focus on the host, the pathogen and/or the environment. Integrated disease management (IDM), which combines crop improvement, biological, cultural, physical and chemical control strategies in a holistic way rather than using a single component strategy proved to be more effective and sustainable. Hence, an ‘Integrated Disease Management’ approach can be helpful in selection and application of a harmonious range of control strategies that would minimize losses and maximize returns. Fields with a history of severe charcoal rot should be rotated for 1–2 years with non-host cereal crops.
Efforts to manage charcoal rot in soybean through adjusting planting dates, crop rotation, planting densities, and irrigation have all been suggested as means of control (Mengistu et al. 2007) as no commercial resistant soybean variety is yet available for effective management of this disease. Managing the population of microsclerotia in the soil is the primary management strategy. Avoiding excessive seed rates and maintaining adequate soil fertility reduces loss from the diseases by maintaining healthy and vigorous plants ecosystem. The best way to avoid issues with charcoal rot is to limit drought stress during the reproductive stages of growth by managing production systems like no-till that conserve soil moisture may also reduce losses by charcoal rot. Planting corn for 3 or more years can decrease disease pressure followed by a yearly rotation to keep populations low. Charcoal rot exhibited a negative and significant relationship with soil organic matter, total N, K and Ca that suggests the soil systems with high levels of biological diversity and activity, and with high internal nutrient cycling, such as no-tillage systems, allow the development of plants with healthier root systems and can avoid the infection by a soil borne pathogen, because this system becomes more resilient to disturbance than conventional tillage systems (Perez-Brandán 2012).
Deshpande and Murumkar (2008) found a reduction in microbial growth and abundance, and at the same time an increase of the pathogen M. phaseolina, which resulted in an increase of root rot in sorghum. High microbial diversity agricultural soils have been associated with suppression of soil-borne plant diseases, and this kind of suppression may be due to general competition or antagonism, which may be non-specific and active against a wide range of soil-borne pathogens (van Bruggen et al. 2006). Patil and Kamble (2011) examined the effect of UV light on the hostile/antagonistic action of Trichoderma koningii against M. phaseolina, using five T. koningii mutants, and found that T. koningii 2 showed maximum antagonistic activity against the charcoal rot pathogen when tested by dual culture method. Seed treatment with P. flourescens along with soil amendment like mustard cake, vermicompost and FYM provided a better protection against Macrophomina root rot of chickpea (Khan and Gangopadhyay 2008). Similarly, soil application of ZnSO4 followed by combined application of T. viride + ZnSO4 significantly reduced root rot incidence (Sundaravadana 2002). Almeida and co-workers (2003) stress that alternative control practices of charcoal rot could be the modification of the soil environment, which would favour antagonists interfering with the biology or survival of the pathogen. The analysis of the fungal and bacterial sequences detected in DS (Direct seeded) treatment showed that the most frequently found fungi are effective biological control agents of plant pathogens (Perez-Brandán et al. 2012). Plectosphaerella cucumerina and Paecilomyces marquandii are nematophagous fungi (Atkins et al. 2003), and Bionectria ochroleuca is a mycoparasite (Chaverri et al. 2011) were recorded under no-till soil. In addition, the most frequent bacterial clone detected is also related to plant protection, such as Bradyrhizobium sp., inducing effective systemic resistance and protecting the host plant against pathogen attacks (Cartieaux et al. 2008). Studies in various pathosystems indicate that auxin signalling is required for host resistance against some necrotrophs, whereas for pathogenic bacteria and biotrophic and hemibitrophic fungi, auxin signalling promotes susceptibility (Karzan and Manners 2009). Auxin is a plant hormone that is involved in many aspects of plant development, and the cross talk between auxin with other plant hormones such as JA and SA is important for balancing plant growth versus defense (Wang et al. 2007; Bari and Jones 2009). These hormones trigger the activation of induced systemic resistance and systemic acquired resistance (SAR) to necrotrophic pathogens (Fey and Parker 2000; Glazebrooki 2005). The SAR is an effective defense mechanism against a broad range of pathogens and insects incurred by host. Genes involved in SA response such as hydroxyl-2-methyl-2(E) butenyl 4-diphosphate. HopW1-1- Interacting protein 1 (WIN1) were identified (Lee et al. 2008). The SA pathway, which is considered one of the major pathways involved in defense against necrotrophic pathogens, regulates the expression of defense defector genes and systemic acquired resistance through the repression of the auxin signaling pathway (Gill et al. 2005). Another hormone that seems to play a role in the resistance of stem rot is abscissa acid (ABA). While ABA was described as a susceptibility factor, other studies (Wiese et al. 2004) showed that it activates plant defense by priming for callose deposition or by restricting the progression of the fungus Cochliobolus miyabeanus in the mesophyll of rice (De Vleesschauwer et al. 2010). Other signaling genes involved in SAR that induce numerous defense genes included apoplectic lipid transfer protein, basic chitinase etc. (Zander et al. 2010). The third category of genes with stem rot tissues includes genes involved in early response as part of the HR. Among these are transcripts encoding proteins such as ATPase transporter, kinases, carbonic anhydrase, AMMECR1, MIPS1, voltage-dependent anion channel, 2-deoxy-D-arabinoheptulosonate 7-phosonate (DAHP) synthase and glutathione peroxidase that were reported previously to be involved in the hypersensitivity resistance (HR) and cell death in plants under pathogenic attack (La Camera et al. 2009). Reactive oxygen species (ROS) seems to be induced following M. phaseolina infection as several genes involved in oxidative stress (alpha-dioxygenase, fumarase, cytosolic GADPH (C subunit), cytosolic ascorbate peroxidase APX1) had more abundant transcripts. Furthermore, several pathogenesis related (PR) genes such as elicitor activated gene 3-1 (EL13), aromatic alcohol: NADP+ oxidoreductase, thaumain, pathogenesis-related and antifungal chitin binding protein had differentially abundant transcripts in diseased versus healthy tissues (Biswas et al. 2014). PR proteins, of which some have antimicrobial functions (Sels et al. 2008) are mainly induced in localized pathogen attack around HR lesion.
Sinclair (1989) examined the effect of thermotherapy on the growth of seed-borne fungi in soybean by immersing infected seeds in heated palm, sunflower and soybean oil as a means of eliminating seed-borne fungi. Glyphosate (N-[phosphonomethyl]glycine) application on glyphosate-resistant crops has been shown to enhance and in a few cases reduce severity (Johal and Huber 2009) of selected soybean diseases. Shahda et al. (1991) studied the in-vitro effect of certain fungicides such as Benlate T, 2-(4-thiazolyl)-1H-benzimidazole (Thiabendazole), N trichtoromethylthio–cyclohexene-1,2-dicarboximide (Captan 75), 5,6-dihydro-2-methyl-N-phenyl-1, 4-oxathiin-3-carboxamide and tetramethylthiuram disulfide (Vitavax 200), 5,6-dihydro-2 methyl-Nphenyl-1,4-oxathiin-3-carboxamide and N-trichtoromethylthio– cyclohexene-1,2-dicarboximide (Vitavax 300), on mycelial growth of seed-borne fungi of sunflower and 5,6-dihydro-2-methyl-N-phenyl- 1,4-oxathiin-3-carboxamide and tetramethylthiuram disulfide (Vitavax 200) was found to be most effective for M. phaseolina. Moreover, Brooker et al. (2007) screened six derivatives of coumarin, for their antifungal activity against M. phaseolina and Pythium species and observed that these derivatives have higher antifungal activities and stability as compared with either the original coumarin or sesamol compounds alone.
Conclusion
During the last five decades, extensive progress has been made by researchers in areas of etiology, epidemiology, biology and biocontrol of the ascomycete fungus M. phaseolina. Charcoal rot epidemics are common under stress conditions such as water scarcity and other biotic and abiotic stresses. The basic knowledge of the biology of M. phaseolina has provided the foundation for developing sustainable strategies to control the disease. Efforts are needed to develop a biocontrol technology for practical use in the management of charcoal rot diseases. In addition, it has become increasingly clear that among integrating several effective control methods, breeding and biological control methods, could be the best strategy for managing this important disease. Compared with major technological, environmental, and socioeconomic changes affecting agricultural production during next century, climate change may be more important; it will however, add another layer of complexity and uncertainty onto a system that is already exceeding difficulty to manage on a sustainable basis. Research on climate change and its interaction with pathogenically different isolates from different geographical regions of M. phaseolina could result in improved understanding and management of pathogen in face of current and future climate extremes.
References
Ahmad I, Burney K, Asad S (1991) Current status of sunflower diseases in Pakistan. In: Ghaffar A, Shahzad S (eds) Proceedings of the national symposium on status of plant pathology in Pakistan, 31 December 1991. Department of Botany, University of Karachi, Karachi, p 53
Almeida AMR, Amorim L, Bergamin A, Torres E, Farias JRB, Benato LC, Pinto MC, Valentim N (2003) Progress of soybean charcoal rot under tillage and no-tillage systems in Brazil. Fitopatol Bras 28:131–135
Ammon V, Wyllie TD, Brown MF Jr (1974) An ultra structural investigation of pathological alterations induced by Macrophomina phaseolina (Tassi)Goidin seedlings of soybean, Glycine max (L.). Merrill Physiol Plant Pathol 4:1–4
Ammon V, Wyllie TD, Brown MF Jr (1975) Investigation of the infection process of Macrophomina phaseolina on the surface of soybean roots using scanning electron microscopy. Mycopathologia 55:77–81
Atkins SD, Clark IM, Sosnowska D, Hirsch PR, Kerry BR (2003) Detection and quantification of Plectosphaerella cucumerina, a potential biological control agent of potato cyst nematodes, by using conventional PCR, Real-Time PCR, selective media, and baiting. Appl Environ Microbiol 69(8):4788–4793
Bari R, Jones J (2009) Role of plant hormones in plant defense responses. Plant Mol Biol 69:473–488
Barrow JR (2003) A typical morphology of dark septate fungal root endophytes of Bouteloua in arid south western USA range lands. Mycorrhiza 13:239–247
Barrow JR, Aaltonen RE (2001) Evaluation of the internal colonization of Atriplex canescens (Pursh) Nutt. roots by dark septate fungi and the influence of host physiological activity. Mycorrhiza 11:199–205
Biswas C, Dey P, Karmakar PG, Satpathy S (2014) Next-generation sequencing and micro RNAs analysis reveal SA/ MeJA1/ ABA pathway genes mediated systemic acquired resistance (SAR) and its master regulation via production of phased, trans-acting siRNAs against stem rot pathogen Macrophomina phaseolina in RIL population of jute (Corchorus capsularis). Physiol Mol Plant Pathol 87:76–85
Brooker NL, Kuzimichev Y, Lass J, Pavlis R (2007) Evaluation of coumarin derivatives as anti fungal agents against soil borne fungal pathogens. Commun Agric Appl Biol Sci 72:785–793
Cartieaux F, Contesto C, Gallou A, Desbrosses G, Kopka J, Taconnat L, Renou JP, Touraine B (2008) Simultaneous interaction of Arabidopsis thaliana with Bradyrhizobium sp. strain ors278 and Pseudomonas syringae pv. Tomato dc3000 leads to complex transcriptome changes. Mol Plant Microbe Interact 21:244–259
Chaverri P, Salgado C, Hirooka Y, Rossman AY, Samuels GJ (2011) Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Stud Mycol 68:57–78
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JL (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253
De Mooy CJ, Burke DW (1990) External infection mechanism of hypocotyls and cotyledons of cowpea seedling by Macrophomina phaseolina. Plant Dis 74:720
De Vleesschauwer D, Yang Y, Cruz CV, Hofte M (2010) Abscisic acid-induced resistance against the brown spot pathogen Cochlibolus miyabeanus in rice involves MAP kinase-mediated repression of ethylene signaling. Plant Physol 154:2036–2052
Deshpande AN, Murumkar DR (2008) Effect of tillage practice and nutrient source on microorganism counts in the rhizosphere of winter sorghum (Sorgum bicolor). Indian J Agron Sci 78:470–472
Dhingra OD, Sinclair JB (1973) Variation among isolates of Macrophomina phaseolina (Rhizoctonia bataticola) from different regions. J Phytopathol 76:200–204
Dhingra OD, Sinclair JB (1978) Biology and pathology of Macrophomina phaseolina. Imprensa Universitária, Universidade Federal de Viçosa, Viçosa
Fernandez RB, De Santiago A, Delgado SH, Perez NM (2006) Characterization of Mexican and non-Mexican isolates of Macrophomina phaseolina based on morphological characteristics, pathogenicity on bean seeds and endoglucanase gene. J Plant Pathol 88:1
Fey BJ, Parker JE (2000) interplay of signaling pathways in plant disease resistance. Trends Genet 16:449–455
Gill MJ, Mauch-Mani B, Jorda L, Vera P (2005) The Arabidopsis csb3 mutant reveals a regulatory link between salicylic acid- mediated disease resistance and the methyl-erythriol-4-phosphate pathway. Plant J 44:155–166
Glazebrooki J (2005) Contrasting mechanism of defense against biotropic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Hamer JE, Farral L, Orbach MJ, Valent B, Chumley FG (1989) Host species-specific conservation of a family of repeated DNA sequences in the genome of a fungal plant pathogen. Proc Natl Acad Sci U S A 86:9981–9985
Howard RJ (1997) Breaching the outer barriers: cuticle and cell wall penetration. In: Carroll GC, Tudzynski P (eds) Themycota. Plant relationships, vol 5, Part A. Springer, Berlin, pp 43–60
Ilyas MB, Sinclair J (1974) Effects of plant age upon development of necrosis and occurrence of intraxylem sclerotiain soybean infected with Macrophomina phaseolina. Phytopathology 64:156–157
Jana TK, Sharma TR, Prasad RD, Arora DK (2003) Molecular characterization of Macrophomina phaseolina and Fusarium species by using single primer RAPD technique. Microbiol Res 158:249–257
Jana T, Sharma TR, Singh NK (2005) SSR-based detection of genetic variability in the charcoal root rot pathogen Macrophomina phaseolina. Mycol Res 109(1):81–86
Johal GS, Huber DM (2009) Glyphosate effects on diseases of plants. Eur J Agron 31:144–152
Karunanithi K, Muthusamy M, Seetharaman K (1999) Cultural and pathogenic variability among the isolates of Macrophomina phaseolina causing root rot of sesame. Plant Dis 14:113–117
Kazan K, Manners JM (2009) Linking development to defense: auxin in plantpathogen interactions. Trends Plant Sci 14:373–382
Khan SN (2007) Macrophomina phaseolina as causal agent for charcoal rot of sunflower. Mycopathology 5:111–118
Khan MA, Gangopadhyay S (2008) Efficacy of Pseudomonas fluorescens in controlling root rot of chick pea caused by Macrophomina phaseolina. J Mycol Plant Pathol 38:580–587
Knox-Davies PS (1967) Mitosis and aneuploidy in the vegetative hyphae of Macrophomina phaesoli. Am J Bot 54:1290–1295
Kulkarni NB, Patil BC (1966) Taxonomy and discussion on the nomenclature of Macrophomina phaseoli (Maubl.) ashby and its isolates from India. Mycopathology 28:257–264
Kunwar IK, Singh T, Machado CC, Sinclair JB (1986) Histopathology of Soybean seed and seedling infection by Macrophomina phaseolina. Phytopathology 76:532–535
La Camera S, Balague C, Gobel C, Geoffroy P, Legrand M, Feussner I (2009) The Arabidopsis paratin-like protein 2(PLP2) plays an essential role in cell death execution and differentially affects biosynthesis of oxylipins and resistance to pathogens. Mol Plant Microbe Interact 22:469–481
Lee MW, Jelenska J, Greenberg JT (2008) Arabidopsis protein important for modulating defense response to Pseudomonas syringe HopW1-1. Plant J 54:452–465
Loganathan M, Sible GV, Maruthasalam S, Saravanakumar D, Raguchander T, Sivakumar R, Samiyappan R (2010) Trichoderma and chitin mixture based bioformulation for the management of head rot (Sclerotinia sclerotiorum (Lip.) deBary)-root knot (Meloidogyne incognita Kofoid and White) chitwood complex disease of cabbage. Arch Phytopathol Plant Proc 43:1011–1024
Mayek-Perez N, Gracia-Espiosa R, Lopez-Castaneda C, Acosta-Gallegis JA, Simpson J (2002) Water relations, histopathology and growth of common bean (Phaseolus vulgaris L.) during pathogenesis of Macrophomina phaseolina under drought stress. Physiol Mol Plant Pathol 60:185–195
Mengistu A, Ray JD, Smith JR, Paris RL (2007) Charcoal rot disease assessment of soybean genotypes using a colony forming unit index. Crop Sci 47:2453–2456
Mengistu A, Smith JR, Ray JD (2011) Seasonal progress of charcoal rot and its impact on soybean productivity. Plant Dis 95:1159–1166
Meyer WA, Sinclair JB, Khare MN (1974) Factors affecting charcoal rot of soybean seedlings. Phytopathology 64:845–849
Miao VP, Covertand SF, Vanetten HD (1991) Fungal gene for antibiotic resistance on a dispensable (‘B’) chromosome. Science 254:1773–1776
Mihail JD (1992) Macrophomina. In: Singleton LS, Mihail JD, Rush CM (eds) Methods for research on soil-borne phytopathogenic fungi. Am Phytopathol Soc Press, St. Paul, pp 134
Mihail JD, Taylor ST (1995) Interpreting variability among isolates of Macrophomina phaseolina in pathogenicity, pycnidium production and chlorate utilization. Can J Bot 73:1596–1603
Mondal SN, Hyakumachi M (1998) Carbon loss and germinability of chlamydospores of Fusarium solani f. sp. phaseoli after exposure to soil at different pH levels, temperatures and matric potentials. Phytopathology 88:148–155
Money NP, Frederick B, Henson JM (1998) Melanin synthesis is associated with changes in hyphopodial turgor, permeability, and wall rigidity in Gaeumannomycesg raminis var. graminis. Fungal Genet Biol 24:240–251
Olaya G, Abawi GS, Barnard J (1996) Influence of water potential on survival of sclerotia in soil and on colonization of bean stem segments by Macrophomina phaseolina. Plant Dis 80:1351–1354
Papavizas GC, Klag NG (1975) Isolation and quantitative determination of Macrophomina phaseolina from soil. Phytopathology 65:182–187
Patil VB, Kamble SS (2011) The influence of ultraviolet light on antagonistic activity of Trichoderma koningii against Macrophomina phaseolina causing charcoal rot of Charcoal rot on sunflower 5 sweet potato. Int J Acad Res 3:702–704
Pearson CAS, Schwenk FW, Crowe FJ, Kelly K (1984) Colonization of soybean roots by Macrophomina phaseolina. Plant Dis 68:1086–1088
Pearson CAS, Leslie JF, Schwenk FW (1987) Host preference correlated with chlorate resistance in Macrophomina phaseolina. Plant Dis 71:828–831
Pecina V, Alvarado MJ, Alanis HW, Almaraz RT, Vandemark GJ (2000) Detection of double-stranded RNA in Macrophomina phaseolina. Mycologia 92:900–907
Pedgaonkar SM, Mayee CD (1990) Stalk water potential in relation to charcoal rot of Sorghum. Indian Phytopathol 43:192–196
Perez-Brandán C, Arzeno JL, Huidobro J, Grümberg B, Conforto C, Hilton S, Bending GD, Meriles JM, Vargas-Gil S (2012) Long-term effect of tillage systems on soil microbiological, chemical and physical parameters and the incidence of charcoal rot by Macrophomina phaseolina (Tassi)Goid in soybean. Crop Prot 40:73–82
Powell NT (1971) Interactions between nematodes and fungi in disease complexes. Annu Rev Phytopathol 9:253–274
Punithalingam E (1983) The nuclei of Macrophomina phaseolina (Tassi) Goid. Nova Hedwig 38:339–367
Purkayastha S, Kaur B, Dilbaghi N, Chaudhury A (2006) Characterization of Macrophomina phaseolina, the charcoal rot pathogen of cluster bean, using conventional techniques and PCRbased molecular markers. Plant Pathol 55:106–116
Ramezani M, Shier WT, Abbas HK, Tonos JL, Baird RE, Sciumbato GL (2007) Soybean charcoal rot disease fungus Macrophomina phaseolina in Mississippi produces the phytotoxin (–)-botryodiplodin but no detectable phaseolinone. J Nat Prod 70:128–129
Reuveni R, Nachmias A, Krikun J (1983) The role of seedborne inoculum on the development of Macrophomina phaseolina on melon. Plant Dis 67:280–281
Ross JP (1965) Predispositions of soybeans to Fusarium wilt by Heterodera glycines and Meloidogyne incognita. Phytopathology 55:361–364
Sels J, Mathys J, De Coninck BM, Cammue BP, De Bolle MF (2008) Plant pathogenesis related (PR) proteins: a focus on PR peptide. Plant Physiology and Biochemistry 46(11):941–950
Shahda WT, Tarabeih AM, Michail SH, Hemeda AAH (1991) Fungi associated with sunflower seeds in Egypt with reference to chemical control measures. J King Saud Univ Agric Sci 3:287–293
Short GE, Wyllie TD, Ammon VD (1978) Quantitative enumeration of Macrophomina phaseolina in soybean tissues. Phytopathology 68:736–741
Sinclair JB (1982) Compendium of Soybean disease. 2nd ed. by American Phytopathology Society, St. Paul, Minnesota, USA.
Sinclair JB (1989) Vegetable oil thermotherapy for soybean seeds. In: Raychaudhri SP, Verma JP (eds) Review of tropical plant pathology: diseases of fiber and oilseed crops, vol 5. Today and Tomorrow’s Printers and Publishers, New Delhi
Sinclair JB, Backman PA (1989) In: Sinclair JB, Backman PA (eds) Compendium of soybean diseases, 3rd edn. APS Press, St. Paul, p 106, Am Phytopathol Soc, EE. UU
Smith GS, Carvil ON (1997) Field screening of commercial and experimental soybean cultivars for their reaction to Macrophomina phaseolina. Plant Dis 81:363–368
Smith GS, Wyllie TD (1999) Charcoal rot. In: Hartman GL, Sinclair JB, Rupe JC (eds) Compendium of soybean disease, 4th edn. American Phytopathological Society, St. Paul, pp 29–31
Solomon PS, Wilson TJG, Rybak K, Parker K, Lowe RGT, Oliver RP (2006) Structural characterization of the interaction between Triticum aestivum and the dothideomycete pathogen Stagono sporanodorum. Eur J Plant Pathol 114:275–282
Suriandraselvan M, Salalrajan F, Aiyyanathan KEA (2006) Relationship between morphological variations and virulence in the isolates of Macrophomina phaseolina causing charcoal rot of sunflower. Madras Agric J 93(1–6):63–67.
Sundaravadana S (2002) Management of blackgram (Vigna mungo (L.) Hepper) root rot Macrophomina phaseolina (Tassi) Goid with bioagents and nutrients. M. Sc. (Ag.) Thesis, Tamil Nadu Agriculture University, Coimbatore
Tesso TT, Claflin LE, Tuinstra MR (2005) Analyses of stalk rot resistance and genetic diversity among drought tolerant sorghum genotypes. Crop Sci 45:645–652
Thilgavathi R, Saravanakumar D, Ragupathy N, Samiyappan R (2007) Integration of biocontrol agents for the management of dry root rot (Macrophomina phaseolina) disease in greengram. Phytopathol Mediterr 46:157–167
Van Bruggen AHC, Semenov AM, Van Diepeningen AD, de Vos OJ, Blok WJ (2006) Relation between soil health, wave-like fluctuations in microbial populations, and soil-borne plant disease management. Eur J Plant Pathol 115:105–122
Vargas Gil S, Pedelini R, Oddino C, Zuza M, Marinelli A, March GJ (2008) The role of potential biocontrol agents in the management of peanut root rot in Argentina. J Plant Pathol 90:35–41
Wang D, Pajerowska-Mukhtar K, Culler AH, Dong XN (2007) Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol 17:1784–1790
Wantanabe T, Smith RS, Snyder WC (1970) Population of Macrophomina phaseoli [sic] in soil as affected by fumigation and cropping. Phytopathology 60:1717–1719
Wiese J, Kranz T, Schubert S (2004) Induction of pathogen resistance in barley by abiotic stress. Plant Biol 6:529–536
Wrather JA, Anderson TR, Arsyad DM, Gai J, Ploper LD, Porta-Puglia A, Ram HH, Yorinori JT (1997) Soybean disease loss estimates for the top 10 soybean producing countries in 1994. Plant Dis 81:107–110
Wrather JA, Anderson TR, Arsyad DM, Tan Y, Ploper LD, Porta-Puglia A, Ram HH, Yorinori JT (2001) Soybean disease loss estimates for the top 10 soybean producing countries in 1998. Can J Plant Pathol 23:115–121
Wyllie TD (1988) Charcoal rot of soybeans-current status. In: Wyllie TD, Scott DH (eds) Soybean diseases of the North Central Region. APS Press, St. Paul, pp 106–113
Wyllie TD (1989) Charcoal rot. In: Sinclair JB, Backman PA (eds) Compendium of soybean diseases, 3rd edn. APS Press, St. Paul, p 30
Xiaojian L, Liu LI, Baidnun O, Derong Z (1988) Geographical distribution of sunflower diseases in China. In: Proc 12th Int Sunfl Conf Novi Sad, Serbia, pp 16–20
Zander M, La Camera S, Lamorte O, Metraux JP, Gatz C (2010) Arabdiopsis thaliana class II TGA transcription factors are essential activator of jasmonic acid/ethylene-induced defense response. Plant J 61:200–210
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Vibha (2016). Macrophomina phaseolina: The Most Destructive Soybean Fungal Pathogen of Global Concern. In: Kumar, P., Gupta, V., Tiwari, A., Kamle, M. (eds) Current Trends in Plant Disease Diagnostics and Management Practices. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-319-27312-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-27312-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27310-5
Online ISBN: 978-3-319-27312-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)